Abstract
Researchers may optimize smoking treatment by addressing three research topics that have been relatively neglected. First, researchers have neglected to intensively explore how counseling contents affect smoking cessation success. Worldwide, millions of smokers are exposed to different smoking cessation contents and messages, yet existing research evidence does not permit strong inference about the value of particular counseling contents or strategies. Research in this area could enhance smoking outcomes and yield new insights into smoking motivation. Second, researchers have focused great attention on inducing smokers to make quit attempts when they contact healthcare systems; the success of such efforts may have plateaued. Also, the vast majority of quit attempts are self-quit attempts, largely unsuccessful, that occur outside such contacts. Researchers should explore strategies for using healthcare systems as conduits for digital- and other population-based interventions independent of healthcare visits. Such resources should be used to graft timely access to evidence-based intervention onto self-quitting, yielding evidence-based, patient-managed quit attempts. Third, most smoking treatments are assembled via selection of components based on informal synthesis of empirical and impressionistic evidence and are evaluated as a package. However, recent factorial experiments show that components of smoking treatments often interact meaningfully; for example, some components may interfere with the effectiveness of other components. Many extant treatments likely comprise suboptimal sets of components; future treatment development should routinely use factorial experiments to permit the assembly of components that yield additive or synergistic effects.
Research in the above three areas should significantly advance our understanding of tobacco use and its treatment.
Implications: A lack of relevant research, and the likely prospect of significant clinical and public health benefit, underscore the importance of performing research on three topics related to smoking intervention: (1) researchers need to identify which contents of smoking counseling are effective; (2) researchers need to devise innovative strategies that use healthcare systems as conduits of smoking treatment delivery outside of clinical contacts; and (3) researchers need to use factorial designs to guide their development of smoking treatments. Research on these topics should yield complementary evidence that guides the development of more effective smoking treatments.
Three Things
This paper, based on the 2016 Ove Ferno Award talk given at the Society for Research on Nicotine and Tobacco Annual Meeting, will highlight three areas of research that I believe have been relatively neglected and that deserve more research attention: counseling content, the timing and routes to treatment access, and the importance of factorial designs as a means of developing treatments and understanding their effects. I believe these topics present notable opportunities to address tobacco use more effectively. My identification of these research needs is not unique, but I wish to encourage researchers to pay greater attention to them.
I discuss these topics together in this paper because I believe that conducting research on this set of knowledge gaps will yield complementary information that can be integrated to enhance net benefit. For example, improving our treatment evaluation methods via factorial designs should yield greater insight into the nature of effective counseling contents, and the latter may influence the selection of the contents of digital interventions that can be disseminated broadly via healthcare. And, coming full circle, the evaluation of the digital interventions will also be enhanced by more efficient research methods.
Counseling Content
We have been investigating counseling for smoking cessation for over 50 years. We should have, by now, developed a good sense of what sorts of information we need to provide smokers to help them quit. In fact, I believe that many in the field of tobacco intervention believe that we do, indeed, possess such knowledge. This sense probably arises from multiple sources. One source is the considerable evidence that counseling works1,2; it is appealing to believe that if counseling works, the content must have some specific efficacy. However, it may be that almost any counseling content would be similarly effective because it is the nonspecific or almost universal features of the counseling contact that are of chief importance.3
Some research does suggest that particular counseling contents are effective. A PHS Guideline meta-analysis performed in 2000 evaluated the relations of multiple different therapeutic strategies or counseling types and found that some were significantly effective and others were not (the results of this meta-analysis were included in the 2008 Clinical Practice Guideline, with minor modification1). In particular, this meta-analysis identified “problem-solving counseling” (or “skill training”) and “intra-treatment support” as being particularly effective.
Unfortunately, as noted in the Guideline itself, this evidence is far from definitive. For instance, it is based on descriptions of the contents of counseling as they were found in the original articles. These descriptions were often brief and unclear. Second, the different types of contents were analytically contrasted with no-contact control conditions; the data do not really permit us to say whether the “active” contents differed from one another. Third, the types of contents were correlated with the number of studies in which they were used and with treatment intensity, and there was considerable overlap among the contents (multiple contents were often used in the same study). Thus, it is difficult to impute associations with the counseling contents per se. There are other limitations; for example, many of the studies are now dated (the great majority over 20–30 years old). Clearly, secular changes (in the population of smokers, exposure to some treatment contents) may have affected effectiveness.
Another factor that may create the illusion of knowledge in this area is that the great majority of smoking cessation trials published today1,4–6 use the same sort of skill-focused counseling (ie, encouragement to avoid smoking cues, suggestions for coping with urges and stressors, along with motivation and support). It is tempting to believe the almost universal adoption of this counseling strategy arises from a sound evidence base. But, of course, tradition and routine often powerfully affect behavior with little evidentiary basis.
There is also the weight of evidence that arises from research on the causal influences on relapse. Through the work of Saul Shiffman and others7–12 we have strong evidence that exposure to smoking cues, drinking, negative affect, and stressors increases the risk for lapsing and relapse. This certainly suggests that training aimed at such risk factors would confer benefit. In addition, West, Michie, and colleagues13 have produced suggestive evidence that links counselors’ use of skill-based intervention strategies with superior outcomes among their patients. For instance, they coded behavior change techniques that were described in clinic-based smoking intervention manuals and found that counselors who used manuals comprising techniques consistent with skill training (eg, praising abstinence, advising a change in routines, advising and assisting with urge coping strategies) tended to produce superior smoking outcomes in their patients. It is certainly encouraging to see an association between such techniques and outcomes, but as the authors note, such data are not conclusive. We do not actually know that the manualized contents were meaningfully related to actual technique use. Moreover, is it possible that clinics that do not mention such basic techniques as coping skill training in their cessation manuals do not take smoking treatment seriously in other ways.
The only route to strong inference that particular counseling contents are especially beneficial is via randomized controlled trials or factorial experiments where counseling content is experimentally manipulated and where other elements of treatment (duration, training, and timing of therapeutic contact) are equated. What do such studies show us? Not much.
For instance, the Lancaster and Stead updated Cochrane Report5 on smoking cessation counseling identified only three such studies. These studies were not combined in a meta-analytic framework because they contrasted different types of content: (1) skill training-relapse prevention content versus information on smoking and health14; (2) motivational interviewing versus health information15; and (3) counseling consistent with the 2008 PHS Guideline recommendation (ie, skill counseling with intra-treatment support) versus counseling that focused on support and encouragement to use cessation medication adherently (this control counseling contained no skill training6). Two of the studies’6,14 RR’s were very close to “1,” indicating no trend toward a significant effect. The third study15 found a significant effect, but the effect was a decrease in abstinence produced by the putative “active” counseling content (motivational interviewing).
The McCarthy study6 may be particularly important because it suggests that the counseling contents identified by the 2008 PHS Guideline were no more effective than a supportive informational control condition. Moreover, the McCarthy et al. study examined the effects of the active counseling on the variables that were thought to mediate its effects (its mechanisms). Participants (N = 403) in this research were randomly assigned to two factors in a 2 × 2 factorial design: active versus placebo medication (bupropion vs. placebo) and active counseling versus control counseling. The active counseling involved eight 10-min sessions focused on sustaining motivation to quit, problem-solving, coping with stress and urges, and provision of intra-treatment support. The control counseling involved the same number of sessions, which were somewhat shorter, and focused on medication management and provision of encouragement and support.
At the end of counseling treatment (EOT: postquit day 28), the 7-day, biochemically confirmed, point-prevalence abstinence rates were 29% versus 26% for the active and control conditions, respectively; a nonsignificant difference.
This study naturally raises questions about why the supposedly effective counseling content was disappointingly ineffective. One possible explanation is that the counseling was poorly executed; that is, it did not affect the key change targets or mechanisms that are thought to mediate improved smoking outcomes. Another explanation might be that it did produce such effects, but, in fact, such effects were not sufficiently determinant of smoking outcomes so as to effect significant long-term change (abstinence). The first explanation is a quality hypothesis; the second a “model of counseling” hypothesis (that counseling is based on an inadequate model of behavior change).
The investigators used 4–7 daily, randomly delivered ecological momentary assessments plus an evening assessment, to gather data on variables thought to mediate skill training benefit: for example, reduction of exposure to smoking triggers (smoking cues, stressors), coping execution, temptation coping, stressor coping, motivation and self-efficacy, withdrawal, and the occurrence of smoking lapses and events surrounding the lapses. Multilevel models calculated the mean level and linear trajectory of ecological momentary assessment data from the quit day to 28 days postquit for those in the two counseling conditions.
Results showed that active versus control counseling produced: (1) reduced likelihood, on average, of being exposed to cigarettes, (2) steeper declines in spending time where cigarettes were available, (3) increased likelihood, on average, that participants would use coping responses in response to urges or stressors, (4) higher self-efficacy (confidence) levels, on average, regarding ability to quit, (5) faster declines in the perceived difficulty of quitting, and (6) greater declines in post-lapse feelings of confidence in quitting across time. Thus, there was evidence that counseling was indeed hitting some of its key, theoretically implicated targets: it was doing much of what it was supposed to do (at least as indicated by real-time self-report). This provides some evidence that it had been competently delivered. But, of course, it did not work, in the sense that its impact on abstinence was both small and nonsignificant.
McCarthy and colleagues then conducted mediational analyses that related the ecological momentary assessment variables gathered (mean levels or trajectories) to the end-of-treatment outcomes. Some putative mediators were significantly related to EOT abstinence: decreased abstinence was directly related to easy access to cigarettes, being bothered by withdrawal, and feeling like giving up after lapsing. Increased abstinence was related to perceived social support and maintaining a higher average level of confidence, motivation, and willingness to work at quitting.
Thus, active counseling affected some of its intended targets, and variables were identified that were strongly related to outcomes. Unfortunately, there was only modest overlap in these two domains; both suggested the importance of exposure to smoking opportunities and self-efficacy or confidence in ability to quit. However, while decreased demoralization after lapses predicted greater likelihood of abstinence, “active” counseling actually accelerated such demoralization. Out of all the potential mediators tested, only two showed significant effects in tests of mediation: confidence in quitting and perceived difficulty in quitting. The problem with these variables is that we do not know whether they really causally increased quitting success versus merely reflected increased quitting success, in a way that was related to counseling. Variables that are typically highly determinant of smoking outcomes, such as coping execution, withdrawal severity, and avoidance of smoking cues, did not, in fact, significantly mediate outcomes. In essence: What mattered, we didn’t change; what we changed, didn’t matter (at least in terms of abstinence). This disjunction between what counseling treatment affects, and what affects abstinence, warrants attention; we essentially replicated this disconnect in a second, more recent study.16 It is worth considering how different the results have been for research on the mediation of pharmacotherapy effects. Studies have consistently shown that pharmacotherapies reliably and meaningfully reduce major determinants of cessation success: that is, craving and withdrawal symptoms.17,18 That is, active cessation medication significantly changes factors that matter.
These findings suggest that our model of counseling may need to be reconsidered. Counseling changes many of its intended targets, but this doesn’t appear to be enough to move the abstinence needle. Of course, it may be that the standard model of change may, in fact, be valid, but our counseling interventions do not create big enough changes in the targeted variables to affect abstinence. However, at present, there is very little evidence that strongly and directly supports such a model. There are, in fact, precious few, well-powered studies that systematically manipulate counseling content (controlling intensity) and that perform mediation research with the targets of counseling (ie, the putative mechanisms) measured in real time, in smokers’ real-world environments. As noted earlier, the Cochrane Report5 identified only three studies that experimentally manipulated counseling content per se (one being McCarthy et al.).
Perhaps more disturbing than our uncertainty regarding counseling mechanisms is the fact that counseling effects on abstinence tend to be so modest. Consider the verdict of the Hajek et al. Cochrane Report19 on behavioral relapse prevention interventions: “At the moment, there is insufficient evidence to support the use of any specific behavioural intervention to help smokers who have successfully quit for a short time to avoid relapse.” (p. 2). Or, consider another recent meta-analysis of the effects of increased counseling intensity when counseling was used as an adjuvant to pharmacotherapy.20 This Cochrane Report examined 38 studies where the active arms of the studies received meaningfully greater levels of counseling intervention than the control arms; the difference produced an estimated risk ratio (RR) of 1.16, with 95% confidence interval (CI) 1.09 to 1.24. This effect fell to nonsignificance if the six studies that did not use biochemical validation were removed from the analysis (RR 1.09, 95% CI 0.99 to 1.21). Of course, if counseling’s net effects are so modest, this unavoidably constrains the potential magnitude of the effects of content per se.
Why does it matter? Why should we invest more time and effort into exploring counseling, especially its content? First, counseling is one of the two major modes of clinical smoking intervention recommended in clinical guidelines published around the world. As such, hundreds of thousands of smokers receive counseling interventions each year, via clinic visits, quitlines, hospital stays, and so on. And, even more are exposed to digital interventions that are based upon the content of counseling interventions. We owe it to smokers to optimize this opportunity.
Right now the impact of smoking counseling is not impressive. One reason for this may be that we don’t know how it works and this thwarts cumulative progress. In the field of counseling in general (beyond smoking counseling) there is great debate about whether the counseling techniques have specific efficacies: that is, whether they work as intended. Considerable evidence shows that a good part of counseling effectiveness is due to nonspecific effects such as therapeutic alliance,3,21–23 which appears to be driven largely by counselor and client characteristics that are fairly intrinsic (eg, personality style) and not easily taught, and by almost universal features of counseling (client perception of teamwork).24–27 Thus, there is a credible alternative to the notion that the specific type of counseling content matters. It seems highly important to determine if content matters and what that content is—otherwise, we may have to abandon the notion of achieving cumulative progress in advancing smoking outcomes, at least via counseling.
Research Opportunities
One could easily say that counseling content has become passe’; that digital health is the future. But, we don’t really know what content works there either, and it tends to ape the content of counseling interventions. Thus, despite the fact that digital technologies offer unique possibilities for innovative real-time intervention tactics, much of the content and approaches deployed on these platforms is highly conventional (see The Evolution of Tobacco Treatment Inside and Outside of Healthcare section). Also, counseling research can serve as a valuable laboratory for testing our theories about the causes of tobacco use and dependence. The field has learned much from examining naturally unfolding relations between stimuli and symptoms on the one hand, and lapses and relapse on the other hand.7–12 While such findings are highly valuable, it is important to complement such findings with results from studies where critical mechanisms are experimentally manipulated via behavioral intervention or counseling: for example, where coping responses are increased, where exposure to smoking cues is reduced, and where conditioned responses are extinguished. Such experimental evidence could strengthen inferences about the determinants of smoking motivation and dependence.
Against this backdrop of disappointing findings regarding counseling research, there are some very promising signs of progress and further research along these lines should be encouraged. First, there is an upsurge in laboratory research that is evaluating specific intervention strategies and their mechanisms under conditions of high internal validity.28,29 In addition, clinical trials are also evaluating multiple novel approaches to behavioral and counseling intervention such as withdrawal symptom extinction and regulation strategies, and innovative strategies to mitigate distress.30–32 Further research is needed to translate the most effective of these strategies into highly disseminable interventions. In other promising efforts, Robert West and his colleagues are using innovative, research approaches to: explore counseling content effectiveness in real-world contexts,12 identify effective content for digital interventions per se,33 and harness machine learning to synthesize existing data in order to tailor smoking intervention strategies across time and person.34,35 These and new developments in analytic approaches to mediational modeling should yield new insights into how treatments work and how they should be used.17,36,37 More powerful mediational modeling should complement the use of efficient factorial designs38; such designs permit the efficient evaluation of multiple counseling contents so that the main and interactive effects of the contents can be accurately traced to clinically significant endpoints.
The Evolution of Tobacco Treatment Inside and Outside of Healthcare
It is clear that a clinic visit is a good time to intervene with smoking. The confluence of smoker availability, the health relevance of smoking, treatment resource availability, and presence of a trusted health advisor certainly make this a teachable moment. This, no doubt, accounts for the tremendous effort that has been invested in research in healthcare settings to identify strategies that improve the identification of smokers, the offer of advice to quit, the offer of assistance, the delivery of assistance, and the arrangement of follow-up support.36 This effort has been somewhat successful as investigators have creatively and tirelessly striven to develop new methods for increasing the involvement of healthcare clinicians and patients in smoking treatment at clinic and hospital visits. There is evidence that through training, support, feedback, and engineering the electronic health record (EHR) and referral systems, we have increased performance along the smoking care continuum. For instance, there is clear evidence that we have increased the rates at which smokers are identified and advised to quit in healthcare settings.39–41 The bottom line issue, though, is the extent to which smokers end up being inducted into treatment at such visits.
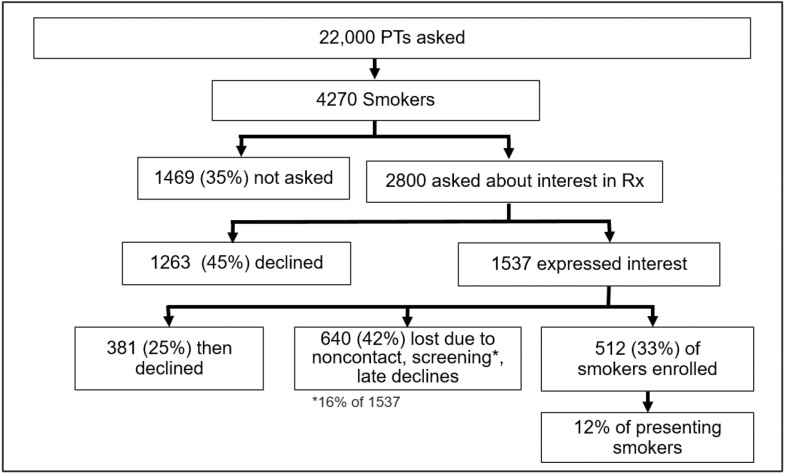
A recent example of what might be a “model system” may illustrate the potential yield of such systems. We have developed a smoking treatment support system for primary care clinics in which the EHR guides the medical assistant or roomer to ask about smoking status and offer assistance, requires recording of these activities via obligatory fields, and then offers 1-click referral (or close to it) to a treatment program. This strategy was used in clinics and healthcare systems that were receptive to it, and it was supported by ongoing training, and performance feedback. Moreover, it referred smokers to a research treatment program that had both smoking reduction and cessation options, that took place in the smoker’s primary care clinic, and that offered free medication and that compensated them for their time. As Figure 1 shows, despite all these advantages, the program only enrolled about 12% of smokers into treatment.42 The major cause of treatment non-entry was treatment refusal at the time of the visit, not because of treatment screening criteria (Figure 1).
Figure 1.
CONSORT for patient flow in EHR recruitment study.
The data depicted in Figure 1 arose from an effort to refer smokers to a research intervention; perhaps smokers would be more willing to accept referral to a low-intensity real-world intervention that demands no face-to-face visits or research assessment? Adsit et al.39 used an EHR guided, primary care smoker identification system similar to that used by Piper et al.42 (whose results are depicted in Figure 1), but this system involved a 1-click referral option to the Wisconsin Tobacco Quitline, which then made proactive calls to the patient. This system also provided closed-loop feedback from the quitline to the primary care clinic and clinician. While 14% of smokers accepted such referral, only 5% were eventually engaged in quitline treatment, and then, of course, only a minority of the enrolled smokers would eventually quit smoking long term.
In sum, it is tough to get smokers into evidence-based smoking treatment in the context of a primary care visit, even under highly favorable circumstances, and it is the case that smokers may make only 1–2 visits to primary care a year. In other words, the opportunities are few (per smoker) and the glean rate, low. It is possible that somewhat higher rates could be obtained via strategies such as opt-out approaches, which arrange treatment for all smokers unless the smoker actively declines it43; it is unclear, at present, whether opt-out strategies will recruit individuals who will actually engage in treatment and benefit from it.
Against this backdrop, consider that about 28 million smokers in the United States want to quit smoking, and about half or slightly more, make a quit attempt each year.44 Also, consider a recent study by Hughes45 in which 152 smokers were recruited who expressed interest in quitting. Smokers were tracked via nightly phone calls over 3 months. Such calls revealed that quitting motivation was ephemeral, often changing on a daily basis, and that 72% of quit attempts were not preceded by a well-articulated or planned decision to quit46; they were fairly spontaneous and often brief.
A recent study of self-quitting47 shows that we have very likely been underestimating the number of quit attempts that smokers make, on average, prior to their quitting successfully. While prior estimates have ranged from about 5–15 attempts or so, the analyses of Chaiton et al.,47 derived from longitudinal tracking of 1277 smokers over 3 years, showed that a more accurate estimate might be close to 30 or more serious attempts (made with intention to quit permanently). This study also showed that, on average, smokers make about 1 quit attempt/year47 and that smokers vary greatly in the number of quit attempts that ultimately lead to success. This variability is consistent with data from genetics research showing that smokers with high genetic loading for dependence (eg, CHRNA5) quit considerably later in life than do other smokers.48,49
In sum, while quit attempts occur frequently over the course of a smoker’s career, they tend to occur at a slow rate, meaning that it is unlikely that the motivation to make such attempts is peaking at the time of aperiodic, episodic healthcare visits. Further, each quit attempt is a crucial resource; each failed attempt translates on average into another year of smoking along with its ensuing harms. And, while many smokers are able to quit on their own with little assistance, a large proportion of them are unsuccessful a daunting number of times.
Therefore, while it is of great importance to induce smokers to make quit attempts when they make clinic visits, we have failed to capitalize on the opportunity afforded by quit attempts that occur outside of healthcare visits. In essence, we have tried to bring the smoker to the treatment versus bringing the treatment to the smoker. A prime goal of tobacco control should be to make effective treatment highly available to smokers whenever they are ready to make a quit attempt. I believe that healthcare systems and other stakeholders should focus on being conduits of treatment versus providers or deliverers of treatment.
What might healthcare systems do to make effective treatment more available and appealing to smokers when they want it and need it? They might pursue such steps as ensuring that smokers have rapid access to cessation pharmacotherapy, including the provision of “on-hand” pharmacotherapy—just like healthcare providers encourage on-hand epi-pens to those at risk for anaphylaxis. They might assume greater responsibility for ensuring their patients have rapid access to multiple forms of eHealth and mHealth intervention resources (apps, websites, texting programs) and provide encouragement to use such resources. These might be made available via EHR/smoker registry services that compile lists of smokers in healthcare systems, and then target smoking treatment outreach via resources such as My Chart in EPIC and healthcare system care managers. Thus, a key research activity of healthcare systems should be the analysis of available digital and “at a distance” interventions so as to identify those whose contents or effects are relatively promising. Also, healthcare systems might provide meaningful incentives to use such additional quitting/reduction aids—after all, one vital goal now becomes not just getting smokers to make quit attempts when they make a visit to the clinic, but increasing the rate of quit attempts outside of such visits. Certainly, incentive interventions have strong track records for affecting quit attempts and outcomes.50–52 Finally, it is important to note that the goal here is different from merely promoting self-quitting; this is an attempt to transform self-quitting into self-directed, aided quitting.53
One concern about the above approach is that many digital interventions have not been validated. The reason for healthcare systems to be key conduits and access points for such interventions is to leverage their perceived expertise and goodwill so as to “push” certain treatment options. Healthcare systems and clinicians will justifiably feel a responsibility to use this influence judiciously. In this regard, researchers and other stakeholders might first acknowledge that researchers and clinicians are no longer gatekeepers or controlling, access points for smoking treatment resources. There are now countless apps and other digital aids for smoking and they will be used by many thousands of smokers with or without our blessing. Much of the change in smoking “treatment” is now bottom-up versus “top-down”; entrepreneurs, smokers and coders are driving a good deal of such change rather than scientists. Fortunately, naturally occurring feedback processes may, over time, encourage the use and development of better and better digital aids—as natural feedback loops occurring via user groups, app forum groups, and economic success, select the best among the digital resources. As Ridley notes in his book The Evolution of Everything,54 such evolution is ubiquitous in language, culture, technological development, and produces change that is gradual, undirected, mutational, inexorable, combinatorial, selective (based on trial and error), and in some sense progressive. It follows “rules” or reflects influences that are enforced locally and repeatedly across a population. However, healthcare systems and other relevant entities (eg, NIH Institutes) still have a vital role to play which they can do in a scientifically grounded manner.
Research and Policy Opportunities
As noted, smokers will obtain and use countless new digital smoking intervention resources with or without researchers’ or clinicians’ guidance or imprimatur. But, researchers can still make huge contributions to the evolving process of smoking treatment by supplying healthcare systems and other providers with data that will help them encourage the use of especially promising resources. Certainly researchers cannot validate the innumerable apps and resources that will be made available to smokers. However, they can take advantage of the naturally occurring, evolutionary sifting and winnowing process that selects some resources as being highly used and valued; researchers should then intensively evaluate those. Such a research plan would focus on tracking the real-world use and naturally occurring outcomes of the various digital resources, and publicizing the results. Use rates per se may provide highly useful information about the engagement potential of a resource, which may communicate a lot about potential population impact. Further, data on use might be linked with data from various rating systems33 and with user experience studies, in order to identify resource features that are particularly related to use. Clinical trials of the most widely used resources would then be feasible and vital.
Researchers should also create new, resources (blogs, websites) for tobacco users who are using, or are interested in, digital interventions for smoking. There are already resources that provide information on, and evaluation of, apps and other digital resources (eg, Freshapps, Appvee, and so on), but researchers can create, modify, and curate resources that provide additional information, including: for example, use rates or popularity, extant research on effectiveness, whether the digital intervention uses evidence-based strategies, and so on. Such resources might hasten the evolution of new technologies and approaches; such data would be ripe of synthesis via artificial intelligence strategies.35
Researchers should also perform more research on the needs and wants of “self-quitters”; what are the preferences of such individuals, what are they looking for? How do their recent use experiences inform the development of new resources or access/delivery methods? Laboratory and clinical research could focus on cognitive-neuroscience strategies that take advantage of technological resources. For instance, strategies that use the information processing power of mobile devices to: forecast for the smoker the likely outcome of choices that s/he might make in response to a given situation; leap the delay-of-gratification deficits of smokers by delivering alternate reinforcers in real time (coupons for products) contingent upon adaptive choices and actions; or that identify distraction strategies that mHealth devices could generate to interrupt urges. Through such research we could develop digital content that takes advantage of the special functionalities of digital resources, rather than relying upon the content of counseling interventions of modest impact/validity.
All of these research directions would be part of the effort to encourage self-quitters to become hybrid quitters; those making self-directed, aided quit attempts.
Nothing about the above discussion suggests that we should abandon the effort to develop and deliver highly effective interventions in the healthcare setting. After all, recent data47 suggest that there are smokers who face a daunting task of quitting without relatively intense support. Steps such as those taken by Rigotti and her colleagues55 to extend the reach of treatment beyond an index healthcare contact constitute a promising step in this direction.
Abandoning Intuitive Treatments
Almost all of our current smoking treatments, in both the research and clinical contexts, have been developed via suboptimal research strategies. In the past, we have developed treatments informally; by unsystematically selecting features or elements (ie, intervention components) from among those that had previously been used in interventions perceived to be successful. Such evidence may derive from syntheses of meta-analyses (Cochrane/PHS), individual studies, personal use, and so on. We then adopt those elements whole cloth, or cobble them together based upon “local” considerations (eg, the resources, time, and personnel available), personal preference, or hunch. Whatever the influences, even if we adopt a treatment exactly as used in a clinical trial, it is highly unlikely that its many elements were originally selected based upon careful analyses of their main and interactive effects.
The Occult Complexity of Treatments
Each treatment comprises numerous potentially modifiable elements. These include the type of intervention component used (eg, skill training, intra-treatment supportive content, distress tolerance training, contingency contracting, or incentive manipulations), and also include the numbers and lengths of treatment contacts, the delivery routes (face-to-face, phone), the timing of contacts (not only intersession interval, but also timing relative to the quit day: for example, motivational, precessation, cessation, and maintenance period interventions56), and the use and types of noncounseling adjuvants, including medication. If the decision is made to use medication, the investigator is faced with questions such as the type(s) of medication to use, whether combination medication is to be used, whether a precessation medication exposure is used, what type of medication adherence adjuvant to use, how long medication is used, and whether a medication taper is used.56 Thus, treatment development requires multiple decisions. These decisions may be made rationally and with due deliberation, or they may be made intuitively, with little consideration. How one approaches the issue of treatment engineering presumably depends, in part, on the perceived consequences of such decisions. In essence, does it matter?
One might adopt the view that “more is better,” and that adding additional treatment elements will only make a treatment more effective (but perhaps not more cost-effective). This view may be based on an implicit underlying model that holds that treatment components exert effects that are additive and that do not meaningfully interact with one another (unless it is to produce multiplicative increases in benefit: synergy). This view may also be based upon the notion that components exert effects via different mechanisms; thus, there need be no concern about a “limited capacity” mechanism that would constrain additive benefit because of ceiling effects. However, because relatively discrete intervention components have so rarely been systematically evaluated in factorial experiments, we have little idea of whether the above assumptions are valid.
My colleagues and I have recently conducted a handful of factorial experiments that systematically evaluate multiple smoking treatment intervention components.57–60 For instance, Cook et al. used a full factorial design to evaluate different intervention components in smokers who were in the Motivation phase of smoking treatment57: that is, who were not willing to make a quit attempt, but were willing to try to reduce their smoking. The chief outcomes were smoking abstinence and amount of smoking reduction. The following four factors were evaluated: smoking reduction counseling versus no reduction counseling, motivational interviewing versus no motivational interviewing, nicotine gum versus no nicotine gum, and nicotine patch versus no nicotine patch. Thus, a total of 517 smokers were randomly assigned to one of 16 treatment conditions that reflected every possible combination of the four factors (see Table 1).
Table 1.
The 16 Treatment Conditions
| Conditions | Nicotine patch | Nicotine gum | Behavioral reduction counseling | Motivational interviewing (MI) strategies |
|---|---|---|---|---|
| 1 | Patch | Gum | Reduction | MI |
| 2 | Patch | Gum | Reduction | No MI |
| 3 | Patch | Gum | No reduction | MI |
| 4 | Patch | Gum | No reduction | No MI |
| 5 | Patch | None | Reduction | MI |
| 6 | Patch | None | Reduction | No MI |
| 7 | Patch | None | No reduction | MI |
| 8 | Patch | None | No reduction | No MI |
| 9 | None | Gum | Reduction | MI |
| 10 | None | Gum | Reduction | No MI |
| 11 | None | Gum | No reduction | MI |
| 12 | None | Gum | No reduction | No MI |
| 13 | None | None | Reduction | MI |
| 14 | None | None | Reduction | No MI |
| 15 | None | None | No reduction | MI |
| 16 | None | None | No reduction | No MI |
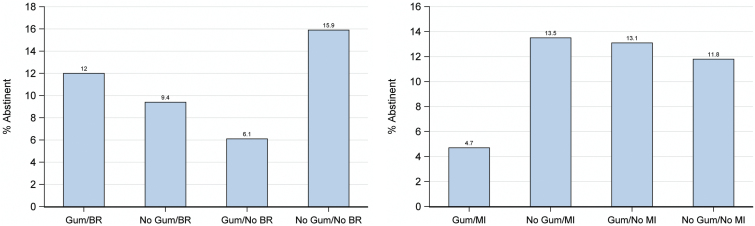
Results showed that the intervention components yielded interaction effects, but no main effects. For instance, in two 2-way interactions, the nicotine gum factor interacted with both the behavioral reduction counseling factor and the motivational interviewing factor in affecting abstinence (see Figure 2). As Figure 2a shows, adding gum to behavioral reduction counseling produced higher abstinence rates at 6-month follow-up than did either intervention by itself, suggesting additive effects, but together the two components still produced lower abstinence rates than the condition that received neither component. Figure 2b shows that either motivational interviewing by itself or nicotine gum by itself produced the highest abstinence rates, but the combination of these two produced the lowest abstinence rate.
Figure 2.
(a) Bar graphs for gum × behavioral reduction (BR). (b) Bar graph for gum × motivational interviewing (MI).
What can we make of such effects? First, it is clear that different smoking intervention components do sometimes significantly interact with one another. Second, the results suggest that combining components may exact costs as well as yield benefits. For instance, in Figure 2b, the combination of motivational interviewing and nicotine gum was meaningfully less effective than when either was used alone. Figure 2a shows that no combination of components performed as well as the subgroup that received neither intervention.
It may be that the net outcomes associated with component combinations reflect several nonorthogonal influences: (1) the benefits of a component, (2) the correlation or redundancy of the mechanisms of the components involved (eg, overlapping or redundant mechanisms, or mechanisms that produce multiplicative effects: antagonism or synergism), and (3) the costs or burden of the components, especially as they are used together (eg, demands on attention, effort, learning, travel). For instance, while nicotine gum and behavioral reduction counseling may produce benefits that sum when they are used together, this is apparently offset by their costs or burden. That is, the burden of their joint use may explain why their combined effects are worse than when neither is used. These are complex issues, however, and space prevents comprehensive discussion. It is important to remember, though, that in addition to the gum and behavioral reduction counseling that created the significant interaction, many of the participants were also getting one or both of the other two components: motivational interviewing or the nicotine patch, which have their own costs or burden. This might add to the net burden faced by participants (the estimation of effects with effect coding does not entirely eliminate influences of other factors in an experiment61).
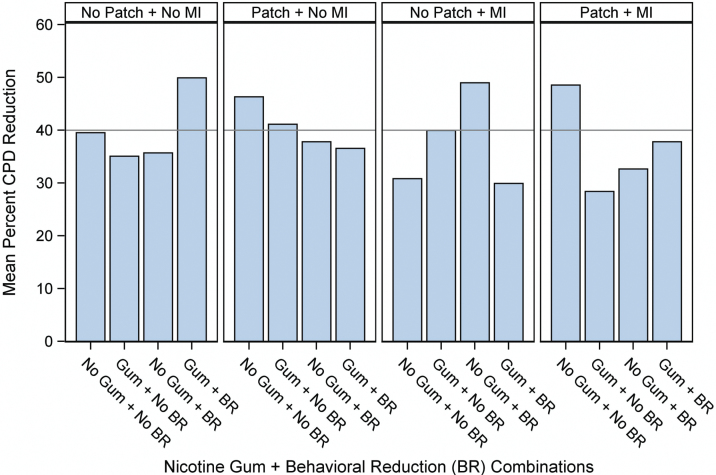
The analysis of the smoking reduction dependent variable in the Cook et al. study revealed a 4-way interaction (Figure 3). Examining this interaction shows that no combination of three or four active components produced as much smoking reduction as did the condition that received no “active” components. In fact, the best outcomes were produced by 2-component combinations—and these all produced similar magnitudes of effects—suggesting that what your get may matter less than how much you get. Finally, the figure shows that an individual component such as nicotine gum can appear to yield very different effects depending on the components with which it is paired.
Figure 3.
Mean percent reduction in cigarettes per day (CPD) at week 26. BR = behavioral reduction; MI = motivational interviewing.
Rather than trying to work through all the factors that might account for the complex patterns observed in this experiment, I will merely make two fundamental points. First, interactions do occur among smoking intervention components. While we did not have enough statistical power in this research to reveal which particular sets of components differed significantly from one another (using simple effects tests), the components did indeed interact. Second, such interactions appear to be quite common; we have found them in all of our factorial experiments of smoking intervention components.
In Piper et al.,59 we found that in-person and phone-based counseling in the cessation phase interacted negatively with one another in their effects on 6-month abstinence.
In Schlam et al.,60 we found a 4-way interaction among intervention components designed to enhance the long-term maintenance of abstinence.
In Fraser et al.,58 provision of a smoking email intervention interacted negatively with provision of a smoking cessation website.
This last study58 is informative since it provided evidence of the mechanism that might account for the relatively poor response to the combined treatment: participants who got both interventions (the email intervention plus the website) used the website less than other participants. Thus, the email intervention interfered with the use of the website, perhaps because it imposed an attentional burden or time constraints that interfered with website use.
Some of the particular interactions that we found may certainly have been fortuitous. Perhaps the most important finding is the fairly consistent pattern in which adding components past a certain point risks degrading the effects of other components.
There are several things to bear in mind when considering the meaning of these findings. First, even when significant negative interactions are found, the combination of the negatively interacting components may still be superior to any subset of the components (the interaction reflects decreased effects of one or more constituent components relative to the component’s effects without an added component(s), but nevertheless the set of components may still produce a net benefit). Second, we have little idea of what is actually producing the interactions we have obtained. For instance, while I have discussed our studies in terms of adding different intervention components, the types of the components or their specific contents may be less important than their correlated features and the specific burdens they impose. Thus, the effects of adding a counseling component may reflect the effects of adding three more person-to-person visits more than adding the counseling content per se. We need to consider carefully all aspects of an intervention in our efforts to understand their effects: for example, the number of visits, duration of contacts, timing of contacts, or attentional demands. Third, the magnitudes of some of the interaction effects are often large relative to the size of the main effects; of sufficient magnitude to have clinical impact. Finally, we selected and designed the components to be compatible with one another; we did not include components that we thought would be competitive. The upshot of this is that we had no inkling of the results that would be obtained. Thus, either we are especially benighted, or other treatment developers might also be misled in the absence of factorial evidence.
Take away messages of our factorial experiments on intervention components are that: (1) more is not necessarily better, (2) that hunches or “expert judgment” about what works are probably poor guides to the state of nature and cannot substitute for formal experimental analysis, and (3) that many of the smoking treatments currently in use for research or clinical purposes may be suboptimal; at the very least comprising elements that little benefit overall outcomes.
Research Opportunities
The use of factorial experiments as per the Multiphase Optimization Strategy seems critical for treatment development, both in terms of efficiency and information yield.38 This recommendation extends not only to new treatments, but also to commonly used treatment packages; experimental analysis may show how to make extant treatments more effective or cost-effective. Further, we need to explore interaction effects; we need to discover which ones replicate and what causes them. In particular, if we could discover the factors that cause negative interactions (eg, some dimension of burden), it might suggest strategies for streamlining treatments, thus reducing their costs and perhaps increasing treatment availability and participation. Finally, since factorial experiments permit the testing of multiple, relatively discrete features of treatment, they can efficiently uncover interactions between person factors and relatively specific intervention components. Thus, such research might advance efforts to match smokers with especially effective treatments.
Overview
The pursuit of the three major topics identified in this paper may yield synergistic effects on knowledge gained and treatment effectiveness. For instance, factorial experiments may be the ideal means of exploring the main and interactive effects of different counseling contents. Further, if we can gain important information on what counseling content is critical to success (vs. nonspecific effects), such content might be incorporated into new digital interventions for smoking. Factorial experiments may also provide a key to efficiently screening multiple digital interventions or their components, and determining the adjuvants that work especially well with them. This evidence should facilitate healthcare systems’ decisions regarding which sorts of low-cost interventions to disseminate to their patients so that they are available to their patients in the course of their daily lives.
Funding
The author received funding from National Cancer Institute (P01 CA180945, R35 CA197573, and K05 CA139871), and National Heart and Lung and Blood Disease Institute (R01 HL109031).
Declarations of Interests
None declared.
Acknowledgments
Timothy B. Baker would like to thank all his colleagues at the Center for Tobacco Research and Intervention at the University of Wisconsin School of Medicine and Public Health, and all his former graduate students and postdoctoral students, for contributing so greatly to his research achievements, and for making his research career so rewarding and enjoyable. Timothy B. Baker would like to thank the Treatment Network Advisory Committee for suggesting that he convert his 2016 Ove Ferno Award talk into a manuscript and for reviewing a draft of the manuscript.
References
- 1. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. 2008. http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Accessed July 6, 2016.
- 2. Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi:10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- 3. Wampold BE, Imel ZE.The Great Psychotherapy Debate: The Evidence for What Makes Psychotherapy Work. 2nd ed. New York, NY: Taylor & Francis; 2015. [Google Scholar]
- 4. Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315(4):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;(2):CD001292. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy DE, Piasecki TM, Lawrence DL, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10(4):717–729. [DOI] [PubMed] [Google Scholar]
- 7. Lam CY, Businelle MS, Aigner CJ, et al. Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res. 2014;16(5):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minami H, Yeh VM, Bold KW, Chapman GB, McCarthy DE. Relations among affect, abstinence motivation and confidence, and daily smoking lapse risk. Psychol Addict Behav. 2014;28(2):376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 10. Shiffman S, Gwaltney CJ, Balabanis MH, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111(4):531–545. [DOI] [PubMed] [Google Scholar]
- 11. Shiftman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004;113(1):166–171. [DOI] [PubMed] [Google Scholar]
- 12. Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34(4):365–373. [DOI] [PubMed] [Google Scholar]
- 13. Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav. 2011;36(4):315–319. [DOI] [PubMed] [Google Scholar]
- 14. Schmitz JM, Spiga R, Rhoades HM, Fuentes F, Grabowski J. Smoking cessation in women with cardiac risk: a comparative study of two theoretically based therapies. Nicotine Tob Res. 1999;1(1):87–94. [DOI] [PubMed] [Google Scholar]
- 15. Ahluwalia JS, Okuyemi K, Nollen N, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction. 2006;101(6):883–891. [DOI] [PubMed] [Google Scholar]
- 16. Piper ME, Cook JW, Schlam TR, et al. Toward the development of precision smoking cessation treatment II: proximal effects of smoking cessation intervention components on putative mechanisms of action. Drug Alcohol Depend. 2017;171(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piper ME, Federmen EB, McCarthy DE, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117(1):94–105. [DOI] [PubMed] [Google Scholar]
- 19. Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2013;(8):CD003999. [DOI] [PubMed] [Google Scholar]
- 20. Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi:10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- 21. Laska KM, Gurman AS, Wampold BE. Expanding the lens of evidence-based practice in psychotherapy: a common factors perspective. Psychotherapy (Chic). 2014;51(4):467–481. [DOI] [PubMed] [Google Scholar]
- 22. Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68(3):438–450. [PubMed] [Google Scholar]
- 23. Murran JC, Barber JP.The Therapeutic Alliance: An Evidence-Based Guide to Practice. New York, NY: Guildford Press; 2011. [Google Scholar]
- 24. Ackerman SJ, Hilsenroth MJ. A review of therapist characteristics and techniques negatively impacting the therapeutic alliance. Psychotherapy. 2001;38(2):171–185. [DOI] [PubMed] [Google Scholar]
- 25. Constantino MJ, Morrison NR, MacEwan G, Boswell JF. Therapeutic alliance researchers’ perspectives on alliance centered training practices. J Psychother Integr. 2013;23(3):284–289. [Google Scholar]
- 26. Baker TB, McFall RM. The promise of science-based training and application in psychological clinical science. Psychotherapy (Chic). 2014;51(4):482–486. [DOI] [PubMed] [Google Scholar]
- 27. Nissen-Lie HA, Havik OE, Høglend PA, Monsen JT, Rønnestad MH. The contribution of the quality of therapists’ personal lives to the development of the working alliance. J Couns Psychol. 2013;60(4):483–495. [DOI] [PubMed] [Google Scholar]
- 28. Beadman M, Das RK, Freeman TP, Scragg P, West R, Kamboj SK. A comparison of emotion regulation strategies in response to craving cognitions: effects on smoking behaviour, craving and affect in dependent smokers [published online ahead of print March 31, 2015]. Behav Res Ther. 2015;69:29–39. doi:10.1016/j.brat.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 29. Hogarth L, Retzler C, Munafò MR, et al. Extinction of cue-evoked drug-seeking relies on degrading hierarchical instrumental expectancies [published online ahead of print June 17, 2014]. Behav Res Ther. 2014;59:61–70. doi:10.1016/j.brat.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown J, Michie S, Geraghty AW, et al. Internet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trial. Lancet Respir Med. 2014;2(12):997–1006. [DOI] [PubMed] [Google Scholar]
- 31. Hendricks PS, Hall SM, Tyus LR, et al. Withdrawal exposure with withdrawal regulation training for smoking cessation: a randomized controlled pilot trial [published online ahead of print April 26, 2016]. Drug Alcohol Depend. 2016;164:28–37. doi:10.1016/j.drugalcdep.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 32. McCarthy DE, Bold KW, Minami H, Yeh VM. A randomized clinical trial of a tailored behavioral smoking cessation preparation program [published online ahead of print December 15, 2015]. Behav Res Ther. 2016;78:19–29. doi:10.1016/j.brat.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ubhi HK, Michie S, Kotz D, Wong WC, West R. A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. J Med Internet Res. 2015;17(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacKinnon DP, Pirlott AG. Statistical approaches for enhancing causal interpretation of the M to Y relation in mediation analysis. Pers Soc Psychol Rev. 2015;19(1):30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michie S.Behaviour change taxonomy (ontology). International Behavioural Trials Network Conference; May 2016; Montreal, QC, Canada. [Google Scholar]
- 36. Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010;51(3–4):199–213. [DOI] [PubMed] [Google Scholar]
- 37. Preacher KJ. Advances in mediation analysis: a survey and synthesis of new developments. Annu Rev Psychol. 2015;66:825–852. [DOI] [PubMed] [Google Scholar]
- 38. Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20(suppl 1):S197–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fraser D, Christiansen BA, Adsit R, Baker TB, Fiore MC. Electronic health records as a tool for recruitment of participants’ clinical effectiveness research: lessons learned from tobacco cessation. Transl Behav Med. 2013;3(3):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Land TG, Rigotti NA, Levy DE, Schilling T, Warner D, Li W. The effect of systematic clinical interventions with cigarette smokers on quit status and the rates of smoking-related primary care office visits. PLoS One. 2012;7(7):e41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piper ME, Baker TB, Mermelstein R, et al. Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Transl Behav Med. 2013;3(3):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richter KP, Ellerbeck EF. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381–386. [DOI] [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention. Quitting smoking among adults - United States 2001–2010. MMWR. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 45. Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine Tob Res. 2014;16(9):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Balmford J, Swift E, Borland R. Reported planning before and after quitting and quit success: retrospective data from the ITC 4-Country Survey. Psychol Addict Behav. 2014;28(3):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaiton M, Diemert L, Cohen JE, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Belsky DW, Moffitt TE, Baker TB, et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013;70(5):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen LS, Hung RJ, Baker T, et al. CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis--a meta-analysis. J Natl Cancer Inst. 2015;107(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55(suppl):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. [DOI] [PubMed] [Google Scholar]
- 52. Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. [DOI] [PubMed] [Google Scholar]
- 53. Borland R, Balmford J, Swift E. Effects of encouraging rapid implementation and/or structured planning of quit attempts on smoking cessation outcomes: a randomized controlled trial. Ann Behav Med. 2015;49(5):732–742. [DOI] [PubMed] [Google Scholar]
- 54. Ridley M.The Evolution of Everything. 1st ed. New York, NY: Harper/Harper Collins Publishers; 2015. [Google Scholar]
- 55. Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41(2):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cook JW, Collins LM, Fiore MC, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111(1):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population-based interventions for smoking cessation: a MOST trial. Transl Behav Med. 2014;4(4):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Piper ME, Fiore MC, Smith SS, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction. 2016;111(1):129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schlam TR, Fiore MC, Smith SS, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111(1):142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baker TB, Smith SS, Bolt DM, et al. Implementing clinical research using factorial designs: a primer. Behav Ther. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]