Abstract
Introduction:
Previous studies in adolescents were not adequately powered to accurately disentangle genetic and environmental influences on smoking initiation (SI) across adolescence.
Methods:
Mega-analysis of pooled genetically informative data on SI was performed, with structural equation modeling, to test equality of prevalence and correlations across cultural backgrounds, and to estimate the significance and effect size of genetic and environmental effects according to the classical twin study, in adolescent male and female twins from same-sex and opposite-sex twin pairs (N = 19 313 pairs) between ages 10 and 19, with 76 358 longitudinal assessments between 1983 and 2007, from 11 population-based twin samples from the United States, Europe, and Australia.
Results:
Although prevalences differed between samples, twin correlations did not, suggesting similar etiology of SI across developed countries. The estimate of additive genetic contributions to liability of SI increased from approximately 15% to 45% from ages 13 to 19. Correspondingly, shared environmental factors accounted for a substantial proportion of variance in liability to SI at age 13 (70%) and gradually less by age 19 (40%).
Conclusions:
Both additive genetic and shared environmental factors significantly contribute to variance in SI throughout adolescence. The present study, the largest genetic epidemiological study on SI to date, found consistent results across 11 studies for the etiology of SI. Environmental factors, especially those shared by siblings in a family, primarily influence SI variance in early adolescence, while an increasing role of genetic factors is seen at later ages, which has important implications for prevention strategies.
Implications:
This is the first study to find evidence of genetic factors in liability to SI at ages as young as 12. It also shows the strongest evidence to date for decay of effects of the shared environment from early adolescence to young adulthood. We found remarkable consistency of twin correlations across studies reflecting similar etiology of liability to initiate smoking across different cultures and time periods. Thus familial factors strongly contribute to individual differences in who starts to smoke with a gradual increase in the impact of genetic factors and a corresponding decrease in that of the shared environment.
Introduction
Smoking remains a serious public health problem. Briefly, tobacco smoking is associated with increased morbidity, mortality, personal, and public cost, even after 50 years since the first Surgeon General’s report.1 Tobacco kills nearly 6 million people each year, of whom more than five million are users and ex-users and more than 600 000 are nonsmokers exposed to secondhand smoke.2 In the United States, smoking is responsible for 69% and 74% of all cancer deaths and 69% and 61% of deaths from cardiovascular disease in female and male smokers, respectively.3 Up to half of current users will eventually die of a tobacco-related cause.2
According to the Surgeon General’s report on “Preventing Tobacco Use Among Youth and Young Adults,” “…evidence is suggestive that tobacco use is a heritable trait, more so for regular use than for onset.” The expression of genetic risk for smoking among young people may be moderated by small group and larger social-environmental factors.4 The editorial from JAMA in 19645 seems like it could have been written today. Its statements “Why some teenagers smoke and others do not is not fully understood” and “…reduction or elimination of cigarette smoking can only be achieved if today’s nonsmokers never start” remain true. Given tobacco use and addiction6—which can occur quickly with smoking as few as 100 cigarettes4—almost always begins before age 18, efforts must be directed toward adolescents and even younger children. Even though substantial reductions in smoking rates have occurred in some countries,7 the number of smokers worldwide is still increasing. That the largest reduction in daily smoking between 1980 and 2012 was among 15- to 19-year olds8 is encouraging but gains are still modest.
Twin studies have consistently found a significant genetic component to the liability to smoking initiation (SI) and nicotine dependence.9 Recent reviews10,11 show heritability estimates for SI from 40% to 70% with family environmental influences more pronounced in adolescence than in adulthood.12–20 Furthermore, there is evidence for overlapping genetic and environmental risk factors between SI and nicotine dependence in adults21,22 suggesting that partly the same genes contribute to liability to SI and nicotine dependence. This evidence for a correlated liability of SI and nicotine dependence makes it more important to study SI as a necessary stage to nicotine addiction.23,24
Previous studies of SI in adolescence have been unable to accurately assess the role of shared environmental factors in the development of smoking behavior for several reasons. First, most studies were underpowered for estimating shared environmental influences in the presence of genetic factors.25 Thus, while evidence for familial resemblance may be strong, sample sizes are often too small to distinguish between shared environmental and genetic factors. Second, given the need for large samples for genetic studies of binary traits, data from different ages and cohorts are often combined, which can overestimate contributions of shared environmental factors for traits correlated with age.23,26 This problem is exacerbated by low prevalence of SI in early adolescence, reducing power of individual studies. We use prevalence here to refer to lifetime prevalence of having initiated smoking.
In this report, we attempt to address these concerns by performing a mega-analysis by pooling data from available adolescent prospective longitudinal twin studies with data on SI. Substantial sample sizes are available for all ages throughout adolescence which allows, for the first time, familial resemblance of SI to be separated into genetic and shared environmental factors. Our aims are to: (1) estimate prevalence of SI across adolescence and test heterogeneity across samples; (2) estimate twin correlations for SI and test their equivalence across samples by age; (3) estimate contributions of genetic, shared environmental, and specific environmental factors to liability of SI at every age across adolescence; and (4) test for sex differences.
Methods
Subjects
Table 1 shows the main characteristics of the participating 11 studies (Supplementary Material). We approached investigators who had published results on the genetic epidemiology of SI in adolescence by the start of the project, all of whom agreed to share individual anonymized data with us. We also had access to local samples with SI data, and a publicly available nationally representative sample. Samples are organized by continent, starting in North America, followed by Europe and Australia, alphabetically by abbreviation. Participating studies were approved by their respective human subjects protection committees. Inclusion criteria were availability of population-based adolescent twin data on smoking.
Table 1.
Years of Assessments, Ages of Participants, Number of Data Collection Waves, Number of Unique Individuals and Geographical Location of Participating Studies
| Years | Ages | Wave | N# | Location | Abbreviationa | |
|---|---|---|---|---|---|---|
| Add health | 1994– | 12–18, 13–19, 18–26, 24–32 | 1–4 | 1556 | United States | US |
| LTS | 1992– | 12–13, 17–19 | 2 | 3166 | United States: Colorado | CO |
| CTS | 13–18 | 1 | ||||
| MFTS | 1990– | 11–12, 14–15, 17–18, 20–21 | 1–6 | 4137 | United States: Minnesota | MN |
| MASATS | 1995–1997 | 11–18 | 1 | 2211 | United States: VA, NC | NC |
| VTSABD-YAFU-TSA | 1986–2007 | 8–16, 18–30, 22–32 | 1–6 | 2832 | United States: VA | VA |
| CVT | 1983–1993 | 9–17 | 1–5 | 1180 | United States: VA | VA2 |
| EFPTS: LLTS | 1986–1999 | 10–16, 18 | 1–8 | 210 | Belgium: EFPTS | BE |
| FTC: FinnTwin16 | 1991–1997 | 16, 17,18 | 1–3 | 14 279 | Finland | FI |
| FTC: FinnTwin12 | 1997–2004 | 14, 17 | 2, 3 | |||
| FTC: Old cohort | 1975 | 18–19 | 1 | |||
| NTR | 1991– | 13–22 | 1–8 | 13 425 | the Netherlands | NL |
| STR: TCHAD | 1993– | 8–9, 13–14, 16–17, 19–20 | 1–4 | 2942 | Sweden: STR | SW |
| ATR: AYATS | 1988–1996 | 13–18 | 1–3 | 2888 | Australia: ATR | AU |
Add Health = National Longitudinal Study of Adolescent Health; ATR: AYATS = Australian Twin Registry: Australian Young Adult Twin Study; CTS = Colorado Twin Registry Community Twin Sample; CVT = Medical College of Virginia CardioVascular Twin Study; EFPTS: LLTS = East Flanders Prospective Twin Survey: Leuven Longitudinal Twin Study; FTC = Finnish Twin Cohort; NTR = Netherlands Twin Registry; LTS = Colorado Longitudinal Twin Sample; MATR: MASATS = Mid-Atlantic Twin Registry: Mid-Atlantic School Age Twin Study; MFTS = Minnesota Family and Twin Studies; STR: TCHAD = Swedish Twin Registry: Twin Study of Child and Adolescent Development; VTSABD-YAFU-TSA = Virginia Twin Study of Adolescent Behavioral Development-Young Adult Follow-up-Transitions to Substance Use.
aTwo-letter abbreviations to be used in tables and graphs.
Measures
Data were collected via questionnaire or personal interview. For the purposes of this report, we focused on ever use of tobacco, thus including those who have experimented with tobacco by trying just one or a few cigarettes. We use the term “smoking initiation” as it has been widely used in genetic epidemiologic studies of smoking behavior. SI was coded 0/1 and defined according to responses to questions like “Have you ever smoked cigarettes or tried any form of tobacco?”. Exact wording of questions, and coding of answers is presented in Supplementary Appendix 1. Two studies (CardioVascular Twin Study and Leuven Longitudinal Twin Study) asked “Have you ever smoked at least 100 cigarettes in your life?” thus requiring a higher threshold for SI.
Statistical Approach
Structural equation models were fit to the twin data, in order to estimate the proportions of variance of additive genetic (A), shared (C), and unique (E) environmental factors contributing to individual differences in liability to SI, using the statistical package OpenMx.27,28 In brief, greater similarity of monozygotic (MZ) than dizygotic (DZ) twins implicates genetic factors, whereas DZ similarity greater than half that of MZ suggests shared environment29 (see Supplementary Material for further detail).
Results
Descriptive Statistics
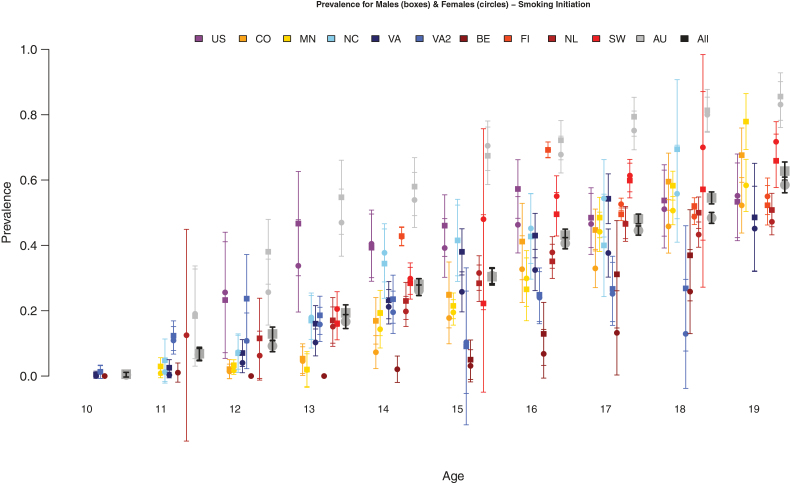
Data from 11 samples were analyzed for SI at each age from 10 to 19. Sample sizes are provided by sample, age, and zygosity in Table 2. Prevalence rates for SI and their standard errors are shown in Figure 1 by age, sex, and sample, with a mean estimate across all samples. Few children (<1%) had initiated smoking by age 10. From age 11 onwards SI rates increased almost linearly to age 19, by which time ~60% of adolescents/young adults have smoked at least one cigarette. There was considerable variability across samples at each age; rates were consistently higher in AYATS compared than all others, and lower in CardioVascular Twin Study and Leuven Longitudinal Twin Study. Variability likely stems from differences in cultural background or in wording between assessments. We formally tested equality of prevalences by twin order, zygosity, sex of co-twin, sex, and sample using structural equation modeling.
Table 2.
Number of Twins Assessed for Smoking Initiation (SI) Across Age and Sample (Top), Including Number of Individual Assessments (IA), Number of Unique Individual Twins per Sample (UI) Between the Ages of 10 and 19, and by Zygosity and Sample (Bottom), Including Number of Unique Individuals (UIZ) and Pairs of Twins (UPZ) With Known Zygosity per Sample
| Age | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | IA | UI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US | 86 | 161 | 346 | 442 | 491 | 448 | 295 | 251 | 2520 | 1480 | ||
| CO | 401 | 363 | 418 | 321 | 298 | 958 | 528 | 510 | 3797 | 2770 | ||
| MI | 646 | 1763 | 105 | 535 | 1629 | 383 | 1024 | 1979 | 474 | 8538 | 4128 | |
| NC | 164 | 335 | 404 | 382 | 277 | 276 | 211 | 122 | 2171 | 2172 | ||
| VA | 565 | 636 | 619 | 755 | 884 | 755 | 829 | 660 | 183 | 5886 | 2710 | |
| VA2 | 476 | 826 | 178 | 712 | 542 | 40 | 434 | 305 | 72 | 3585 | 1148 | |
| BE | 124 | 187 | 187 | 186 | 186 | 196 | 181 | 114 | 181 | 1542 | 210 | |
| FI | 4709 | 5729 | 10 683 | 6490 | 1133 | 28 744 | 13 262 | |||||
| NL | 10 | 132 | 494 | 993 | 1090 | 1410 | 1536 | 2089 | 1944 | 9698 | 6036 | |
| SW | 853 | 1352 | 43 | 924 | 1395 | 41 | 672 | 5280 | 2561 | |||
| AU | 112 | 328 | 329 | 507 | 496 | 956 | 761 | 709 | 399 | 4597 | 2869 | |
| Total | 1165 | 2581 | 4029 | 4362 | 10 854 | 5289 | 11 911 | 18 095 | 12 506 | 5566 | 76 358 | 39 346 |
| MZm | MZf | DZm | DZf | DZo | UIZ | MZm | MZf | DZm | DZf | DZo | UPZ | |
| US | 268 | 282 | 246 | 220 | 380 | 1396 | 138 | 143 | 127 | 113 | 201 | 722 |
| CO | 613 | 729 | 440 | 457 | 529 | 2768 | 309 | 365 | 222 | 229 | 266 | 1391 |
| MI | 1166 | 1226 | 642 | 728 | 0 | 3762 | 583 | 613 | 321 | 364 | 0 | 1881 |
| NC | 329 | 560 | 283 | 393 | 497 | 2062 | 179 | 301 | 159 | 209 | 286 | 1134 |
| VA | 623 | 805 | 345 | 370 | 559 | 2702 | 312 | 403 | 173 | 185 | 281 | 1354 |
| VA2 | 288 | 308 | 146 | 164 | 242 | 1148 | 144 | 154 | 73 | 82 | 121 | 574 |
| BE | 42 | 44 | 42 | 40 | 42 | 210 | 21 | 22 | 21 | 20 | 21 | 105 |
| FI | 1828 | 2241 | 2436 | 2393 | 3542 | 12 440 | 919 | 1125 | 1235 | 1208 | 1775 | 6262 |
| NL | 939 | 1535 | 793 | 1009 | 1646 | 5922 | 494 | 816 | 421 | 551 | 913 | 3195 |
| SW | 475 | 515 | 337 | 388 | 754 | 2469 | 241 | 258 | 170 | 195 | 387 | 1251 |
| AU | 574 | 751 | 395 | 441 | 704 | 2865 | 288 | 376 | 199 | 225 | 356 | 1444 |
| Total | 7145 | 8996 | 6105 | 6603 | 8895 | 37 744 | 3628 | 4576 | 3121 | 3381 | 4607 | 19 313 |
MZm = monozygotic male; MZf = monozygotic female; DZm = dizygotic male; DZf = dizygotic female; DZo = dizygotic opposite sex.
Figure 1.
Prevalence rates of smoking initiation (SI) by sex (males in boxes, females in circles), age and sample.
Model Assumptions Testing
Models were fitted by maximum likelihood to the combined SI data, separately at each age from 12 to 19 years, allowing prevalence of SI to differ by twin order, zygosity, sex of co-twin, sex and sample, and correlations to differ by zygosity and sample (Supplementary eTable 1). Prevalences of SI at ages 10–11 were too low to permit meaningful analysis. Model assumptions, including equality of prevalences across twin order (model T1), zygosity (model T2), and sex of co-twin (model T3) were met across all samples at each age. However, prevalences for males were higher than those for females at all ages between 12 and 19, and significantly so at ages 16, 18, and 19 (model T4). Furthermore, prevalences could not be constrained to be equal across samples at any age (model T5).
We further tested whether twin correlations could be equated across all 11 samples. Tests were performed separately for MZ (model C1) and DZ (model C2) twins, for all same sex twins (model C3) and including opposite sex twins (model C4). Correlations could be equated across samples at most ages, except for MZ correlations at age 16 and DZ correlations at age 14 (Supplementary eTable 1). When applying Bonferroni or false discovery rate corrections, no equality tests were significant, except at age 16, which was borderline significant. Testing equality of correlations and prevalences across datasets simultaneously (models CT1 and CT2) suggested that twin correlations could be equated across samples, when allowing for differences in prevalence by sex and sample. Twin correlations by zygosity for each sample are presented in Supplementary eFigure 1, a and b, along with joint estimates after equating correlations across samples, for ages 12–19 (see Supplementary eFigure 2, a and b for correlations by sample).
MZ correlations were consistently high during adolescence. Like-sex DZ correlations varied more and gradually decreased towards young adulthood. Opposite-sex DZ correlations showed a more pronounced decreasing trend. This pattern of correlations over adolescence is broadly consistent with a changing role of sources of SI familial resemblance from shared environmental to genetic factors. The nature and/or magnitude of these effects possibly differ by sex.
Genetic Analyses
Twin models were fitted to data from all samples at ages 12–19. DZ twin correlations for SI were invariably greater than half those of MZ twins, consistent with A, C, and E factors contributing to individual differences in liability to SI. Based on results from testing model assumptions, prevalences for SI were equated across twin order, zygosity, and sex of co-twin, but allowed to differ by sex and sample. Models were tested for heterogeneity across samples by equating variance components (ACE), genetic correlations (rg) and shared environmental correlations (rc) parameters (Supplementary eTable 2). Parameters could be equated across all samples at all ages, as indicated by more parsimonious fits of D1–D4 models compared to the corresponding S1–S4 models.
We fitted alternative models testing whether different genetic or different shared environmental factors contributed in males and females, and whether the magnitude of ACE contributions was the same across sexes. At ages 12, 13, 15, and 19, neither type of sex difference was significant. At remaining ages (14, 16, 17, and 18), models including different proportions and different types of genetic and shared environmental factors in males and females—by estimating correlations between them (rc) across sex—performed better suggesting sex differences in etiology of liability to SI at later ages. Neither genetic (model D5) nor shared environmental (model D6) parameters could be dropped from models at any age.
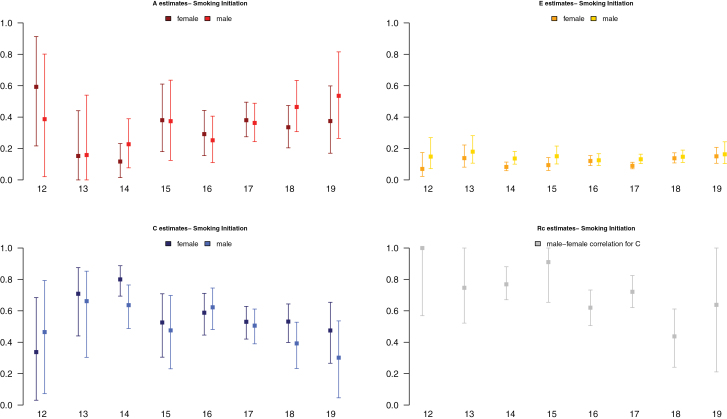
We present results for models with separate parameters for males and females. Estimates and confidence intervals for A, C, E, and rc from the best fitting models, with parameters constrained across samples, are presented for ages 12–19 in Figure 2. Results showed an increase in proportion of liability to SI explained by additive genetic factors from 15% to 45%, and a corresponding decrease in proportion of variance accounted for by shared environmental factors, with unique environmental factors explaining a small stable amount of variance in liability to SI. Gender differences in factors influencing SI increased across adolescence: the shared environmental correlation between males and females decreased from 1.0 to about 0.6 from ages 12 to 19, suggesting that in early adolescence environmental factors that increase similarity between twins are mostly the same in males and females, whereas in later adolescence, only about half are. Although the magnitude of genetic and environmental factors was allowed to differ by sex, the trend of increasing contributions of genetic factors and decreasing contributions of shared environmental factors was observed for both.
Figure 2.
Estimates of proportions of variance of additive genetic (A estimates), shared environmental (C estimates), specific environmental (E estimates) factors, and of the correlation between male and female shared environmental factors (Rc estimates) to liability of SI by sex and age.
Discussion
We set out to combine SI data across 11 primarily longitudinal twin datasets to obtain stable and reliable estimates of additive genetic, shared, and unique environmental contributions to liability of SI across adolescence. Previous studies on individual samples and review articles indicated a trend towards increasing contributions of additive genetic factors and decreasing contributions of shared environmental factors from early to late adolescence and into young adulthood.13,15–17,19,20,30–33 However, no single study was large enough to estimate genetic and shared environmental variance components accurately at every age across adolescence, especially at younger ages where prevalence of smoking is relatively low which limits statistical power. We successfully combined data from 11 samples, primarily of European descent, comprising five samples from the United States, four European, and one Australian, resulting in sample sizes ranging from ~4000 to >18 000 individuals at each age from 12 to 19 years.
We draw five main conclusions. First, smoking prevalence increased rapidly and almost linearly between ages 10 and 19 from zero to >60% of adolescents having tried smoking cigarettes. Furthermore there appeared to be substantial consistency in longitudinal trends as well as variability within each age in smoking prevalence by sample. There were significant sex differences in prevalence, however, with higher rates in males than in females, consistent with epidemiological literature on smoking in adolescents from European, North American, and global surveys.34–37
Second, model fitting confirmed that prevalence of SI could not be equated across samples. This could reflect differences in assessment of SI, availability, access, and attitudes towards smoking across cultures and variation in stage of the tobacco epidemic.37 Assumptions of the classical twin method were met in that prevalences could be equated across twin order, zygosity, and sex of co-twin. Differences in prevalence by zygosity could be interpreted as sibling cooperation or sibling competition.38 Our results suggest little role for sibling interaction, because prevalences were similar across zygosity. Prevalence of SI did not differ significantly if the twin was the same or opposite sex of their twin. While we did not have singletons in the analysis, these analyses indicate the absence of twin-specific effects on SI and support the generalization of our results to the populations from which they were drawn.39
Third, even though prevalences could not be equated across samples, twin correlations could. This suggests that etiology of liability to initiate smoking is broadly consistent across samples—of primarily European descent—collected on three continents. To our knowledge, this is the first report to show this notable similarity in twin correlations across cultures for smoking in adolescence. It suggests that similar etiological factors operate within families in high-income countries of mostly European ancestry. The implication is that preventive measures found to be effective in one such country would likely work well in others. Although twin correlations, and thus heritability of SI, could be equated across samples collected on different continents, this does not imply that social factors at the school, state, population composition level do not moderate aspects of smoking behavior.40–42 However, this type of genotype by environment effect appeared stronger for regular smoking than for initiation. Furthermore, changing policies across time, such as the Surgeon General’s Report about the effects of smoking on health, has also been shown to affect the magnitude of genetic influences on regular smoking.43 As the majority of the samples in the current report were collected in the 1990s, we did not take genotype by cohort effects into account. Future analyses, however, should investigate whether macro and micro environmental factors cause variation in liability to SI.
Fourth, at each age across adolescence both additive genetic and shared environmental factors contribute significantly to variance in liability to SI. Consistent with prior literature on SI from early to late adolescence, the influence of additive genetic factors appears to increase while that of the shared environment decreases.10,31 Although at first sight these results may not appear novel, the current study is the first to be sufficiently powered to detect a contribution of additive genetic factors of 20% of the variance in early adolescence, and a modest contribution of shared environmental factors in later adolescence, thus stressing the importance of both sources throughout adolescence. While assortative mating could mimic effects of shared environmental influences, and given significant spousal correlations for smoking behavior,13 it seems unlikely that assortment would account for the shared environment found here. Assuming a marital correlation for SI of 0.20, shared environmental contributions would be overestimated by 2% and 15% for MZ correlations of 0.85 and DZ correlations of 0.50 and 0.70 respectively. Correspondingly, heritability would be underestimated.
Fifth, while sex differences in genetic and environmental factors were nonsignificant in early adolescence, they were postpuberty, suggesting that to some degree environmental factors that contribute to SI liability in males differ from those in females. Models allowing for different shared environmental factors across sex fitted marginally better than those allowing different genes across sex, although power is limited in comparing these alternative explanations, even with current sample sizes. While it seems plausible that girls are exposed to partially different environmental factors than boys, we cannot exclude sex-specific genetic factors. A possible source of such genetic differences between boys and girls across adolescence would be differential rates of maturation, which is known to be partly influenced by genetic factors.44
Results from this study increase the evidence beyond suggestive4 that smoking behavior, and in particular SI in adolescence, is a heritable trait. However, genome-wide studies of smoking behavior have only identified some genes underlying SI in contrast to major findings underlying variability in consumption of cigarettes.45–48 The strongest association for SI was reported for SNPs in the BDNF gene.46 A handful of other SNPs have been found to be genome-wide significant for SI, but require replication.49,50 Given the broad age range of individuals in the large scale consortia, selective attrition by genotype due to smoking related mortality could have obscured signals for SI.51 Furthermore, evidence for shared environmental contributions, especially in early adolescence, is strong. Even though a recent review of behavioral genetics research suggests that “most environmental effects are not shared by children growing up in the same family,”52 evidence is accumulating that shared environmental factors contribute significantly to behavior in early adolescence, and especially for externalizing behaviors and substance use.53–55 These shared environmental effects may result from: parents and older siblings (including secondhand smoke and effects of assortative mating); peers56,57; or social environment factors such as advertising controls, tobacco pricing, smoke-free regulations and tobacco availability.58
Heritability of SI is significant even at young ages and jumps at ages 14–15, the transition from middle to high school in the United States, which therefore appears to be a critical period to target prevention measures. Most individual differences in SI in adolescence are accounted for by familial factors, although they shift from being more shared environmental to more genetic. It would seem justified to target prevention at the whole family, rather than solely at teenagers.59 These results are consistent with a review of the effectiveness of family-based interventions to prevent children and adolescent from starting to smoke,60 which report a moderately positive effect of high intensity programs that address family functioning. Furthermore, office-based interventions by pediatric providers engaged in delivering prevention and cessation counseling to both patients and parents/caregivers show great promise.61 We also believe that providing personalized information—including genetic information—about smoking risks could improve smoking prevention.62 Finally, prevention efforts might be especially effective if targeted at children with both high genetic and environmental risk, as they are at greatest risk of nicotine addiction. Our results suggest an increase in risk for children having one or two parents who smoke which could be considered when evaluating the cost-benefit ratio of targeted (families, high-risk children) versus whole population intervention campaigns.
In summary, this study showed that even though substantial differences exist in prevalence of SI across samples, etiology of SI liability is markedly similar across different populations of Western European descent.
Limitations
This study should be interpreted in the context of four potential limitations. First, items used to query participants about their SI differed across studies. While most samples included questions about lifetime SI, two samples only recorded SI when participants had smoked at least 100 cigarettes. This difference likely accounts for differences in prevalence across studies. A re-analysis excluding these samples showed almost identical results; the largest difference was <1 change in A and C estimates at ages 12–13 (results available upon request). Second, results may not generalize to the entire US population as over 95% of participants were of European ancestry. Results from analyses limited to European ancestry twins were very similar to those that included twins of other ethnicities (results available upon request), thus strengthening generalizability of results. Furthermore, available samples are from high-income countries, so results may not generalize to low- and middle-income countries. Third, sample sizes at different ages differed, which affected power to detect certain effects, including sex differences at younger ages. Fourth, the current study only included data on twins, thus limiting to three the sources of variance to be estimated. Future modeling including other relatives such as parents and siblings would allow estimation of the effects of assortative mating, parent–child environmental transmission, and the action and interaction of additional types of genetic and environmental factors.
Supplementary Material
Supplementary Appendix 1, eFigures 1 and 2, and eTables 1 and 2 can be found online at http://www.ntr.oxfordjournals.org
Funding
Data analysis supported by the National Institutes of Health (DA025109, DA024304 and DA018673). Add Health: This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis. LTS-CTS: Data collection was supported by DA011015. MFTS: Data collection was supported by DA05147 (male MFTS twins), AA09367 (female MFTS twins), DA013240 (OS twins) and DA036216. MATR: MASATS: Data collection was supported by the Templeton Foundation and the Keck Foundation (K01 DA000386). VTSABD-YAFU-TSA: Data collection was supported by MH045268, MH068521, DA024413, and Virginia Tobacco Settlement Foundation grant 8520012. CVT: Data collection was supported by HL031010. EFPTS: LLTS: Data collection was supported by Research Fund K.U.Leuven (OT/86/80), the National Bank of Belgium, the Fund for Medical Research (Belgium) (3.0038.82, 3.0008.90, 3.0098.91) and (NATO 860823). FTC: Data collection in the Finnish twin cohorts was supported by National Institute of Alcohol Abuse and Alcoholism (AA-08315, AA-12502, AA-00145, and AA-09203 to RJR, and AA018755 and AA015416 to DD), the Academy of Finland Center of Excellence in Complex Disease Genetics (213506, 129680 to JK), and the Academy of Finland (100499, 205585, 118555, 141054, 265240, 263278 and 264146 to JK). NTR: Data collection was supported by Netherlands Organization for Scientific Research (NWO: 463-06-001, 451-04-034) and (MagW/ZonMW: 904-61-090, 985-10-002, ZonMw 912-10-020, ZonMw 904-61-193, 451-04-034, 400-05-717, ZonMw Addiction-31160008, ZonMw Middelgroot 911-09-032), Spinozapremie (SPI 56-464-14192); BBMRI–NL (184.021.007): Biobanking and Biomolecular Resources Research Infrastructure and from European Science Council (ERC) Genetics of Mental Illness (230374) and Beyond the Genetics of Addiction (284167). VU University: Institute for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam (NCA). STR: TCHAD: Data collection supported by the Swedish Council for Working Life and Social Research (2004-0383) and the Swedish Research Council (2004-1415). ATR: AYATS: Data collection in the Australia twin cohorts was supported by National Health and Medical Research Council grants, Victorian Health Promotion Foundation, and the NHMRC Australian Twin Registry. Prof J. Hopper established the cohort and received the funding grants. JMV is supported by the European Research Council (ERC) starting grant (284167 to PI JMV).
Declaration of Interests
All authors reviewed and approved the final manuscript before its submission. JK has consulted for Pfizer on nicotine dependence in 2012–2014.
Supplementary Material
Acknowledgments
We thank Amir Toor for his helpful comments on the manuscript.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Tobacco. www.who.int/mediacentre/factsheets/fs339/en/. Accessed October 28, 2016. [Google Scholar]
- 3. Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 5. Childish habit. JAMA. 2014;311(2):205. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. Prezmting Tobacco Use Among Young People: A Report of the Surge & General. Atlanta, GA: U.S. Department of Health and Human Services,Public Health Service, Centers for DiseaseControl and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1994. [Google Scholar]
- 7. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–192. [DOI] [PubMed] [Google Scholar]
- 8. Vink JM, Boomsma DI. Interplay between heritability of smoking and environmental conditions? A comparison of two birth cohorts. BMC Public Health. 2011;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(suppl 2):S51–57; discussion S69–70. [DOI] [PubMed] [Google Scholar]
- 10. Maes HH, Neale MC. Genetic modeling of tobacco use behavior and trajectories. In: Swan GE, Baker TB, Chassin Let al. , eds. National Cancer Institute. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence. Tobacco Control Monograph No. 20. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; NIH Publication No. 09-6366; 2009. [Google Scholar]
- 11. Rose RJ, Broms U, Korhonen T, Dick DM, Kaprio J. Genetics of smoking behavior. In: Kim Y, ed. Handbook of Behavior Genetics. New York, NY: Springer; 2009:411–432. [Google Scholar]
- 12. Langinvainio H, Kaprio J, Koskenvuo M, Tarkkonen L. Structural analysis of smoking, alcohol use, and personality factors in MZ and DZ twin pair relationships. Prog Clin Biol Res. 1981;69(pt C):23–35. [PubMed] [Google Scholar]
- 13. Boomsma DI, Koopmans JR, Van Doornen LJ, Orlebeke JF. Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction. 1994;89(2):219–226. [DOI] [PubMed] [Google Scholar]
- 14. Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29(6):383–393. [DOI] [PubMed] [Google Scholar]
- 15. Maes HH, Woodard CE, Murrelle L, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60(3):293–305. [DOI] [PubMed] [Google Scholar]
- 16. Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94(7):981–993. [DOI] [PubMed] [Google Scholar]
- 17. McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96(5):671–677. [DOI] [PubMed] [Google Scholar]
- 18. Hopfer CJ, Stallings MC, Hewitt JK. Common genetic and environmental vulnerability for alcohol and tobacco use in a volunteer sample of older female twins. J Stud Alcohol. 2001;62(6):717–723. [DOI] [PubMed] [Google Scholar]
- 19. White VM, Hopper JL, Wearing AJ, Hill DJ. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98(8):1087–1100. [DOI] [PubMed] [Google Scholar]
- 20. Rhee SH, Hewitt JK, Young SE, et al. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60(12):1256–1264. [DOI] [PubMed] [Google Scholar]
- 21. Maes HH, Sullivan PF, Bulik CM, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34(7):1251–1261. [DOI] [PubMed] [Google Scholar]
- 22. Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35(4):397–406. [DOI] [PubMed] [Google Scholar]
- 23. Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav Genet. 2006;36(4):507–524. [DOI] [PubMed] [Google Scholar]
- 24. Neale MC, Røysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G x E interaction. Twin Res Hum Genet. 2006;9(4):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behav Genet. 2002;32(3):221–227. [DOI] [PubMed] [Google Scholar]
- 26. Neale MC, Martin NG. The effects of age, sex, and genotype on self-report drunkenness following a challenge dose of alcohol. Behav Genet. 1989;19(1):63–78. [DOI] [PubMed] [Google Scholar]
- 27. Boker S, Neale M, Maes H, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mx: Statistical Modelling (6th ed) [computer program]. Richmond, VA: Department of Psychiatry; 2006. [Google Scholar]
- 29. Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers BV; 1992. [Google Scholar]
- 30. Kaprio J, Hammar N, Koskenvuo M, et al. Cigarette smoking and alcohol use in Finland and Sweden: a cross-national twin study. Int J Epidemiol. 1982;11(4):378–386. [DOI] [PubMed] [Google Scholar]
- 31. Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42(6):710–719. [DOI] [PubMed] [Google Scholar]
- 32. Kaprio J. Genetic epidemiology of smoking behavior and nicotine dependence. COPD. 2009;6(4):304–306. [DOI] [PubMed] [Google Scholar]
- 33. Baker JH, Maes HH, Larsson H, Lichtenstein P, Kendler KS. Sex differences and developmental stability in genetic and environmental influences on psychoactive substance consumption from early adolescence to young adulthood. Psychol Med. 2011;41(9):1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harrell JS, Bangdiwala SI, Deng S, Webb JP, Bradley C. Smoking initiation in youth: the roles of gender, race, socioeconomics, and developmental status. J Adolesc Health. 1998;23(5):271–279. [DOI] [PubMed] [Google Scholar]
- 35. WHO Europe. The European Tobacco Control Report 2007. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2007. [Google Scholar]
- 36. Okoli C, Greaves L, Fagyas V. Sex differences in smoking initiation among children and adolescents. Public Health. 2013;127(1):3–10. [DOI] [PubMed] [Google Scholar]
- 37. Lopez AD, Collishaw NE, Piha T. A descriptive model of the tobacco epidemic in developed countries. Tob Control. 1994;3(3):242–247 [Google Scholar]
- 38. Eaves L. A model for sibling effects in man. Heredity (Edinb). 1976;36(2):205–214. [DOI] [PubMed] [Google Scholar]
- 39. Andrew T, Hart DJ, Snieder H, et al. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4(6):464–477. [DOI] [PubMed] [Google Scholar]
- 40. Boardman JD. State-level moderation of genetic tendencies to smoke. Am J Public Health. 2009;99(3):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boardman JD, Blalock CL, Pampel FC, et al. Population composition, public policy, and the genetics of smoking. Demography. 2011;48(4):1517–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, Hewitt JK. Do schools moderate the genetic determinants of smoking? Behav Genet. 2008;38(3):234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boardman JD, Blalock CL, Pampel FC. Trends in the genetic influences on smoking. J Health Soc Behav. 2010;51(1):108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eaves L, Silberg J, Foley D, et al. Genetic and environmental influences on the relative timing of pubertal change. Twin Res. 2004;7(5):471–481. [DOI] [PubMed] [Google Scholar]
- 45. Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loukola A, Hällfors J, Korhonen T, Kaprio J. Genetics and smoking. Curr Addict Rep. 2014;1(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vink JM, Smit AB, de Geus EJ, et al. Genome-wide association study of smoking initiation and current smoking. Am J Hum Genet. 2009;84(3):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoon D, Kim YJ, Cui WY, et al. Large-scale genome-wide association study of Asian population reveals genetic factors in FRMD4A and other loci influencing smoking initiation and nicotine dependence. Hum Genet. 2012;131(6):1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taylor AE, Morris RW, Fluharty ME, et al. Stratification by smoking status reveals an association of CHRNA5-A3-B4 genotype with body mass index in never smokers. PLoS Genet. 2014;10(12):e1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Top 10 replicated findings from behavioral genetics. Perspect Psychol Sci. 2016;11(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buchanan JP, McGue M, Keyes M, Iacono WG. Are there shared environmental influences on adolescent behavior? Evidence from a study of adoptive siblings. Behav Genet. 2009;39(5):532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. [DOI] [PubMed] [Google Scholar]
- 55. Korhonen T, Latvala A, Dick DM, et al. Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genet. 2012;42(4):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: associations between and within families across late adolescence. Dev Psychol. 2000;36(2):180–189. [PubMed] [Google Scholar]
- 57. Rose RJ, Viken RJ, Dick DM, et al. It does take a village: nonfamilial environments and children’s behavior. Psychol Sci. 2003;14(3):273–277. [DOI] [PubMed] [Google Scholar]
- 58. Do E, Maes H. Narrative review of genes, environment, and cigarettes. Ann Med. 2016;48(5):337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hudziak JJ, Bartels M. Genetic and environmental influences on wellness, resilience, and psychopathology: a family-based approach for promotion, prevention and intervention. In: Hudziak JJ, ed. Developmental Psychopathology and Wellness. Washington, DC: American Psychiatric Publishing, Inc; 2008:267–286. [Google Scholar]
- 60. Thomas RE, Baker PR, Thomas BC, Lorenzetti DL. Family-based programmes for preventing smoking by children and adolescents. Cochrane Database Syst Rev. 2015;2:CD004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pbert L, Farber H, Horn K, et al. State-of-the-art office-based interventions to eliminate youth tobacco use: the past decade. Pediatrics. 2015;135(4):734–747. [DOI] [PubMed] [Google Scholar]
- 62. Vandenbergh DJ, Schlomer GL, Cleveland HH, et al. An adolescent substance prevention model blocks the effect of CHRNA5 genotype on smoking during high school. Nicotine Tob Res. 2016;18(2):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.