Abstract
Background
Occult metastatic tumors, below imaging thresholds, are a limitation of staging systems that rely on cross-sectional imaging alone and are a cause of the routine understaging of pancreatic ductal adenocarcinomas (PDACs). We investigated circulating tumor cells (CTCs) as a preoperative predictor of occult metastatic disease and as a prognostic biomarker for PDAC patients.
Experimental Design
A total of 126 patients (100 with cancer, 26 with benign disease) were enrolled in our study and CTCs were identified and enumerated from 4 mL of venous blood using the microfluidic NanoVelcro assay. CTC enumeration was correlated with clinicopathologic variables and outcomes following both surgical and systemic therapies.
Results
CTCs were identified in 78% of PDAC patients and CTC counts correlated with increasing stage (ρ = 0.42, ρ < 0.001). Of the 53 patients taken for potentially curative surgery, 13 (24.5%) had occult metastatic disease intraoperatively. Patients with occult disease had significantly more CTCs than patients with local disease only (median 7 vs. 1 CTC, p < 0.0001). At a cutoff of three or more CTCs/4 mL, CTCs correctly identified patients with occult metastatic disease preoperatively (area under the receiver operating characteristic curve 0.82, 95% confidence interval (CI) 0.76–0.98, p < 0.0001). CTCs were a univariate predictor of recurrence-free survival following surgery [hazard ratio (HR) 2.36, 95% CI 1.17–4.78, p = 0.017], as well as an independent predictor of overall survival on multivariate analysis (HR 1.38, 95% CI 1.01–1.88, p = 0.040).
Conclusions
CTCs show promise as a prognostic biomarker for PDAC patients at all stages of disease being treated both medically and surgically. Furthermore, CTCs demonstrate potential as a preoperative biomarker for identifying patients at high risk of occult metastatic disease.
Pancreatic ductal adenocarcinoma (PDAC) ranks fourth in cancer-related mortality in the US due to late presentation and resistance to systemic therapy; however, the recent success of multidrug regimens has begun to change this paradigm.1,2 Tumor staging by cross-sectional imaging alone, the current practice standard, routinely understages PDAC patients, as evidenced by high recurrence rates and the low survival benefit of curative-intent surgery.3–5 Due to the historic inadequacies of systemic therapy in PDAC, a ‘surgery first’ mentality for all patients with even a remote chance of cure could be justified. However, the success of multidrug regimens calls into question the survival benefit of pancreatic resections for patients who almost certainly have occult metastatic disease below the detection limit of cross-sectional imaging. Recent studies have even demonstrated a potential survival benefit for neoadjuvant therapy in patients with very early-stage disease.3,6 These studies highlight the need for better pretreatment biomarkers for staging and prognostication in PDAC to inform first-line therapy decision making.
Circulating tumor cells (CTCs), cells of tumor origin circulating in the blood, are thought to represent the intravasated tumor stage, between the formation of an invasive cancer and its eventual distant metastasis.7,8 CTCs are found in the blood of patients with all epithelial tumors and have been studied as a biomarker in numerous cancer types.8 We have previously used the single-cell sequencing ability of the microfluidic NanoVelcro CTC assay to validate our immunocytochemical (ICC) CTC definition by confirming the tumor origin of PDAC CTCs through demonstration of mutational congruence between the primary tumor and the isolated CTCs.9 We then used our PDAC CTC assay to show that CTCs are present in the majority of PDAC patients, but not patients with non-malignant pancreatic disease, and that CTCs may have utility as a diagnostic biomarker at the time of disease presentation.10
In this study, we evaluated CTC enumeration as a pretreatment biomarker for prognosis and therapeutic planning in PDAC patients. Specifically, we investigated if CTCs could help identify patients with occult metastatic disease, defined as the presence of metastatic disease that is not detected by cross-sectional imaging. Separately, we investigated the strength of association between CTC enumeration and survival, both alone and in conjunction with other variables previously identified as prognostic.
MATERIALS AND METHODS
Between December 2012 and March 2015, we approached patients presenting to our multispecialty pancreatic clinic with suspicion for, or diagnosis of, malignant pancreatic disease and enrolled them under IRB #11-002112. Exclusion criteria included any malignancies within the past 5 years, active inflammatory bowel disease, or suspicion of pancreatic neuroendocrine tumor. A database of demographic, laboratory, and relevant clinicopathologic variables was prospectively maintained. Diagnosis was confirmed by biopsy or surgical pathology whenever possible, and radiographically when a tissue diagnosis was not available. Patients were assessed as having early-stage, locally advanced, or metastatic disease preoperatively using cross-sectional imaging via a pancreas protocol, contrast-enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) scan. TNM stage was assessed using the American Joint Committee on Cancer (AJCC) staging manual.11
Peripheral venous blood (VB) was collected and CTC enumeration performed using the NanoVelcro CTC assay, as previously described and detailed in the electronic supplementary methods.12 Each NanoVelcro chip can process 2 mL of VB, and samples were run on two chips in parallel. Thus, CTC counts are reported per 4 mL of blood throughout the manuscript. CTC enumeration was performed on an Eclipse 90i fluorescent microscope using NAS Elements 4.1 software (Nikon, Tokyo, Japan). White blood cells were defined as round/ovoid (DAPI+/CD45+/CK−), and CTCs were defined as round/ovoid, size ≥ 6 μm (DAPI+/CD45−/CK+). Any CD45 positivity greater than 2 × background discounted a cell as being a CTC. Final CTC counts are represented as a total count per 4 mL of VB.
Statistical Methods
Differences in CTC number or cancer antigen (CA) 19-9 levels were evaluated using the non-parametric Mann–Whitney U test, cut-off points for CTC enumeration were evaluated using Cutoff Finder,13 and diagnostic performance of CTCs was evaluated using receiver operating characteristic curves (ROCs) for determination of the area under the curve (AUROC) in addition to sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) calculations. Survival curves were computed using Kaplan–Meier methods and compared by log-rank (Mantel–Cox) tests. Univariate analysis of predictors was performed using a Cox regression model, and significant (p < 0.15) factors were subsequently entered into a multivariate Cox model to identify independent predictors of overall survival (OS) and recurrence-free survival (RFS). CA19-9 and CTC values had skewed distributions originally and were log-transformed to a normal distribution for use in survival analysis. A two-tailed p value < 0.05 was considered statistically significant throughout the study. All statistical analyses and calculations were performed with the assistance of R version 3.3.2 (The R Project, Vienna, Austria).
RESULTS
Patient Characteristics
We approached 138 patients, of whom 126 were enrolled successfully, as outlined in the Methods section (Fig. 1). The clinical, laboratory, radiologic, and treatment characteristics of the 126 patients are summarized in Table 1. For the 100 patients with a diagnosis of PDAC, on cross-sectional imaging at the time of blood draw, the median tumor diameter was 3.5 cm [interquartile range (IQR) 2.2–4.0]. Of patients with local disease only (n = 71), 31 (43.7%) demonstrated involvement of the mesenteric vessels and were classified as locally advanced. Thus, by cross-sectional imaging, 9% had AJCC stage 1 disease, 31% had stage 2 disease, 31% had stage 3 disease, and 29% had stage 4 disease (Fig. 1).
FIG. 1.
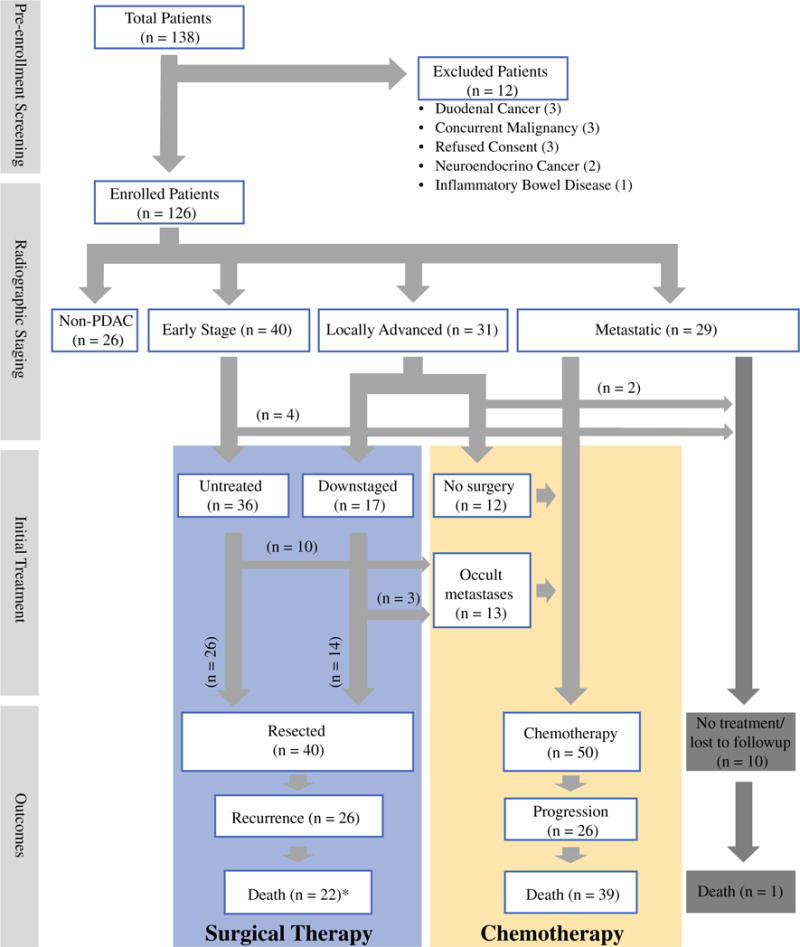
Patient enrollment process, radiographic staging, initial treatment decisions, and outcomes for all patients. *Of the 22 patients who died, 20 had experienced a recurrence prior to death. PDAC pancreatic ductal adenocarcinoma
TABLE 1.
Clinical and pathologic characteristics of patients (n = 126)
| Characteristics | Data | CTC count [median (IQR)] |
|---|---|---|
| Patient | ||
| Age, years [median (IQR)] | 67 (61–73) | – |
| Female [n (%)] | 59 (46.8) | – |
| Diagnosis [n (%)] | ||
| PDAC | 100 (79.4) | 2 (1–6) |
| IPMN [SB/MD] | 13 [5/8] (10.3) | 0 |
| Serous cyst | 4 (3.2) | 0 |
| Chronic pancreatitis | 3 (2.4) | 0 |
| MCN | 2 (1.6) | 0 |
| PANIN | 2 (1.6) | 0 |
| Pseudocyst | 1 (0.8) | 0 |
| Complex cyst | 1 (0.8) | 1 |
| JCC stage at draw [n (%)]a | ||
| 1 | 9 (9) | 0 (0–1) |
| 2 | 31 (31) | 5 (1–8) |
| 3 | 31 (31) | 3 (0–7) |
| 4 | 29 (29) | 8 (4–12) |
| CA19-9 [median (IQR)]b | 139 (24–1010) | – |
| Surgical characteristicsc | ||
| Downstaged [n (%)] | 18 (36.7) | 2 (1–5) |
| Planned procedure [n (%)] | ||
| Whipple | 37 (69.8) | 2 (1–3) |
| Distal pancreatectomy | 16 (30.2) | 1 (0–2) |
| Occult metastatic disease [n (%)] | 13 (24.5) | 7 (3–13) |
| Metastatic location [n (%)] | ||
| Liver | 6 (46.2) | 10 (4–17) |
| Intraperitoneal | 7 (53.9) | 4 (1–9) |
| Pathologic characteristicsd | ||
| Tumor diameter [median (IQR)] | 3.5 (2.2–4.0) | – |
| LVI [n (%)] | 21 (52.5) | 2 (0–3) |
| PNI [n (%)] | 36 (90.0) | 2 (1–4) |
| Grade | ||
| Well | 4 (10.3) | 3 (2–4) |
| Moderate | 27 (69.2) | 1 (0–3) |
| Poor | 8 (20.5) | 1 (1–4) |
| Indeterminate | 1 (2.5) | 0 (0–0) |
| Lymph node positive [n (%)] | 28 (70.0) | 1 (1–3) |
CTC circulating tumor cell, IQR interquartile range, PDAC pancreatic ductal adenocarcinoma, IPMN intraductal papillary mucinous neoplasm, SB side branch, MD main duct, AJCC American Joint Committee on Cancer, CA19-9 cancer antigen 19-9 LVI lymphovascular invasion, PNI perineural invasion, MCN mucinous cystic neoplasm, PANIN pancreatic intraepithelial neoplasia
n = 100 with PDAC
n = 90 with CA19-9 available
n = 53 taken to surgery
n = 40 with pathologic information
Of the 53 patients taken to the operating room for potentially curative surgery, 17 (32.1%) had received chemotherapy prior to the operation. The blood draws for all surgical patients occurred on the day of surgery prior to induction of anesthesia. The operative plan included a Whipple procedure in 37 (69.8%) patients and a distal pancreatectomy in 16 (30.2%) patients. Furthermore, 13 (24.5%) of the 53 patients were found to have metastatic disease not detected on cross-sectional imaging intraoperatively, referred to as occult metastatic disease (Fig. 1). Of these 13 patients, 3 (23.1%) cases were discovered during diagnostic laparoscopy and the rest during exploratory laparotomy. The site of metastasis was the liver in 6 (46.2%) patients, and other peritoneal sites in 7 (53.9%) patients. All 13 patients subsequently underwent chemotherapy. Thus, 50 patients were treated with palliative chemotherapy as their primary treatment, 25 with metastatic disease, 13 with occult metastatic disease, and 12 with locally advanced disease.
Enumeration of Circulating Tumor Cells (CTCs)
CTC counts were assessed from 4 mL of VB for all patients. CTCs were found in 78/100 (78%) PDAC patients, and one or more CTCs per 4 mL of VB were detected in 4/9 (44.4%) stage I, 23/31 (74.2%) stage II, 24/31 (77.4%) stage III, and 27/29 (93.1%) stage IV PDAC patients (Fig. 2a). CTC count correlated with increasing stage of disease (ρ = 0.42, p < 0.001) For patients with non-malignant pancreatic disease, 1/26 (3.8%) patients was found to have a single CTC. This patient had a 6 cm complex cyst, non-diagnostic cyst fluid analysis, and a lack of malignant cells on fine-needle aspiration. The patient was followed for over 4 years and did not demonstrate any signs of invasive disease. No other patients with benign pancreatic disease developed PDAC over the course of the study. CTC counts did not correlate with CA19-9 levels (ρ = 0.20, p = 0.064), or with age or sex (data not shown).
FIG. 2.
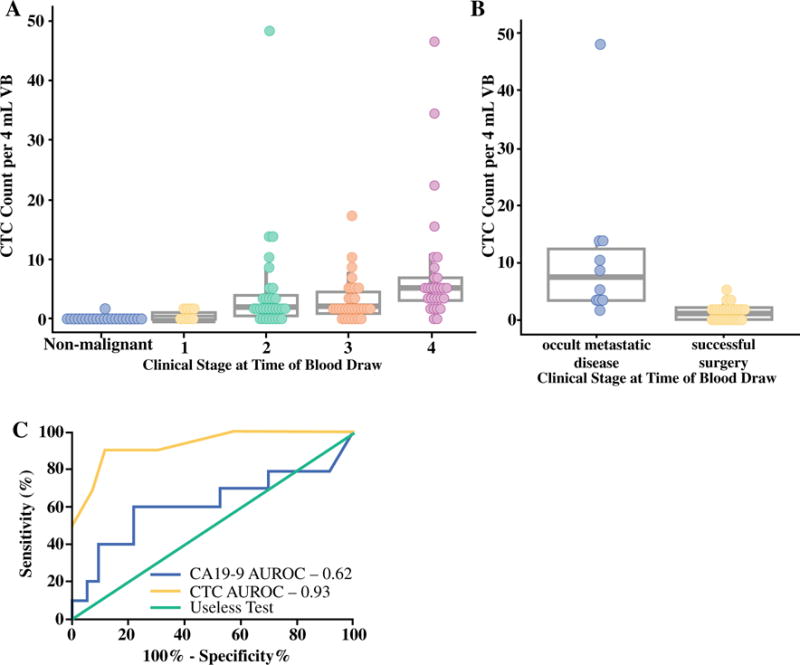
a CTC count by pretreatment radiographic AJCC stage of disease. Non-malignant refers to the 26 patients with non-malignant pancreatic diseases outlined in Table 1. b Performance of CTCs as a preoperative predictor of occult metastatic disease in the subset of patients with untreated tumors (n = 36). CTC enumeration is displayed for patients with successfully resected tumors versus those found to have occult metastatic disease intraoperatively. c Comparison of the performance of CTCs and CA19-9 in the preoperative detection of patients with occult metastatic disease in the subset of patients with untreated tumors (n = 36). CTC circulating tumor cell, AJCC American Joint Committee on Cancer, VB venous blood, CA19-9 cancer antigen 19-9, AUROC area under the receiver operating characteristic curve
CTCs as a Predictive Biomarker for Surgical Resection
We evaluated CTC enumeration as a preoperative biomarker predicting the presence of occult metastatic disease among the 53 patients taken for potentially curative surgery. Occult metastatic disease was found in 13 (24.5%) patients intraoperatively, and these patients had a median of seven CTCs (IQR 3–13), versus one CTC (IQR 0–2) in patients with localized disease (p < 0.0001). Among the 36 patients without prior chemotherapy, a median of eight CTCs (IQR 3–12) were found in the 10 patients with occult metastatic disease, versus one CTC (IQR 0–2) in the 26 patients with localized disease (p < 0.0001) (Fig. 2b). For patients who received downstaging chemotherapy, the results were similar, with a median CTC count of seven (IQR 4–12) for patients with occult disease, versus one CTC (IQR 1–5) for those with localized tumors.
For all surgical patients, at the optimized cut-off of three or more CTCs/4 mL of VB, CTCs were able to preoperatively distinguish patients with occult metastatic disease from those with potentially curable, localized tumors, with a sensitivity of 85%, specificity of 80%, PPV of 94%, NPV of 58%, and AUROC of 0.82 (95% confidence interval [CI] 0.76–0.98, p < 0.0001). Similarly, for the untreated patients only (n = 36), three or more CTCs/4 mL of VB distinguished patients with occult metastatic disease, with a sensitivity of 90%, specificity of 88%, PPV of 96%, NPV of 75%, and AUROC of 0.93 (95% CI 0.83–1.00, p < 0.001) (Fig. 2c). For comparison, CA19-9, at the optimum cut-off of 500 U/mL, distinguished patients with potentially curable tumors from those with advanced disease, with a sensitivity of 60.0%, specificity of 77.3%, PPV of 81.0%, NPV of 54.5%, and AUROC of 0.62 (95% CI 0.37–0.87, p = 0.084) (Fig. 2c).
Outcomes and Survival Analysis
Patients were followed for a minimum of 24 months after blood draw, and the median survival time for all PDAC patients was 16.8 months (IQR 7.4–33.3). At the time of analysis, 38/100 (38.0%) remained alive. OS for patients with occult metastatic disease was nearly identical to patients with radiologically apparent metastatic disease, and was significantly shorter than that of patients who underwent successful resection [hazard ratio (HR) 3.6, 95% CI 1.75–7.73, p < 0.001] (Fig. 3a). We performed a univariate analysis of potential preoperative indicators of prognosis, including CTC count, CA19-9 level, and radiographic indicators (Table 2). For all patients, regardless of stage, CTC count was strongly associated with survival from the time of blood draw (HR 1.69, 95% CI 1.28–2.25, p < 0.001) (Fig. 3b). Additional factors associated with shorter survival on univariate analysis included age, presence of metastatic disease, and CA19-9 level (Table 2). To determine the subset of factors that provided independent information on survival time, a Cox proportional hazards model was developed. We used multivariate Cox regression analysis at a stringency level of p < 0.15 on univariate analysis to include factors. The factors that produced the strongest independent association with survival time were age (per year; HR 1.04, 95% CI 1.01–1.07, p = 0.009), presence of metastatic disease (HR 2.04, 95% CI 1.11–3.74, p = 0.022), and CTC count (per log unit; HR 1.38, 95% CI 1.01–1.88, p = 0.040) (Table 2). We next limited our analysis to just the 53 patients taken for potentially curative surgery. Again, CTCs were found to be an independent predictor of OS (HR 2.48, 95% CI 1.32–4.67, p = 0.0048).
FIG. 3.
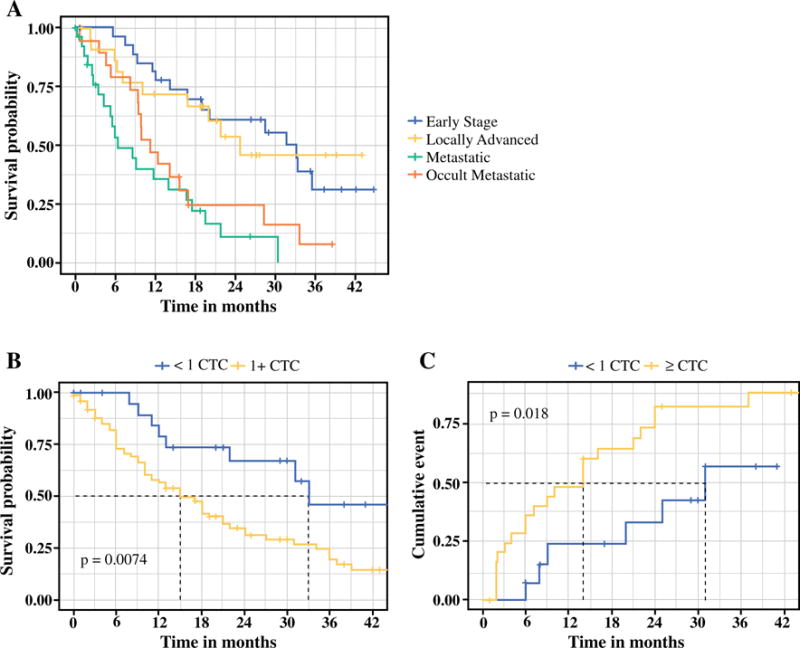
a Overall survival for all patients diagnosed with PDAC in the study, separated into subsets of patients with resected tumors (n = 40, blue), locally advanced disease treated palliatively (n = 12, yellow), occult metastatic disease (n = 12, orange), or metastatic disease (n = 25, green). Patients with occult metastatic disease had survival similar to patients with radiologically visible metastatic disease, and significantly different from patients with early-stage or locally advanced disease. b Overall survival for all patients stratified by CTC count (n = 100). c Time to recurrence for patients after surgical resection stratified by CTC count (n = 40). CTC circulating tumor cell, PDAC pancreatic ductal adenocarcinoma
TABLE 2.
Univariate and multivariate analysis for predictors of overall survival (n = 100)
| Predictor | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Clinical predictors | ||||||
| Age, per year | 1.03 | 1.01–1.06 | 0.011 | 1.04 | 1.01–1.07 | 0.009 |
| Male sex | 0.89 | 0.54–1.47 | 0.64 | – | – | – |
| Preoperative predictors | ||||||
| Radiographic | ||||||
| Mesenteric vessel involvement | 2.25 | 0.51–9.84 | 0.28 | – | – | – |
| Metastatic disease | 6.61 | 1.55–28.12 | 0.011 | 2.04 | 1.11–3.74 | 0.022 |
| Tumor location | ||||||
| Head | – | – | – | – | – | – |
| Tail | 1.48 | 0.66–3.30 | 0.34 | – | – | – |
| Tumor diameter, per cma | 1.00 | 0.86–1.17 | 0.96 | – | – | – |
| Laboratory | ||||||
| CA19-9, per log unitb | 1.09 | 0.97–1.21 | 0.14 | 1.09 | 0.98–1.22 | 0.12 |
| CTC count, per log unit | 1.69 | 1.28–2.25 | < 0.001 | 1.38 | 1.01–1.88 | 0.040 |
HR hazard ratio, CI confidence interval, CA19-9 cancer antigen 19-9, CTC circulating tumor cell
n = 91
n = 90
Recurrence
Of the 40 patients successfully resected, 26 (65%) developed a recurrence (Fig. 3c) and 22 (55%) died, with a median survival for all resected patients of 28.5 months. Median survival was 33.1 months in preoperatively untreated patients versus 21.7 months in patients receiving neoadjuvant chemotherapy prior to resection; however, the difference in median survival was not statistically significant (p = 0.33). Univariate preoperative predictors of PDAC recurrence when accounting for the competing risk of non-cancer-related death are shown in electronic supplementary Table 1 On univariate analysis, tumors were more likely to recur in patients who received downstaging chemotherapy (HR 3.34, p = 0.001) and patients with higher CTC counts (per log unit; HR 2.65, p = 0.017). When the analysis was limited to the subset of early-stage patients who did not receive neoadjuvant therapy, CTC counts were no longer associated with shorter time to recurrence (HR 2.57, p = 0.08). On multivariate analysis, CTC counts were not found to be independently predictive of shorter time to recurrence (electronic supplementary Table 1).
DISCUSSION
The combination of late presentation and resistance to systemic therapy makes PDAC one of the most deadly malignancies; however, the recent successes of multidrug regimens and the anecdotal success of early-phase trials of new targeted and immune therapies may finally change this paradigm.14 Tumor staging by cross-sectional imaging alone routinely understages PDAC patients, as evidenced by high recurrence rates and low survival benefit of curative-intent surgery. Furthermore, multiple studies have demonstrated that a ‘surgery first’ paradigm for borderline resectable, and even early-stage, patients does not result in a survival benefit and may in fact decrease survival.3,6 Additionally, for borderline resectable patients who have undergone neoadjuvant chemotherapy, studies have demonstrated that cross-sectional imaging is no longer an accurate predictor of the extent of local disease.15 Thus, there is a pressing need for new biomarkers to complement cross-sectional imaging, in particular for the identification of patients likely to benefit from non-surgical treatments first.
In the present study, we investigated CTCs as a biomarker for staging and prognostication of PDAC. We enrolled patients at all stages of disease undergoing a variety of treatment modalities and determined how CTC enumeration correlated with clinicopathologic variables, surgical therapy, and survival outcomes. The current study builds on our previous investigation of CTCs as a diagnostic biomarker10 by demonstrating the prognostic and predictive ability of CTCs for PDAC patients. We believe that our most significant finding was the predictive ability of CTCs for occult metastatic disease in the preoperative setting. Using the cut-off of three or more CTCs, we could differentiate patients with occult metastatic disease from those with localized, potentially curable tumors (AUROC 0.93 for untreated patients). This represents a significant improvement over the only widely available biomarker in PDAC, serum CA19-9 (AUROC 0.619). Radiographic criteria, including tumor size and mesenteric vessel involvement, were also not predictive of occult metastatic disease. Importantly, the survival curves of patients with occult metastatic disease reflected that of patients with radiographically detectable metastatic disease and not that of early-stage patients or even locally advanced patients who underwent downstaging chemotherapy (Fig. 3a). While our study is small, the rate of occult metastatic disease in our patient cohort (13/53, 24.5%) was similar to the average rate for all patients taken to surgery at our institution between 2008 and 2013 (53/192, 27.6%). Thus, we believe our cohort is representative and that CTCs may prove useful as a predictive biomarker in the difficult decision of pursuing local versus systemic therapy as an initial treatment modality. However, our study was not able to determine if CTC counts predicted patients who were likely to respond to neoadjuvant therapy, or if neoadjuvant therapy provided a survival benefit for patients in our study. In addition to their predictive significance for occult metastatic disease, we found CTCs to have prognostic value at all stages of disease and for both surgical and systemic therapies. Like previous studies in PDAC, as well as other solid tumors, CTCs were associated with worse outcomes and were an independent predictor of OS for all patients in our cohort.16–20
To our knowledge, our study is the largest to date utilizing a microfluidic platform for CTC enumeration in PDAC; however, its size is still limited for subgroup analyses of surgical outcomes. Our median CTC count for PDAC patients was 2 (IQR 1–6), lower than that found using other techniques such as filters or flow cytometry.16,20–22 One possibility is that our use of only 4 mL of VB may be too low to accurately establish the number of CTCs, especially in early-stage patients. In future studies we will assess the utility of larger blood volumes.
CONCLUSIONS
CTCs detected using the NanoVelcro platform show promise as a prognostic biomarker for PDAC patients at all stages of disease. Furthermore, CTCs demonstrate potential as an adjunctive biomarker to detect potential occult metastatic disease in patients with local disease being considered for surgical resection. Given the high recurrence rate after surgical resection, we see a potential application for surgical decision making, especially in the subset of patients already at increased risk of recurrence, such as those with borderline resectable tumors at high risk of having occult metastatic disease.
Supplementary Material
Acknowledgments
This work was funded in part by a University of California Los Angeles (UCLA) Jonsson Comprehensive Cancer Center Impact Grant, as well as an award provided by NantOmics. The authors would like to thank Dr. William Isacoff, Dr. Howard Reber, Dr. Joe Hines, and Dr. Timothy Donahue for assistance in obtaining patient samples. The NanoVelcro chips used in this research were supported by research Grant (R33 CA174562 and U01 CA198900) and a Small Business Innovation Research (SBIR) grant (R44 CA180482) from the National Institutes of Health.
Footnotes
Electronic supplementary material: The online version of this article (https://doi.org/10.1245/s10434-017-6290-8) contains supplementary material, which is available to authorized users.
DISCLOSURES
The intellectual property that is associated with this study has been licensed to CytoLumina Technologies Corp. Hsian-Rong Tseng has financial interests in this company, given his roles as one of the company’s co-founders. Colin M. Court, Jacob S. Ankeny, Shonan Sho, Paul Winograd, Shuang Hou, Min Song, Zev A. Wainberg, Mark D. Girgis, Thomas G. Graeber, Vatche G. Agopian, and James S. Tomlinson have no disclosures to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubert CR, Bergquist JR, Groeschl RT, Habermann EB, Wilson PM, Truty MJ, et al. Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: an intention to treat analysis of the National Cancer Database. Surgery. 2016;160(4):1080–96. doi: 10.1016/j.surg.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest. 2009;13(7):1337–44. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.5081. https://doi.org/10.1200/JCO.2016.68.5081. [DOI] [PubMed]
- 7.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 8.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–31. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 9.Court CM, Ankeny JS, Sho S, Hou S, Li Q, Hsieh C, et al. Reality of single circulating tumor cell sequencing for molecular diagnostics in pancreatic cancer. J Mol Diagn. 2016;18(5):688–96. doi: 10.1016/j.jmoldx.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankeny JS, Court CM, Hou S, Li Q, Song M, Wu D, et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114(12):1367–75. doi: 10.1038/bjc.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin M, Edge S, Greene F. AJCC cancer staging manual. 8th. Cham: Springer; 2017. [Google Scholar]
- 12.Lu YT, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, et al. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods. 2013;64(2):144–52. doi: 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–34. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 15.Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg. 2011;146(7):836–43. doi: 10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]
- 16.Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–16. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidard FC, Huguet F, Louvet C, Mineur L, Bouche O, Chibaudel B, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24(8):2057–61. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann K, Kerner C, Wilfert W, Mueller M, Thiery J, Hauss J, et al. Detection of disseminated pancreatic cells by amplification of cytokeratin-19 with quantitative RT-PCR in blood, bone marrow and peritoneal lavage of pancreatic carcinoma patients. World J Gastroenterol. 2007;13(2):257–63. doi: 10.3748/wjg.v13.i2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Albuquerque A, Kubisch I, Breier G, Stamminger G, Fersis N, Eichler A, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology. 2012;82(1):3–10. doi: 10.1159/000335479. [DOI] [PubMed] [Google Scholar]
- 20.Poruk KE, Blackford AL, Weiss MJ, Cameron JL, He J, Goggins M, et al. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(11):2681–90. doi: 10.1158/1078-0432.CCR-16-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamande JW, Hupert ML, Witek MA, Wang H, Torphy RJ, Dharmasiri U, et al. Modular microsystem for the isolation, enumeration, and phenotyping of circulating tumor cells in patients with pancreatic cancer. Anal Chem. 2013;85(19):9092–9100. doi: 10.1021/ac401720k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Court CM, Ankeny JS, Hou S, Tseng HR, Tomlinson JS. Improving pancreatic cancer diagnosis using circulating tumor cells: prospects for staging and single-cell analysis. Expert Rev Mol Diagn. 2015;15(11):1491–1504. doi: 10.1586/14737159.2015.1091311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


