Abstract
The Androgen Receptor (AR), a member of the steroid hormone receptor family, plays important roles in the physiology and pathology of diverse tissues. AR ligands, which include circulating testosterone and locally synthesized dihydrotestosterone, bind to and activate the AR to elicit their effects. Ubiquitous expression of the AR, metabolism and cross reactivity with other receptors limit broad therapeutic utilization of steroidal androgens. However, the discovery of selective androgen receptor modulators (SARMs) and other tissue-selective nuclear hormone receptor modulators that activate their cognate receptors in a tissue-selective manner provides an opportunity to promote the beneficial effects of androgens and other hormones in target tissues with greatly reduced unwanted side-effects. In the last two decades, significant resources have been dedicated to the discovery and biological characterization of SARMs in an effort to harness the untapped potential of the AR. SARMs have been proposed as treatments of choice for various diseases, including muscle-wasting, breast cancer, and osteoporosis. This review provides insight into the evolution of SARMs from proof-of-concept agents to the cusp of therapeutic use in less than two decades, while covering contemporary views of their mechanisms of action and therapeutic benefits.
Keywords: Androgens, Selective androgen receptor modulators (SARMs), coactivators, kinase, muscle wasting, Duchenne muscular dystrophy (DMD), breast cancer, stress urinary incontinence
Introduction
The androgen receptor (AR) is one of the 49 members of the steroid receptor family of ligand-activated transcription factors (1). The steroid or nuclear hormone receptors play pivotal roles in the organogenesis, physiology, and pathology of a variety of tissues (1,2). These receptors are promiscuously activated by wide-ranging ligands, including natural hormones, growth factors, peptides, or synthetic molecules (3–5). The steroid receptor family is organized into three classes (1). Class I is comprised of receptors for hormones such as androgens, progestins, estrogens, and corticosteroids. Receptors for vitamins and thyroid hormones belong to class II, while receptors for bile acids and for which natural ligands have not yet been identified are relegated to class III.
Hormone signaling is under tight control with several context specific means of regulating both the potency of a hormone response and the cellular outcome of hormone-receptor interactions. Hormone signaling is modulated by the expression of various metabolic enzymes (6–8), expression of coactivators and corepressors (9,10), and the activity of various kinases and growth factors. For example, the estrogens have beneficial effects in bone and brain, while having growth-promoting effects in uterus and breast (11–14). Activation of the estrogen receptor (ER) by the principal circulating estrogen, estradiol, may not only result in the prevention or treatment of osteoporosis, but may concurrently cause mammary and uterine hyperplasia. Although both the beneficial and the growth-promoting effects arise from agonistic activities of estrogens, the tissue of action determines whether the effect is beneficial or detrimental. As it is ideal to have targeted therapeutic effects, the ubiquitous expression of receptors presents a therapeutic challenge and precludes wider use of exogenous hormone administration. To circumvent the limitations resulting from global receptor activation, researchers sought ligands, referred to as Selective Receptor Modulators (SRMs) that activate receptors in a tissue-specific manner. Selective Estrogen Receptor Modulators (SERMs) were the first SRMs to be characterized and developed (15). Tamoxifen and raloxifene are classical examples of SERMs that function as antagonists in the breast, but as agonists in the uterus or bone, respectively, either directly or through metabolic conversion (16–19). Decades after the discovery of SERMs, Selective Androgen Receptor Modulators (SARMs) (20) were first described and subsequently developed to facilitate tissue-selective activation of the AR. This was followed by the discovery of Selective Glucocorticoid Receptor Modulators (SGRMs) (21), Selective Progesterone Receptor Modulators (SPRMs) (22) and others. Recently, a tissue-selective Farnesoid X receptor modulator was discovered with potential as a treatment for metabolic diseases (23), further increasing the number of tissue-selective nuclear receptor modulators available for therapeutic purposes.
Structure of the AR
Eight exons code for a 90 KB AR gene located in the X chromosome (24). The AR is expressed in diverse tissues such as skeletal muscle, testes, prostate, breast, uterus, and others (25). Unlike the Progesterone Receptor (PR) and the ER, the AR does not have endogenously expressed well-characterized isoforms. The AR is comprised of four distinct domains. The N-terminal domain (NTD) of the AR (spanning from amino acids 1–559) is the least homologous domain among the class I members, with a homology of less than 15–20% (26,27). The activation function-1 (AF-1) domain located in the NTD plays a pivotal role in AR’s function (27). Deletion of the AR-AF-1 leads to a significant loss in AR’s transcriptional capacity (26,28–30). The AF-1 of AR contains all, save three, of AR’s phosphorylation sites, and hence is the target of various growth factors (31) that phosphorylate the sites and activate the AR ligand independently (32). The DNA Binding Domain (DBD) is highly conserved between receptors, has two zinc finger motifs that are responsible for DNA recognition and dimerization, and plays a role in AR binding to Androgen Responsive Elements (ARE) within the regulatory regions of androgen responsive genes. The hinge region that lies between the DBD and the Ligand Binding Domain (LBD) is a lysine rich region and is important for the nuclear localization of the receptor (33). Deletion of this domain eliminates nuclear localization and transcriptional activity of the AR in the presence of ligand (34). The LBD of the AR is responsible for ligand binding, is only moderately conserved among the receptors, and contains AF-2 which is important for the ligand-dependent full activation of the receptor.
Selective Androgen Receptor Modulators (SARMs)
The AR and its endogenous ligands, androgens, are important for development and maintenance of muscle and bone, secondary sexual organs, and development of other tissues (35). Although androgens are important for normal development of various tissues, under certain circumstances they also promote pathology of the prostate, heart, and the liver. Risks of testosterone therapy such as dyslipidemia, benign prostatic hypertrophy, and uterine hyper-proliferation preclude its use. These pathological roles of testosterone and its 5α-reduced form (DHT) led to the search for tissue-selective agonists of the AR that could potentially activate the AR in selected tissues while sparing other tissues such as prostate, heart, and liver. Such an agonist would provide an opportunity to fully realize the therapeutic benefits of androgens. Most of the SARMs developed thus far are non-steroidal and have the ability to activate the AR in muscle and bone, without accompanying activation or minimal activation of the AR in prostate or seminal vesicles.
In a similar vein, SARM development has also sought to overcome the potential virilizing effects of steroidal androgens (36). Considering that females, like males, are also affected by osteoporosis, sarcopenia, and cachexia, a non-virilizing SARM could treat these pathological states in women, without the virilizing side-effects accompanying steroidal androgens. The putative beneficial effects of testosterone therapy in certain female populations appear to be outweighed by the risks of virilization and poorly characterized cardiovascular risk. Recent clinical trials, although highlighting testosterone’s ability to improve sexual function and muscle mass in older men, corroborated concerns that testosterone’s cardiac risks outweighed its therapeutic benefits (37,38).
Discovery of SARMs and structural diversity: (20)
The first preclinical evidence for tissue-selective activation of the AR was that arylpropionamide SARMs increased levator ani muscle weight in castrated rats to the level of sham-operated rats, but only partially increased the prostate and seminal vesicles weight (39,40). This model, called the Hershberger assay, has been the primary means of demonstrating tissue selectivity in SARM discovery. Although the use of levator ani muscle as a surrogate end point for anabolic activity in skeletal muscle is criticized due to its unparalleled expression of the AR, it provides a sensitive and rapid assessment of anabolic effects. Over the next decade structure-activity relationship studies were conducted on the arylpropionamide class of SARMs that culminated in two clinical candidates, with enobosarm being the most advanced in clinical development (40,41). In addition to their effects on muscle, enobosarm and other arylpropionamide SARMs also demonstrated beneficial effects on bone (42). Enobosarm has been or is being evaluated in several phase II and phase III clinical trials for multiple indications (43–45).
Ligand Pharmaceuticals developed tricyclic quinolinones that coincided with the discovery of arylpropionamide SARMs (46,47). Similar to the arylpropionamide SARMs, these quinolinones also bind to and activate the AR in low nanomolar concentrations while eliciting tissue-selective activation of the AR in muscle.
Based on structure activity relationship of several SARM templates, Ligand Pharmaceuticals chose LGD2226 as their first clinical candidate (48). Although LGD2226 demonstrated myo- and osteo-anabolic activity and maintenance of sexual function in various preclinical models, the development of LGD2226 was discontinued. Ligand advanced another molecule LGD2941 to phase I clinical trials for frailty and osteoporosis in collaboration with Takeda Abbott pharmaceuticals. To date, there isn’t any evidence that this and other SARMs developed by Ligand have advanced further.
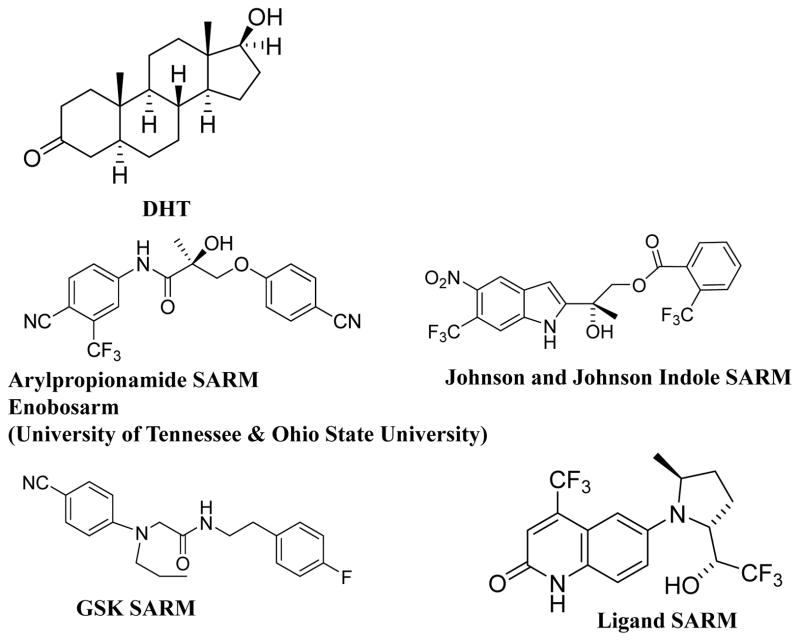
Other companies such as Merck (49), Glaxo, Johnson and Johnson, Orion, and Pfizer all pursued SARM development during the late 1990s and the first decade of 2000s. Preclinical results from each of the optimized scaffolds demonstrated potent tissue-selectivity and anabolic activity. However, most, if not all, of these compounds failed to advance to clinical development either due to toxicity or lack of efficacy, or other undisclosed reasons. The structure of various SARM templates is provided in Figure 1.
Figure 1.
Structures of selected SARMs from various pharmaceutical companies.
Mechanisms for SARM tissue-selectivity
The molecular mechanisms underlying the separation of the detrimental androgenic activities (e.g., virilization/prostatic hypertrophy) from the desired anabolic effects remain unknown. While the SERMs have been investigated for decades, studies to characterize the molecular mechanisms of action of SARMs have only been initiated more recently. Several mechanisms such as the coactivator-corepressor ratio and tissue-selective modulation of signaling pathways that have been established as mechanisms for the tissue-selectivity of SERMs are also applicable to the SARMs (50,51). The tissue-selective expression of ER-isoforms, (ER-α and ER-β), that often have distinct responses to ligand and thereby provide tissue-selective modulation of ER function by SERMs is unique to SERMs (52). A variety of other molecular mechanisms likely contribute to the observed tissue-selectivity of SARMs.
Enzymes
Although the tissue-specific expression of various steroidogenic and metabolic enzymes does not sufficiently explain characterized differences between the pharmacology of steroidal androgens and SARMs, it can account for the amplification and inactivation of steroidal androgens observed in some tissues (53). One of the enzymes, 5α-reductase, converts testosterone into more potent DHT in some tissues (e.g., prostate and skin) but not others (e.g., muscle and bone), representing a tissue specific amplification of steroidal ligands in prostate and other tissues that does not contribute to nonsteroidal SARM action.
Expression of other enzymes belonging to hydroxysteroid dehydrogenase (HSD) class regulates the availability of steroidal androgens to the AR in a variety of tissues. The enzymes 3-α HSD and 3-β HSD convert DHT to 3-α and 3-β diols, respectively (54). Both of these DHT metabolites are weaker AR ligands compared to testosterone or DHT. Considering that the 3-α and 3-β HSDs are bifunctional enzymes, the tissue-specific expression and directionality of function ultimately regulate AR activity. Other androgen-synthesizing enzymes 17-β HSD types 3 and 5 are highly expressed in testes and prostate and they play important roles in synthesizing testosterone from precursor androstenedione (55,56). Interestingly, most SARMs developed are non-steroidal and are not thought to be susceptible to enzymatic metabolism in target tissues and hence retain their activity in all the tissues wherever they are bioavailable.
Coregulator Function
More than 200 AR-interacting proteins (both coactivators and corepressors) (57,58), including those with intrinsic functions such as histone acetyl transferase activity (SRCs, CBP) (50), histone deacetylase activity (NCoR, SMRT) (50), and other chromatin modifying functions (SWI/SNF/BRG) (50), have been identified. In addition to several coactivators that are shared by other steroid receptors, a few coactivators such as the ARA family members that exclusively activate the AR have been reported to be expressed in tissues such as prostate (59,60). The AR differs from other receptors in its interaction with coregulators. The LBD of the AR has 11 anti-parallel helices, unlike other receptors that contain 12 helices as the AR is devoid of helix 2, that undergo significant rearrangement upon ligand binding creating a shallow hydrophobic pocket to facilitate association with the NTD of the AR or coactivators containing an LxxLL motif (61,62). While the coactivator interaction surface in other class I receptors exists in the AF-2 in the LBD, the coactivator interaction surface in the AR exists in the AF-1 (58,63).
Earlier studies with SERMs identified that distinct chemical scaffolds have the ability to induce unique conformational changes in the ER, resulting in interaction with different cofactor subsets. The same has been demonstrated with SARMs, which induce distinct conformational changes compared to testosterone and DHT, resulting in complexes containing different coregulator complexes (64). Using combinatorial peptide-phage display, McDonnell and his colleagues elegantly showed that different ligands induce distinct AR and ER conformations leading to their association with varying coactivator peptides (64,65). The SARMs RTI-018 and RTI-001 possessed a spectrum of agonist activities and altered kinetics of response and these differences were attributed to SARM-mediated structural differences leading to the association of the AR with coactivator peptides distinct from DHT (66).
In another study, the antagonist bicalutamide recruited corepressors SMRT and NCoR, butin the presence of IL-8 recruited coactivators thus adopting agonist features (67). This study confirms the findings that changes in conformation induced by an external stimulus can result in a shift in the transcriptional complex formed by the AR resulting in a change in activity.
The role of coregulators in the tissue-selective action of synthetic molecules was deduced by O’Malley and colleagues. RU486 or Mifepristone, a PR antagonist, demonstrated cell-line-specific agonist/antagonistic activities that resulted from the coactivator/corepressor ratio available in the respective cell lines (68). The same was also shown with tamoxifen, whose function was governed by the coactivator/corepressor ratio in breast cancer and uterine cell lines (51,69).
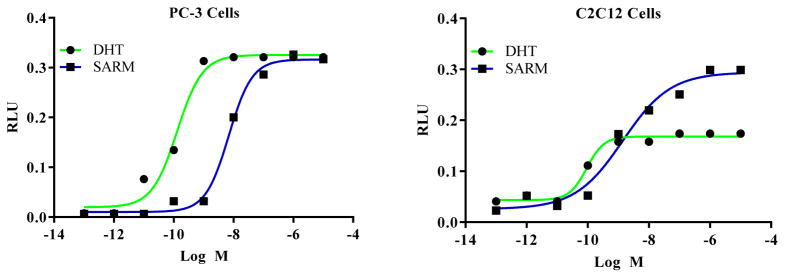
Previously unreported data from our group supports the role of coactivators in the tissue-selective function of arylpropionamide SARMs (Figure 2). Transactivation studies performed in C2C12 muscle cells and in prostate cancer PC3 cells in the presence of the coactivator SRC-1indicate that while SRC-1 had a similar impact on SARM- and DHT-induced AR transactivation in PC-3 cells, SRC-1 robustly increased SARM but not DHT-induced AR transactivation in C2C12 cells. This data suggests that the conformational change induced by the SARM facilitates the interaction of the AR with coactivators in muscle cell environments, while DHT-induced conformation facilitates the interaction of coactivators with the AR in the prostate cellular environment.
Figure 2.
PC-3 (prostate cancer cells) or C2C12 (muscle cells) cells were transfected with CMV-hAR, GRE-LUC, CMV-LUC, and coactivator SRC-1 using Amaxa electroporator. Cells were treated 24 hours after transfection and luciferase assay was performed 48 hours after transfection.
The results show that while SARM increased the AR transactivation significantly in C2C12 cells even at lower concentrations (1 nM), it increased the AR transactivation in prostate cancer cells only at higher concentrations (10 nM).
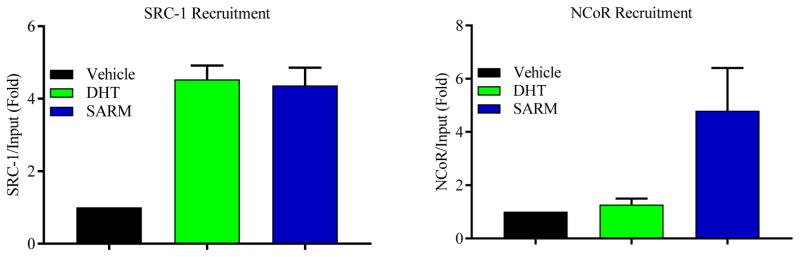
We also evaluated the recruitment of the AR, coactivators, and corepressors to the androgen response elements (AREs) located on the PSA enhancer in LNCaP cells (Figure 3). While DHT robustly recruited the coactivator SRC-1, but not corepressor NCoR, to the PSA enhancer, an arylpropionamide SARM recruited both SRC-1 and NCoR to the PSA enhancer. This suggests that the SARMs form a complex in the prostate cellular environment comprised of both coactivators and corepressors that will prevent maximal activation of the AR in this androgenic tissue. Alternatively, the SARMs and steroidal androgens utilize coactivators in anabolic tissues to promote maximal activation of the AR. These results and associated literature evidence are summarized in Figure 4 as a model to demonstrate the role of coregulators in SARM action.
Figure 3. Recruitment of both coactivator and corepressor in a SARM complex.
LNCaP cells were serum starved for 2 days and treated with vehicle, 10 nM DHT, or 100 nM arylpropionamide SARM for 4 hours. Cells were fixed with 1% formaldehyde to cross-link protein:DNA complex and immunonoprecipitation was performed with SRC-1 or NCoR antibodies. DNA:protein complex crosslink was reversed, DNA extracted, and realtime PCR with primers and probes specific to the PSA enhancer ARE was performed.
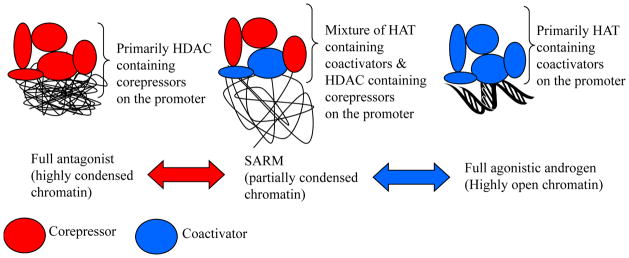
Figure 4. Model.
While a full agonist such as DHT promotes a full agonistic conformation in prostate environment by interacting with coactivators, a SARM promotes a partial agonistic conformation by promoting a complex that contains coactivators and corepressors.
Differences in the transcriptional complex formed in response to ligand binding also modulate the rate of nuclear translocation and nuclear export. We identified that within the same structural scaffold, SARMs with minor structural modifications have completely distinct nuclear translocation potential, demonstrating that the conformational change induced by these SARMs has resulted in varying nuclear translocation rate (70).
Intracellular Signaling Cascades
From ligand sensitization to translation of genes, all cellular processes are dependent on the intracellular levels and activity of partners involved in the signaling pathway. These signaling events lead to critical post-translational modifications including phosphorylation, sumoylation, ubiquitination and others that are important for the function of the receptor. The intracellular levels of kinases and phosphatases and the availability of growth factors promote the genomic, non-genomic, and ligand-independent activation of the AR.
Similar to the coactivators, distinct signaling cascades regulate the function of transcription factors. These are due to multiple players acting in an orchestrated fashion to mediate their effects. Testosterone signals through inhibition of p38 MAPK, Notch-1, Notch-2 and Jagged-1 signaling pathways in macrophages, but utilize PI3K-Akt pathway in bone cells (71–73). This suggests that each ligand uniquely influences distinct pathways depending on cell and tissue type to mediate its pharmacologic and physiological response (74). AR phosphorylation is also affected ligand- dependently and -independently through growth factor alterations leading to divergent physiological responses (75).
Literature evidence suggests that non-genomic effects are also important for the anabolic effects of androgens and estrogens (76), whereas nuclear genomic effects are critical for the development of sexual organs. In addition to the nuclear AR, AR is also thought to be located at the plasma membrane to mediate rapid non-genomic effects. For example, the progression of meiosis that requires ERK occurs without the requirement for transcription. The ability of a ligand to promote non-genomic activation in cells will also determine its cell-type-specific function (77–79).
We demonstrated that the SARMs and DHT utilize distinct signaling pathways to promote their genomic and non-genomic effects. An arylproprionamide SARM mediated its actions through Src kinase, MEK-1/2 or MEK-3, ERK and p38 MAPK pathways, while DHT primarily affected the IP3, PLC, PI3K, PKC, ERK, and JNK pathways (80). Adding complexity, modulation of these pathways altered the recruitment of the AR and its cofactors to the PSA enhancer in a ligand-dependent manner. In addition, both SARM and DHT induced Xenopus laevis oocyte maturation and rapid phosphorylation of several kinases through distinct signaling pathways. These results support the potential utilization of signaling pathways available in a tissue microenvironment to promote maximal stimulation of the AR by various ligands.
Although these are some potential mechanistic explanations for the tissue-selective function of the SARMs, other potential mechanisms including conformation change of the AR resulting in differential nucleo-cytoplasmic shuttling, the role of metabolizing enzymes and tissue availability of SARMs, and/or inhibition of the hypothalamus-pituitary-gonadal axis that leads to reduced synthesis of testosterone might also contribute in part to tissue-selective SARM function.
Potential clinical uses of SARMs
SARMs could be used in diseases where steroidal androgens have been proposed as therapeutics. The initial focus of SARM clinical development was their use for muscle wasting conditions. However, the use of SARMs is now expanding to other diseases such as breast cancer.
Muscle-wasting disorders
Adults over 40 years of age lose about 1% muscle mass each year (81). With life expectancy increasing around the globe, the number of people with compromised muscle mass and accordingly deficits in physical function has increased in the last decade. Age-related muscle wasting or sarcopenia (82) and muscle wasting due to cancer, also called cancer cachexia (44), are two serious muscle wasting disorders with no treatment options. Sarcopenia is a major cause of frailty and carries with it an increase in physical disability as well as morbidity and mortality (83). The demographic that is widely affected by cancer is adults over 60 years of age. This age-group, already at higher risk to be deficient in muscle due to age-related decline, is then at high risk to lose additional muscle due as their cancer progresses and they receive anti-cancer therapy. Advanced cancer patients lose up to 1.5 kg of lean mass per year (84). Studies have also demonstrated that muscle mass directly correlates with survival in cancer patients (85,86). Androgens are important for building and maintaining skeletal muscle, and due to their anabolic effects on muscle are considered front-runners in the potential treatment of cancer cachexia and sarcopenia (43,45). SARMs are particularly relevant in this regard due to their tissue-selectivity and potential to provide therapeutic increases in muscle mass with reduced side-effects.
With wide-spread use of corticosteroids to combat inflammation and allergies, even children are susceptible to corticosteroid-induced muscle wasting. Although non-steroidal SGRMs that spare muscle and bone, but have significant anti-inflammatory effects, have been preclinically developed and tested, they have not successfully entered clinical trials, making steroidal corticosteroids the only available option for a number of indications (87). SARMs have been shown to be effective in ameliorating multiple preclinical models of muscle wasting including glucocorticoid mediated muscle atrophy. (88).
Duchenne muscular dystrophy (DMD)
DMD is a genetic disorder that arises due to mutations in the cytoskeletal protein dystrophin (89,90). The dystrophin gene is located in the X chromosome and a number of its mutations cause truncated proteins that manifest clinically in the form of muscular dystrophy. Boys with DMD suffer from progressive muscle wasting and weakness and will become wheel-chair-bound often before reaching puberty. Boys with DMD suffer from cardiac and respiratory failures due to weakness in the heart and lung muscles, respectively, resulting in premature death (91,92). Recently, therapies to correct mutations using exon-skipping strategies have been developed with one of these molecules receiving approval from the Food and Drug Administration (FDA) (93). Although corticosteroids are the standard of care to combat inflammation in DMD, with the exception of the novel exon skipping drug there are currently no disease-modifying therapeutic agents, available to treat DMD. Regrettably prolonged use of corticosteroids results in undesirable side-effects such as muscle wasting.
One of the strategies suggested to combat DMD is the use of SARMs. Recently, the SARM GLPG0492 was tested in dystrophin-mutated mdx mouse preclinical models of DMD. GLPG0492 increased body weight, muscle mass and function in mdx mice that were either sedentary or were stressed by exercise (94). These data support the use of SARMs for the treatment of DMD either alone or as combination with exon-skipping drugs or other strategies such as NFkB or myostatin inhibitors. Combination therapies capable of increasing muscle mass could potentially extend the survival of the DMD afflicted boys. Considering the function of AR on secondary sexual tissues, any SARM considered for clinical evaluation in children with DMD should exhibit a broad tissue-selectivity and impeccable safety profile.
Stress urinary incontinence (SUI)
The levator ani muscle in the pelvic floor is an AR-enriched muscle. Preclinical screening of SARMs often includes determining their ability to increase levator ani muscle weight, which is used as a surrogate for anabolic activity. The levator ani muscle and the pelvic floor muscles in women become weak due to ageing, child birth, and depletion of circulating hormones post-menopausally. Currently no treatment options are available for SUI. Since women are highly susceptible to uterine hyperplasia and virilization, steroidal androgens are not an appropriate choice to treat SUI. SARMs capable of selectively building pelvic floor muscles with reduced virilizing and uterine proliferative side-effects would be preferred and better tolerated for SUI. Preclinically, we demonstrated that treatment of ovariectomized mice with SARMs resulted in restoration of the pelvic muscles to their sham-operated weight (95). Additionally, the induction of several genes associated with muscle catabolism was inhibited. This study supported the clinical evaluation of enobosarm for SUI.
Osteoporosis
The ability of SARMs to increase both muscle and bone strength in animal models suggests that they may provide a unique dual approach to osteoporosis therapy (96) (40,42,97)(98). Currently osteoporosis is primarily treated with anti-resorptive agents that prevent further breakdown of bone by the body. Anti-resorptive agents potentially prevent further bone turn-over, but will be unable to increase bone mass. In preclinical models, AR agonists such as DHT and SARMs have prevented bone loss in both castrated male rats and ovariectomized female rats. They also increased cortical and trabecular bone mineral density above baseline in these experimental conditions (99). SARMs have been shown not only to prevent loss of bone (i.e., treatment begins at time of surgery) in ovariectomized and castrated rats, but also to increase bone strength (42,97).
Breast Cancer
Although androgens have been considered to be a risk factor in prostate cancer they have been recommended to treat breast cancers. Even before the discovery of SERMs and aromatase inhibitors, steroidal androgens such as medroxyprogesterone and fluoxymesterone were used to treat breast cancer (100). Expression of the AR in breast cancer has been consistently correlated with better disease-free- and overall- survival. In addition, combined expression of the AR with steroidogenic enzymes that result in increased synthesis of androgens have been shown to be extremely beneficial in breast cancer. However, these steroidal androgens, as indicated above, caused virilization, resulting in their discontinuation.
With the discovery of SARMs, women with breast cancer may have another safe and effective treatment option. Although the mechanism underlying the role of the AR in breast cancer is not completely understood, experimental evidence suggests that the AR inhibits ER function to inhibit the growth of ER-positive breast cancers. While the mechanism of action in ER-positive breast cancer is somewhat clearer, the mechanism of action in ER-negative or triple negative breast cancer (TNBC) is complex and has not been elucidated. Both a SARM, enobosarm, and an antagonist, enzalutamide, are in clinical trials to treat breast cancers. Recent press release from GTx, Inc., which is currently conducting a clinical trial with enobosarm in women with ER-positive breast cancer, indicated that the SARM slowed further growth of cancer (partial response and stable disease) in a subset of patients (101). Although no clinical data have been published on enzalutamide in ER-positive breast cancer, a phase I clinical trial to evaluate safety and bioavailability of enzalutamide in women with ER-positive breast cancer showed that enzalutamide reduced the serum concentration of anastrozole and exemestane by 90% and 50%, respectively, which could impact the efficacy of the aromatase inhibitors (102). However, enzalutamide has shown early promise in triple-negative breast cancer (TNBC). With TNBC being further sub-classified into several subsets, it may be possible that a SARM and an antagonist might provide anti-proliferative effects to distinct subsets of TNBC. This concept will be evaluated in future clinical trials. The reader is referred to a recent review for detailed information on the potential role of AR in breast cancer (103).
Most of the above indicated diseases require prolonged treatment with anabolic agents, which suggests that a strong safety profile will be required. Prolonged SARM administration is expected to be safer than steroidal agents. As such SARM stand poised to replace steroidal androgens for the treatment of these diseases.
Current clinical development paradigm of SARMs
Currently, to the best of our knowledge, the only SARM that is in clinical trials is enobosarm (GTx, Inc., Memphis, TN). Other companies that have previously pursued SARMs clinically are Ligand Pharmaceuticals, Merck, Glaxo, and Radius, Inc. A search of the clinicaltrials.gov database returned a small number of trials, many of which are phase I studies and investigator-initiated exploratory trials. While several other SARMs have been tested clinically for various diseases, they have not advanced beyond phase II proof-of-concept. Enobosarm was tested in two double-blind placebo-controlled phase III clinical trials in lung cancer patients at risk for cachexia. While enobosarm significantly improved muscle mass in both the trials, it only marginally increased the physical function. Similarly, two phase III trials with anamorelin, a non-peptide, orally-active, centrally-penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor, in subjects with lung cancer and cachexia showed that it increased muscle mass but failed to improve hand grip strength (104). However, the anamorelin results have been submitted to the European Medicines Agency (EMA) as part of a package for regulatory approval. We speculate that the results of the phase III trials with enobosarm and anamorelin and the complexity in conducting clinical trials in this space have discouraged other companies from pursuing SARMs in cancer-associated muscle wasting and that the field is seeking regulatory clarity before proceeding (105).
GTx Inc. is currently conducting three phase II proof-of-concept clinical trials. One each for ER-positive breast cancer, triple-negative breast cancer, and SUI. While the results from ER-positive breast cancer trials (one completed and another ongoing) were largely positive, the TNBC trial is still recruiting patients. The SUI trial is a novel and interesting study. Since the levator ani muscle is enriched in the AR, it responds quickly when exposed to androgens. Since SUI, in many cases, is associated with damaged and therefore weakened pelvic floor muscles, androgen-based approaches, as a result of their ability to increase muscle mass, might provide a potential therapeutic opportunity. The tissue-selective properties of SARMs position them as an ideal treatment option for SUI.
Summary and future
SARMs of several structural templates and a variety of potencies are described in peer-reviewed and patent literature. SARMs represent a new generation of tissue-selective androgens with an as yet unrealized potential to treat several diseases. Unfortunately, the development path for diseases such as cachexia and sarcopenia is difficult due to apparent regulatory requirements to show both increases in muscle mass and improvement in physical function (e.g., stair climb power, hand grip strength), with the later endpoint being highly variable and dependent on multiple factors. The future of SARMs currently hinges on the ongoing phase II clinical trials for breast cancer, SUI, and osteoporosis. Although strong preclinical data and rationale suggest a positive outcome for a SARM in SUI, the ongoing trial with a SARM for SUI is the first-in-human trial and its outcome will determine the utility of SARMs in SUI. Ongoing phase II trials of enobosarm in breast cancer are similarly positioned to set the course for the use of SARMs in this disease, while regulatory decisions regarding other muscle building agents will likely set the course forward, if any, for the use of SARMs in acute or chronic muscle wasting. The future of SARMs for the treatment of these and other conditions should be decided in the next five years either way. We anxiously await the results.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power RF, Conneely OM, O’Malley BW. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992;13:318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 4.Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O’Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 5.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 6.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imperato-McGinley J, Guerrero L, Gautier T, German JL, Peterson RE. Steroid 5alpha-reductase deficiency in man. An inherited form of male pseudohermaphroditism. Birth Defects Orig Artic Ser. 1975;11:91–103. [PubMed] [Google Scholar]
- 8.Schindler AE. Metabolism of androstenedione and testosterone in human fetal brain. Prog Brain Res. 1975;42:330. doi: 10.1016/s0079-6123(08)63689-4. [DOI] [PubMed] [Google Scholar]
- 9.Liao G, Chen LY, Zhang A, Godavarthy A, Xia F, Ghosh JC, Li H, Chen JD. Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J Biol Chem. 2003;278:5052–5061. doi: 10.1074/jbc.M206374200. [DOI] [PubMed] [Google Scholar]
- 10.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 11.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 12.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 13.Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Reprod. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- 14.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles D, Barr W, Bell ET, Brown JB, Fotherby K, Loraine JA. Clomiphene in the Treatment of Oligomenorrhea and Amenorrhea. Am J Obstet Gynecol. 1963;86:913–922. doi: 10.1016/s0002-9378(16)35246-2. [DOI] [PubMed] [Google Scholar]
- 16.Kedar RP, Bourne TH, Powles TJ, Collins WP, Ashley SE, Cosgrove DO, Campbell S. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet. 1994;343:1318–1321. doi: 10.1016/s0140-6736(94)92466-x. [DOI] [PubMed] [Google Scholar]
- 17.Lahti E, Blanco G, Kauppila A, Apaja-Sarkkinen M, Taskinen PJ, Laatikainen T. Endometrial changes in postmenopausal breast cancer patients receiving tamoxifen. Obstet Gynecol. 1993;81:660–664. [PubMed] [Google Scholar]
- 18.Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000;78:181–186. doi: 10.1006/gyno.2000.5859. [DOI] [PubMed] [Google Scholar]
- 19.Mincey BA, Moraghan TJ, Perez EA. Prevention and treatment of osteoporosis in women with breast cancer. Mayo Clin Proc. 2000;75:821–829. doi: 10.4065/75.8.821. [DOI] [PubMed] [Google Scholar]
- 20.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Discovery of nonsteroidal androgens. Biochem Biophys Res Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 21.Link JT, Sorensen B, Patel J, Grynfarb M, Goos-Nilsson A, Wang J, Fung S, Wilcox D, Zinker B, Nguyen P, Hickman B, Schmidt JM, Swanson S, Tian Z, Reisch TJ, Rotert G, Du J, Lane B, von Geldern TW, Jacobson PB. Antidiabetic activity of passive nonsteroidal glucocorticoid receptor modulators. J Med Chem. 2005;48:5295–5304. doi: 10.1021/jm050205o. [DOI] [PubMed] [Google Scholar]
- 22.Tabata Y, Iizuka Y, Shinei R, Kurihara K, Okonogi T, Hoshiko S, Kurata Y. CP8668, a novel orally active nonsteroidal progesterone receptor modulator with tetrahydrobenzindolone skeleton. Eur J Pharmacol. 2003;461:73–78. doi: 10.1016/s0014-2999(02)02958-8. [DOI] [PubMed] [Google Scholar]
- 23.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubahn DB, Joseph DR, Sar M, Tan J, Higgs HN, Larson RE, French FS, Wilson EM. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988;2:1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- 25.Tan JA, Joseph DR, Quarmby VE, Lubahn DB, Sar M, French FS, Wilson EM. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol Endocrinol. 1988;2:1276–1285. doi: 10.1210/mend-2-12-1276. [DOI] [PubMed] [Google Scholar]
- 26.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- 27.Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270:7341–7346. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- 28.Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5:1396–1404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 29.Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol. 1999;19:8383–8392. doi: 10.1128/mcb.19.12.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alen P, Claessens F, Verhoeven G, Rombauts W, Peeters B. The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol Cell Biol. 1999;19:6085–6097. doi: 10.1128/mcb.19.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors. 2009;35:528–536. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 33.Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. Embo J. 1992;11:3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–13123. [PubMed] [Google Scholar]
- 35.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 36.Holterhus PM, Piefke S, Hiort O. Anabolic steroids, testosterone-precursors and virilizing androgens induce distinct activation profiles of androgen responsive promoter constructs. J Steroid Biochem Mol Biol. 2002;82:269–275. doi: 10.1016/s0960-0760(02)00220-0. [DOI] [PubMed] [Google Scholar]
- 37.Permpongkosol S, Khupulsup K, Leelaphiwat S, Pavavattananusorn S, Thongpradit S, Petchthong T. Effects of 8-Year Treatment of Long-Acting Testosterone Undecanoate on Metabolic Parameters, Urinary Symptoms, Bone Mineral Density, and Sexual Function in Men With Late-Onset Hypogonadism. J Sex Med. 2016;13:1199–1211. doi: 10.1016/j.jsxm.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Traish AM. Testosterone therapy in men with testosterone deficiency: are the benefits and cardiovascular risks real or imagined? Am J Physiol Regul Integr Comp Physiol. 2016;311:R566–573. doi: 10.1152/ajpregu.00174.2016. [DOI] [PubMed] [Google Scholar]
- 39.Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]
- 40.Gao W, Reiser PJ, Coss CC, Phelps MA, Kearbey JD, Miller DD, Dalton JT. Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–4897. doi: 10.1210/en.2005-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinath R, Dobs A. Enobosarm (GTx-024, S-22): a potential treatment for cachexia. Future Oncol. 2014;10:187–194. doi: 10.2217/fon.13.273. [DOI] [PubMed] [Google Scholar]
- 42.Kearbey JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT. Selective Androgen Receptor Modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res. 2007;24:328–335. doi: 10.1007/s11095-006-9152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, Dalton JT. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials) Curr Oncol Rep. 2016;18:37. doi: 10.1007/s11912-016-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, Johnston MA, Steiner MS. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–345. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards JP, West SJ, Pooley CL, Marschke KB, Farmer LJ, Jones TK. New nonsteroidal androgen receptor modulators based on 4-(trifluoromethyl)-2(1H)-pyrrolidino[3,2-g] quinolinone. Bioorg Med Chem Lett. 1998;8:745–750. doi: 10.1016/s0960-894x(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi RI, Edwards JP, Caferro TR, Ringgenberg JD, Kong JW, Hamann LG, Arienti KL, Marschke KB, Davis RL, Farmer LJ, Jones TK. 4-Alkyl- and 3,4-dialkyl-1,2,3,4-tetrahydro-8-pyridono[5,6-g]quinolines: potent, nonsteroidal androgen receptor agonists. Bioorg Med Chem Lett. 1999;9:1335–1340. doi: 10.1016/s0960-894x(99)00186-9. [DOI] [PubMed] [Google Scholar]
- 48.Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, Lopez FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A. An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology. 2007;148:363–373. doi: 10.1210/en.2006-0793. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt A, Kimmel DB, Bai C, Scafonas A, Rutledge S, Vogel RL, McElwee-Witmer S, Chen F, Nantermet PV, Kasparcova V, Leu CT, Zhang HZ, Duggan ME, Gentile MA, Hodor P, Pennypacker B, Masarachia P, Opas EE, Adamski SA, Cusick TE, Wang J, Mitchell HJ, Kim Y, Prueksaritanont T, Perkins JJ, Meissner RS, Hartman GD, Freedman LP, Harada S, Ray WJ. Discovery of the selective androgen receptor modulator MK-0773 using a rational development strategy based on differential transcriptional requirements for androgenic anabolism versus reproductive physiology. J Biol Chem. 2010;285:17054–17064. doi: 10.1074/jbc.M109.099002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 51.Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 52.Madeira M, Mattar A, Logullo AF, Soares FA, Gebrim LH. Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer. 2013;13:425. doi: 10.1186/1471-2407-13-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao W, Dalton JT. Ockham’s Razor and Selective Androgen Receptor Modulators (SARMs): Are We Overlooking the Role of 5{alpha}-Reductase? Mol Interv. 2007;7:10–13. doi: 10.1124/mi.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, Biron S, Luu-The V, Tchernof A. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. 2006;191:637–649. doi: 10.1677/joe.1.06365. [DOI] [PubMed] [Google Scholar]
- 55.Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Belanger A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 56.Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang CY, McDonnell DP. Androgen receptor-cofactor interactions as targets for new drug discovery. Trends Pharmacol Sci. 2005;26:225–228. doi: 10.1016/j.tips.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 59.Fujimoto N, Yeh S, Kang HY, Inui S, Chang HC, Mizokami A, Chang C. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J Biol Chem. 1999;274:8316–8321. doi: 10.1074/jbc.274.12.8316. [DOI] [PubMed] [Google Scholar]
- 60.Kang HY, Yeh S, Fujimoto N, Chang C. Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 61.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 62.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 63.He B, Minges JT, Lee LW, Wilson EM. The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J Biol Chem. 2002;277:10226–10235. doi: 10.1074/jbc.M111975200. [DOI] [PubMed] [Google Scholar]
- 64.Chang CY, McDonnell DP. Evaluation of ligand-dependent changes in AR structure using peptide probes. Mol Endocrinol. 2002;16:647–660. doi: 10.1210/mend.16.4.0818. [DOI] [PubMed] [Google Scholar]
- 65.Chang C, Norris JD, Gron H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP. Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors alpha and beta. Mol Cell Biol. 1999;19:8226–8239. doi: 10.1128/mcb.19.12.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kazmin D, Prytkova T, Cook CE, Wolfinger R, Chu TM, Beratan D, Norris JD, Chang CY, McDonnell DP. Linking ligand-induced alterations in androgen receptor structure to differential gene expression: a first step in the rational design of selective androgen receptor modulators. Mol Endocrinol. 2006;20:1201–1217. doi: 10.1210/me.2005-0309. [DOI] [PubMed] [Google Scholar]
- 67.Baek SH, Ohgi KA, Nelson CA, Welsbie D, Chen C, Sawyers CL, Rose DW, Rosenfeld MG. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3100–3105. doi: 10.1073/pnas.0510842103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Auboeuf D, Wong J, Chen JD, Tsai SY, Tsai MJ, O’Malley BW. Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486. Proc Natl Acad Sci U S A. 2002;99:7940–7944. doi: 10.1073/pnas.122225699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng Q, O’Malley BW. Nuclear receptor modulation--role of coregulators in selective estrogen receptor modulator (SERM) actions. Steroids. 2014;90:39–43. doi: 10.1016/j.steroids.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narayanan R, Yepuru M, Szafran AT, Szwarc M, Bohl CE, Young NL, Miller DD, Mancini MA, Dalton JT. Discovery and mechanistic characterization of a novel selective nuclear androgen receptor exporter for the treatment of prostate cancer. Cancer Res. 2010;70:842–851. doi: 10.1158/0008-5472.CAN-09-3206. [DOI] [PubMed] [Google Scholar]
- 71.Guo D, Zhang H, Liu L, Wang L, Cheng Y, Qiao Z. Testosterone influenced the expression of Notch1, Notch2 and Jagged1 induced by lipopolysaccharide in macrophages. Exp Toxicol Pathol. 2004;56:173–179. doi: 10.1016/j.etp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res. 2004;19:1181–1190. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- 73.Liu L, Wang L, Zhao Y, Wang Y, Wang Z, Qiao Z. Testosterone attenuates p38 MAPK pathway during Leishmania donovani infection of macrophages. Parasitol Res. 2006;99:189–193. doi: 10.1007/s00436-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 74.Huber DM, Bendixen AC, Pathrose P, Srivastava S, Dienger KM, Shevde NK, Pike JW. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142:3800–3808. doi: 10.1210/endo.142.9.8402. [DOI] [PubMed] [Google Scholar]
- 75.Dehm SM, Tindall DJ. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem. 2006;281:27882–27893. doi: 10.1074/jbc.M605002200. [DOI] [PubMed] [Google Scholar]
- 76.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 77.Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR. Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Mol Endocrinol. 2003;17:1106–1116. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 78.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci U S A. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz LB, Kim B, Jahani D, Hammes SR. G protein beta gamma subunits inhibit nongenomic progesterone-induced signaling and maturation in Xenopus laevis oocytes. Evidence for a release of inhibition mechanism for cell cycle progression. J Biol Chem. 2000;275:41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 80.Narayanan R, Coss CC, Yepuru M, Kearbey JD, Miller DD, Dalton JT. Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol Endocrinol. 2008;22:2448–2465. doi: 10.1210/me.2008-0160. [DOI] [PubMed] [Google Scholar]
- 81.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 82.Bosy-Westphal A, Eichhorn C, Kutzner D, Illner K, Heller M, Muller MJ. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr. 2003;133:2356–2362. doi: 10.1093/jn/133.7.2356. [DOI] [PubMed] [Google Scholar]
- 83.Muhlberg W, Sieber C. Sarcopenia and frailty in geriatric patients: implications for training and prevention. Z Gerontol Geriatr. 2004;37:2–8. doi: 10.1007/s00391-004-0203-8. [DOI] [PubMed] [Google Scholar]
- 84.Wallengren O, Iresjo BM, Lundholm K, Bosaeus I. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015;23:79–86. doi: 10.1007/s00520-014-2332-y. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Motoyama S, Sato Y, Wakita A, Kawakita Y, Saito H, Minamiya Y. Decreased Skeletal Muscle Mass After Neoadjuvant Therapy Correlates with Poor Prognosis in Patients with Esophageal Cancer. Anticancer Res. 2016;36:6677–6685. doi: 10.21873/anticanres.11278. [DOI] [PubMed] [Google Scholar]
- 86.Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, Sangha R, Turner RA, Baracos VE, Sawyer MB. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Lierop MJ, Alkema W, Laskewitz AJ, Dijkema R, van der Maaden HM, Smit MJ, Plate R, Conti PG, Jans CG, Timmers CM, van Boeckel CA, Lusher SJ, McGuire R, van Schaik RC, de Vlieg J, Smeets RL, Hofstra CL, Boots AM, van Duin M, Ingelse BA, Schoonen WG, Grefhorst A, van Dijk TH, Kuipers F, Dokter WH. Org 214007-0: a novel non-steroidal selective glucocorticoid receptor modulator with full anti-inflammatory properties and improved therapeutic index. PLoS One. 2012;7:e48385. doi: 10.1371/journal.pone.0048385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151:3706–3719. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 89.Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 90.Rahimov F, Kunkel LM. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. 2013;201:499–510. doi: 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–386. doi: 10.1016/s0046-8177(76)80053-6. [DOI] [PubMed] [Google Scholar]
- 92.Politano L, Nigro V, Nigro G, Petretta VR, Passamano L, Papparella S, Di Somma S, Comi LI. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA. 1996;275:1335–1338. [PubMed] [Google Scholar]
- 93.Lim KR, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther. 2017;11:533–545. doi: 10.2147/DDDT.S97635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cozzoli A, Capogrosso RF, Sblendorio VT, Dinardo MM, Jagerschmidt C, Namour F, Camerino GM, De Luca A. GLPG0492, a novel selective androgen receptor modulator, improves muscle performance in the exercised-mdx mouse model of muscular dystrophy. Pharmacol Res. 2013;72:9–24. doi: 10.1016/j.phrs.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Ponnusamy S, Sullivan RD, Thiyagarajan T, Tillmann H, Getzenberg RH, Narayanan R. Tissue Selective Androgen Receptor Modulators (SARMs) Increase Pelvic Floor Muscle Mass in Ovariectomized Mice. J Cell Biochem. 2016 doi: 10.1002/jcb.25751. [DOI] [PubMed] [Google Scholar]
- 96.Mohler ML, Nair VA, Hwang DJ, Rakov IM, Patil R, Miller DD. Nonsteroidal Tissue Selective Androgen Receptor Modulators: A Promising Class of Clinical Candidates. Expert Opinion in Therapeutic Patents. 2005;15(11):1565–1585. [Google Scholar]
- 97.Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N. Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol Pharm Bull. 2003;26:1563–1569. doi: 10.1248/bpb.26.1563. [DOI] [PubMed] [Google Scholar]
- 98.Hamann LG. Discovery and Preclinical Profile of a Highly Potent and Muscle Selective Androgen Receptor Modulator (SARM). Abstract MEDI 11 from the 227th National American Chemical Society Meeting; Anaheim, CA. March 2004. [Google Scholar]
- 99.Mason RA, Morris HA. Effects of dihydrotestosterone on bone biochemical markers in sham and oophorectomized rats. J Bone Miner Res. 1997;12:1431–1437. doi: 10.1359/jbmr.1997.12.9.1431. [DOI] [PubMed] [Google Scholar]
- 100.Coss CC, Jones A, Dalton JT. Selective androgen receptor modulators as improved androgen therapy for advanced breast cancer. Steroids. 2014;90:94–100. doi: 10.1016/j.steroids.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 101.GTx Reports Results from Ongoing Enobosarm Phase 2 Clinical Trial in ER+/AR+ Breast Cancer. 2016 [Google Scholar]
- 102.Schwartzberg LS, Yardley D, Elias A, Patel M, LoRusso PM, Burris HA, Gucalp A, Peterson A, Blaney M, Steinberg J, Gibbons J, Traina TA. A Phase I/Ib Study of Enzalutamide Alone and in Combination with Endocrine Therapies in Women with Advanced Breast Cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2339. [DOI] [PubMed] [Google Scholar]
- 103.Narayanan R, Dalton JT. Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers (Basel) 2016:8. doi: 10.3390/cancers8120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–531. doi: 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 105.Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, Deutz NE, Doehner W, Evans WJ, Ferrucci L, Garcia JM, Gralla RJ, Jatoi A, Kalantar-Zadeh K, Lainscak M, Morley JE, Muscaritoli M, Polkey MI, Rosano G, Rossi-Fanelli F, Schols AM, Strasser F, Vellas B, von Haehling S, Anker SD. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6:272–274. doi: 10.1002/jcsm.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]