Abstract
Objective
Adolescents with Type 1 diabetes are at risk for poorer adherence, lower quality of life (QOL), and poorer glycemic control (HbA1c). Authoritative parenting (AP) along with youth adherence and QOL was hypothesized to relate to better HbA1c.
Methods
Parent–youth dyads (N = 257) completed baseline measures of adherence and QOL. Youth completed an AP questionnaire, and HbA1c samples were evaluated. Structural equation modeling determined relations among AP, adherence, QOL, and glycemic control.
Results
AP indirectly linked to better HbA1c (β = −.15, p = .021) through both better adherence and higher QOL. AP also was associated directly with better adherence (β = .26, p = .001), which in turn was linked to better HbA1c (β = −.35, p = .021). In addition, adherence was associated directly with QOL (β = −.56, p = .001).
Conclusions
Together, better youth adherence and higher QOL are two mechanisms by which more AP indirectly relates to better glycemic control during the early adolescent years.
Keywords: adherence, adolescence, parenting glycemic control, QOL, type 1 diabetes
Type 1 diabetes (T1D) is one of the most common chronic pediatric conditions with >15,000 new diagnoses each year in the United States (Imperatore et al., 2012; Torpy, Campbell, & Glass, 2010). Given that T1D is most commonly diagnosed during childhood, parents play an integral role in T1D management. This complex medical condition requires a multifaceted treatment regimen to which youth must adhere with assistance and guidance from parents.
Regimen adherence is complicated during the transition into adolescence when adherence often decreases and more erratic blood glucose (BG) control results as youth struggle to manage diabetes care amidst other developmentally normative activities (Amed et al., 2013). Poorer adherence and glycemic control often occur. A decline also can occur in diabetes-related quality of life (QOL; Lawrence et al., 2012) based on the perceived impact that diabetes has on a youth’s physical, emotional, mental, and social well-being (Wallander, Schmitt, & Koot, 2001). Better adherence and higher diabetes QOL are facilitated by appropriate parental involvement and guidance during this challenging transition as youth assume more responsibility for complex diabetes management. The effectiveness of parental involvement can be understood, in part, by parenting style or the amount of warmth and control that parents exhibit during child rearing (Maccoby & Martin, 1983). Authoritative parenting (AP), in particular, is related to better adherence and youth QOL, as supportive parents foster positive views of T1D and its associated regimen (Lawrence et al., 2012; Mlynarczyk, 2013).
Authoritative Parenting
Recent literature indicates that parental style may buffer the difficulties faced by youth during the adolescent transition. Chronic illness in youth can change the parent/youth relationship and can impact the role of parents in child rearing (Case-Smith, 2004; Coffey, 2006). Some medical conditions require greater parent involvement, which can result in increased parental regulation (Pinquart, 2013). Higher levels of warmth generally yield better youth outcomes; conversely, parents who exhibit lower levels of warmth and higher levels of controlling behaviors have youth with poorer medical outcomes (Holmbeck et al., 2002; Pinquart, 2013). In diabetes, youth must navigate the challenges associated with complex regimen management of BG monitoring, calculation and timing of insulin doses, dietary consumption, and energy expenditure. Throughout this process, parents provide varying levels of guidance and support which relates to different adolescent outcomes. An AP style, with higher levels of warmth and moderate levels of control, relates to better youth regimen adherence and QOL (Davis et al., 2001; Monaghan, Horn, Alvarez, Cogen, & Streisand, 2012; Mlynarczyk, 2013). Authoritative parents are more likely to allow their children autonomy to explore and to practice self-care while providing safety, structure, and support. Thus, higher levels of AP should relate to better glycemic control through both better youth adherence and better youth QOL.
Indeed, adolescents with parents who have higher levels of AP have better regimen adherence, higher QOL, and better glycemic control or lower glycosylated hemoglobin (HbA1c; Greene, Mandleco, Roper, Marshall, & Dyches, 2010; Mlynarczyk, 2013; Monaghan et al., 2012). Initial studies reveal that AP is the only parenting style related to better diabetes outcomes; permissive parenting relates to poorer outcomes (Davis et al., 2001). However, earlier studies have not evaluated levels of AP. Further, earlier studies of parenting style may be susceptible to social desirability biases because parents self-reported their parenting styles.
Adherence
A complex diabetes care regimen can make adherence challenging for some youth. Adherence is described as a youth’s ability to follow a diabetes treatment plan by checking BG levels, taking insulin, as well as general care behaviors, such as diet and exercise (Iannotti, Nansel, et al., 2006). In fact, the frequency of youths’ BG monitoring is predictive of how closely youths adhere to their regimen, with more BG checks related to better adherence (Kichler, Kaugars, Maglio, & Alemzadeh, 2012). Closer adherence to a prescribed regimen results in lower HbA1c levels and fewer health complications (Mulvaney et al., 2012; Rausch et al., 2012). However, more than half of youth with T1D do not meet the adherence guidelines prescribed by their endocrinologists (Amed et al., 2013). Many factors relate to adherence declines in adolescence, most notably, reduced parental monitoring and poorer communication, as well as more erratic extracurricular schedules and correspondingly less predictable adherence behaviors exacerbated by more time outside the home (Amed et al., 2013; Amiel, Sherwin, Simonson, Lauritano, & Tamborlane, 1986; Chisholm et al., 2007; Hilliard, Wu, Rausch, Dolan, & Hood, 2013; Rustad et al., 2013; Wysocki & Greco, 2006). In contrast, AP fosters T1D self-efficacy and can help prevent a decline in adolescent adherence (Wiebe et al., 2005). However, source bias and halo effects may skew some of these findings because studies often have parents report their parenting style and youth report their adherence. Use of multiple informants could provide greater stability and reliability in diabetes constructs.
Quality of Life
Management of a complex T1D regimen can have a significant impact on a youth’s QOL. AP and successful transition from greater parent to more youth responsibility during adolescence relates to better youth QOL (Naughton et al., 2008; Reynolds & Helgeson, 2011). Better adherence also is associated with higher QOL, positive health-related outcomes, better family relationships, and less psychological distress (Lawrence et al., 2012; Naughton et al., 2008; Pereira, Berg-Cross, Almeida, & Machado, 2008; Reynolds & Helgeson, 2011). In contrast, lower QOL relates to poorer general health outcomes including increased comorbidities and complications at 10-year follow-up (Naughton et al., 2008; Wikblad, Leksell, & Wibell, 1996).
A number of factors are related to both QOL and physical health outcomes (Lawrence et al., 2012; Naughton et al., 2008; Mlynarczyk, 2013). As noted, better adherence is associated with better youth diabetes-related QOL and better glycemic control (Lawrence et al., 2012). Further, parents who exhibit higher levels of AP have youth who experience better adherence and better diabetes-related QOL (Mlynarczyk, 2013). Surprisingly, a relation between QOL and better glycemic control is not always found, suggesting this relation may not be straightforward.
To better understand the role of AP in youth glycemic control, co-occurring QOL and adherence may need to be simultaneously studied with a statistical approach that models the interrelations of these factors. More complex or subtle relations could be revealed among key components of diabetes management with concurrent modeling of constructs through structural equation modeling (SEM). SEM also provides an opportunity to combine parent and youth data for more stability and greater construct reliability, particularly with important constructs such as adherence and QOL. A parent/youth mixed-source method can provide important information about reporter dyads that minimize individual biases (Holmbeck et al., 2002). Specifically, as youth in the current study were old enough, 11–14 years, to provide impartial reports of AP this source was chosen to prevent parental rater bias (Holmbeck, Li, Schurman, Friedman, & Coakley, 2002). Similarly, combined parent and youth reports of adherence and QOL were used to avoid individual rater bias. Further, the combination of parent and youth reports of QOL ensures a complete representation of both internal and external aspects of well-being (Varni et al., 2003).
In addition to multisource data, a multimethod approach was used to obtain study information. Prior evaluations of adherence in the context of AP often rely on a sole questionnaire to describe diabetes behaviors. Questionnaires can provide a useful overall summary of adherence. However, a relatively global estimate of behavior over a relatively long time interval of a week or more can exhibit pronounced halo effects (Clifford, Perez-Nieves, Skalicky, Reany, & Coyne, 2014). Inclusion of an additional adherence measure of BG checks over multiple, brief 24-hr intervals should strengthen the adherence component of the study. Incorporation of multiple methods further reduces biases in reporting and provides richer information about a construct (Holmbeck et al., 2002).
As a statistical approach, SEM allows both adherence and QOL to be tested simultaneously as paths that may relate AP to HbA1c and allows evaluation of whether AP has a direct or indirect relation with HbA1c. Simultaneous analysis of AP, QOL, adherence, and HbA1c also can characterize the interrelations among these factors that naturally co-occur while controlling the effects of relevant demographic variables of socioeconomic status (SES) and diabetes factors, such as type of insulin regimen, that relate to HbA1c. Using SEM, the current study hypothesizes that higher levels of AP will relate to higher levels of adherence and to better QOL, each of which may relate to one another. Further, higher levels of adherence and youth QOL should relate to lower (better) HbA1c. Through SEM, both direct and indirect paths of AP to HbA1c will be evaluated simultaneously with connections through adherence and QOL.
Method
Participants
Participants were 257 parent–youth dyads (youth aged 11–14 years) enrolled in a randomized clinical trial (RCT) at two Mid-Atlantic children’s hospitals. Enrollment in the trial required willingness to participate in four brief coping or education intervention sessions at routine endocrinology visits over the course of a year. Psychological measures from the baseline assessment were evaluated for this study, in addition to HbA1c data that were collected at baseline and at 3 months. Both HbA1c data points occurred before the start of intervention. Eligibility requirements for the trial included diabetes duration of at least 1 year, absence of severe complications or other medical diagnoses, and English fluency.
Procedure
The psychological data for these analyses were baseline data collected from an RCT approved by institutional review boards at each hospital and university. Eligible families were identified from endocrinology clinic rosters. Families were mailed an informational letter, which was followed by a phone call from trained research assistants. At a regularly scheduled endocrinology clinic visit, each youth and one parent provided assent and consent, respectively, and completed self-report questionnaires. Total testing time was approximately 1 hr. Youth received a $25 gift card for participation.
Measures
Disease and Demographic Information
Background information was collected from parents with a questionnaire developed by the research team that included information about youth age, gender, ethnicity, date of diagnosis, insulin regimen (pump vs. number of injections), and caregiver marital status. SES was calculated using the Hollingshead Four-Factor Index, based on parental education and employment (Hollingshead, 1975).
Authoritative Parenting
The Parenting Styles Index (PSI) is a youth report measure of AP (Steinberg, Lamborn, Dornbusch, & Darling, 1992) that consists of three scales of Acceptance/Involvement, Strictness/Supervision, and Autonomy Granting. Youth report the frequency of AP behaviors on this 26-item questionnaire. Internal consistency for the PSI has been previously established (Steinberg et al., 1992). Internal reliability for study participants was Acceptance/Involvement (α = .75), Strictness/Supervision (α = .57), and Autonomy Granting (α = .62). Scores from each scale were entered into an AP construct for the SEM measurement model initially, but ultimately only the Acceptance/Involvement scale was retained in the final measurement model and in the SEM analysis.
Adherence
A multimethod approach to the measurement of adherence was undertaken. First, youth and parent reported BG checks per day on an adapted version of the 24-hr Diabetes Interview (DI; Holmes et al., 2006). During the DI, youth and parents are interviewed separately and asked to report the number of BG checks completed on the previous day, using their glucose meters brought to clinic to enhance accuracy of report. Reports from two occasions during the same 2-week span were averaged for each reporter. Previous literature has indicated that number of BG checks per day is a reliable proxy for diabetes management in youth, with more BG checks related to better glycemic control (Hilliard et al., 2013).
Next, parents and youth also completed the Diabetes Behavior Rating Scale (DBRS; Iannotti, Schneider et al., 2006). The DBRS questionnaire asks respondents to rate the frequency with which routine T1D care behaviors (e.g., prevention, modification, and intervention behaviors) occurred over the previous week. Two forms of the DBRS exist: one for youth on a regimen of insulin injections (36 items) and one for youth who an insulin pump (37 items). Higher scores indicate better adherence. Internal consistency for the DBRS has been well established (Iannotti, Nansel, et al., 2006). Total scores were analyzed. For the current study, good internal consistency was found for each version (DBRS parent report for pump α = .69, DBRS youth report for pump α = .80, DBRS parent report for insulin injection α = .79, DBRS youth report for insulin injections α = .81).
Quality of Life
Youth and parents completed the Pediatric QOL—T1D Module questionnaire (Varni et al., 2003). The QOL Diabetes Module is a self-report measure that examines QOL in areas of problems, barriers, adherence, worry, and communication. Participants rated each of the 28 items on a 5-point scale (Never = 0 to Almost Always = 4) with higher scores indicating higher QOL. Scores are summed to provide a total QOL index for youth and another for parents such that higher scores indicate a better QOL and fewer problems. Internal consistency for the QOL has been well established (Varni et al., 2003). Total youth and total parent scores were used to create a QOL construct. Internal consistency was calculated for both parent and youth report (youth report α = .86; parent report α = .83).
Glycemic Control
To assess glycemic control (HbA1c), HbA1c values were assayed from blood samples (DCA 2000, Bayer, Inc., Tarrytown, NY) during a routine endocrinology visit. Baseline HbA1c was collected from youths’ medical charts to coincide with participant entrance into the study. To provide greater stability to the glycemic control measure, a second HbA1c was recorded, 3 months later, but before the initiation of psychological treatment. HbA1c assays provide historical information about average glucose levels over the previous 3 months. Lower values reflect better glycemic control.
Data Analytic Plan
Descriptive analyses were conducted with SPSS 21 (IBM Corp., 2012). Pearson’s correlation coefficients evaluated relations among demographic factors, AP, adherence, QOL, and glycemic control. SEM analyses were conducted with Mplus 6 software (Muthén & Muthén, 1998-2010). Post hoc power analysis used the root-mean-square error of approximation (RMSEA) parameter from the SEM model (MacCallum, Browne, & Cai, 2006; MacCallum, Browne, & Sugarwara, 1996) and revealed 0.831 power, which is adequate. G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) was used to conduct a sample size estimate based on the correlations of the direct effects in the SEM model. The G*Power sample size indicates N < 200 for each relation to reach at least 80% power. The full information maximum likelihood procedure was used to include participants who had missing individual data points, presumed to be missing at random. This procedure, which is the default in Mplus 6, imputes missing data values based on the current estimate of known parameters and then re-estimates the parameters based on known and imputed data (Collins, Schafer, & Kam, 2001). This method is a preferred way to handle missing data, as it includes all available data in statistical analyses (Collins et al., 2001). Demographic data with missing values were not estimated.
The fit and indicator factor loadings of each latent variable were examined using Confirmatory Factor Analysis. Model fit and standardized path loadings were examined within the SEM (Kline, 2011; MacCallum & Austin, 2000). To account for contextual factors that may affect the constructs of interest, correlated medical and demographic variables were considered in the model. Modification indices were accepted one at a time until model fit was deemed acceptable. Direct and indirect effects among the latent and observed variables in the model were analyzed using the MacKinnon method for SEM (MacKinnon, 2008).
Overall model fit was assessed using five empirically established value indicators. A chi-square value closer to zero with a p value >.05 indicates good fit (Hu & Bentler, 1999). However, owing to the large sample size of the current study, the chi-square statistic is not the best assessment of fit because it is closely related to sample size. A RMSEA value <0.06 indicates good fit (Hu & Bentler, 1998, 1999) and a standardized root mean square residual (SRMSR) value <0.08 indicates acceptable fit (Kline, 2011). Additionally, a comparative fit index (CFI) and Tucker–Lewis index (TLI) value >0.90 indicate acceptable fit (Hu & Bentler, 1998).
Results
Descriptive Results
Participants included 257 parent–youth dyads (51% male) aged 11–14 years (M = 12.84, SD = 1.24) with T1D and their primary caregivers (91% mothers; Table I). Youth were primarily Caucasian (69%) and from middle-class families (42% upper-middle, 39% middle). At baseline, the mean HbA1c was 8.81% (SD = 1.63) and at 3 months 8.93% (SD = 1.54), higher than recommended by the American Diabetes Association (ADA) for this age but consistent with other published studies (Chiang et al., 2014). An insulin pump was the most common type of regimen (44%), while 20% had an intensive basal/bolus regimen.
Table I.
Demographic Information of Study Participants
| Mean (SD) | Percentage (%) | |
|---|---|---|
| Age (years) | 12.8 (1.2) | |
| Gender: Male | 50.6 | |
| Race/Ethnicitya | ||
| Caucasian | 69.9 | |
| African American | 19.1 | |
| Hispanic American | 5.3 | |
| Asian American | 1.9 | |
| Other | 3.8 | |
| Hollingshead Index of SESb | 46.6 (11.7) | |
| Category score | 2.45 (.87) | |
| Duration of disease (years) | 5.1 (3.1) | |
| Regimen (insulin pump) | 44.0 |
Parent report of ethnicity.
SES = socioeconomic status, higher scores indicate higher SES.
Parents and youth reported somewhat low, though normative, levels of diabetes adherence on a global questionnaire (DBRS; Iannotti, Nansel, et al., 2006). Data from the 24-hr DI revealed an average of 4.3 BG checks per day. This average falls below the six BG checks per day recommended by the ADA but is comparable with that of other adolescent samples (Chiang et al., 2014; Holmes, et al., 2006). With respect to diabetes-related QOL, both parents and youth reported scores within 1 SD of normative data (Varni et al., 2003). Normative data for AP were not available.
Preliminary Analyses
Means, standard deviations, and correlations of key study variables are presented in Table II. As expected, results showed that all indicator variables for each latent construct (i.e., AP, Adherence, QOL, and Glycemic Control) were intercorrelated except that the PSI Strictness/Supervision subscale was not significantly correlated with the PSI Autonomy Granting subscale.
Table II.
Correlation Matrix, Means, and Standard Deviations for Key Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. PSI Involvement/Acceptance | – | ||||||||
| 2. QoL, parent | .283*** | – | |||||||
| 3. QoL, youth | .239*** | .489*** | – | ||||||
| 4. DBRS, parent | .209** | .274*** | .251*** | – | |||||
| 5. DBRS, youth | .275*** | .207** | .103† | .394*** | – | ||||
| 6. Parent BG checks | .272*** | .251*** | .248*** | .380*** | .267*** | – | |||
| 7. Youth BG checks | .258*** | .200** | .257*** | .351*** | .261*** | .794*** | – | ||
| 8. Baseline HbA1c | −.208** | −.288*** | −.301*** | −.302*** | −.169** | −.328*** | −.287*** | – | |
| 9. 3-Month HbA1c | −.136† | −.290*** | −.224** | −.369*** | −.214** | −.317*** | −.302*** | .790*** | – |
| Mean | 3.28 | 62.38 | 70.39 | .67 | .63 | 4.3 | 4.34 | 8.81 | 8.63 |
| SD | .50 | 13.06 | 14.14 | .11 | .13 | 1.51 | 1.53 | 1.63 | 1.53 |
Note. ***p < .001; **p < .01; *p < .05; †p < .10.
More intensive insulin regimens coded as higher values.
SES = socioeconomic status, higher scores indicate higher SES.
Structural Equation Model
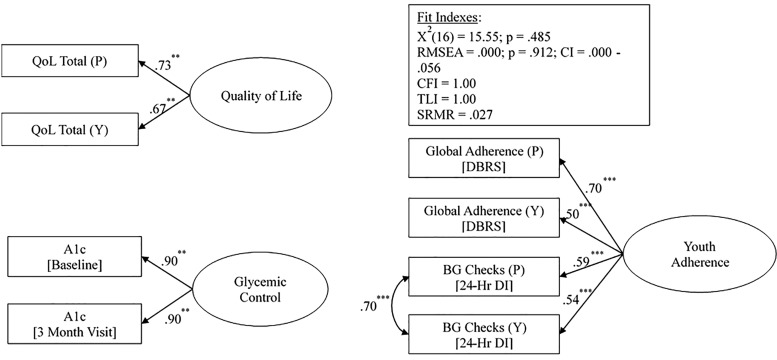
The initial measurement model consisted of four latent variables measuring parenting style (three subscales), adherence (parent- and youth-reported DBRS, parent- and youth-reported BG checks on the 24-hr DI), QOL (parent and youth reports), and glycemic control (HbA1c at baseline and at 3 months). The fit and indicator factor loadings of each latent variable were examined using confirmatory factor analysis. All indicators sufficiently loaded onto the hypothesized latent constructs (β > .50, p < .001), except the PSI Autonomy Granting subscale (β =.20, p = .012) and PSI Strictness/Supervision subscales (β = .32, p < .001). Accordingly, these two subscales were deleted and AP was reconstructed as an observed variable based on the Acceptance/Involvement subscale alone.
The final measurement model consisted of three latent variables measuring adherence (parent- and youth-reported DBRS), parent- and youth-reported BG checks (on the 24-hr DI), QOL (parent and youth report), and glycemic control (HbA1c at baseline and at 3 months). The proposed measurement model demonstrated good fit with the data, x2(16) = 15.55, p = .485; CFI = 1.000; TLI = 1.001; RMSEA = 0.000 (90% CI 0.000–0.056), p = .912; SRMSR = 0.027. Standardized path coefficient with error variance correlations are reported in Figure 1. All indicators sufficiently loaded onto the hypothesized latent constructs (β > .50, p < .001). The resulting measurement model fit the data.
Figure 1.
Standardized path coefficients with error variance correlations for latent variable measurement model.
Note. *p < .05, **p < .01, ***p < .001. Y = youth report; P = parent report.
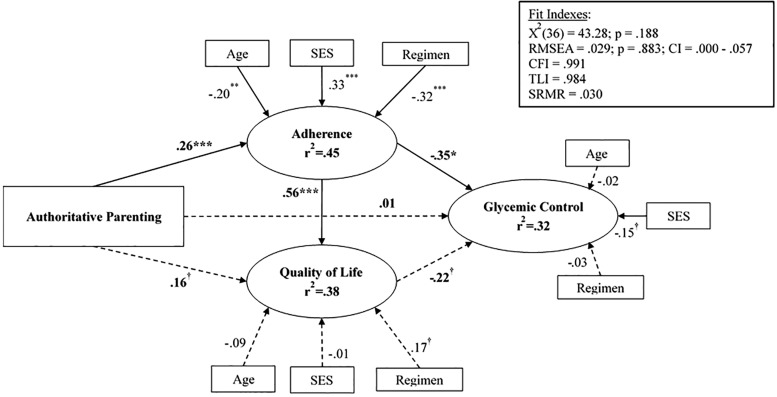
The proposed structural equation model also demonstrated good fit with the data, x2(36) = 43.28, p = .188; CFI = 0.991; TLI = 0.984; RMSEA = 0.029 (90% CI 0.000–0.057), p = .883; SRMSR = 0.030. The Acceptance/Involvement subscale of the PSI was an observed exogenous variable, and child age, insulin regimen, and SES were included in the model as covariates. Standardized coefficients are presented in Figure 2. The pathways from AP acceptance/involvement to glycemic control through adherence and QOL was examined with the indirect effects and their standard errors (SE) computed by Mplus. Greater AP was related to greater adherence (β = .26, p < .001; SE = 0.070) but not directly related to QOL (β = .16, p = .065; SE = 0.086) nor to glycemic control (β = .01, p = .860; SE = 0.072). The direct path from adherence to glycemic control was significant (β = −.35, p = .021; SE = 0.154), while the direct path from QOL to glycemic control was not (β = −.22, p = .053; SE = 0.115). Further, greater adherence was directly related to higher QOL (β = .56, p < .001; SE = 0.139). A moderate correlation was observed between QOL and adherence (β = .47, p < .001; SE = 0.109). The indirect individual pathways from AP acceptance/involvement to glycemic control via adherence (β = −.09, p = .055; SE = 0.049) and from AP acceptance involvement to glycemic control via QOL (β = −.04, p = .209; SE = 0.028) were not significant. However, the combined total indirect effects from parental acceptance/involvement to glycemic control were significant (β = −.15, p = .021; SE = 0.065) and suggest that adherence and QOL together link parental acceptance and involvement to lower HbA1c. Overall, the model accounted for 45% of the variance in adherence, 38% of the variance in QOL, and 32% of the variance in glycemic control.
Figure 2.
The relation of authoritative parenting to diabetes QOL, adherence, and glycemic control.
Note. Numbers are standardized path estimates. †p < .10, *p <. 05, **p < .01, ***p < .001.
Discussion
AP is connected to better glycemic control directly through better adherence. Further, paths from better adherence and from higher QOL together indirectly link AP to better glycemic control. This indirect link likely arises from the moderate correlation between better adherence and higher QOL such that although adherence alone is sufficient to directly link AP to glycemic control, it also combines with QOL and indirectly connects to glycemic control as well. Novel use of SEM to study AP in the T1D literature was able to detect these direct and subtler indirect relations between AP and better glycemic control. Consistent with prior literature, established relations were found between more AP and better youth adherence (Greene et al., 2010, Mlynarczyk, 2013) as well as between better adherence and better glycemic control (Lawrence et al., 2012; Nansel et al., 2008; Naughton et al., 2008). However, contrary to earlier reports in the literature (Anderson et al., 2002; Greene et al., 2010; Sherifali et al., 2009), a direct path between AP and glycemic control was not obtained when factors of adherence and QOL were simultaneously considered.
New to the literature, the use of SEM showed that AP is directly related to glycemic control through better youth adherence when interrelated components of diabetes management are considered, as occurs naturalistically. Higher levels of AP, specifically parental acceptance and involvement, relate to better regimen adherence, which in turn relates to better glycemic control. This chain of associations suggests that better adherence may be an integral component of the link between higher levels of AP and better glycemic control (Greene et al., 2010, Mlynarczyk, 2013). This assertion is further supported by the present results because, like others, the present study found a direct association between the Parental Involvement subscale of the AP measure and glycemic control in the simple bivariate correlations (Table II). However, with more complex modeling that simultaneously considers the inextricably linked roles of youth adherence and QOL, the direct association of AP to glycemic control is no longer significant in the SEM (β =.01). Instead of a direct relation, AP is linked to glycemic control through adherence. In the present study, children with more authoritative and involved parents have correspondingly better adherence and better glycemic control.
Also new to the literature, an indirect relation was found between AP and glycemic control through the two avenues of youth adherence and diabetes QOL. Not surprisingly, better youth adherence relates to better QOL (β =.52) and in fact the two factors are moderately correlated (β =.47). Their intercorrelation may explain why both together link AP to glycemic control indirectly. In essence, the indirect paths of AP to glycemic control through both adherence and QOL asserts the importance of adherence and better QOL. Longitudinal study of this relation could determine whether there is a dynamic interplay between the two factors. Interestingly, higher QOL may serve as a proxy indicator of the quality of parental involvement during parent–youth adherence interactions, although presently studied variables only hint, and cannot confirm, this possibility. Alternatively, QOL may serve as an overall indicator of the day-to-day successfulness or smoothness of parent/youth adherence efforts. These possibilities necessarily are speculative, pending additional investigation. Nonetheless, these findings show favorable relations among AP and youth diabetes functioning, as measured by better adherence, higher QOL, and better glycemic control.
In the present study, parental involvement from the PSI drives the AP findings. As noted in a preliminary measurement model with all AP subscales, the Acceptance/Involvement subscale loaded the highest (β =.76) on the AP latent construct, possibly an artifact of families who enrolled into a yearlong study to improve youth diabetes adherence. Owing to the lower loadings of the Strictness/Supervision (β =.32) and Autonomy Granting (β =.20) subscales, these were removed from the final measurement model. These lower loadings may reflect the young adolescent age of the present sample, which coupled with a complex daily medical regimen, necessarily requires more parental structure to ensure good health and a potentially lower level of youth autonomy for age. Nonetheless, parental involvement that is warm and supportive, characteristic of AP, is related throughout adolescence to better youth adherence and glycemic control (King, Berg, Butner, Butler & Wiebe, 2014; Wiebe, et al., 2005).
Selection of multiple or optimal reporters for each construct in the study was designed to minimize source bias. Parent and youth reports of adherence and QOL along with youth report of AP was designed to measure each construct adequately and to minimize well-known single-source error effects and bias (Kline, 2011). A mixed-source method can manage reporting biases and provide detailed information about the functioning of parent–youth dyads (Holmbeck et al., 2002). Further, multiple reporters and multiple measures of observable behavior in a latent construct can account more accurately for the data as a whole. Inclusion of ratings from multiple reporters allows analysis of family-level data, accounts for within-family nonindependence, and more accurately measures each construct (Kenny, 1995).
The present SEM findings provide novel information about the interrelation of youth adherence and QOL and their association to AP and better glycemic control. Few studies have examined QOL, as it relates to either AP or glycemic control or examined QOL as a function of the multiple reporters. In addition to a path from better adherence to better QOL, youth regimen adherence and QOL are significantly correlated. Both constructs were reported by both youths and parents, providing more stability and increased confidence in the strength of these findings.
The validity of the present model is supported by the diabetes and demographic relations found in the bivariate correlations. Consistent with the literature, youth with more intensive insulin regimens or those from higher SES families exhibited better glycemic control. Youth who checked their BG levels more frequently reported higher QOL and better glycemic control. In this sample of 11–14-year-olds, older youth was related to less parental involvement, lower levels of general adherence, less BG monitoring, lower QOL, and poorer glycemic control. These latter associations underscore the ongoing clinical need for programs that assist parents and their young adolescents with successful diabetes management strategies.
Limitations of the current study include a preponderance of middle- and higher-SES families who tend to report higher levels of AP such that range restriction should be considered in these results (Lamborn et al., 1991). Research with a broader range of SES would enhance generalization of the findings. Also, the present study of baseline psychological data collected from a RCT does not allow causal inferences to be made.
Strengths of this study include a large sample of youths and parents who are representative of other published samples of adolescents with T1D. The large sample size (n = 257 dyads) provides sufficient statistical power to conduct the current SEM model. Further, inclusion of a sizable minority population (30%) also should enhance the generalization of the study results. This study examined adherence and QOL as potential mechanisms by which more AP is related to better glycemic control. With a deeper understanding of the interrelations among AP and beneficial aspects of diabetes management, clinicians should be better able to better assist parents and youth navigate the transition into adolescence.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (Grant R01DK070917) awarded to C.H.
Conflicts of interest: None declared.
References
- Amed S., Nuernberger K., McCrea P., Reimer K., Krueger H., Aydede S. K., Ayers D., Collet J. (2013). Adherence to clinical practice guidelines in the management of children, youth, and young adults with type 1 Diabetes—A prospective population cohort study. The Journal of Pediatrics, 163, 543–548.e1. doi:http://dx.doi.org/10.1016/j.jpeds.2013.01.070 [DOI] [PubMed] [Google Scholar]
- Amiel S. A., Sherwin R. S., Simonson D. C., Lauritano A. A., Tamborlane W. V. (1986). Impaired insulin action in puberty. The New England Journal of Medicine, 315, 215–219. doi:10.1056/NEJM198607243150402 [DOI] [PubMed] [Google Scholar]
- Anderson B. J., Vangsness L., Connell A., Butler D., Goebel-Fabbri A., Laffel L. M. (2002). Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association, 19, 635–642. doi:752 [pii] [DOI] [PubMed] [Google Scholar]
- Case-Smith J. (2004). Parenting a child with a chronic medical condition. American Journal of Occupational Therapy, 58, 551–560. [DOI] [PubMed] [Google Scholar]
- Chiang J. L., Kirkman M. S., Laffel L. M. B., Peters A. L. (2014). Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care, 37, 2034–2054. doi: 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm V., Atkinson L., Donaldson C., Noyes K., Payne A., Kelnar C. (2007). Predictors of treatment adherence in young children with type 1 diabetes. Journal of Advanced Nursing, 57, 482–493. doi:10.1111/j.1365-2648.2006.04137.x [DOI] [PubMed] [Google Scholar]
- Clifford S., Perez-Nieves M., Skalicky A. M., Reany M., Coyne K. S. (2014). A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Current Medical Research and Opinion, 30, 1071–1085. doi:10.1185/03007995.2014.884491 [DOI] [PubMed] [Google Scholar]
- Coffey J. S. (2006). Parenting a child with chronic illness: A metasynthesis. Pediatric Nursing, 32, 51–59. [PubMed] [Google Scholar]
- Collins L. M., Schafer J. L., Kam C. M. (2001). A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods, 6, 330–351. [PubMed] [Google Scholar]
- Davis C. L., Delamater A. M., Shaw K. H., La Greca A. M., Eidson M. S., Perez-Rodriguez J., Nemery R. (2001). Parenting styles, regimen adherence, and glycemic control in 4- to 10-year-old children with diabetes. Journal of Pediatric Psychology, 26, 123–129. doi:10.1093/jpepsy/26.2.123 [DOI] [PubMed] [Google Scholar]
- Faul F.,M., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Greene M. S., Mandleco B., Roper S. O., Marshall E. S., Dyches T. (2010). Metabolic control, self-care behaviors, and parenting in adolescents with type 1 diabetes: A correlational study. The Diabetes Educator, 36, 326–336. [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Wu Y. P., Rausch J., Dolan L. M., Hood K. K. (2013). Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. Journal of Adolescent Health, 52, 28–34. doi:http://dx.doi.org/10.1016/j.jadohealth.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor scale of social index. Unpublished manuscript. [Google Scholar]
- Holmbeck G. N., Johnson S. Z., Wills K. E., McKernon W., Rose B., Erklin S., Kemper T. (2002). Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: The mediational role of behavioral autonomy. Journal of Consulting and Clinical Psychology, 70, 96–110. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Li S. T., Schurman J. V., Friedman D., Coakley R. M. (2002). Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology, 27, 5–18. [DOI] [PubMed] [Google Scholar]
- Holmes C. S., Chen R., Streisand R., Marschall D. E., Souter S., Swift E. E., Peterson C. C. (2006). Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology, 31, 770–784. [DOI] [PubMed] [Google Scholar]
- Hu L., Bentler P. (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 34, 424–453. [Google Scholar]
- Hu L., Bentler P. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi: http://dx.doi.org/10.1080/10705519909540118 [Google Scholar]
- Iannotti R. J., Nansel T. R., Schneider S., Haynie D. L., Simons-Morton B., Sobel D. O., Zeitzoff L., Plotnick L. P., Clark L. (2006). Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care, 29, 2263–2267. doi:10.2337/dc06-0685 [DOI] [PubMed] [Google Scholar]
- Iannotti R. J., Schneider S., Nansel T. R., Haynie D. L., Plotnick L. P., Clark L. M., Sobel D. O., Simons-Morton B. (2006). Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. Journal of Developmental and Behavioral Pediatrics, 27, 98–105. doi:10.1097/00004703-200604000-00003 [DOI] [PubMed] [Google Scholar]
- Imperatore G., Boyle J. P., Thompson T. J., Case D., Dabelea D., Hamman R. F., Lawrence J. M., Liese A. D., Liu L. L., Mayer-Davis E. J., Rodriguez B. L., Standiford D.; SEARCH for Diabetes in Youth Study Group. (2012). Projections of type 1 and type 2 diabetes burden in the U.S. population aged. Diabetes Care, 35, 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny D. A. (1995). The effect of nonindependence on significance testing in dyadic research. Personal Relationships, 2, 67–75. [Google Scholar]
- Kichler J. C., Kaugars A. S., Maglio K., Alemzadeh R. (2012). Exploratory analysis of the relationships among different methods of assessing adherence and glycemic control in youth with type 1 diabetes mellitus. Health Psychology, 31, 35–42. doi:10.1037/a0024704 [DOI] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Butler J. M., Wiebe D. J. (2014). Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents' adherence. Health Psychology, 33, 424–432. doi:10.1037/a0032804 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. B. (2011). Principles and practice of structural equation modeling, 3rd ed New York, NY: The Guilford Press. [Google Scholar]
- Lamborn S. D., Mounts N. S., Steinberg L., Dornbusch S. M. (1991). Patterns of competence and adjustment among adolescents from authoritative, authoritarian, indulgent, and neglectful families. Child Development, 62, 1049–1065. doi:10.2307/1131151 [DOI] [PubMed] [Google Scholar]
- Lawrence J. M., Yi-Frazier J. P., Black M. H., Anderson A., Hood K., Imperatore G., Klingensmith G. J., Naughton M., Mayer-Davis E., Seid M.; SEARCH for Diabetes in Youth Study Group. (2012). Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. The Journal of Pediatrics, 161, 201–207.e2. [Google Scholar]
- MacCallum R. C., Austin J. T. (2000). Applications of structural equation modeling in psychological research. Annual Review of Psychology, 51, 201–226. doi:10.1146/annurev.psych.51.1.201 [DOI] [PubMed] [Google Scholar]
- MacCallum R. C., Browne M. W., Cai L. (2006). Testing differences between nested covariance structure models: Power analysis and null hypotheses. Psychological Methods, 11, 19–35. doi:10.1037/1082-989X.11.1.19; 10.1037/1082-989X.11.1.19.supp [DOI] [PubMed] [Google Scholar]
- MacCallum R. C., Browne M. W., Sugawara H. M. (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1, 130–149. [Google Scholar]
- Maccoby E. E., Martin J. A. (1983). Socialization in the context of the family: Parent-child interaction In Hetherington E. M., Mussen P.H. (Eds.), Handbook of child psychology (Vol. IV, 4th ed., pp. 1–101). New York, NY: John Wiley & Sons. [Google Scholar]
- MacKinnon D. P. (2008). Introduction to statistical mediation analysis. New York, NY: LEA. [Google Scholar]
- Mlynarczyk S. M. (2013). Adolescents' perspectives of parental practices influence diabetic adherence and quality of life. Pediatric Nursing, 39, 181–189. [PubMed] [Google Scholar]
- Monaghan M., Horn I. B., Alvarez V., Cogen F. R., Streisand R. (2012). Authoritative parenting, parenting stress, and self-care in pre-adolescents with type 1 diabetes. Journal of Clinical Psychology in Medical Settings, 19, 255–261. doi:10.1007/s10880-011-9284-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney S. A., Rothman R. L., Dietrich M. S., Wallston K. A., Grove E., Elasy T. A., Johnson K. B. (2012). Using mobile phones to measure adolescent diabetes adherence. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 31, 43–50. doi:10.1037/a0025543; 10.1037/a0025543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998-2011). Mplus, 6th ed Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nansel T. R., Weisberg-Benchell J., Wysocki T., Laffel L., Anderson B.; for the Steering Committee of the Family Management of Diabetes Study. (2008). Quality of life in children with type 1 diabetes: A comparison of general and diabetes-specific measures and support for a unitary diabetes quality-of-life construct. Diabetic Medicine, 25, 1316–1323. doi:10.1111/j.1464-5491.2008.02574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M. J., Ruggiero A. M., Lawrence J. M., Imperatore G., Klingensmith G. J., Waitzfelder B., McKeown R. E., Standiford D. A., Liese A. D., Loots B.; SEARCH for Diabetes in Youth Study Group. (2008). Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for diabetes in youth study. Archives of Pediatrics & Adolescent Medicine, 162, 649–657. doi:10.1001/archpedi.162.7.649; 10.1001/archpedi.162.7.649 [DOI] [PubMed] [Google Scholar]
- Pereira M. G., Berg-Cross L., Almeida P., Machado J. C. (2008). Impact of family environment and support on adherence, metabolic control, and quality of life in adolescents with diabetes. International Journal of Behavioral Medicine, 15, 187–193. doi:10.1080/10705500802222436; 10.1080/10705500802222436 [DOI] [PubMed] [Google Scholar]
- Pinquart M. (2013). Do the parent–child relationship and parenting behaviors differ between families with a child with and without chronic illness? A meta-analysis. Journal of Pediatric Psychology, 38, 708–721. doi:10.1093/jpepsy/jst020 [DOI] [PubMed] [Google Scholar]
- Rausch J. R., Hood K. K., Delamater A., Shroff Pendley J., Rohan J. M., Reeves G., Dolan L., Drotar D. (2012). Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care, 35, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K. A., Helgeson V. S. (2011). Children with diabetes compared to peers: Depressed? distressed? A meta-analytic review. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 42, 29–41. doi:10.1007/s12160-011-9262-4; 10.1007/s12160-011-9262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad J. K., Musselman D. L., Skyler J. S., Matheson D., Delamater A., Kenyon N. S., Cáceda R., Nemeroff C. B. (2013). Decision-making in diabetes mellitus type 1. The Journal of Neuropsychiatry and Clinical Neurosciences, 25, 40–50. doi:10.1176/appi.neuropsych.12010016; 10.1176/appi.neuropsych.12010016 [DOI] [PubMed] [Google Scholar]
- Sherifali D., Ciliska D., O’Mara L. (2009). Parenting children with diabetes: Exploring parenting styles on children living with type 1 diabetes mellitus. The Diabetes Educator, 35, 476–483. doi:10.1177/0145721709333268 [DOI] [PubMed] [Google Scholar]
- Steinberg L., Lamborn S. D., Dornbusch S. M., Darling N. (1992). Impact of parenting practices on adolescent achievement: Authoritative parenting, school involvement, and encouragement to succeed. Child Development, 63, 1266–1281. doi:10.2307/1131532 [DOI] [PubMed] [Google Scholar]
- Torpy J. M., Campbell A., Glass R. M. (2010). JAMA patient page. chronic diseases of children. JAMA, 303, 682. doi:10.1001/jama.303.7.682 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Jacobs J. R., Gottschalk M., Kaufman F., Jones K. L. (2003). The PedsQL™ in type 1 and type 2 diabetes: Reliability and validity of the pediatric quality of life inventory™ generic core scales and type 1 diabetes module. Diabetes Care, 26, 631–637. doi:10.2337/diacare.26.3.631 [DOI] [PubMed] [Google Scholar]
- Wallander J. L., Schmitt M., Koot H. M. (2001). Quality of life measurement in children and adolescents: Issues, instruments, and applications. Journal of Clinical Psychology, 57, 571–585. doi:10.1002/jclp.1029 [DOI] [PubMed] [Google Scholar]
- Wiebe D. J., Berg C. A., Korbel C., Palmer D. L., Beveridge R. M., Upchurch R., Lindsay R., Swinyard M. T., Donaldson D. L. (2005). Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology, 30, 167–178. doi:10.1093/jpepsy/jsi004 [DOI] [PubMed] [Google Scholar]
- Wikblad K., Leksell J., Wibell L. (1996). Health-related quality of life in relation to metabolic control and late complications in patients with insulin dependent diabetes mellitus. Quality of Life Research, 5, 123–130. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Greco P. (2006). Social support and diabetes management in childhood and adolescence: Influence of parents and friends. Current Diabetes Reports, 6, 117–122. [DOI] [PubMed] [Google Scholar]