Abstract
Objectives To examine the feasibility/acceptability of a parent-delivered Active Music Engagement (AME + P) intervention for young children with cancer and their parents. Secondary aim to explore changes in AME + P child emotional distress (facial affect) and parent emotional distress (mood; traumatic stress symptoms) relative to controls. Methods A pilot two-group randomized trial was conducted with parents/children (ages 3–8 years) receiving AME + P (n = 9) or attention control (n = 7). Feasibility of parent delivery was assessed using a delivery checklist and child engagement; acceptability through parent interviews; preliminary outcomes at baseline, postintervention, 30 days postintervention. Results Parent delivery was feasible, as they successfully delivered AME activities, but interviews indicated parent delivery was not acceptable to parents. Emotional distress was lower for AME + P children, but parents derived no benefit. Conclusion Despite child benefit, findings do not support parent delivery of AME + P.
Keywords: cancer, coping, music therapy, parents, traumatic stress symptoms, young children.
Introduction
Emotional distress in parents and their young child with cancer during acute treatment is a prevalent and persistent problem that interferes with parent–child interaction, life quality, and family function (Bennett, English, Rennoldson, & Starza-Smith, 2013; Kazak & Barakat, 1997; Kazak & Baxt, 2007; Kazak, Boeving, Alderfer, Hwang, & Reilly, 2005; Myers et al., 2014; Rodriguez et al., 2012). In the longer term, this acute emotional distress is related to traumatic stress symptoms after treatment ends. Although some young children and parents are resilient and experience positive growth after treatment, as many as 58% of parents and 40% of childhood cancer survivors have later reported traumatic stress symptoms in the moderate to severe range (Best, Streisand, Catania, & Kazak, 2001; Graf, Bergstraesser, & Landolt, 2013; Kazak & Barakat, 1997; Lindahl Norberg, Poder, Ljungman, & von Essen, 2012). These parents and young children require interventions to manage acute treatment distress and prevent traumatic stress symptoms in survivorship, and yet, a recent systematic review revealed a glaring absence of empirically validated interventions that address the interrelated needs of parents and young children with cancer (Robb & Hanson-Abromeit, 2014).
Music-based play is a particularly viable intervention modality for young children and parents because it is a pervasive, spontaneous, and normal aspect of family life (Flohr, 2005; Imberty, 1996). Music and music play influence mood, focus on wellness of the child and family, structure and support meaningful interactions, and are a primary means by which young children cope with potentially traumatic experiences (Barrera, Rykov, & Doyle, 2002; Bradt, Dileo, Grocke, & Magill, 2011; Ginsburg, 2007). Our earlier research found that an Active Music Engagement (AME) intervention delivered by a board-certified music therapist (MT-BC) increased coping-related behaviors including active engagement, initiation, and positive facial affect in hospitalized children with cancer (Robb, 2000; Robb et al., 2008). To date, all of our studies have been delivered directly to the child by a Board Certified Music Therapist. In this study, we were interested in translating the AME for parent delivery as we hypothesized a parent-delivered intervention, if feasible and acceptable, could have benefits of reducing both child and parent distress during cancer treatment.
The conceptual framework for our study is based on Robb’s Contextual Support Model of Music Therapy (CSM-MT; Robb, 2000), which informed parent-delivered Active Music Engagement (AME + P) content, and Kazak’s Pediatric Medical Traumatic Stress Model (Kazak & Baxt, 2007), which provides a useful heuristic for understanding short- and long-term consequences of pediatric cancer treatment for children and parents. In our framework, recurring cancer treatment events (e.g., hospitalizations, procedures) are viewed as potentially traumatic events. Parent appraisal of events as traumatic are influenced by preexisting factors. Higher distress has been related to: (1) demographics including younger parent/child age, female parent gender, and less education (Kazak et al., 2003; Rodriguez et al., 2012), (2) higher parent/child distress with prior hospitalizations (Barrera et al., 2004; Best et al., 2001; Kazak & Barakat, 1997; Myers et al., 2014), and (3) disease and treatment characteristics including diagnosis, related disease, and greater treatment intensity (Kazak et al., 2005; Norberg & Boman, 2013).
Grounded in motivational and developmental coping theory, the CSM-MT explains how music can be used to create supportive environments that encourage learning and enactment of active coping strategies to reduce distress (Robb, 2000). Essential intervention elements, which include three forms of contextual support, included (1) structure: age-appropriate, music-based activities to create a structured, predictable environment that supports the actions of children and parents, leading to experiences of competence, (2) autonomy support: opportunities for children to choose materials, and music composed/arranged to support actions initiated by children/parents, and (3) relationship support: music-based play experiences designed to support and sustain reciprocal parent–child interactions.
In this pilot study, we examined potential benefits of AME for parents, and whether we were successful in translating AME activities into an easily taught and more sustainable format where parents could learn to use AME activities with their child during treatment. If successful, AME translation for parent delivery (AME + P) would result in a single intervention with potential to reduce both parent and child distress. We hypothesized AME + P would increase child engagement (an indicator of AME parent delivery feasibility and a potential mediator), and improve proximal outcomes of child emotional distress (i.e., positive facial affect) and parent emotional distress (i.e., mood disturbance, traumatic stress symptoms).
Our primary aim was to examine feasibility (assessed through observational coding on delivery and child engagement) and acceptability (assessed through parent interviews) of AME parent delivery during a short, in-patient admission for chemotherapy. Our secondary aim was to explore changes in AME + P child and parent emotional distress (assessed using direct observation and self-report, respectively) relative to attention control participants at postintervention and 30 days postintervention.
Methods
Participants
Institutional review board approval was received before implementation. The oncology clinic coordinator at a large Midwestern children’s hospital provided study introduction to eligible parents/children, who were enrolled as a dyad. Inclusion criteria were (a) children aged 3–8 years inclusive; (b) expected hospitalization ≥ 3 days for chemotherapy; and (c) a consistent parent who could be present for all study sessions. Exclusion criteria were (a) child and/or parent did not speak English; (b) child had significant cognitive impairment, based on physician judgment. Participants were enrolled over 9 months (May–July 2011; September 2012–January 2013).
Study Design and Procedures
We used a pilot randomized trial design with a 1:1 allocation ratio. Following parent/child dyad consent/assent, on the first day of admission, parents completed baseline (T1) measures and dyads were immediately randomized to AME + P or attention control group. Randomization assignments in blocks of 2 or 4 were created by the biostatistician using a computer algorithm. Assignments were made by the project manager at enrollment using sealed numbered opaque envelopes. Our a priori target sample was 12 parent/child dyads per group, based on the idea that 12 per group is minimally sufficient to estimate effect sizes (ESs) for future randomized studies when outcomes are normally distributed (Julious, 2005). Owing to time constraints, as a single site we did not meet our accrual goal.
Conditions
To address risk for unblinding evaluators, we trained three MT-BCs to deliver both study conditions and addressed risks for diffusion by developing study-specific quality assurance monitoring procedures including field notes, manualized protocols, quality assurance checklists, and video monitoring. Children/parents randomly assigned to AME + P or attention control received three sessions of similar duration (45 min AME + P; 35 min attention control), one session daily over the first three consecutive days of inpatient chemotherapy. The number and duration of sessions were based on our prior studies and average length of stay for in-patient chemotherapy. Session one was delivered within 24 hr of hospital admission, and a consistent therapist delivered all sessions.
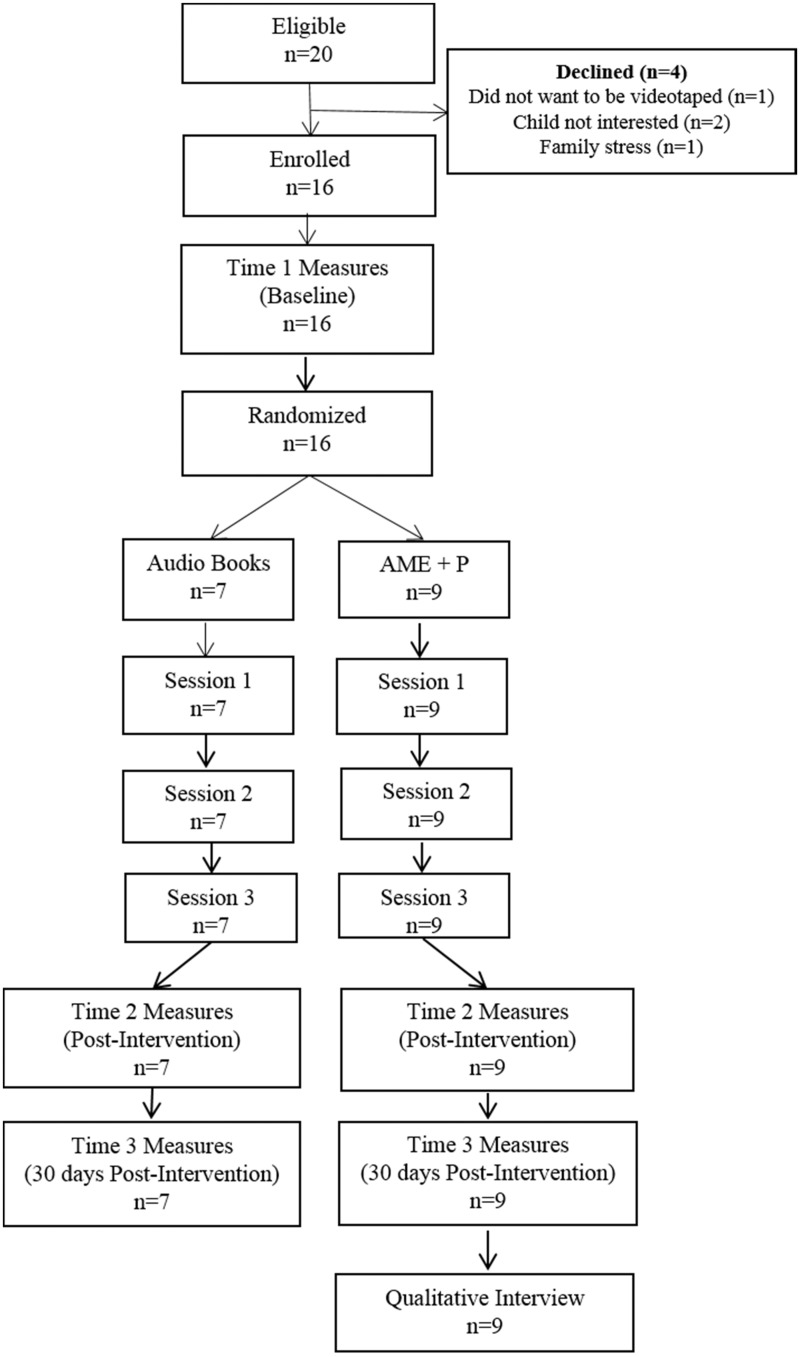
All sessions were video recorded. Sessions 1 and 2 were video recorded to desensitize parents/children to being videotaped; we analyzed Session 3 for behavioral data (i.e., child distress; engagement; parent implementation). Follow-up evaluations occurred after Session 3 (T2) and 30 days postintervention (T3). Data collectors, masked to group assignment, administered measures. The first author conducted AME + P parent interviews immediately following T3 measures (Figure 1).
Figure 1.
Study schema and CONSORT diagram.
Active Music Engagement + Parent Delivery Intervention
For AME + P we added a parent education/coaching component to previously tested AME activities for young children with cancer (Robb, 2000; Robb et al., 2008). Through tip-sheets and MT-BC modeling/instruction, parents learned (1) common behavioral responses of young children to cancer treatment, and (2) how to deliver AME activities to support their child and sustain a sense of family normalcy while hospitalized and as they transition home. We also developed an AME resource kit (activity cards, play materials, music CD) to support between-session and at-home use by parents and children. Materials were designed to accommodate the developmental needs and interests of both younger and older children. Below is a summary of session content:
Session 1: Information/Modeling Session. MT-BC discussed a child behavior tip sheet with the parent and facilitated a joint session with parent/child, modeling AME activities. Parent/child received AME resource kit, a second tip sheet, and tailored suggestions for independent use between sessions.
Session 2: Parent Delivery/Coaching Session. MT-BC faded modeling and assumed a coaching role, as parent delivered/engaged in AME activities with child. MT-BC provided support and made recommendations for use between sessions based on his/her observations and conversations about third tip sheet content on other ways to use music play during hospitalization (e.g., invasive procedures, sleep).
Session 3: Parent Delivery/Observation Session. MT-BC observed parent deliver AME activities. At session closure, MT-BC provided tailored suggestions for home use using fourth tip sheet.
Audio-Storybooks Attention Control Condition
Audio-Storybooks (ASB) Attention control condition controlled for attention from a trained clinician, shared parent–child experience, and audio-visual stimulation. In each session the MT-BC offered children/parents a choice of several illustrated children’s books with audio recorded narration (Walt Disney Group, 2010). The MT-BC was present while parents/children listened to the selected audio storybook, and at the close of sessions provided an audio-storybook kit containing two story collections and a CD player, and encouraged between-session listening. Previous data indicate the ASB was acceptable to children/parents and had no significant behavioral benefit (Robb, 2000; Robb et al., 2008).
Measures
The AME Parent Delivery Checklist
The AME Parent Delivery Checklist is a 10-item observational tool of parent behaviors important for effective AME delivery. Trained observers scored each item using a 4-point Likert-type scale (1 = never observed; 4 = always observed). Total mean scores range from 1.0 to 4.0, with higher scores indicating more successful parent delivery. Two trained coders independently completed checklists for all participants based on Session 3 videos. Initial training continued until intra- and interobserver reliability reached a minimum criterion of 0.85, using an index of concordance (sum of agreements/sum of agreements + disagreements). To ensure consistency among observers, we conducted periodic interobserver reliability checks that ranged from 0.91 to 0.99; 44% of videos were assessed for reliability. To assess AME activity use, parents also reported use between sessions (duration), and at home following discharge (frequency/duration).
Child Engagement
Child engagement was also used to assess feasibility of parent delivery by examining how well parents were able to engage their child in AME activities. A behavioral coding form, used in our previous studies, allowed for objective measurement of child engagement (Robb, 2000; Robb et al, 2008). Four molecular behaviors indicative of active engagement (physical activity, focus of attention, choice making, and following directions) are coded, and were selected for inclusion based on motivational coping theory (Skinner & Wellborn, 1994).
Independent observers viewed Session 3 videos and coded the presence or absence of each behavior using 10-s time intervals for observation, followed by 5-s time intervals to record observed responses. Observers completed training in coding procedures using sample videotapes. Training continued until intra- and interobserver reliability reached a minimum criterion of 0.85. To ensure consistency, we conducted periodic interobserver reliability checks that ranged from 0.94 to 0.99; 38% of videos were assessed for reliability.
Behavioral coding was done using 3-min time intervals (nine probes/discrete observations per time interval). Seven time intervals were coded for AME + P conditions, resulting in 63 probes of coded material. Owing to its shorter duration, three time intervals were coded for the attention control condition, resulting in 27 probes of coded material. Frequency scores for each condition were calculated across time intervals and used to compare outcomes for each condition. For Active Engagement, scores were computed by averaging the frequencies of “active” responses under “physical activity,” “focused” responses under “focus of attention,” “yes” responses under “follows directions,” and “yes” responses under “makes a choice.” Definitions for each behavior are provided in Table I. Noting from our prior work that average coding scores did not change over the length of a session (Robb et al., 2008), control means were multiplied by 63 probes/27 probes to account for the unequal number of probes/time of observation between the two groups.
Table I.
Behavioral Coding Definitions for Positive Facial Affect and Child Engagement
| Behavior | Definition |
|---|---|
| Positive facial affect | “Positive” was coded when child smiled or laughed during the time interval. |
| “Negative” was coded when child cried, whined, or raised voice during the time interval. Also coded if child verbalized fear, unhappiness, or pain. | |
| “Neutral” was coded when child exhibited no overt behavioral response indicative of an affect state (e.g., flat affect) during the time interval. | |
| Active engagement | |
|
“Active” responses were defined as the child engaging in a play activity during the coding interval (e.g., playing an instrument, turning pages of a book). The child’s engagement in an activity had to involve active manipulation of materials or active use of self (e.g., moving body to music) to be considered an active response. |
|
“Focused” responses were defined as the child’s eyes (attention) being fixated on the central activity that he/she was engaged in or that was being presented by another adult. |
|
“Yes” for choice making was coded when the child either physically (i.e., pointing or reaching) or verbally indicated a choice. |
|
“Yes” for follows directions was coded when the child verbally or physically responded to a verbal direction. |
Parent Interviews
Parent interviews were conducted to assess AME + P acceptability. Parent interviews occurred in private rooms during routine clinic appointments. Parents were asked to discuss the meaningfulness and helpfulness of the intervention. All interviews were audio-recorded, transcribed, and de-identified for subsequent analysis.
Facial Affect
Facial affect is an indicator of emotional distress, which is an important variable related to stress appraisals and self-regulation in young children (Compas & Boyer, 2001). A behavioral coding form, used in our previous studies, allowed for objective measurement of child facial affect (Robb, 2000; Robb et al, 2008). Observer training and coding procedures were identical to those used for Child Engagement (see above). To ensure consistency among observers, we conducted periodic interobserver reliability checks that ranged from 0.93 to 0.97; 38% of videos were assessed for reliability. Mean frequency scores for each condition were calculated across time intervals and used to compare outcomes for each condition. For Positive Facial Affect, scores were computed based on the frequency of facial affect scores coded as “positive.” Coding definitions for positive facial affect are provided in Table I. As with child engagement, control means were multiplied by 63/27 order to account for the unequal time of observation between the two groups.
Profile of Mood States-Short Form
Profile of Mood States-Short Form (POMS-SF), used as a measure of parent emotional distress, is a 37-item self-report measure of mood disturbance. Respondents are given 37 adjectives used to describe feelings during the past week and asked to respond to each item using a 5-point Likert scale (0 = not at all; 4 = extremely). Total negative mood scores are calculated by summing the 37 item scores (range = 0–148), with higher scores indicating greater mood disturbance. Construct validity has been supported by numerous validity studies. The POMS-SF strongly correlates with the original 65-item POMS (r = .99), and is one of the most commonly used measures for parent emotional distress in pediatric cancer research (Shacham, 1983).
Impact of Events Scale-Revised
Impact of Events Scale-Revised (IES-R), used as a second measure of parent emotional distress, is a 22-item self-report measure of traumatic stress symptoms in response to a traumatic event specified in the instructions. In our study, the child’s cancer treatment was the specified stressor. The scale includes three subscales: intrusion, avoidance, and hyperarousal. Parents respond to items using a 5-point Likert scale (0 = not at all; 4 = extremely). Higher scores indicate greater traumatic stress symptoms. The IES-R has been used in studies of childhood cancer patients and their parents. The internal reliabilities of the Intrusion, Avoidance, and Hyperarousal scales have been reported as 0.91, 0.84, and 0.90, respectively (Weiss, 1997).
Data Analyses
Analyses were performed in SAS Version 9.3. p-values < .05 were considered statistically significant. Two-sample t tests and Fisher’s exact tests were used to compare demographics and clinical characteristics between AME + P and control groups.
Feasibility
We used descriptive statistics to examine parent delivery scores using the AME Parent Delivery Checklist and self-reported frequency and duration of AME kit use between scheduled sessions, and at home after discharge. Descriptive statistics are also reported for child engagement. A two-sample t test was used to compare child engagement between the two groups. The ES was estimated by Cohen’s d (0.2 = small, 0.5 = medium, 0.8 = large).
Acceptability
AME + P parent interviews occurred 30 days postintervention. Interview questions began with open-ended questions about the parent/child’s experience and any perceived benefits, followed by directive questions about the intervention and other ways we can help parents during treatment. Audio-recorded interviews were transcribed and de-identified. Two authors (S.L.R., A.K.H.) analyzed qualitative data as follows: (1) transcriptions were reviewed for accuracy and revised as needed; (2) after listening to and reading each interview, the two raters independently extracted significant statements, then compared statements and resolved any discrepancies through consensus; 3) significant statements were reviewed and used to develop a list of coding categories; (4) using MAX QDA software, raters coded parents’ statements. Coded statements were reviewed to identify and describe common themes and sub-themes characterizing parents’ experiences.
Preliminary Outcomes
Means and standard deviations for each outcome were calculated by group and time. For Child distress, a two-sample t test with Satterthwaite approximation owing to unequal variance was used to compare the group means. Effect size was estimated by Cohen’s d. For parent distress, analysis of covariance (ANCOVA) was used to test for group differences on each outcome variable. Separate models were fit at data collection T2 and data collection T3. Each model was adjusted for the baseline value of the outcome measure. Partial η2 was used to estimate ESs for ANCOVA (small: ≤ .08; medium: .09–.24; large: ≥ .25). Partial η2 represents the percent variance in the outcome variable explained by group, while controlling for baseline value of the outcome variable.
Results
Sample
Figure 1 summarizes study accrual, intervention delivery, and data collection. Table II contains demographic and clinical characteristics. Children were primarily White and non-Hispanic. Although no significant group differences were found, percentage-wise, there were more children with a leukemia diagnosis rather than tumor in the control group (71% vs. 44%), and more married parents in the control group (86% vs. 33%).
Table II.
Comparison of Baseline Participant Characteristics by Randomization Group
| Participant characteristics | AME+P n = 9 | Control n = 7 | p |
|---|---|---|---|
| Child age at enrollment (mean (SD); range) | 5.4 (1.9); 3–8 | 5.6 (1.7); 3–8 | 0.8913a |
| Child gender, n (%) | 1.000b | ||
| Female | 4 (44.4) | 4 (57.1) | |
| Male | 5 (55.6) | 3 (42.9) | |
| Child ethnicity, n (%) | 0.7000b | ||
| Hispanic or Latino | 1 (11.1) | 1 (14.3) | |
| Not Hispanic or Latino | 8 (88.9) | 5 (71.4) | |
| Unknown | 0 | 1 (14.3) | |
| Child race, n (%) | 1.0000b | ||
| African-American | 1 (11.1) | 0 | |
| White | 7 (77.9) | 6 (85.7) | |
| Other | 1 (11.1) | 1 (14.3) | |
| Unknown or not reported | 0 | 0 | |
| Child diagnosis, n (%) | 0.3575a | ||
| Leukemia | 4 (44.4) | 5 (71.4) | |
| Tumor | 5 (55.6) | 2 (28.6) | |
| Time from diagnosis or relapse and start of study (months) (mean (SD); range) | 4.3 (2.4); 1–8 | 5.1 (1.5); 4–8 | 0.4387a |
| Intensity of treatment, n (%) | 0.7231 | ||
| 2 | 1 (11.1) | 1 (14.3) | |
| 3 | 6 (66.7) | 6 (85.7) | |
| 4 | 2 (22.2) | 0 | |
| Parent age at enrollment (mean (SD); range) | 35 (8.5); 25–48 | 32.7 (4.9); 27–36 | 0.6837a |
| Parent ethnicity, n (%) | 1.0000b | ||
| Hispanic or Latino | 1 (11.1) | 0 | |
| Not Hispanic or Latino | 4 (44.4) | 3 (42.9) | |
| Unknown | 4 (44.4) | 4 (57.1) | |
| Parent race n (%) | 1.0000b | ||
| African-American | 0 | 0 | |
| White | 4 (44.4) | 3 (42.9) | |
| Other | 1 (11.1) | 0 | |
| Unknown or not reported | 4 (44.4) | 4 (57.1) | |
| Parent education n (%) | 0.3832b | ||
| Below high school | 0 | 0 | |
| High school graduate | 2 (22.2) | 1 (14.3) | |
| Some college or certification courses | 3 (33.3) | 0 | |
| College graduate | 4 (44.5) | 4 (57.1) | |
| Graduate or professional degree | 0 | 1 (14.3) | |
| Unknown | 0 | 1 (14.3) | |
| Parents marital status n (%) | 0.2364b | ||
| Single | 2 (22.2) | 0 | |
| Married | 3 (33.3) | 6 (85.7) | |
| Divorced or separated | 3 (33.3) | 1 (14.3) | |
| Unknown | 1 (11.1) | 0 |
aTwo-sample t test.
bFisher’s exact test.
Feasibility
Parent Delivery/AME Activity Use
Of the nine parents randomized to AME + P, the average AME checklist score was 3.94 (SD = 0.2; range: 3.5–4.0). Highest score possible was a 4.0, indicating parents had little difficulty delivering AME activities.
Parents reported higher between-session use of activities for AME + P (n = 9; M = 27 min, SD = 43.22) versus ASB (n = 7; M = 13.6 min, SD = 14.36). Postintervention use at home or during subsequent hospital admissions was also higher for AME + P (mean frequency = 5.8, SD = 3.78; mean duration = 28.1 min, SD = 16.03) versus ASB (mean frequency = 1.5, SD = 2.51; mean duration = 12.86 min, SD = 16.04).
Child Engagement
Mean engagement scores were similar in AME + P and control groups (Table III). Effect size was small (Cohen’s d = 0.19) and p-value was not significant (p = .711).
Table III.
Means (SDs) in Session 3 for Child Engagement and Child Distress (n = 9 for AME + P; n = 7 for Control)
| Group | Mean (SD) | t (df)a | p | Cohen’s-d | |
|---|---|---|---|---|---|
| Child engagement | AME+P | 20.61 (10.59) | 0.38 (14) | 0.711 | 0.19 |
| Control | 18.89 (6.39) | ||||
| Child distress (positive facial affect) | AME+P | 13.56 (10.17) | 2.34 (10.7) | 0.040 | 1.07 |
| Control | 4.93 (3.86) |
at-statistic and degrees of freedom (df). Satterthwaite approximation used for child engagement owing to unequal group variances.
Acceptability
We briefly describe the five theme categories that emerged from our parent interview analysis. See Table IV for themes, subthemes, and representative statements.
Table IV.
Parent Interview Themes, Subthemes, Representative Statements on Helpfulness and Meaningfulness of the AME + P
| Themes | |
|---|---|
| Subthemes | Data Samples Illustrative of Subthemes |
| The music play experience | |
| New and Normalizing Experiences Are Important During Cancer Treatment | “…our whole definition of normal has changed…and our goal is to get it back to normal, old normal, or at least something closely resembling old normal… so I think [programs like this] that give them some semblance of a normal routine or a normal childhood are really beneficial…” Parent A (Mother, 7 y/o son) |
| Provides Success Experiences and Accommodates Limitations Resulting From Cancer Treatment | “It was nice to have an activity that was engaging and different that he could in bed. Even when he wasn’t feeling well he was still able to do it.” Parent A (Mother, 7 y/o son) |
| Provided Opportunities for Child to Be in Control and Make Choices | “…that’s good that he can handle what he wants to do with us instead of, [us saying to him], do this. He likes to have the control so that way was good for him.” Parent C (Mother, 6 y/o son) |
| Materials Were Age Appropriate, Highly Engaging, and Flexibly Supported Child’s Creativity | “They have games in the playroom, but it’s the same games, so you’re playing the same thing over and over…with the music kit … [there were] so many different games that you could play.” Parent F (Grandmother, 8 y/o granddaughter) |
| Music Is Valued as an Enjoyed, Shared Family Experience | “…music is a big part of [my son’s] life. It always has been. Dad is a huge music fan…” Parent A (Mother, 7 y/o son) |
| Having Something to Look Forward To Is Important | “She really enjoyed it. It gave her something to look forward to every day.” Parent G (Mother, 4 y/o daughter) |
| Music play benefit for child | |
| Provided Developmental Stimulation | “…because they don’t get to go to preschool. They’re missing out on all of that learning. So … any activities that can be implemented with music would be great….” Parent D (Mother, 4 y/o daughter) |
| Helped Child Relax and Sleep | “…the [music] helped her a lot to relax and fall asleep.” Parent F (Grandmother, 8 y/o granddaughter) |
| Encouraged Activity during Confining Treatment | “She got up and was active. Usually she will just lay in bed…or just wants to watch movies…so it was nice to see her get up and move around …” Parent G (Mother, 4 y/o daughter) |
| Improved Mood & Lessened Pain | “…that day before [the music therapist came] he was with pain and he was in a really bad mood… So when the music time gets and he started dancing I was like oh my, because he was feeling bad…it was a good thing…” Parent C (Mother, 6 y/o son) |
| Takes Mind Off Treatment and Fear | “…it’s something she enjoyed and kept her mind going and busy and she didn’t have [to] lay there and think about things” Parent F (Grandmother, 8 y/o granddaughter) |
| Meaningful Way to Connect With Family Members, Peers, and Healthcare Providers | “It gave us another outlet, another way to interact with each other.” Parent A (Mother, 7 y/o son) |
| Music play benefit for parent | |
| Something Special We Can Do Together | “So it was fun to have that one on one time, just me and her to do it.” Participant G (Mother, 4 y/o daughter) |
| Brightened the Day and Lifted Mood | “You can be having a really…crummy day and it becomes hey, I’m going to beat my triangle, we’re going to sing a song, and it does change your mood. [It] can just lighten the heaviness in the room, so that’s always good.” Parent B (Mother, 8 y/o son) |
| Relief of Child Distress Positively Impacts Parent | “So when the music time gets and he started dancing I was like oh my, because he was feeling bad [before the music therapy session]. When he’s like that it’s like for some reason we’re here and that makes me feel that there is something more that makes me feel better to make him smile and dance.” Parent C (Mother, 6 y/o son) |
| Parents need support during cancer treatment | |
| Hospital Stays and Treatment are Hard to Get Through | “…you don’t have time to think about why you're feeling it or how you’re feeling it, or what it means, you’re just going through, trying to get to the end of treatment.” Parent A (Mother, 7 y/o son) |
| Engaging Child can be Hard and Exhausting-Parents Need Breaks | “Like I said, we constantly interact with [our]kids…I love him, but you know we could all use a break sometimes here and there.” (Mother, 5y/o son) |
| The music play kit | |
| Parents Supported Child Initiated Use in Hospital and Home | “…when we didn’t have things going on, she would go grab her [music] bag and pull it out. Come on, let’s play, Mom.” Parent D (Mother, 4 y/o daughter) |
Theme I: The Music Play Experience
Parents described qualities of music play that were of particular value for their child during hospitalization. Qualities included having something to look forward to during treatment, and opportunities for normalizing, successful, and engaging experiences where their child could be in control and share the experience with family members. Parent comments centered more on the child experience, and less on the parent experience.
Theme II: Music Play Benefit for Child
When asked to describe any perceived benefit, parent comments focused on their child. Parents described six benefits including developmental stimulation, relaxation, increased physical activity, improved mood, taking mind off treatment, and a meaningful way to connect with family, peers, and healthcare providers.
Theme III: Music Play Benefit for Parent
When asked if they perceived any benefit for themselves, parents shared that music play activities offered something special they could do with their child, that this shared time lifted their mood, and that seeing positive change in their child made them feel better.
Theme IV: Parents Need Support During Cancer Treatment
In addition, we asked how to best support parents during their child’s cancer treatment. Parents shared that hospital stays are stressful and hard to get through, and that engaging their child during cancer treatment hospitalizations can be challenging and exhausting. Having a break from parental responsibilities is helpful, but not always available.
Theme V: Music Play Kit
When asked about using kit materials beyond scheduled sessions, parents indicated use was most often initiated by their child, and was used between scheduled sessions to relax, share with family members, or manage long periods of waiting. Parents also indicated bringing their kit for use during subsequent hospital admissions.
Preliminary Outcomes
Child Behavioral Coding scores indicate that AME + P child participants showed less distress with respect to positive facial affect relative to control participants (Table III). The ES was large (Cohen’s d = 1.07) and p-value significant (p = .040). As shown in Table V, ESs for parent-report measures tended to be small. A medium ES was noted for parent mood disturbance (ES = 0.17); however, this effect indicated improvement in the control group relative to AME + P group. In addition, relative to control group standard deviation, the AME + P group had scores almost 1 SD worse at baseline for parent mood disturbance.
Table V.
Means (SDs) Over Time With Effect Size Estimates for Parent Outcomes (n = 9 for AME + P; n = 7 for Control)
| Outcome | Group | Time point |
T2 data collection |
T3 data collection |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | F | p | Partial η2 | F | p | Partial η2 | ||
| Parent mood disturbance (POMS) | AME+P | 51.1 (18.8) | 47.9 (16.1) | 45.3 (19.7) | 2.58 | 0.132 | 0.166a | 1.22 | 0.289 | 0.086a |
| Control | 43.3 (9.1) | 32.6 (15.5) | 32.7 (12.9) | |||||||
| Parent traumatic distress (IES-R) | AME+P | 2.1 (1.3) | 2.1 (1.2) | 1.9 (1.2) | 0.25 | 0.625 | 0.019a | 0.08 | 0.783 | 0.006a |
| Control | 1.7 (1.0) | 1.5 (1.5) | 1.8 (1.7) | |||||||
aEffect sizes indicate greater improvement in control relative to AME + P.
Discussion
High parent delivery scores indicate parents were able to deliver AME activities without difficulty, and parent delivery resulted in lower child emotional distress. However, child engagement scores were similar across both conditions, and parent interview data indicated that AME parent delivery did not offer any reprieve from stress associated with keeping their young child occupied and engaged during cancer treatment hospitalizations. In addition, outcome data indicate parents did not derive the intended benefit and in some cases, experienced greater distress than control group parents.
AME + P parents shared that cancer treatment admissions are “hard to get through” and keeping their child engaged is both “challenging” and “exhausting.” These statements are consistent with findings that the most stressful aspect of having a child with cancer may be related to caregiving responsibility, including feelings of uncertainty and diminished ability to help manage child distress (Rodriguez et al., 2012). During AME + P, the expectation was for parents to “lead” their child in music-play activities; the goal being to empower parents with strategies and resources to help their child cope with in-patient hospitalization. Although parents were able to deliver the music-play activities and saw benefits for their child, they also shared that engaging their child is a responsibility from which they have no reprieve. Given that child emotional distress scores for affect were better relative to the control group, we were successful in decreasing this indicator of child distress. But our intention was to also diminish parent distress, and this did not occur.
One explanation for diminished mood disturbance observed in control parents, but not AME + P parents, may be that control group parents were able to relax and enjoy an audio-recorded storybook with their child. This activity did not require much cognitive or physical energy. Consistent with our previous studies, this condition did not result in child benefit, but is acceptable and enjoyable (Robb, 2000; Robb et al., 2008). In contrast, AME + P parents took on the role of facilitating play sessions and found them enjoyable and beneficial for their child, but required a higher level of energy to sustain child engagement. This active role may have increased parent distress and perhaps increased awareness of child limitations related to treatment, which may explain the lack of change on parent mood scores. This explanation is supported by studies establishing caregiving as a significant source of parent stress, that parents report using a limited number of strategies to manage child distress, and that learning is less efficient during times of stress (Hildenbrand et al., 2011; Rodriguez et al., 2012; Shors & Shors, 2006).
Our parent interview data also suggest that removing parents from the role of primary facilitator, and returning to a therapist-delivered intervention model, may provide more benefit for parents because they would be able to relax and enjoy what they reported as a normalizing, shared experience. Parent-reported use of the music-play resource kit between sessions and after return home indicate parents and children were able to use activities outside sessions, and found them helpful, suggesting the kit functioned as intended and may potentially help with promoting carry-over effects from therapist-led sessions.
Although absolute comparison of mean frequencies from this study and our 2008 study are not possible owing to slight differences in timing and number of probes, it is informative to compare the ES data for child engagement and distress observed in each study. Based on the CSM-MT, engagement is an important, potential mediator for reducing child distress.
Interestingly, in the therapist-delivered intervention study, the ES for child engagement was 2.41 (compared with 0.19 for parent delivery), and the ES for reduced child distress in the therapist-delivered intervention was 1.80 (compared with 1.07 for parent delivery). The ESs for child engagement and distress were much larger for the therapist-delivered intervention, and although not powered for a mediational analysis, these findings offer preliminary evidence that child engagement may mediate changes in child distress warranting subsequent examination of their relationship. This information would offer greater understanding about the mechanisms of action responsible for AME outcomes and help identify the amount of therapist support needed by parents and children to sustain benefits observed in therapist-delivered sessions.
Here we describe five main study limitations. First, risk for bias in condition delivery increased because music therapists delivered both conditions, which was done to control for individual therapist qualities and to mask data collectors to parent/child group assignment. To address this risk, we used study-specific procedures to monitor treatment fidelity across conditions. Second, findings must be cautiously interpreted owing to the small sample, and cannot be generalized beyond our sample. For example, our observation that the AME + P group had worse baseline scores for parent mood disturbance may have impacted our ES estimates. Third, measurement of child distress was limited to one behavioral indicator during intervention sessions. The addition of a parent-proxy measure would allow for a broader conceptualization of child distress that could be measured across a wider range of time. Fourth, we asked parents to recall use of AME activities over a 30-day period and this likely affected reporting accuracy. Fifth, at study closure we noticed the timeframe for recall on two of our T2 measures (IES-R, POMS) was over the “past week.” Given that the period between T1 and T2 was approximately three to four days, this may have influenced our ability to detect meaningful change.
Based on our findings and study limitations, we offer three recommendations for subsequent studies. First, examine whether parent distress decreases under the traditional AME. Second, conduct an adequately powered study to further investigate whether child engagement is a mediator for child distress outcomes. Third, add a parent-proxy report of child emotional distress so this outcome can be measured across all time points, with a broader conceptualization of distress.
In summary, few supportive care interventions address the unique needs of young children with cancer (<8 years); even fewer address interrelated parent and young child needs (Robb & Hanson-Abromeit, 2014). Consistent with previous AME studies, data support the intervention as potentially viable in addressing child distress, and may have the potential to improve parent outcomes. Future studies will examine child engagement as a potential mediator, and efficacy of the AME to manage shared parent and child distress.
Acknowledgements
The authors thank Tiffany Randle, Phillip Mauskapf, Courtney Spiegel, Marcie Sherman, Melissa Lee, Anne Bubnick, Anna Winfrey, Kristen Edmondson, April Roser, Johnna Ross, Caitlin Krater, and Kellie Schallert for their assistance with this study. We also extend our gratitude to the patients and families who participated and the care team at Riley Children’s Hospital at IU Health.
Funding
This work was funded by the first author’s institutional CTSI career award NIH/PHS (NCCR) KL2RR025760-02 and IUPUI RESPECT center pilot study grant.
Conflicts of interest: None declared.
References
- Barrera M., D'agostino N. M., Gibson J., Gilbert T., Weksberg R., Malkin D. (2004). Predictors and mediators of psychological adjustment in mothers of children newly diagnosed with cancer. Psycho-Oncology, 13, 630–641. [DOI] [PubMed] [Google Scholar]
- Barrera M. E., Rykov M. H., Doyle S. L. (2002). The effects of interactive music therapy on hospitalized children with cancer: A pilot study. Psycho-Oncology, 11, 379–388. [DOI] [PubMed] [Google Scholar]
- Bennett E., English M. W., Rennoldson M., Starza-Smith A. (2013). Predicting parenting stress in caregivers of children with brain tumours. Psycho-Oncology, 22, 629–636. [DOI] [PubMed] [Google Scholar]
- Best M., Streisand R., Catania L., Kazak A. E. (2001). Parental distress during pediatric leukemia and posttraumatic stress symptoms (PTSS) after treatment ends. Journal of Pediatric Psychology, 26, 299–307. [DOI] [PubMed] [Google Scholar]
- Bradt J., Dileo C., Grocke D., Magill L. (2011). Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database of Systematic Reviews, CD006911. [DOI] [PubMed] [Google Scholar]
- Flohr J. (2005). The musical lives of young children. Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- Ginsburg K. (2007). The importance of play in promoting health child development and maintaining strong parent-child bonds. Pediatrics, 119, 182–191. [DOI] [PubMed] [Google Scholar]
- Graf A., Bergstraesser E., Landolt M. A. (2013). Posttraumatic stress in infants and preschoolers with cancer. Psycho-Oncology, 22, 1543–1548. doi:10.1002/pon.3164 [DOI] [PubMed] [Google Scholar]
- Hildenbrand A. K., Clawson K. J., Alderfer M. A., Marsac M. L. (2011). Coping with pediatric cancer: Strategies employed by children and their parents to manage cancer-related stressors during treatment. Journal of Pediatric Oncology Nursing, 28, 344–354. [DOI] [PubMed] [Google Scholar]
- Imberty M. (1996). Linguistic and musical development in preschool and school-age children In Deliege I., Sloboda J. A. (Eds.), Musical beginnings: Origins and development of musical competence (pp. 191–213). New York, NY: Oxford University Press. [Google Scholar]
- Julious S. A. (2005). Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics, 4, 287–291. [Google Scholar]
- Kazak A. E., Barakat L. P. (1997). Brief report: Parenting stress and quality of life during treatment for childhood leukemia predicts child and parent adjustment after treatment ends. Journal of Pediatric Psychology, 22, 749–758. [DOI] [PubMed] [Google Scholar]
- Kazak A. E., Baxt C. (2007). Families of infants and young children with cancer: A post-traumatic stress framework. Pediatric Blood and Cancer, 49, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Kazak A. E., Boeving C. A., Alderfer M. A., Hwang W. T., Reilly A. (2005). Posttraumatic stress symptoms during treatment in parents of children with cancer. Journal of Clinical Oncology, 23, 7405–7410. [DOI] [PubMed] [Google Scholar]
- Kazak A. E., Cant M. C., Jensen M. M., McSherry M., Rourke M. T., Hwang W. T., Alderfer M.A., Beele D., Simms S., Lange B. J. (2003). Identifying psychosocial risk indicative of subsequent resource use in families of newly diagnosed pediatric oncology patients. Journal of Clinical Oncology, 21, 3220–3225. [DOI] [PubMed] [Google Scholar]
- Lindahl Norberg A., Poder U., Ljungman G., von Essen L. (2012). Objective and subjective factors as predictors of post-traumatic stress symptoms in parents of children with cancer–a longitudinal study. PLoS ONE [Electronic Resource], 7, e36218.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Balsamo L., Lu X., Devidas M., Hunger S. P., Carroll W. L., Winick N. J., Maloney K.W., Kadan-Lottick N. S. (2014). A prospective study of anxiety, depression, and behavioral changes in the first year after a diagnosis of childhood acute lymphoblastic leukemia: A report from the Children's Oncology Group. Cancer, 120, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg A. L., Boman K. K. (2013). Mothers and fathers of children with cancer: Loss of control during treatment and posttraumatic stress at later follow-up. Psycho-Oncology, 22, 324–329. [DOI] [PubMed] [Google Scholar]
- Robb S. L. (2000). The effect of therapeutic music interventions on the behavior of hospitalized children in isolation: Developing a contextual support model of music therapy. Journal of Music Therapy, 37, 118–146. [DOI] [PubMed] [Google Scholar]
- Robb S. L., Clair A. A., Watanabe M., Monahan P. O., Azzouz F., Stouffer J. W., Ebberts A., Darsie E., Whitmer C., Walker J., Nelson K., Hanson-Abromeit D., Lane D., Hannan A. (2008). Non-randomized controlled trial of the active music engagement (AME) intervention on children with cancer. Psycho-Oncology, 17, 957.. [DOI] [PubMed] [Google Scholar]
- Robb S. L., Hanson-Abromeit D. (2014). A review of supportive care interventions to manage distress in young children with cancer and parents. Canc Nursing, 37, E1–E26. [DOI] [PubMed] [Google Scholar]
- Rodriguez E. M., Dunn M. J., Zuckerman T., Vannatta K., Gerhardt C. A., Compas B. E. (2012). Cancer-related sources of stress for children with cancer and their parents. Journal of Pediatric Psychology, 37, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Version 9.3 [computer program]. Carey, NC: SAS Institue, Inc.
- Shacham S. (1983). A shortened version of the profile of mood states. Journal of Personality Assessment, 47, 305–306. [DOI] [PubMed] [Google Scholar]
- Shors T. J., Shors T. J. (2006). Stressful experience and learning across the lifespan. Annual Review of Psychology, 57, 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner E. A., Wellborn J. G. (1994). Coping during childhood and adolescence: A motivational perspective In Fetherman D. L., Lerner R. M., Perlmutter M. (Eds.), Life-span development and behavior (Vol. 12, pp. 91–133). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Walt Disney Group. (2010a). 3-in-1 read-along storybook and CD: Enchanting tales. New York, NY: Disney Press. [Google Scholar]
- Weiss D.M.C.R. (1997). The impact of events scale-revised In Keane J. W. T. (Ed.), Assessing psychological trauma and PTSD (pp. 399–411). New York, NY: Guilford. [Google Scholar]