Abstract
Objective Impairments in executive function (EF) skills have been observed in youth with type 1 diabetes (T1D), and these skills are critical for following the complex treatment regimen. This study examines parent reports of EF in relation to measures of adherence, glycemic control (A1c), and psychosocial outcomes (depression and quality of life) in adolescents with T1D. Methods A total of 120 adolescents (aged 13–17 years, 52.5% female, 87.5% White) with T1D and their parents completed questionnaires. Glucometers were downloaded and A1c was obtained during clinical visits at the time of enrollment. Results The prevalence of clinically significant elevated scores on specific EF skills ranged from 11 to 18.6%. In multivariate analyses, parent-reported EF deficits were associated with poorer adherence and lower quality of life, explaining 13 and 12% of the variance, respectively. Conclusions Adolescents with T1D exhibit specific EF deficits that may negatively impact their quality of life and their ability to engage in self-management activities.
Keywords: adolescents, adherence, diabetes, psychosocial functioning, quality of life
Introduction
Type 1 diabetes (T1D) ranks among the most prevalent pediatric chronic illnesses, with estimates of >18,000 new annual cases in the United States in youth <20 years of age (Center for Disease Control and Prevention, 2014; SEARCH for Diabetes in Youth Study Group, 2007). Compared with other age-groups, adolescents with T1D are at a particular risk for deteriorating glycemic control and poor treatment adherence because of psychosocial and physiological changes (Clements et al., 2015; Hilliard, Wu, Rausch, Dolan, & Hood, 2013). Furthermore, increased depressive symptoms and decreased quality of life are more prevalent in adolescents with T1D as compared with healthy populations (Grey, Whittemore, & Tamborlane, 2002; Varni et al., 2003), with differences more pronounced among females than males (Hood et al., 2006; Naughton et al., 2014). The negative impact of these psychosocial issues further elevates the likelihood of poorly controlled diabetes (Baucom et al., 2015; Hilliard et al., 2013; Lawrence et al., 2012).
The risks associated with poor glycemic control are well documented. Long-term complications such as retinopathy, cardiovascular disease, and neuropathy have been linked to suboptimal glycemic control, particularly hyperglycemia (DCCT Group, 2000; Wood et al., 2013). The complex treatment regimen recommended to achieve and maintain optimal glycemic control includes frequent blood glucose checks, monitoring carbohydrate consumption, and multiple daily administrations of insulin (American Diabetes Association, 2016). Following this regimen requires careful planning (e.g., remembering to bring supplies) and complex decision-making (e.g., making adjustments to insulin based on activity level or food intake), and treatment adherence often presents a challenge for adolescents. During adolescence, responsibility for completing these tasks typically shifts from parents to adolescents, which may further compromise diabetes management (Anderson et al., 2002). Given the complexity of treatment management, the influence of adolescent cognitive factors has recently been explored in relation to adherence behaviors and outcomes. Specifically, previous studies in youth with T1D have identified associations between treatment-related variables and executive function (EF), or the ability to plan, organize, and monitor goal-directed behavior (Duke & Harris, 2014).
Given the complex tasks involved in T1D management, and the potential implications of suboptimal cognitive function, EF is an important component to consider when researching treatment adherence. To guide our understanding of EF, we used a widely accepted, three-component framework for EF, consisting of three cognitive tasks: updating (i.e., keeping track of and adjusting working memory content), shifting (i.e., alternating between tasks or cognitive sets), and inhibition (i.e., behavioral self-control; Miyake et al., 2000). Recent evidence suggests that this structure emerges as children advance through adolescence (Lee, Bull, & Ho, 2013). Generally, substantial gains in inhibition occur early in childhood (Best & Miller, 2010; Boelema et al., 2014), while working memory and shifting abilities improve gradually throughout adolescence (Best & Miller, 2010; Boelema et al., 2014; Lee et al., 2013). Evidence from neuroimaging literature indicates that brain development continues during adolescence through axonal myelination and changes in synaptic organization and connectivity in the prefrontal cortex (Blakemore & Choudhury, 2006; Spear, 2013). These changes that take place during adolescence may play a contributing role in the development and honing of EF skills (Spear, 2013). Impairments in EF may be the result of variations in brain function or structure (i.e., slower gray and white matter growth) in youth with T1D in response to glycemic variability (Lin, Northam, Rankins, Werther, & Cameron, 2010; Mauras et al., 2015; Ohmann et al., 2010). On the other hand, adolescents who have problems with EF may have greater difficulty engaging in treatment management, resulting in suboptimal glycemic control.
Despite the considerable neurodevelopmental changes that mark adolescence, and the increased risk of suboptimal glycemic control characteristic of adolescents with T1D, the role of EF in adolescents with T1D remains an understudied area. The limited number of studies that have been conducted in adolescents with T1D have identified associations between general constructs of EF and adherence (Bagner, Williams, Geffken, Silverstein, & Storch, 2007; Berg et al., 2014; McNally, Rohan, Pendley, Delamater, & Drotar, 2010; Smith et al., 2014); however, these studies have relied on subjective measures of adherence (i.e., questionnaires and interviews), and at least two included younger children, who may not be responsible for treatment management (McNally et al., 2010; Smith et al., 2014). The literature examining EF and glycemic control includes mixed findings, with some studies indicating a significant association (e.g., Berg et al., 2014; Nylander et al., 2013), but others finding no relationship between these variables (e.g., Smith et al., 2014). One study examined EF in relation to both adherence and glycemic control and found significant associations only among males (Graziano et al., 2011), while a recent study piloted a novel diabetes-specific measure of EF and found significant associations for both adherence (adolescent and parent report) and glycemic control (Duke, Raymond, & Harris, 2014). These findings have not been replicated, further indicating a need for additional research to explore these factors. Finally, none of these studies examined EF in relation to psychosocial outcomes in adolescents with T1D, such as quality of life or depression.
Current Study
To address these limitations and current gaps in the existing literature, the current study examined the relationship between specific aspects of parent-reported EF, glycemic control, and adherence, captured through subjective reports (parent and adolescent questionnaires) and objective glucometer data. The current study focused on the specific components of EF discussed above (i.e., shifting, updating, and inhibition), which may provide important clinical information. We hypothesized that poorer EF would be associated with lower adherence across measures. In line with previous studies, we also predicted that poorer EF would be associated with worse glycemic control (i.e., higher hemoglobin A1c). In addition, we sought to describe the relationship between EF and psychosocial outcomes, including depression and quality of life in adolescents of studies with T1D. Finally, given that sex differences in outcomes of studies with adolescents with T1D have been inconsistent (Graziano et al., 2011; Hood et al., 2006; Naughton et al., 2014; Nylander et al., 2013), we explored sex as a potential moderator of the associations between EF, adherence, glycemic control, and psychosocial outcomes.
Methods
The current study was a cross-sectional analysis of baseline data collected in a pilot randomized trial of a positive psychology intervention for adolescents with T1D (ClinicalTrials.gov NCT02746627). Adolescents were approached at their routine diabetes appointments at an academic medical center. Adolescents were eligible if they met the following criteria: (1) age 13–17 years, (2) diagnosed with T1D for at least 6 months, (3) no other major health problems, (4) not participating in other intervention studies, and (5) A1c level between 8.0 and 12.0% at the time of recruitment. Of the adolescents approached (n = 183), 63 (34%) declined participation (most common reasons were lack of time and interest). There were no significant differences in those who did and did not participate related to age, mean A1c, sex, or race/ethnicity. Data analyses included a sample of 120 participants (see Table I for demographic and clinical characteristics).
Table I.
Demographics and Baseline Clinical Characteristics (n = 120)
| Adolescent baseline variables | Range | Mean (SD) | N (%) elevateda |
|---|---|---|---|
| Age (years) | 13–17 | 14.83 (1.44) | – |
| Duration of diabetes (years) | 1–16 | 5.84 (3.64) | – |
| A1c (%) | 8–11.5 | 9.16 (0.90) | – |
| Daily BGM | 0.21–9.30 | 3.29 (1.75) | – |
| PHQ-9 | 0–24 | 4.14 (4.02) | 7 (5.8) |
| SCI—overall | |||
| Parent report | 1.29–5.0 | 3.53 (0.73) | – |
| Child report | 1.71–4.86 | 3.50 (0.74) | – |
| BRIEF T scores | |||
| GEC | 35–78 | 51.30 (10.76) | 16 (13.6) |
| Initiate | 36–80 | 52.35 (11.25) | 22 (18.6) |
| Inhibit | 40–84 | 48.86 (10.42) | 13 (11.0) |
| Shift | 38–91 | 50.94 (11.67) | 19 (16.1) |
| Working Memory | 38–85 | 53.51 (11.68) | 21 (17.8) |
| PedsQL diabetes | 26.85–97.22 | 70.87 (12.05) | – |
| Adolescent demographic variables | |||
| N (%) | |||
| Gender | |||
| Male | – | – | 57 (47.5) |
| Female | – | – | 63 (52.5) |
| Race/ethnicity | |||
| White, Non-Hispanic | – | – | 105 (87.5) |
| Other | – | – | 14 (11.7) |
| Unknown | – | – | 1 (0.8) |
| Annual family income (USD) | |||
| <39,000 | – | – | 32 (26.7) |
| 40,000–79,000 | – | – | 45 (37.5) |
| >80,000 | – | – | 43 (35.8) |
| Treatment type | |||
| Insulin pump | – | – | 61 (50.8) |
| Injection | – | – | 59 (49.2) |
| ADHD diagnosis | |||
| Yes | – | – | 9 (7.5) |
| No | – | – | 111 (92.5) |
Note. Daily BGM = average daily blood glucose checks from meter download, blood glucose monitoring; PHQ-9 = Patient Health Questionnaire (adolescent report); SCI = Self-Care Inventory; BRIEF = Behavior Rating Inventory of Executive Function (parent report); GEC = Global Executive Composite; PedsQL Diabetes = Pediatric Quality of Life Inventory 3.0 Diabetes Module (adolescent report); ADHD = attention deficit/hyperactivity disorder; USD = U.S. Dollar.
Executive function = BRIEF T score ≥65; Clinically significant depressive symptoms = PHQ-9 ≥ 11.
Procedures
All study procedures and measures were approved by the university’s institutional review board/Human Research Protection Program. Parents and adolescents provided consent/assent and completed electronic questionnaires on a HIPAA-protected secure site (REDCap; Harris et al., 2009). Glucometer data were downloaded if available, but if participants forgot their meters, attempts to capture glucometer data were made at subsequent clinic visits (meter data were available from 93%). Parents and adolescents were compensated for their time.
Adolescents and parents agreed to participate in a randomized trial comparing an 8-week education intervention (n = 60) with a positive psychology intervention (n = 60). Adolescents in both groups completed brief measures of mood every 2 weeks by phone or text during the active phase of the intervention, and adolescents and their parents completed questionnaire data at 3 and 6 months.
Measures
Demographic and Clinical Variables
Parents provided demographic information, such as race/ethnicity and annual family income, as well as diabetes-related variables, including adolescents’ date of diagnosis and treatment type (i.e., insulin pump or injections).
Executive Function
Parents completed the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000), which assessed the adolescents’ cognitive ability to plan, organize, and manage time in the school and home environments. The 86-item BRIEF questionnaire includes eight scales that form an overall Global Executive Composite (GEC). Each item is rated on a 3-point Likert scale from 1 = never a problem to 3 = often a problem, with higher scores signifying poorer EF. Age- and sex-adjusted T scores are used to compare individuals’ EF to normative data, with a mean of 50 and SD of 10; the clinical cutoff T score of ≥65 indicates impaired EF. The GEC was used to assess general EF, to allow for the comparison of results to other studies of youth with T1D (Bagner et al., 2007; Berg et al., 2014; Smith et al., 2014). Additionally, four of the eight scales (Inhibit, Initiate, Working Memory, and Shift) were included in the analyses, as these scales are most representative of the domains of EF captured in the aforementioned framework (Miyake et al., 2000). Similar to other studies (e.g., Limbers & Young, 2015), we selected the Inhibit scale to represent the Inhibition function, the Working Memory and Initiate scales to represent the Updating function, and the Shift scale to represent the Shifting function. In our sample, the overall GEC had excellent reliability (Cronbach’s alpha = .97), as did the four scales (Inhibit α = .91, Working Memory α = .91, Initiate α = .83, and Shift α = .85). The BRIEF has two validity scales: Inconsistency and Negativity. In our sample, five parents scored in the Questionable range for Inconsistency, and no one scored in the Inconsistent range. Only one of the parents scored in the Elevated range for Negativity, and no one scored in the Highly Elevated range, meaning that ratings of EF were reasonably consistent and not overly negative. Additionally, adolescents were asked to report if they have ever been diagnosed with attention deficit/hyperactivity disorder (ADHD), to account for the impact on reported EF.
Adherence
Adolescents and their parents each reported on adolescents’ adherence to the diabetes regimen using the Self-Care Inventory (SCI; Lewin et al., 2009), which assesses key elements of T1D management, including diet, exercise, blood glucose monitoring (BGM), and insulin administration. The mean of seven items scored on a scale of 1 (never do it) to 5 (always do this as recommended without fail) is used to calculate an overall adherence score. Higher scores indicate better adherence. In our sample, Cronbach’s alpha was .78 for both teen (C-SCI) and parent (P-SCI) reports of adherence. As an objective proxy measure of adherence (Guilfoyle, Crimmins, & Hood, 2011), frequency of BGM (average number of blood glucose checks over a 28 ± 2-day period from glucometers, daily BGM) was used.
Glycemic Control
The A1c test reflects average blood glucose level over the past 2–3 months, measured quarterly in adolescents with T1D. A1c level was obtained from each participant’s medical record at the time of enrollment, with analyses conducted using a Bayer Diagnostics DCA2000® Analyzer. The American Diabetes Association recommends a target A1c of <7.5% for adolescents with T1D (American Diabetes Association, 2016).
Depression
Adolescents reported on depressive symptoms experienced over the past 2 weeks, measured using the Patient Health Questionnaire (PHQ-9; Kroenke, Spitzer, & Williams, 2001). Each of nine items is assigned a score of 0–3 and contributes to an overall score between 0 and 27, with scores of >11 indicating clinically significant symptoms of depression in adolescents (Richardson et al., 2010). Cronbach’s alpha was .79 in our sample.
Quality of Life
The Pediatric Quality of Life Inventory (PedsQL) 3.0 Diabetes Module was used to measure adolescent report of diabetes-specific health-related quality of life. The 28-item module assesses how often adolescents encounter problems with diabetes-related symptoms, treatment barriers, adherence, worry, and communication. The total scaled score was used, as recommended in the literature (Nansel et al., 2008), ranging from 0 to 100, with higher values indicating better quality of life. Cronbach’s alpha was .87 in our sample.
Statistical Plan
Statistical analyses were performed using IBM SPSS Statistics 23. Descriptive analyses were conducted to describe the sample (Table I), and further analyses with the Kruskal–Wallis H test and Mann–Whitney U test were performed to examine potential demographic differences in components of EF (Table II). Bivariate correlations were calculated to test the strength of associations between EF and adherence, using parent report, self-report, and glucometer data, as well as EF and glycemic control, depressive symptoms, and quality of life. Finally, linear regression models were conducted to assess general EF as a predictor of adherence and diabetes-related outcomes, adjusting for preselected covariates (i.e., adolescent’s sex, age, race/ethnicity, and family income). To examine potential effects of child sex, we tested child sex as a moderator by creating an interaction term (GEC centered × Child sex), which was added to the final step of each model.
Table II.
Differences in Parent-Reported Executive Function by Demographic and Clinical Characteristics
| GEC | Initiate | Inhibit | Shift | WM | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 50.00 | 50.85 | 47.84 | 49.50 | 52.44 |
| Male | 52.73 | 54.00 | 50.00 | 52.54 | 54.70 |
| Race/ethnicity | |||||
| White, non-Hispanic | 51.99 | 53.01 | 49.32 | 51.37 | 53.98 |
| Other | 46.07 | 47.43 | 45.64 | 48.00 | 49.29 |
| Income (USD) | |||||
| <40,000 | 55.47* | 55.09 | 53.88* | 56.28* | 56.94 |
| 40,000–80,000 | 47.34 | 48.50 | 46.36 | 47.70 | 49.73 |
| >80,000 | 52.26 | 54.29 | 47.67 | 50.26 | 54.86 |
| Treatment type | |||||
| Insulin pump | 51.51 | 53.61 | 48.42 | 50.49 | 53.61 |
| Injection | 51.08 | 51.08 | 49.31 | 51.39 | 53.41 |
| ADHD diagnosis | |||||
| Yes | 56.00 | 58.44 | 49.78 | 51.22 | 63.67* |
| No | 50.87 | 51.81 | 48.85 | 50.95 | 52.57 |
| PHQ-9 | |||||
| Yes | 53.43 | 52.86 | 50.14 | 56.71 | 55.43 |
| No | 51.16 | 52.32 | 48.78 | 50.58 | 53.39 |
Note. ADHD = attention deficit/hyperactivity disorder; BRIEF =Behavior Rating Inventory of Executive Function; GEC = Global Executive Composite, WM = Working Memory scale; PHQ-9 = Pediatric Health Questionnaire; USD = U.S. Dollar; T scores are presented.
p < .01;
p < .001.
Results
Descriptive Results
Descriptive statistics for demographic and clinical variables are presented in Table I. Mean T scores for overall EF (GEC) and the BRIEF scales ranged from 48.9 to 53.5, which is within the normal range of functioning. As seen in Table I, 11.0% of adolescents demonstrated elevated scores on the Inhibit scale, 16.1% on the Shift scale, 17.8% on the Working Memory scale, and 18.6% on the Initiate scale. In addition, 13.6% of adolescents demonstrated elevated scores on the GEC. Compared with the published norms, males in our sample scored significantly higher on the Initiate Scale (t = 2.80, p = .008) and Working Memory scales (t = 2.87, p = .006), as well as the GEC (t = 2.21, p = .033), and females in our sample scored significantly higher on the Inhibit scale (t = 2.24, p = .021; Gioia et al., 2000).
As seen in Table II, there were no significant differences on any of the BRIEF scales related to sex (male vs. female), race/ethnicity (White, non-Hispanic vs. Other), or treatment type (insulin pump vs. injections), but there were some income-related differences in EF. The lowest income group demonstrated significantly higher scores (i.e., greater impairment) than the middle-income group on the GEC and Shift scale, and the lowest income group had significantly higher scores than the middle- or highest-income groups on the Inhibit scale (all p < .01). This is similar to findings in the normative sample, in which small but significant correlations between socioeconomic status and BRIEF scale scores were evident; however, socioeconomic status only accounted for 5% of the variance and was not considered a major factor in the interpretation of scores in the normative sample (Gioia et al., 2000). There were no differences in EF related to clinically significant depressive symptoms, and the only significant difference related to adolescents’ self-reported diagnosis of ADHD was the Working Memory scale (adolescents with ADHD had significantly higher scores than those who did not).
Bivariate Associations Between EF, Adherence, and Diabetes-Related Outcomes
Bivariate correlations are presented in Table III. Higher scores on the GEC and Initiate, Shift, and Working Memory scales were significantly associated with lower levels of parent-reported adherence (r’s range from −.26 to −.36, all p < .01). However, none of the EF scores was significantly related to adolescents’ self-reported adherence, or to glucometer data (mean daily BGM). In terms of diabetes-related outcomes, higher GEC, Initiate, Inhibit, and Shift scores were significantly related to lower diabetes-related quality of life (r’s range from −.24 to −.32, all p < .01). However, there was no significant association between EF and glycemic control or depressive symptoms.
Table III.
Correlations Between Adherence, Outcomes, and Executive Function Measures
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | ||||||||||||
| 2. Duration of diabetes | .18 | – | |||||||||||
| 3. P-SCI | −.09 | −.21 | – | ||||||||||
| 4. C-SCI | −.10 | −.08 | .38** | – | |||||||||
| 5. Daily BGM | −.27* | −.20 | .16 | .25* | – | ||||||||
| 6. A1c | .14 | .11 | −.19 | −.39** | −.26* | – | |||||||
| 7. GEC | −.11 | −.06 | −.32** | −.10 | .06 | .06 | – | ||||||
| 8. Initiate | −.06 | .01 | −.26* | −.09 | .03 | .07 | .84** | – | |||||
| 9. Inhibit | −.22 | −.07 | −.20 | −.10 | .06 | .03 | .76** | .50** | – | ||||
| 10. Shift | −.13 | −.08 | −.36** | −.10 | .05 | .02 | .80** | .55** | .68** | – | |||
| 11. Working Memory | −.07 | −.11 | −.27* | −.05 | .11 | −.04 | .85** | .77** | .48** | .57** | – | ||
| 12. PHQ-9 | .26* | −.02 | −.17 | −.30** | −.04 | .08 | .15 | .09 | .11 | .15 | .10 | – | |
| 13. PedsQL | −.13 | −.10 | .26* | .31** | .02 | −.25* | −.31** | −.24* | −.27* | −.32** | −.17 | −.39** | – |
Note. P-SCI = parent-reported Self-Care Inventory; C-SCI = adolescent self-reported Self-Care Inventory; Daily BGM = average daily blood glucose monitoring; GEC = Global Executive Composite; PedsQL = Pediatric Quality of Life Inventory (adolescent self-report) Diabetes Module; PHQ-9 = Pediatric Health Questionnaire (adolescent self-report).
p < .01;
p < .001.
Multivariate Analyses
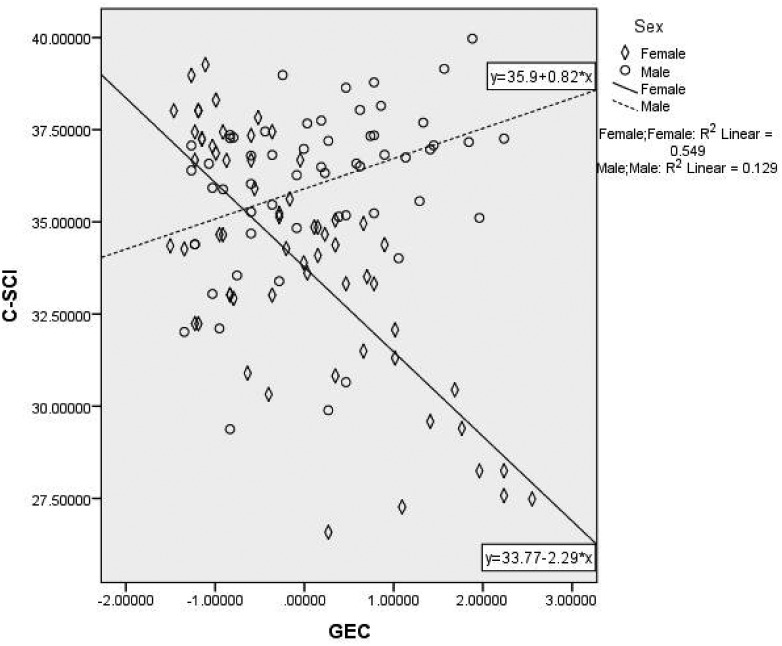
Finally, we conducted linear regression models to test general EF (GEC) as a predictor of parent-reported adherence, adolescent-reported adherence, and quality of life, adjusting for child age, sex, family income, and race/ethnicity. The model predicting parent report of adherence (P-SCI) was significant (F(6, 112) = 3.77, p = .002). As seen in Table IV, in the final model, race/ethnicity and GEC were significant predictors, and the addition of GEC explained 13% of the variance in adherence. The interaction term was not significant, indicating that child sex was not a significant moderator of the effect of GEC on parent-reported adherence. The overall model for adolescent report of adherence (C-SCI) was also significant (F(6, 111) = 3.42, p = .004), and in the final model, both GEC and the GEC × Sex interaction term were significant predictors (see Table IV). As seen in Figure 1, the association between GEC and adherence was stronger for girls than for boys. Finally, the model for quality of life was also significant (F(6, 112) = 3.71, p = .002). In the final model predicting PedsQL, child age, sex, and GEC were significant predictors (see Table IV), and the addition of GEC explained 12% of the variance in quality of life. The interaction term was not significant, indicating that child sex was not a significant moderator of the effect of GEC on adolescent quality of life.
Table IV.
Summary of Regression Analyses Predicting Adolescent Adherence and Quality of Life
| Parent-reported adherence |
Self-reported adherence |
Quality of life |
||||
|---|---|---|---|---|---|---|
| Predictor | ΔR2 | β | ΔR2 | β | ΔR2 | β |
| Step 1: | .02 | .10* | .05 | |||
| Child age | −.07 | −.11 | −.16 | |||
| Family income | −.02 | .19* | .04 | |||
| Race/ethnicity | −.11 | −.14 | .08 | |||
| Child sex | .00 | .16 | .16 | |||
| Step 2: | .13*** | .02 | .12*** | |||
| Child age | −.11 | −.12 | −.20* | |||
| Family income | −.07 | .17 | −.01 | |||
| Race/ethnicity | −.18* | −.17 | .01 | |||
| Child sex | .04 | .18 | .20* | |||
| GEC | −.38*** | −.15 | −.36*** | |||
| Step 3: | .02 | .04* | .00 | |||
| Child age | −.11 | −.13 | −.20* | |||
| Family income | −.06 | .15 | −.01 | |||
| Race/ethnicity | −.19* | −.17 | .01 | |||
| Child sex | .04 | .17 | .20* | |||
| GEC | −.26* | −.33** | −.41*** | |||
| GEC × Sex | −.18 | .26* | .08 | |||
| Total R2 | .12 | .11 | .12 | |||
Note. GEC = Global Executive Composite; raw score was centered. Total R2 is adjusted R2.
p < .05;
p < .01;
p < .001.
Figure 1.
Moderating effect of sex on the association between executive function and adolescents’ self-reported adherence. GEC = Global Executive Composite from the Behavior Rating Inventory of Executive Function; C-SCI = Child Self-Care Inventory.
Discussion
In the current study, adolescents’ parent-reported EF skills were significantly related to adherence and quality of life. While the adolescents in our sample were within the normal range of functioning on a measure of EF, scores on the Working Memory, Shift, and Initiate scales differed significantly from normative values. Our finding that greater deficits in EF were significantly associated with poorer parent-reported adherence, after adjusting for adolescent age, sex, income, and race/ethnicity, provides partial support for the hypothesized relationship between EF and adherence and is in line with previous reports (Bagner et al., 2007; Berg et al., 2014). Contrary to our hypothesis, a significant association between EF and an objective measure of adherence (glucometer data) was not found. It is possible that the influence of EF varies across diabetes tasks, as the adherence questionnaire captured behaviors related to lifestyle and insulin use, in addition to BGM patterns; the task of testing blood glucose levels may not carry a cognitive load high enough to result in detectable differences between varying EF abilities and adherence. Instead, it may be that other diabetes-related tasks require more skill and planning (e.g., correct dosing and timing of insulin use and monitoring food intake) and therefore could be more sensitive to problems in EF.
There was no significant direct relationship between EF and glycemic control in our sample, similar to previous findings in preadolescents; however, our finding that adherence behaviors (as measured by parent report, self-report, and objective meter download) were significantly associated with glycemic control, support the proposed model of an indirect association, in which deficits in EF affect adherence behaviors, which in turn may affect glycemic control (McNally et al., 2010). It is important to note that the cross-sectional nature of our study does not allow for tests of the direction of effects. Further, there is some evidence that assessing EF abilities with a diabetes-specific measure may have a stronger relationship with glycemic control (Duke et al., 2014). Finally, it may be possible that parents who recognize poor EF skills in their adolescent with T1D maintain a more present role in diabetes management, mitigating the effects on outcomes such as glycemic control (Smith et al., 2014).
In multivariate analyses adjusting for covariates, parent reports of adolescents’ overall EF also emerged as a significant predictor of adolescent-reported quality of life. Maintaining health-related quality of life while practicing good diabetes care is a goal for diabetes management in youth and their families (Lawrence et al., 2012), suggesting that EF may also play a role in the amount that T1D influences the psychosocial well-being of adolescents. It is possible that adolescents demonstrating problems in EF struggle to face challenges related to their diabetes, negatively impacting quality of life. Alternatively, poorer quality of life may reflect other problems in the adolescent’s life that could interfere with their ability to draw on EF skills.
It is important to note that, while adolescents in our sample generally displayed age-appropriate EF skills, certain domains of EF were characterized by nearly double the amount of elevated symptoms found in normative samples (Gioia et al., 2000); these included EF skills captured by the Working Memory, Initiate, and Shift scales. These findings are similar to the small but significant deficits observed in youth with T1D using objective neurocognitive tests (Naguib, Kulinskaya, Lomax, & Garralda, 2009). The demanding nature and heightened stress/emotions accompanying diabetes management may undermine frontal cortical activity necessary for regulating cognition and behavior, impacting EF performance (Spear, 2013). Alternatively, it could be that poor diabetes management, resulting in greater glycemic variability and hyperglycemia, may have a negative impact on brain structures associated with EF (Lin et al., 2010; Mauras et al., 2015; Ohmann et al., 2010). Additionally, this finding highlights the need for further research to understand which specific EF skills are critical to diabetes care. For example, working memory may be needed to follow the necessary steps of checking blood glucose, calculating insulin dose, and administering insulin, while the ability to shift attention and behavior may be important when tolerating changes to routines that may affect diabetes care, such as dining out at an unfamiliar restaurant (Wasserman, Hilliard, Schwartz, & Anderson, 2015).
Finally, we found evidence for a moderating effect of child sex in the association between general EF with self-reported adherence. Specifically, for boys, there was a negative association with self-reported adherence, such that fewer problems with EF were related to lower perceptions of adherence to treatment regimens, and greater problems with EF was related to better self-reported adherence. For girls, the association between problems with EF and adherence was positive; greater problems with EF were associated with lower self-reported adherence. In the only other study that examined sex differences in EF among adolescents with T1D (Graziano et al., 2011), boys’ EF and emotional regulation deficits were associated with worse adherence and glycemic control, but there were no significant associations for girls. Our findings suggest that girls may be more accurate reporters of diabetes management; however, larger studies are needed to better understand the impact of adolescent sex on the relationships between EF, adherence, and outcomes.
While this study contributes to the literature on EF in adolescents with T1D, several limitations should be considered. First, participants were targeted because of having poor glycemic control (A1c > 8.0%), and this may have limited our ability to detect an association between glycemic control exhibit and EF skills. Second, because of the structure of clinic visits, parents were typically informed of their adolescent’s A1c value before completing study materials, which could have impacted their reporting on adolescents’ behavior (i.e., BRIEF and P-SCI). As such, these findings should be replicated in multimethod studies. Third, because of the use of cross-sectional data, the direction of effects between EF and diabetes management is impossible to discern, and a longitudinal study design is recommended. Finally, EF was measured through questionnaire data; although the BRIEF is a well-established instrument to measure EF, it is based on parent report of the child’s everyday EF ability. Because parents also reported on adherence, it is possible that the apparent relation between these two variables may be attributed at least in part to shared method variance, or potential bias related to the reporter, and direct measurement of EF is a necessary next step (i.e., neuropsychological testing). However, similar studies using the BRIEF Parent Form have found this association to exist across reporters (Berg et al., 2014; McNally et al., 2010; Smith et al., 2014). Additionally, it is important to note that performance-based tests and rating scales may provide valuable and nonoverlapping information about EF skills, with the former capturing EF ability, and the latter describing success in goal achievement or the application of EF skills (Isquith, Roth, & Gioia, 2013; Toplak, West, & Stanovich, 2013).
If these findings are replicated in multimethod and longitudinal studies, several potential clinical implications are suggested. First, adolescents with poor EF skills may be adherent to some specific aspects of diabetes care, such as monitoring blood glucose satisfactorily, but failing in other areas that require greater application of cognitive skills, such as making adjustments to insulin dose. It remains essential, therefore, for future research to determine whether specific EF skills, rather than overall EF, are stronger predictors of diabetes management tasks and outcomes, and warrant skill-specific intervention. Second, it is important to consider that problems in EF (whether they are caused by diabetes or as the result of poor management) may have a negative impact on adolescents’ quality of life. Finally, two studies have demonstrated that self-care autonomy disproportionate to psychological maturation is associated with glycemic dysregulation and inadequate adherence (King et al., 2012; Wysocki et al., 1996), indicating that adolescents may assume excessive autonomy in their diabetes care without psychological readiness. Providers should collaborate with adolescents and their parents in understanding their planning, organization, and monitoring skills to discuss a more structured plan that optimizes individual diabetes treatment, while allowing for the assessment of adolescents’ maturity in EF skills when transitioning responsibility over diabetes management. For example, Wasserman et al. (2015) provide practical implications of EF skills in diabetes management and ways to strengthen them in adolescents with T1D. Still, a subset of adolescents may experience more serious difficulties in EF that reach beyond the scope of diabetes management (e.g., a diagnosis of ADHD), and may require a comprehensive formal evaluation of cognitive function and other disorders with coinciding symptoms (Wasserman et al., 2015).
The current study highlights specific problems with EF in adolescents with T1D, and demonstrates that parent-reported EF is related to adherence and quality of life. Furthermore, results highlight that these associations can be further influenced by demographic variables such as adolescent sex, illustrating that the relationships between EF, adherence, and outcomes in adolescents with T1D are complex and multifaceted. The adolescent with T1D should continue to be studied comprehensively, with the focus expanding beyond traditional measures such as medical outcomes (e.g., complications and A1c) to include cognitive abilities (i.e., EF) and other important diabetes-related variables such as health-related quality of life, depression, and other psychosocial variables, such as coping and self-efficacy.
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NIH. This study is registered with ClinicalTrials.gov (NCT02746627). We would like to thank the families who participated in our study, and acknowledge the work of Emily Hamburger, MEd, on the creating the figure.
Funding
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DP3DK097678).
Conflicts of interest: None declared.
References
- American Diabetes Association. (2016). Standards of medical care in diabetes - 2016. Diabetes Care, 39, S1–S106.26696671 [Google Scholar]
- Anderson B. J., Vangsness L., Connell A., Butler D., Goebel-Fabbri A., Laffel L. (2002). Family conflict, adherence, and glycaemic control in youth with short duration Type 1 diabetes. Diabetic Medicine, 19, 635–642. [DOI] [PubMed] [Google Scholar]
- Bagner D. M., Williams L. B., Geffken G. R., Silverstein J. H., Storch E. A. (2007). Type diabetes in youth: The relationship between adherence and executive functioning. Child Health Care, 36, 169–179. doi:10.1080/02739610701335001 [Google Scholar]
- Baucom K. J., Queen T. L., Wiebe D. J., Turner S. L., Wolfe K. L., Godbey E. I., Fortenberry K. T., Mansfield J. H., Berg C. A. (2015). Depressive symptoms, daily stress, and adherence in late adolescents with type 1 diabetes. Health Psychology, 34, 522–530. doi:10.1037/hea0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. A., Wiebe D. J., Suchy Y., Hughes A. E., Anderson J. H., Godbey E. I., Butner J., Tucker C., Franchow E. I., Pihlaskari A. K., King P. S., Murray M. A., White P. C. (2014). Individual differences and day-to-day fluctuations in perceived self-regulation associated with daily adherence in late adolescents with type 1 diabetes. Journal of Pediatric Psychology, 39, 1038–1048. doi:10.1093/jpepsy/jsu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J. R., Miller P. H. (2010). A developmental perspective on executive function. Child Development, 81, 1641–1660. doi:10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S. J., Choudhury S. (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47, 296–312. doi:10.1111/j.1469-7610.2006.01611.x [DOI] [PubMed] [Google Scholar]
- Boelema S. R., Harakeh Z., Ormel J., Hartman C. A., Vollebergh W. A. M., van Zandvoort M. J. E. (2014). Executive functioning shows differential maturation from early to late adolescence: Longitudinal findings from a TRAILS study. Neuropsychology, 28, 177–187. doi:10.1037/neu0000049 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services. [Google Scholar]
- Clements M. A., Foster N. C., Maahs D. M., Schatz D. A., Olson B. A., Tsalikian E., Lee J. M., Burt-Solorzano C. M., Tamborlane W. V., Chen V., Miller K. M., Beck RW; T1D Exchange Clinic Network. (2015). Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatric Diabetes, doi:10.1111/pedi.12295. [DOI] [PubMed] [Google Scholar]
- Duke D. C., Harris M. A. (2014). Executive function, adherence, and glycemic control in adolescents with type 1 diabetes: A literature review. Current Diabetes Reports, 14, 1–10. doi:10.1007/s11892-014-0532-y. [DOI] [PubMed] [Google Scholar]
- Duke D. C., Raymond J. K., Harris M. A. (2014). The diabetes related executive functioning scale (DREFS): Pilot results. Children's Health Care, 43, 327–344. doi:10.1080/02739615.2013.870040. [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. (2000). Behavior Rating Inventory of Executive Function: BRIEF. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Graziano P. A., Geffken G. R., Williams L. B., Lewin A. B., Duke D. C., Storch E. A., Silverstein J. H. (2011). Gender differences in the relationship between parental report of self‐regulation skills and adolescents' management of type 1 diabetes. Pediatric Diabetes, 12(4 pt 2), 410–418., doi:10.1111/j.1399-5448.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- Grey M., Whittemore R., Tamborlane W. (2002). Depression in type 1 diabetes in children: natural history and correlates. Journal of Psychosomatic Research, 53, 907–911. doi:10.1016/S0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Guilfoyle S. M., Crimmins N. A., Hood K. K. (2011). Blood glucose monitoring and glycemic control in adolescents with type 1 diabetes: meter downloads versus self‐report. Pediatric Diabetes, 12, 560–566. doi:10.1111/j.1399-5448.2010.00735. [DOI] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Wu Y. P., Rausch J., Dolan L. M., Hood K. K. (2013). Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. Journal of Adolescent Health, 52, 28–34. doi:10.1016/j.jadohealth.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood K. K., Huestis S., Maher A., Butler D., Volkening L., Laffel L. M. (2006). Depressive symptoms in children and adolescents with type 1 diabetes association with diabetes-specific characteristics. Diabetes Care, 29, 1389–1389. doi:10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- Isquith P. K., Roth R. M., Gioia G. (2013). Contribution of rating scales to the assessment of executive functions. Applied Neuropsychology: Child, 2, 125–132. doi:10.1080/21622965.2013.748389. [DOI] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Drew L. M., Foster C., Donaldson D., Murray M., Swinyard M., Wiebe D. J. (2012). Longitudinal trajectories of metabolic control across adolescence: Associations with parental involvement, adolescents’ psychosocial maturity, and health care utilization. Journal of Adolescent Health, 50, 491–496. doi:10.1016/jadohealth.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2001). The PHQ‐9. Journal of General Internal Medicine, 16, 606–613. doi:10.1046/j.1525-1497.2001.016009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. M., Joyce P., Black M. H., Anderson A., Hood K., Imperatore G., Klingensmith G. J., Naughton M., Mayer-Davis E. J., Seid M.; SEARCH for Diabetes in Youth Study Group. (2012). Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. The Journal of Pediatrics, 161, 201–207. doi:10.1016/j.jpeds.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Bull R., Ho R. M. (2013). Developmental changes in executive functioning. Child Development, 84, 1933–1953. doi:10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- Lewin A. B., LaGreca A. M., Geffken G. R., Williams L. B., Duke D. C., Storch E. A., Silverstein J. H. (2009). Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI). Journal of Pediatric Psychology, 34, 999–1007. doi:10.1093/jpepsy/jsp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbers C. A., Young D. (2015). Executive functions and consumption of fruits/vegetables and high saturated fat foods in young adults. Journal of Health Psychology, 20, 602–611. doi:10.1177/1359105315573470. [DOI] [PubMed] [Google Scholar]
- Lin A., Northam E. A., Rankins D., Werther G. A., Cameron F. J. (2010). Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatric Diabetes, 11, 235–243. doi:10.1111/j.1399-5448.2009.00588.x. [DOI] [PubMed] [Google Scholar]
- Mauras N., Mazaika P., Buckingham B., Weinzimer S., White N. H., Tsalikian E., Hershey T., Cato A., Cheng P., Kollman C., Beck R. W., Ruedy K., Aye T., Fox L., Arbelaez A. M., Wilson D., Tansey M., Tamborlane W., Peng D., Marzelli M., Winer K. K., Reiss A. L. (2015). Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes, 64, 1770–1779. doi:10.2337/db14-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K., Rohan J., Pendley J. S., Delamater A., Drotar D. (2010). Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care, 33, 1159–1162. doi:10.2337/dc09-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Naguib J. M., Kulinskaya E., Lomax C. L., Garralda M. E. (2009). Neuro-cognitive performance in children with type 1 diabetes—a meta-analysis. Journal of Pediatric Psychology, 34, 271–282. doi:10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- Nansel T. R., Weisberg‐Benchell J., Wysocki T., Laffel L., Anderson B. (2008). Quality of life in children with Type 1 diabetes: a comparison of general and diabetes‐specific measures and support for a unitary diabetes quality‐of‐life construct. Diabetic Medicine, 25, 1316–1323. doi:10.1093/jpepsy/jsn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M. J., Joyce P., Morgan T. M., Seid M., Lawrence J. M., Klingensmith G. J., Waitzfelder B, Standiford D. A., Loots B.; SEARCH for Diabetes in Youth Study Group. (2014). Longitudinal associations between sex, diabetes self-care, and health-related quality of life among youth with type 1 or type 2 diabetes mellitus. The Journal of Pediatrics, 164, 1376–1383. doi:10.1016/j.jpeds.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander C., Toivonen H., Nasic S., Söderström U., Tindberg Y., Fernell E. (2013). Children and adolescents with type 1 diabetes and high HbA1c–a neurodevelopmental perspective. Acta Paediatrica, 102, 410–415. doi:10.1111/apa.12128. [DOI] [PubMed] [Google Scholar]
- Ohmann S., Popow C., Rami B., König M., Blaas S., Fliri C., Schober E. (2010). Cognitive functions and glycemic control in children and adolescents with type 1 diabetes. Psychological Medicine, 40, 95–103. doi:10.1017/S0033291709005777. [DOI] [PubMed] [Google Scholar]
- Richardson L. P., McCauley E., Grossman D. C., McCarty C. A., Richards J., Russo J. E., Rockhill C., Katon W. (2010). Evaluation of the Patient Health Questionnaire (PHQ-9) for detecting major depression among adolescents. The Journal of Pediatrics, 126, 1117–1123. doi:10.1542/peds.2010-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARCH for Diabetes in Youth Study Group. (2007). Incidence of diabetes in youth in the United States. JAMA, 297, 2716–2724. doi:10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Smith L. B., Kugler B. B., Lewin A. B., Duke D. C., Storch E. A., Geffken G. R. (2014). Executive functioning, parenting stress, and family factors as predictors of diabetes management in pediatric patients with type 1 diabetes using intensive regimens. Children's Health Care, 43, 234–252. doi:10.1080/02739615.2013.839383. [Google Scholar]
- Spear L. P. (2013). Adolescent neurodevelopment. Journal of Adolescent Health, 52, S7–S13. doi:10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. (2000). Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The New England Journal of Medicine, 342, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak M. E., West R. F., Stanovich K. E. (2013). Practitioner review: Do performance-based measures and ratings of executive function assess the same construct?. The Journal of Child Psychology and Psychiatry, 54, 131–143. doi:10.1111/jcpp.12001 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Jacobs J. R., Gottschalk M., Kaufman F., Jones K. L. (2003). The PedsQL™ in type 1 and type 2 diabetes reliability and validity of the pediatric quality of life inventory™ generic core scales and type 1 diabetes module. Diabetes Care, 26, 631–637. doi:10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- Wasserman R. M., Hilliard M. E., Schwartz D. D., Anderson B. J. (2015). Practical strategies to enhance executive functioning and strengthen diabetes management across the lifespan. Current Diabetes Reports, 15, 1–9. doi:10.1007/s11892-015-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. R., Miller K. M., Maahs D. M., Beck R. W., DiMeglio L. A., Libman I. M., Quinn M., Tamborlane W. V., Woerner S. E.; T1D Exchange Clinic Network. (2013). Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care, 36, 2035–2037. doi:10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T., Taylor A., Hough B. S., Linscheid T. R., Yeates K. O., Naglieri J. A. (1996). Deviation from developmentally appropriate self-care autonomy: Association with diabetes outcomes. Diabetes Care, 19, 119–125. doi:10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]