Abstract
Objective
Explore interrelationships between domains of child health-related quality of life (HRQL) and parent emotional functioning using parent-proxy and child report in the context of hematopoietic stem cell transplant (HSCT).
Methods
Data on 258 parent–child dyads were used from two longitudinal studies. Domains of HRQL included physical, emotional, and role functioning, and HSCT-related worry. We used structural equation modeling to model the outcome of parent emotional functioning using primary and alternative conceptual models.
Results
Parent-proxy raters reported lower child HRQL than child raters. Structural equation models demonstrated relationships between child emotional functioning, child HSCT-related worry, and parent emotional functioning, with some differences by raters.
Conclusions
Relationships between child HRQL and parent emotional functioning within the context of HSCT are complex. To optimize the child’s health outcomes, providing psychosocial support for children and their families may be necessary, especially for those experiencing distress or facing treatment complications.
Keywords: cancer and oncology, parent stress, quality of life, structural equation modeling
Many parents of children with serious illness, and cancer in particular, face psychological distress, such as anxiety, depression, and posttraumatic stress syndrome (Boman, Viksten, Kogner, & Samuelsson, 2004; Packman, Weber, Wallace, & Bugescu, 2010; Riva et al., 2014), and decreased emotional functioning (Boman et al., 2004; McGrath, 2002; Rodday, Terrin, Chang, & Parsons, 2013a; Young, Dixon-Woods, Findlay, & Heney, 2002). In addition to the psychological distress associated with having a child with cancer, the parent caregiver must deal with the uncertainty of high-risk treatments. Although treatments offer a potential cure for these life-threatening pediatric medical conditions, they are not without risks, including short- and long-term morbidity and treatment failure, relapse, or death.
Parents of children undergoing hematopoietic stem cell transplant (HSCT), which is a treatment for malignant and nonmalignant diseases, represent one example of parents facing uncertainty and distress about their child’s health. Malignancies treated by HSCT may include cancers of the blood or bone marrow, such as leukemia or lymphoma, or as “rescue therapy” for aggressive treatment of solid tumors, including neuroblastomas, bone cancers, and brain tumors. Nonmalignancies may include disorders of the blood or bone marrow, such as bone marrow failure syndromes or hemoglobinopathies, or disorders of the immune system or metabolism.
The HSCT course can be divided into the pretransplant phase, the peritransplant hospitalization, and the postdischarge follow-up. As part of the pretransplant phase, the child and a parent caregiver may be relocated to the transplant center to undergo extensive pretransplant evaluation and preparation. The patient receives a preparative conditioning regimen that may include chemotherapy with or without radiation. Following the transplant infusion/reinfusion, the child remains hospitalized, awaiting successful engraftment and reconstitution of normal blood cells. During this time, the child is at risk for acute complications, such as infection, organ toxicity, and acute graft versus host disease (aGVHD). In the postdischarge follow-up, the child is at risk for chronic graft versus host disease (cGVHD) and late-onset infection. The possibility of relapse or death exists throughout the entire HSCT course.
Parent emotional functioning is influenced by the child’s HSCT course, including such complications as aGVHD, organ toxicity, cGVHD, and infection (Terrin et al., 2013). As a result, the parent may have difficulty being an effective caregiver in the presence of this psychological distress (Pennarola et al., 2012, 2015). Both parent and child health-related quality of life (HRQL) are impacted by the HSCT course (Barrera, Atenafu, & Hancock, 2009; Barrera, Boyd-Pringle, Sumbler, & Saunders, 2000; Clarke, Eiser, & Skinner, 2008; Packman et al., 2010; Parsons et al., 2006; Parsons, Tighiouart, & Terrin, 2013). Research has shown that parent emotional functioning and distress is associated with child HRQL and has explored differences and similarities in child and parent-proxy report of child HRQL (Eiser & Morse, 2001; Panepinto, Hoffmann, & Pajewski, 2010; Parsons et al., 2005, 2006, 2013; Parsons, Fairclough, Wang, & Hinds, 2012; Rodday, Terrin, & Parsons, 2013b). However, the role that child worry about his/her future health following HSCT (i.e., infection, rehospitalization, relapse) plays in the relationship between parent emotional functioning and child HRQL has not been studied. Furthermore, the extent to which the relationships between these factors may vary by rater has also not been explored.
Wilson and Cleary’s model that links biological features of disease to symptoms, functioning, and quality of life (Wilson & Cleary, 1995) provides the framework for our two conceptual models that aim to understand the relationship between child HRQL, child worry, and parent emotional functioning. Although their model has been used to conceptualize the link between disease and quality of life, especially in adult clinical populations (Bakas et al., 2012; Mayo et al., 2015; Shahrbanian, Duquette, Ahmed, & Mayo, 2016; Villalonga-Olives et al., 2014), it has been used less frequently in the pediatric setting and not in the context of a severe clinical state, such as HSCT. Our interpretation of the Wilson and Cleary model would suggest that the HSCT course, and its resulting complications, may cause the child to worry about his/her future health, which may result in decreased child functioning and HRQL (Packman et al., 2010; Rodday et al., 2013b). As a result, the parent’s HRQL, and notably their emotional functioning, may be affected. These relationships linking child worry about their future health to child HRQL and finally, to parent emotional functioning form the basis of our primary conceptual model. Our alternative conceptual model switches the position of child worry about his/her future health and child functioning and HRQL. In this model, the complications of HSCT can cause decreased child functioning and HRQL, which may lead the child to worry more about his/her future health, and finally affect the parent’s emotional functioning. Understanding which conceptualization captures the relationship between parent and child HRQL, and differences by child and parent-proxy raters, will allow clinicians to tailor their care, including setting patient and parent expectations about the HSCT course and providing psychological support if needed.
Given the parent’s important role as the child’s primary caregiver, describing the relationship between the child’s HRQL and the parent’s emotional functioning is paramount to understanding how to support the parent, which should lead to improved clinical and HRQL outcomes in their child. As such, this study aims to explore which components of the child’s HRQL are associated with parent emotional functioning at the acute posttransplant phase, a period when the risk for complications is high and HRQL is near its nadir.
Method
Participants
This analysis used data from two longitudinal studies: (1) the Journeys to Recovery (JTR) study, which described the 12-month HRQL trajectory following HSCT, and (2) the HSCT-Comprehensive Health Enhancement Support Study (CHESSTM) (Parsons et al., 2011), which was a randomized, controlled trial of a web-based intervention designed to improve the health-related knowledge, skills, and HRQL of parents of children undergoing HSCT. Together, these studies were conducted at eight HSCT centers across the United States from 2003 to 2011 and included 363 parent–child dyads. A total of 965 patients scheduled to undergo HSCT were initially screened and 634 (66%) met eligibility criteria, which included a working knowledge of English and having a parent/legal guardian who could consent on behalf of the child. Parental consent and age-appropriate assent were obtained. The studies were approved by the institutional review board at each clinical center and at Tufts Medical Center. Because the HRQL measures used in these studies were developed for children and adolescents aged 5–18 years, parent and child dyads were removed from the analysis if the child was <5 years old (n = 53). Only data from the 45-day assessment were used in the current analysis because this period captures the acute phase following HSCT while still maintaining a large sample size not available at later periods. After excluding an additional 52 dyads that were missing the 45-day assessment, our final sample size was 258 dyads. Most parents were mothers (84.5%) with a mean age of 39.7 (SD = 6.9) years. Mean child age was 10.9 (SD = 4.1) years, and 49.6% were female (Table I). By 45 days, 185 (71.7%) children had a serious transplant-related complication (aGVHD ≥ grade 2, systemic infection, or moderate to severe end organ toxicity) and 6 children had died.
Table I.
Baseline Parent, Child, and Disease Characteristics
| Characteristic | M (SD), median (25th–75th percentile), or n (%) |
|---|---|
| Parent demographics | |
| Parent age in years, M (SD) | 39.7 (6.9) |
| Parent female, n (%) | 218 (84.5%) |
| Parent education, n (%) | |
| High school graduate or less | 79 (30.6%) |
| Some college or more | 179 (69.4%) |
| Household income, n (%) | |
| <$40,000 | 74 (29.3%) |
| $40,000–$59,999 | 50 (19.8%) |
| $60,000–$79,999 | 36 (14.2%) |
| >$80,000 | 93 (36.8%) |
| Race/ethnicity, n (%) | |
| Non-Hispanic White | 173 (67.6%) |
| Non-Hispanic non-White | 30 (11.7%) |
| Hispanic | 49 (19.4%) |
| Refused, unknown | 4 (1.6%) |
| Child demographics | |
| Child age n years, M (SD) | 10.9 (4.1) |
| Child female, n (%) | 128 (49.6%) |
| Child disease characteristics | |
| Transplant type, n (%) | |
| Autologous | 54 (20.9%) |
| Allogeneic, related | 74 (28.7%) |
| Allogeneic, unrelated | 130 (50.4%) |
| Causal malignancy, n (%) | 195 (75.6%) |
| Duration of illness in months, median (25th–75th) | 11.5 (5, 40) |
| Study, n (%) | |
| JTR | 134 (51.9%) |
| HSCT-CHESS™, control | 61 (23.6%) |
| HSCT-CHESS™, intervention | 63 (24.4%) |
Note. JTR = Journeys to Recovery; HSCT-CHESS = hematopoietic stem cell transplant–Comprehensive Health Enhancement Support Study.
Measures
Child Health Ratings Inventories
This validated measure has rater- and age-specific versions and separate modules for generic and HSCT-specific HRQL (Parsons et al., 2005, 2006). The parent version of the Child Health Ratings Inventories (CHRIs) includes two sections: (1) parent-proxy report of the child’s health and functioning and (2) parent report of their own functioning. The school-aged child version (ages 5–12 years) of the CHRIs elicits child report of their own health and functioning using pictorial response sets, while the adolescent version (ages 13–18 years) is text based with parallel wording to the parent version. The 20 items from the CHRIs-General module form three domains of generic HRQL: physical, emotional, and role functioning. To supplement the CHRIs-General, a 10-item HSCT module was developed and validated in the 1990s to measure three domains of child HRQL related to the HSCT: hassles, worry about transplant-related outcomes (infection, rehospitalization, relapse), and body image. See Parsons et al. (2005, 2006) for item content. All CHRIs items use an acute, 1-week recall period because of the rapidly changing health status of patients undergoing HSCT. All response sets have five options with scores ranging from 1 to 5. Using established conventions for generic and disease-specific scales, higher scores on the CHRIs-General domains indicate better HRQL, whereas higher scores on the HSCT module domains indicate more of that construct (e.g., more worry).
With the widening variability in clinical practice in protective isolation practices and medication use over time and by clinical site, the domains of hassles and body image from the HSCT module show less reliability across clinical sites (Cronbach’s α = .45–.63) in our sample. However, HSCT-related worry remains reliable (Cronbach’s α ≥ .79), regardless of clinical site, indication for transplant, or treatment era. Thus, the domains of hassles and body image were excluded from the analysis. Within our sample, the Cronbach’s α for the included parent-proxy reported domains ranged from 0.80 to 0.93, while the Cronbach’s α for the included child reported domains ranged from 0.79 to 0.84 (Table II). Except for child-rated HSCT-related worry (α = .79), these Cronbach’s α exceed .8, which demonstrates internal consistency reliability for established scales (Nunnally & Bernstein, 1994).
Table II.
HRQL Domain Properties and Correlations by Rater
| Domain | M (SD) | Cronbach’s α | Correlationsa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parent EF | Parent-proxy PF | Parent-proxy EF | Parent-proxy RF | Parent-proxy worry | Child PF | Child EF | Child RF | |||
| Parent emotional functioning | 56.1 (18.2) | .86 | ||||||||
| Parent-proxy report | ||||||||||
| Physical functioning | 44.1 (32.8) | .93 | .27 | |||||||
| Emotional functioning | 55.7 (20.8) | .88 | .46 | .59 | ||||||
| Role functioning | 64.5 (26.6) | .82 | .30 | .46 | .62 | |||||
| HSCT-related worry | 25.8 (23.8) | .80 | −.20 | −.15 | −.19 | −.17 | ||||
| Child report | ||||||||||
| Physical functioning | 64.1 (25.7) | .82 | .18 | .36 | .37 | .23 | −.21 | |||
| Emotional functioning | 69.3 (19.6) | .84 | .25 | .38 | .57 | .38 | −.25 | .61 | ||
| Role functioning | 74.7 (24.3) | .80 | .19 | .17 | .30 | .23 | −.19 | .47 | .51 | |
| HSCT-related worry | 27.3 (25.7) | .79 | −.17 | −.18 | −.15 | −.12 | .25 | −.30 | −.36 | −.32 |
Note. EF = emotional functioning; PF = physical functioning; RF = role functioning; HSCT = hematopoietic stem cell transplant; HRQL = health-related quality of life.
All correlations had p < .05.
Demographic and Medical Information
As part of the baseline assessment, demographic variables on the patients and their parents were obtained. Research staff collected clinical data from medical records, including the following baseline variables: transplant type (autologous, related allogeneic, unrelated allogeneic), causal diagnosis (malignancy vs. nonmalignancy), and duration of illness in months. Throughout the transplant course, data on clinical outcomes, including infection (based on the Common Toxicity Criteria of Adverse Events, v. 3.0), treatment toxicity (Bearman et al., 1988), aGVHD, and cGVHD were collected. A binary variable for the presence of any serious transplant-related complications by Day 45 was created to indicate aGVHD grade ≥ 2, systemic infection, or moderate to severe end organ toxicity.
Statistical Analysis
Demographic and clinical variables and HRQL scores were summarized with means (SD) or medians (25th–75th percentiles) for continuous variables or using frequencies and percentages for categorical variables. Using chi-square tests or two-sample t-tests, we compared baseline characteristics of parents and children by completion status of the 45-day assessment to detect for differences that could affect generalizability. We reported Pearson correlations between domains and considered <0.30 as low, 0.30–0.60 as moderate, and >0.60 as high.
Structural equation modeling (SEM) was used to assess the relationship between the different domains of child HRQL and parent emotional functioning. Unlike traditional regression techniques, SEM allows variables to be both dependent and independent, and allows examination of indirect pathways leading to the dependent variable. Given the larger number of parameters estimated in SEM, a sample size of at least 200 observations is recommended (Boomsma & Hoogland, 2001; Tomarken & Waller, 2005). Separate sets of models were constructed using child report of their own HRQL and parent-proxy report of the child’s HRQL. Parents reported their own emotional functioning for all models. HRQL domains (i.e., latent variables) were scored using the solution to the confirmatory factor analysis (CFA). Rather than weighting items equally, as is done when averaging items together to create a domain score, this method uses a weighted combination of the item scores based on the factor loadings (MPlus, 2016). We used the untransformed 5-point item scoring for this method, and resulting scores had a mean of 0.
Before constructing the SEMs, CFA was used to assess the measurement models and calculate factor scores. The fit of the models was assessed using the root mean square error of approximation (RMSEA), the comparative fit index (CFI), the Tucker–Lewis Index (TLI), and the standardized root mean square residual (SRMR). The following criteria are indicative of acceptable model fit: RMSEA <0.06; CFI and TLI close to 0.95 or greater; and SRMR <0.08 (Hu & Bentler, 1999). Model chi-square statistics were also reported. Nonsignificance indicates acceptable model fit; however, given the large sample sizes used in SEM, the chi-square statistic is often statistically significant, limiting its usefulness as a fit statistic (Kelloway, 2014). To improve fit of the measurement models, additional parameters representing covariance between items were added to the model based on modification indexes >10 and the face validity of the relationship between the items. Items were not allowed to load onto other factors and no items were removed to maintain consistency with the original CHRIs domains.
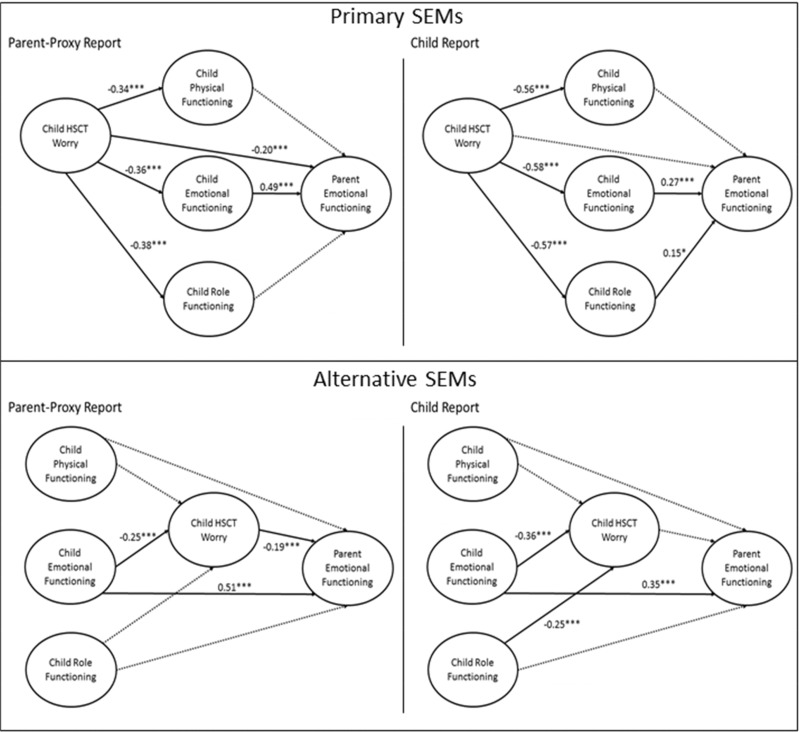
SEMs using maximum likelihood estimation were built with parent emotional functioning as the outcome and the child HRQL domains as latent factors (Figure 1), with child HSCT-related worry domain preceding the generic child HRQL domains, according to our primary conceptual model. Paths were eliminated one-by-one according to the largest p-value until all p-values for the paths were <.1. Alternative SEMs were explored that placed the generic child HRQL domains before the HSCT-related worry domain in association with parent emotional functioning (Figure 1). This represents an alternative conceptual model where decreased child HRQL and functioning could lead to child HSCT-related worry. Akaike information criterion (AIC) values were reported for the different models allowing for comparisons of their fit, with lower AIC values indicating better fit. To control for potential confounding, the SEMs adjusted for child and parent age, child and parent gender, complications by 45 days, and study/intervention assignment. Of note, in the intention-to-treat analysis of the web-based intervention from the HSCT-CHESS study, no statistically significant intervention effect on the primary outcome of parent emotional functioning at 6 months was found, making confounding by this factor less likely. Both unstandardized and standardized coefficients were also reported from the SEMs. For standardization, the variances of dependent and independent variables were standardized to equal one; coefficients are interpreted as the number of SDs the dependent variable changes given a change of 1 SD in the independent variable. MPlus (MPLUS (Version 7) [Computer Software], 2015) was used to fit the measurement and structural models. All remaining analyses were conducted in SAS Version 9.4 (SAS Institute, Inc., Cary, NC). The type I error rate was set to 0.05.
Figure 1.
Primary and alternative structural equation models assessing relationship between child health-related quality of life and parent emotional functioning by rater. Note. *p < .1, **p < .05, ***p < .01. Standardized estimates are reported. Models adjusted for child and parent age, child and parent gender, complications by 45 days, and study/intervention assignment. Dashed lines indicate nonsignificant pathways that were removed from the model.
Results
Parent-proxy raters reported their child’s generic HRQL lower than child raters reported their own generic HRQL (p-values from paired t-tests <.01), but there was no systematic difference in child HSCT-related worry scores by rater (Table II). Parent emotional functioning was more highly correlated with parent-proxy report of the child’s HRQL than with child report of their HRQL, but all were significantly different than 0 (p < .05). Of note, within rater, the highest correlated domains were parent-proxy-reported physical and emotional functioning (r = .59), parent-proxy-reported emotional and role functioning (r = .62), and child-reported physical and emotional functioning (r = .61). For child-reported HSCT-related worry, there were moderate correlations with child-reported physical (r = −.30), emotional (r = −0.36), and role functioning (r = −0.32), but correlations of the parent-proxy reported domains were lower.
Confirmatory Factor Analysis
The initial CFA measurement models for both parent-proxy report and child report did not meet the acceptable fit criteria. Accordingly, item covariances were added based on measurement indexes >10 provided that the item relationships had face validity (e.g., exercising moderately and exercising hard). The revised measurement models for both parent-proxy and child report met fit criteria for RMSEA and SRMR, although the CFI and TLI were slightly lower than desired (Table III).
Table III.
SEM Performance and Fit Statistics by Rater
| Model | Model chi-square | CFI | TLI | AIC | RMSEA (90% CI) | SRMR |
|---|---|---|---|---|---|---|
| Parent-proxy report | ||||||
| Measurement model + item-level covariance | 510.8 (df = 282)* | 0.943 | 0.934 | 17,258.722 | 0.056 (0.048, 0.064) | 0.058 |
| Primary SEM with significant paths and adjustment | 907.593 (df = 419)* | 0.876 | 0.857 | 16,990.196 | 0.068 (0.062, 0.074) | 0.131 |
| Alternative SEM with significant paths and adjustment | 718.364 (df = 430)* | 0.927 | 0.918 | 16,778.967 | 0.052 (0.045, 0.058) | 0.061 |
| Child report | ||||||
| Measurement model + item-level covariance | 463.329 (df = 285)* | 0.937 | 0.928 | 18,106.070 | 0.049 (0.041, 0.057) | 0.056 |
| Primary SEM with significant paths and adjustment | 743.799 (df = 422)* | 0.888 | 0.872 | 17,661.994 | 0.055 (0.049, 0.062) | 0.075 |
| Alternative SEM with significant paths and adjustment | 698.101 (df = 433)* | 0.908 | 0.897 | 17,594.297 | 0.049 (0.043, 0.056) | 0.070 |
Note. SEM = structural equation modeling; CFI = comparative fit index; TLI = Tucker–Lewis Index; AIC = Akaike information criterion; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual.
Adjustment was for child and parent age, child and parent gender, complications by 45 days, and study/intervention assignment.
p < .001.
Primary SEM by Rater
The primary SEM for parent-proxy report indicated statistically significant relationships between higher child HSCT-related worry and lower child HRQL in the domains of physical, emotional, and role functioning (Table IV, Figure 1). Higher child HSCT-related worry was also associated with lower parent emotional functioning. Child emotional functioning was the only generic child HRQL domain that was associated with parent emotional functioning. In addition to the direct effect that child HSCT-related worry had on parent emotional functioning, there was also an indirect effect through child emotional functioning. This parent-proxy report model explained 40% of the variability in parent emotional functioning. However, fit statistics for this model fell outside the acceptable range (Table III).
Table IV.
Results From Primary SEM Assessing Relationship Between Child HRQL and Parent Emotional Functioning by Rater
| Pathway | Parent-proxy report |
Child report |
||
|---|---|---|---|---|
| Unstandardized estimate (SE) | Standardized estimate (SE) | Unstandardized estimate (SE) | Standardized estimate (SE) | |
| Direct effects | ||||
| Child worry → Child PF | −0.46 (0.11)*** | −0.34 (0.07)*** | −0.61 (0.11)*** | −0.56 (0.06)*** |
| Child worry → Child EF | −0.36 (0.08)*** | −0.36 (0.07)*** | −0.39 (0.08)*** | −0.58 (0.06)*** |
| Child worry → Child RF | −0.41 (0.1)*** | −0.38 (0.08)*** | −0.59 (0.1)*** | −0.57 (0.06)*** |
| Child worry → Parent EF | −0.18 (0.07)*** | −0.20 (0.07)*** | – | – |
| Child PF → Parent EF | – | – | – | – |
| Child EF → Parent EF | 0.45 (0.07)*** | 0.49 (0.06)*** | 0.37 (0.14)*** | 0.27 (0.09)*** |
| Child RF → Parent EF | – | – | 0.13 (0.08)* | 0.15 (0.09)* |
| Indirect effects | ||||
| Child worry → Child PF → Parent EF | – | – | – | – |
| Child worry → Child EF → Parent EF | −0.16 (0.04)*** | −0.18 (0.04)*** | −0.15 (0.06)*** | −0.16 (0.06)*** |
| Child worry → Child RF → Parent EF | – | – | −0.08 (0.05)* | −0.08 (0.05)* |
Note. Models adjusted for child and parent age, child and parent gender, complications by 45 days, and study/intervention assignment.
EF = emotional functioning; PF = physical functioning; RF = role functioning; SEM = structural equation modeling; HRQL = health-related quality of life.
p < .1, **p < .05, ***p < .01.
Similar to the parent-proxy report model, the child report model indicated statistically significant relationships between higher child HSCT-related worry and lower child HRQL in the domains of physical, emotional, and role functioning (Table IV, Figure 1). Higher child emotional functioning was also significantly associated with higher parent emotional functioning. Unlike in the parent-proxy report model, child HSCT-related worry was not directly associated with parent emotional functioning, but was associated indirectly through child emotional functioning. This model also indicated a borderline relationship between higher child role functioning and higher parent emotional functioning. This child report model explained 14% of the variability in parent emotional functioning. However, fit statistics for this model, except for the RMSEA, fell outside the acceptable range (Table III).
Alternative SEM by Rater
The alternative SEM for parent-proxy report indicated a statistically significant relationship between higher child emotional functioning and lower HSCT-related worry (Table V, Figure 1). Child physical and role functioning were not associated with child HSCT-related worry. A relationship between higher child emotional functioning and higher parent emotional functioning was observed, as was an association between higher child HSCT-related worry and lower parent emotional functioning. There was also an indirect relationship between child emotional functioning, child HSCT-related worry, and parent emotional functioning. This parent-proxy report model explained 38% of the variability in parent emotional functioning. This model had acceptable fit criteria for RMSEA and SRMR, but was slightly below the threshold for CFI and TLI (Table III).
Table V.
Results From Alternative SEM Assessing Relationship Between Child HRQL and Parent Emotional Functioning by Rater
| Pathway | Parent-proxy report |
Child report |
||
|---|---|---|---|---|
| Unstandardized estimate (SE) | Standardized estimate (SE) | Unstandardized estimate (SE) | Standardized estimate (SE) | |
| Direct effects | ||||
| Child PF → Child worry | – | – | – | – |
| Child EF → Child worry | −0.23 (0.07)*** | −0.25 (0.07)*** | −0.53 (0.15)*** | −0.36 (0.09)*** |
| Child RF → Child worry | – | – | −0.25 (0.1)*** | −0.25 (0.09)*** |
| Child PF → Parent EF | – | – | – | – |
| Child EF → Parent EF | 0.45 (0.07)*** | 0.51 (0.06)*** | 0.46 (0.11)*** | 0.35 (0.07)*** |
| Child RF → Parent EF | – | – | – | – |
| Child worry → Parent EF | −0.18 (0.07)*** | −0.19 (0.07)*** | – | – |
| Indirect effects | ||||
| Child PF → Child worry → Parent EF | – | – | – | – |
| Child EF → Child worry → Parent EF | 0.04 (0.02)** | 0.05 (0.02)** | – | – |
| Child RF → Child worry → Parent EF | – | – | – | – |
Note. Models adjusted for child and parent age, child and parent gender, complications by 45 days, and study/intervention assignment.
EF = emotional functioning; PF = physical functioning; RF = role functioning; SEM = structural equation modeling; HRQL = health-related quality of life.
p < .1, **p < .05, ***p < .01.
For the child report model, higher child emotional and role functioning were associated with significantly lower child HSCT-related worry (Table V, Figure 1). Child emotional functioning was the only factor that was significantly associated with parent emotional functioning. This child report model explained 18% of the variability in parent emotional functioning. This model had acceptable fit criteria for RMSEA and SRMR, but was slightly below the threshold for CFI and TLI (Table III).
Discussion
Among children who are 45 days post-HSCT, the relationship between child HRQL and parent emotional functioning is complex. Across all generic domains of child HRQL, parent-proxy report of child HRQL was lower than child report. The SEM based on the alternative conceptual model, which placed generic child HRQL before child HSCT-related worry in relation to parent emotional functioning, met more of the model fit criteria (e.g., RMSEA, SRMR) than the primary model, which placed child HSCT-related worry first. Interestingly, the pathways differed slightly by parent-proxy and child report. Our analyses indicate relationships between child emotional functioning, child HSCT-related worry, and parent emotional functioning.
The link between parent emotional functioning and child emotional functioning and worry is not surprising, but the directionality of the relationships is complex. Following an illness or high-risk treatment, a parent witnessing his/her child’s decreased HRQL, emotional distress, and worry about future health may experience emotional distress himself/herself, which may affect the parent’s ability to function as a caregiver in optimizing his/her child’s health outcomes (Pennarola et al., 2012, 2015). Adding complexity is that parents in more distress may perceive their child’s HRQL as lower (Panepinto et al., 2010). Furthermore, children of parents with lower emotional functioning may experience decreased HRQL and clinical outcomes themselves as the result of their parent’s distress. Our findings confirmed prior research that the child’s HSCT affects both the child’s HRQL and parent’s emotional functioning, that parent emotional functioning and child HRQL are associated, and that there are differences in child HRQL scores by raters (Barrera et al., 2000, 2009; Clarke et al., 2008; Eiser & Morse, 2001; Packman et al., 2010; Panepinto et al., 2010; Parsons et al., 2005, 2006, 2012, 2013; Rodday et al., 2013b). The testing of several conceptual models allowed us to make further insights into these relationships between child HRQL and parent emotional functioning, as well as to incorporate child worry about his/her future health into these established relationships, which has not been previously studied. Both the primary and alternative conceptual models were developed based on the Wilson and Cleary framework (Wilson & Cleary, 1995), where factors resulting from the disease, such as symptoms or complications, precede functioning and HRQL. The better fit of the alternative model supports the idea that disease and transplant impact child HRQL and functioning, which affect child HSCT-related worry, and parent emotional functioning. As children experienced decreased functioning in the period immediately following their HSCT, we found that this was associated with or manifested by worrying about their future health (i.e., infection, rehospitalization, relapse), which also took an emotional toll on the parent.
One interesting finding from our SEMs was that role functioning played a more prominent role in the child versus parent-proxy report. For children, their role as a child may define who they are, including the ability to spend time with family and friends, and may be closely linked to their emotional functioning. On the other hand, parents may focus more on the child’s emotional functioning, rather than focusing on whether their child is able to fulfill his/her role as a child. In the case of HSCT, or in other complex health conditions, collecting child HRQL data from multiple raters may provide the most comprehensive picture of how the child is doing. Another interesting finding was that relationships with child emotional functioning, rather than physical functioning, were more prominent. Based on prior research, we might expect that HSCT-related complications would have a large impact on child physical functioning (Parsons et al., 2013); our adjustment for complications may partly explain the smaller role of physical functioning. Furthermore, the domains of child emotional functioning and worrying about future disease may be more closely linked than child physical functioning and worry. In addition, prior research has shown that physical conditions can also affect emotional functioning and well-being (Rothrock et al., 2010). When considering how to best support the child and parent caregiver, focusing on the child’s psychological symptoms and how to best manage them may be more important than focusing on their physical functioning (e.g., ability to walk or climb), at least during the acute posttransplant phase.
These findings have several implications for supporting child recipients of HSCT and their parent caregivers. Results from both the primary and alternative conceptual models highlight that clinicians need to be cognizant of the relationship between the HSCT course, including possible complications, on children’s and parent’s emotional distress to ensure that support is available for both members of the parent–child dyad. The better fit of the alternative conceptual model lends support to theory that the child’s HRQL, and his/her emotional functioning in particular, provides the background or context in which he/she interprets the uncertainty of the HSCT course. For example, children with lower emotional functioning may be more likely to experience worry about the post-HSCT health, regardless of whether it is clinically justified. The primary conceptual model may have lent support to the theory that the child’s worry about his/her future health impacts his/her HRQL. While pediatric psychologists or psychosocial providers are typically part of the care team, their involvement varies by practice site and family. Given our findings, additional psychosocial support could be targeted to children with lower HRQL to address issues before the child begins worrying about his/her future health. To facilitate this, the child’s HRQL could be assessed intermittently throughout the HSCT course to detect low or decreasing levels of HRQL. Children could then be asked about how certain aspects of their HRQL and worry are affected by the HSCT course. Acknowledging and validating the child’s feelings may provide opportunity to educate and inform the parent and child about possible future outcomes in a sensitive, age-appropriate, and realistic way. Oncology clinicians could work with psychosocial providers on the best approaches. In addition to improving the child’s HRQL, we hope this would improve parent’s emotional functioning and their ability to care for their child. Our prior research has also highlighted the importance of compassionate communication based on the parent’s outlook of how the HSCT will precede in hopes of addressing his/her hopes, fears, expectations, and goals of care (Ullrich et al., 2016).
We acknowledge this study’s limitations. Unfortunately, the SEMs did not reach all of the desired model fit statistics, particularly for the primary model. There are several plausible explanations. First, the conceptual model may not fully explain the relationships between the different factors. However, we did find that the alternative model was a better fit to the data. Second, key factors that are necessary to better explain the relationships, such as measures of the child’s symptoms, the parent’s coping patterns, social support, or resilience, may be missing. Although we collected data on many of these constructs, this was a secondary analysis, and so, we did not have data on the same measures in each study. We did have complete HRQL data, as that was related to the primary outcome of each study. This is one of the challenges of evaluating a conceptual model with SEM as a secondary analysis of an existing data set as opposed to designing a study specifically for evaluating a conceptual model. Third, determining the direction of these relationships may not be possible, and we may need to accept that their interrelatedness is complex. This analysis is not meant to establish a causal relationship between child HRQL and parent emotional functioning, but to better understand potential pathways. Another limitation is that we focused only on a cross-sectional assessment at 45 days. This period was selected because of the magnitude of clinical complications and also the large number of participants still enrolled in the study. Although we have longitudinal data from these studies, we chose to analyze a single time point because we wanted to understand the relationship between child HRQL and parent emotional functioning while they were both influenced by similar factors (i.e., clinical complications), as opposed to exploring the relationship between child HRQL at baseline and parent emotional functioning 45 days later when the child’s HRQL may have changed from baseline levels. However, the use of cross-sectional data limits the establishment of temporality in the relationships.
Despite these limitations, this study highlights the complex relationships between child HSCT-related worry, generic child HRQL, and parent emotional functioning within the context of pediatric HSCT, and especially the domains relating to child worry and child and parent emotional functioning. Next steps may be to design a study that would collect primary data that correspond to all factors in the conceptual model, such as symptom scales. The post-HSCT period can be challenging for children and their parent caregivers, especially when the child experiences psychological distress and complications from their treatment. To optimize the child’s outcomes, the parent caregiver must be an active and engaged caregiver, which can be difficult in the face of psychological distress. Therefore, acknowledging the child’s and parent’s distress and worry and providing psychosocial support may be necessary for ensuring the mental and physical health of the child and his/her parent caregiver.
Funding
This work was supported by a Predoctoral Fellowship in Health Outcomes from the Pharmaceutical Research and Manufacturers of America Foundation (PhARMA) [to A.M.R]; the American Cancer Society Research Scholars (Grant Number PB02-186-01-PBP [to S.K.P.]), the National Cancer Institute (Grant Number R01 CA 119196 [to S.K.P.]), and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (Grant Number UL1 TR001064).
Conflicts of interest: None declared.
Reference
- Bakas T., McLennon S. M., Carpenter J. S., Buelow J. M., Otte J. L., Hanna K. M., Ellett M. L., Hadler K. A., Welch J. L. (2012). Systematic review of health-related quality of life models. Health and Quality of Life Outcomes, 10, 134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera M., Atenafu E., Hancock K. (2009). Longitudinal health-related quality of life outcomes and related factors after pediatric SCT. Bone Marrow Transplantation, 44, 249–256. [DOI] [PubMed] [Google Scholar]
- Barrera M., Boyd-Pringle L. A., Sumbler K., Saunders F. (2000). Quality of life and behavioral adjustment after pediatric bone marrow transplantation. Bone Marrow Transplantation, 26, 427–435. doi:10.1038/sj.bmt.1702527. [DOI] [PubMed] [Google Scholar]
- Bearman S. I., Appelbaum F. R., Buckner C. D., Petersen F. B., Fisher L. D., Clift R. A., Thomas E. D. (1988). Regimen-related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology, 6, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Boman K. K., Viksten J., Kogner P., Samuelsson U. (2004). Serious illness in childhood: The different threats of cancer and diabetes from a parent perspective. Journal of Pediatrics, 145, 373–379. doi:10.1016/j.jpeds.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Boomsma A., Hoogland J. J. (2001). The robustness of LISREL modeling revisited. In Cudeck R., du Toit S., Sorbom D. (Eds.), Structural equation models: Present and future. A Festschrift in honor of Karl Jöreskog (pp. 139–168). Lincolnwood, IL: Scientific Software International. [Google Scholar]
- Clarke S. A., Eiser C., Skinner R. (2008). Health-related quality of life in survivors of BMT for paediatric malignancy: A systematic review of the literature. Bone Marrow Transplantation, 42, 73–82. doi:10.1038/bmt.2008.156. [DOI] [PubMed] [Google Scholar]
- Eiser C., Morse R. (2001). Can parents rate their child’s health-related quality of life? Results of a systematic review. Quality of Life Research, 10, 347–357. [DOI] [PubMed] [Google Scholar]
- Hu L., Bentler P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. doi: 10.1080/10705519909540118. [Google Scholar]
- Kelloway E. K. (2014). Using Mplus for structural equation modeling: A researcher’s guide. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Mayo N. E., Scott S. C., Bayley M., Cheung A., Garland J., Jutai J., Wood-Dauphinee S. (2015). Modeling health-related quality of life in people recovering from stroke. Quality of Life Research, 24, 41–53. [DOI] [PubMed] [Google Scholar]
- McGrath P. (2002). Beginning treatment for childhood acute lymphoblastic leukemia: Insights from the parents’ perspective. Oncology Nursing Forum, 29, 988–996. doi: 10.1188/02.ONF.988-996. [DOI] [PubMed] [Google Scholar]
- MPLUS (Version 7) [Computer Software]. (2015). Los Angeles, CA, Muthen & Muthen.
- MPlus. (2016). Retrieved from https://www.statmodel.com/techappen.shtml. Retrieved 1 July 2016.
- Nunnally J., Bernstein I. (1994). Psychometric theory (3rd ed) New York, NY: McGraw-Hill, Inc. [Google Scholar]
- Packman W., Weber S., Wallace J., Bugescu N. (2010). Psychological effects of hematopoietic SCT on pediatric patients, siblings and parents: A review. Bone Marrow Transplantation, 45, 1134–1146. doi:10.1038/bmt.2010.74. [DOI] [PubMed] [Google Scholar]
- Panepinto J. A., Hoffmann R. G., Pajewski N. M. (2010). The effect of parental mental health on proxy reports of health-related quality of life in children with sickle cell disease. Pediatric Blood & Cancer, 55, 714–721. doi: 10.1002/pbc.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. K., Fairclough D. L., Wang J., Hinds P. S. (2012). Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: The value of both raters’ perspectives. Quality of Life Research, 21, 915–923. doi: 10.1007/s11136-011-9986-4. [DOI] [PubMed] [Google Scholar]
- Parsons S. K., Ratichek S. J., Rodday A. M., Davies S., Bingen K., Kupst M. J., Schwartz L., Guinan E. C., Syrjala K. L., Patel S., Mayer D. K., McTavish F., Gustafson D. H. (2011). Caring for the caregiver: EHealth interventions for parents of pediatric hematopoietic stem cell transplant recipients. Pediatric Blood & Cancer, 56, 1159. [Google Scholar]
- Parsons S. K., Shih M. C., DuHamel K. N., Ostroff J., Mayer D. K., Austin J., Martini D. R., Williams S. E., Mee L., Sexson S., Kaplan S. H., Redd W. H., Manne S. (2006). Maternal perspectives on children’s health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. Journal of Pediatric Psychology, 31, 1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]
- Parsons S. K., Shih M. C., Mayer D. K., Barlow S. E., Supran S. E., Levy S. L., Greenfield S., Kaplan S. H. (2005). Preliminary psychometric evaluation of the Child Health Ratings Inventories (CHRIs) and Disease-Specific Impairment Inventory-HSCT (DSII-HSCT) in parents and children. Quality of Life Research, 14, 1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- Parsons S. K., Tighiouart H., Terrin N. (2013). Assessment of health-related quality of life in pediatric hematopoietic stem cell transplant recipients: Progress, challenges and future directions. Expert Review of Pharmacoeconomics & Outcomes Research, 13, 217–225. doi: 10.1586/erp.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarola B. W., Rodday A. M., Bingen K., Schwartz L. A., Patel S. K., Syrjala K. L., Mayer D. K., Ratichek S. J., Guinan E. C., Kupst M. J., Hibbard J. H., Parsons S. K. (2015). Changing factors associated with parent activation after pediatric hematopoietic stem cell transplant. Supportive Care in Cancer, 23, 1997–2006. doi: 10.1007/s00520-014-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarola B. W., Rodday A. M., Mayer D. K., Ratichek S. J., Davies S. M., Syrjala K. L., Patel S., Bingen K., Kupst M. J., Schwartz L., Guinan E. C., Hibbard J. H., Parsons S. K.; for the HSCT-CHESS Study. (2012). Factors associated with parental activation in pediatric hematopoietic stem cell transplant. Medical Care Research and Review, 69, 194–214. doi: 10.1177/1077558711431460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva R., Forinder U., Arvidson J., Mellgren K., Toporski J., Winiarski J., Norberg A. L. (2014). Patterns of psychological responses in parents of children that underwent stem cell transplantation. Psycho-Oncology, 23, 1307–1313. doi: 10.1002/pon.3567. [DOI] [PubMed] [Google Scholar]
- Rodday A. M., Terrin N., Chang G., Parsons S. K. (2013a). Performance of the parent emotional functioning (PREMO) screener in parents of children undergoing hematopoietic stem cell transplantation. Quality of Life Research, 22, 1427–1433. doi: 10.1007/s11136-012-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodday A. M., Terrin N., Parsons S. K. (2013b). Measuring global health-related quality of life in children undergoing hematopoietic stem cell transplant: A longitudinal study. Health and Quality of Life Outcomes, 11, 26. doi: 10.1186/1477-7525-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock N. E., Hays R. D., Spritzer K., Yount S. E., Riley W., Cella D. (2010). Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). Journal of Clinical Epidemiology, 63, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrbanian S., Duquette P., Ahmed S., Mayo N. E. (2016). Pain acts through fatigue to affect participation in individuals with multiple sclerosis. Quality of Life Research, 25, 477–491. [DOI] [PubMed] [Google Scholar]
- Terrin N., Rodday A. M., Tighiouart H., Chang G., Parson S. K.; Journeys to Recovery Study. (2013). Parental emotional functioning declines with occurrence of clinical complications in pediatric hematopoietic stem cell transplant. Supportive Care in Cancer, 21, 687–695. doi: 10.1007/s00520-012-1566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken A. J., Waller N. G. (2005). Structural equation modeling: Strengths, limitations, and misconceptions. Annual Review of Clinical Psychology, 1, 31–65. doi: 10.1146/annurev.clinpsy.1.102803.144239. [DOI] [PubMed] [Google Scholar]
- Ullrich C. K., Rodday A. M., Bingen K., Kupst M. J., Patel S. K., Syrjala K. L., Harris L. L., Recklitis C. J., Schwartz L., Davies S., Guinan E. C., Chang G., Wolfe J., Parsons S. K. (2016). Parent outlook: How parents view the road ahead as they embark on hematopoietic stem cell transplantation for their child. Biology of Blood and Marrow Transplantation, 22, 104–111. doi: 10.1016/j.bbmt.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalonga-Olives E., Kawachi I., Almansa J., Witte C., Lange B., Kiese-Himmel C., von S. N. (2014). Pediatric health-related quality of life: A structural equation modeling approach. PLoS.One, 9, e113166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. B., Cleary P. D. (1995). Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Journal of the American Medical Association, 273, 59–65. doi:10.1001/jama.1995.03520250075037. [PubMed] [Google Scholar]
- Young B., Dixon-Woods M., Findlay M., Heney D. (2002). Parenting in a crisis: Conceptualising mothers of children with cancer. Social Science and Medicine, 55, 1835–1847. doi:10.1016/S0277-9536(01)00318-5. [DOI] [PubMed] [Google Scholar]