Abstract
Objective
To extend existing research on the pain burden experienced by youth with inflammatory bowel disease (IBD) by examining the complexity of psychosocial factors involved in pain-related distress.
Methods
Parents completed measures of family stress and their child’s pain-related expressions of distress and coping. Youth with IBD rated their depressive symptoms (n = 183 dyads). Mediation analyses were performed using regression-based techniques and bootstrapping.
Results
Greater family stress was positively related to children’s pain-related expressions of distress and passive coping. Significant indirect effects were found in the relationship between family stress and expressed pain-related distress through parent-reported passive coping, depressive symptoms, and both passive coping and depressive symptoms sequentially.
Conclusions
Results suggest that family stress can place children at risk for greater expressed pain-related distress through effects on coping and depressive symptoms. Addressing psychosocial difficulties is important for closing the gap between disability and health in youth with IBD.
Keywords: coping, depression, inflammatory bowel disease, pain
Abdominal pain is a hallmark symptom of the inflammatory bowel diseases (IBD: Crohn’s disease, ulcerative colitis, and indeterminate colitis), with 50–70% of patients presenting with pain as one of the primary complaints at the time of IBD onset or disease flare (Bielefeldt, Davis, & Binion, 2009). Multiple factors impact pain perception in patients with IBD, including bowel inflammation that causes pain through the release of inflammatory cytokines and mediators, the development of strictures or adhesions within the intestines, dysmotility or changes in the normal motility of the intestines, small intestinal bacterial overgrowth, and medication side-effects (Srinath, Young, & Szigethy, 2014). However, direct physical causes do not adequately account for the pain experience in patients with IBD (Gracie et al., 2017). Approximately 20% of adult patients continue to experience pain despite demonstrating clinical and endoscopic remission of their disease (Cross, Wilson, & Binion, 2005; Edwards, Radford‐Smith, & Florin, 2001). In a recent pediatric sample, 13% of patients with Crohn’s disease reported abdominal pain despite clinical remission (Zimmerman et al., 2013). Taken together, findings highlight the high pain burden born by patients with IBD, both during periods of active disease as well as for a subset of patients during periods of clinical disease remission. There is a critical need to identify factors beyond disease activity associated with pain perception to identify patients at risk for greater pain burden and to inform intervention development.
Pain perception and related distress are subjective experiences, influenced by emotional functioning and cognitive processing (Bielefeldt et al., 2009). Increased symptoms of depression are reported by pediatric patients with IBD and current abdominal pain, regardless of disease activity ratings (Zimmerman et al., 2013). In adults with IBD, self-reported depressive coping style explained more variance in disease-related concerns than demographic and disease variables (Mussell, Bocker, Nagel, & Singer, 2004). Ondersma and colleagues demonstrated that subjective symptom reporting including abdominal pain was associated with negative affect but not with an objective indicator of inflammation (erythrocyte sedimentation rate) in adolescents with IBD (Ondersma, Lumley, Corlis, & Tojek, 1997). There is some evidence that both anxiety and gastrointestinal (GI) symptom severity are associated with brain regions commonly activated in response to fear, suggesting a potential mechanism by which negative emotions may impact GI symptoms (Drossman, 2005; Van Oudenhove et al., 2016). It is unclear, however, why some patients with IBD seem to be at increased risk for pain-related distress and mood problems, while others experience typical adjustment in the face of chronic illness.
Disease activity has repeatedly been examined in relation to emotional difficulties and adjustment, though findings have been mixed. While some studies have found significant relationships between disease severity indicators and symptoms of psychosocial distress (Ondersma et al., 1997; Schuman, Graef, Janicke, Gray, & Hommel, 2013; Szigethy et al., 2004; Wood et al., 1987), multiple studies have reported no significant relationship between behavioral/emotional functioning and disease factors such as validated disease activity scores, growth delay, and/or frequency of relapse (Mackner & Crandall, 2005, 2006; Ondersma et al., 1997; Steinhausen & Kies, 1982; Walker, Smith, Garber, & Van Slyke, 1997). Though findings may seem initially counterintuitive, they are consistent with the larger body of child health research showing that psychosocial factors such as stress coping strategies and emotional functioning are better predictors of distress than illness or physical functioning factors (Thompson & Gustafson, 1996). Findings are consistent with Wallander and Varni’s (1992) disability-stress-coping model, a comprehensive model of adjustment for children managing diagnosis with a chronic physical condition. Based on this model, a child’s adjustment is the product of stress and protective factors interacting across levels of influence, from disease-related factors to family and environmental context.
The perception of stress, stressful life events, and depression are psychosocial factors that have repeatedly been associated with increased disease symptoms in adults with IBD (Targownik et al., 2015), including disease relapse (Bernstein et al., 2010; Bitton et al., 2008; Langhorst, Hofstetter, Wolfe, & Hauser, 2013). Psychoneuroimmunological research demonstrates that chronic life stress is associated with immunosuppression and increases in inflammation (Mawdsley & Rampton, 2005), which suggest potential biological pathways by which stress may affect disease outcomes. Such pathways offer incomplete explanations for relationships seen between life stress and disease outcomes in adults with IBD, however. Psychological mechanisms explaining these relationships are poorly understood and pediatric populations have been largely ignored.
The disability-stress-coping model (Wallander & Varni, 1992) suggests that children’s adjustment or distress associated with disease factors is impacted by patients’ coping resources and emotional functioning. A well-established body of literature demonstrates that greater exposure to childhood stressors can affect future methods of coping, including greater use of maladaptive, passive coping strategies and poorer mental health outcomes (Edwards, Holden, Felitti, & Anda, 2003; Leitenberg, Gibson, & Novy, 2004). Further, reliance on active, adaptive coping strategies has been shown to buffer the relationship between perceived stress and poorer emotional functioning in patients with chronic, immune-mediated disease (Treharne, Lyons, Booth, & Kitas, 2007). Coping refers to purposeful efforts to manage stressful events or experiences (Lazarus & Folkman, 1984). Coping with pain and related distress can take many different forms, including more helpful, adaptive coping strategies such as support-seeking or problem-solving, as well as less helpful, maladaptive strategies such as escape (Zimmer-Gembeck & Skinner, 2011). Without intervention, patients with IBD have been shown to rely more on passive coping strategies compared with controls (Jones, Wessinger, & Crowell, 2006). A bias to rely more heavily on passive coping strategies (e.g., catastrophizing thinking, self-isolation, disengaging from typical activities) is concerning for overall well-being of patients with IBD, as these strategies are consistently associated with poorer outcomes compared with more adaptive coping strategies (Langer, Romano, Mancl, & Levy, 2014; McCombie, Mulder, & Gearry, 2013; van Tilburg et al., 2015). In adults with IBD, maladaptive coping strategies have been associated with greater anxiety and depressive symptoms over the first 6 months from diagnosis (Jones et al., 2006). As pain and pain-related distress are common for youth with IBD, a better understanding of related factors may be helpful in identifying children at risk for poor adjustment and in designing clinical intervention.
The present study sought to extend existing research on the pain burden experienced by youth with IBD by examining the complexity of psychosocial and contextual factors involved in expressed pain-related distress. Specifically, we aimed to examine the experience of family stressors, coping, and depressive symptoms as predictors of parent-observed pain-related distress behaviors. Based on previous research examining these predictors in isolation as well as Wallander and Varni’s (1992) disability-stress-coping model, we hypothesized that greater experience of family stressors would relate to greater expressed pain-related distress. Further, we tested whether coping and depressive symptoms contributed to explaining how the experience of stressors could relate to expressed pain-related distress in patients with IBD.
Methods
Participants
The sample consisted of 183 children diagnosed with IBD and their parents. Participants were enrolled in a randomized controlled trial (RCT) of a cognitive-behavioral intervention for pediatric IBD patients (Levy et al., 2016). The present data were collected at baseline before randomization or intervention. Of the 210 dyads enrolled in the RCT, 27 were excluded from the current analysis subsample for the following reasons: baseline assessment not complete (20 dyads), parent data missing (1 dyad), child data missing (1 dyad), and non-independence among siblings, as the family had two children with IBD enrolled in the RCT (5 dyads).
Participants were recruited from the gastroenterology departments of Seattle Children’s Hospital in Seattle, WA, and Mary Bridge Children’s Hospital in Tacoma, WA. All procedures were approved by the Institutional Review Boards of both institutions. Inclusion criteria consisted of (1) child age of 8–18 years, (2) child diagnosed with IBD for at least 3 months, (3) child medically approved to engage in normal daily activities at the time of recruitment, (4) child and parent participant had cohabitated for at least the past 3 months, and (5) child and parent English fluency. Exclusion criteria included 1) child chronic disease other than IBD, and 2) developmental disability requiring full-time special education or impairing the ability to participate in study procedures.
Measures
Parent-Reported Measures
Parents completed measures online or, if preferred, via paper and pencil. Study variables and corresponding questionnaires are described below.
Observed Pain-Related Distress
The Affective Distress subscale from the Pain Behavior Check List (PBCL; Kerns et al., 1991) was used to assess parents’ reports of observable expressions of their child’s pain-related distress. The Affective Distress subscale consists of items measuring child irritability, anger, the child telling others not to bother her/him, and asking, “Why did this happen to me?”. Parents rated frequency of observing these behaviors when their child had abdominal pain on a 0–4 (never to always) scale. Though originally developed for use with adult chronic pain patients, the PBCL was adapted for use in the current study, as most pediatric pain behavior measures were developed for assessing behavior in the context of acute procedural pain or to be completed by trained observers. A Cronbach’s alpha of 0.85 for the Total score on the PBCL was found within the study sample; Cronbach’s α = .78 for the Affective Distress subscale. In the current sample, validity was supported through positive correlations on PBCL Total scores with parent-reported child functional disability (r = .32, p < .001) and child-reported functional disability (r = .35, p < .001) using the Functional Disability Inventory; child-reported GI symptom severity (r = .17, p < .001) using the Children’s Somatization Inventory; and child-reported massaging/guarding the painful area (r = .18, p = .014) using a subscale of the Pain Response Inventory. Conversely, total PBCL scores were inversely correlated with parent-reported child quality of life (r = −.42, p < .001) and child-reported quality of life (r = −.34, p < .001) using the Pediatric Quality of Life Inventory. Our group has previously published findings from this measure demonstrating that parent report on the PBCL predicted parental protective responses, mediated by parental catastrophizing about child pain (Langer, Romano, Mancl, & Levy, 2014).
Family Stress
The Family Inventory of Life Events and Changes (FILE; McCubbin, Patterson, & Wilson, 1996) is a 70-item parent-reported measure to account for the stressful life events experienced by a family in the past year. Parents were asked to indicate whether their family experienced each of the possible 67 stressors (e.g., divorce, increased strain on family money, family member lost or quit a job) and are provided with 3 blank items to generate unique stressors not listed. A total score is obtained by summing the number of stressors endorsed. The FILE demonstrated adequate internal consistency within the study sample, with a Cronbach’s α of .78 for the total scale.
Coping
Parent report of children’s coping with stomachaches or other GI problems was measured with the Pain Response Inventory (PRI; Walker et al., 1997). The PRI consists of 3 higher-order scales (active, passive, and accommodative coping). As passive coping has been associated with poorer outcomes in patients with abdominal pain in past research (van Tilburg et al., 2015; Walker, Smith, Garber, & Claar, 2005), the current study focused on this domain. The passive coping scale consists of Catastrophizing (5 items such as “Think to himself or herself that it’s never going to stop”), Self-Isolation (5 items such as “Try to be alone.”), and Behavioral Disengagement (5 items such as “Give up trying to feel better”). Parents rated how often their child used each of these coping strategies on a 0–4 (never to always) scale when experiencing a stomachache or other GI symptoms. Items on the Passive Coping scale are summed and averaged to obtain a mean subscale score. Cronbach’s α = .91 for the Passive Coping scale within the study sample.
Child-Reported Measure
Children completed a battery of questionnaires via phone for the baseline assessment, administered by a nurse researcher. To facilitate comprehension, answer choices were mailed to children in advance of the phone session. For the current study, we were interested in child-reported depressive symptoms.
Depression
Depressive symptoms were measured via child report on the Children’s Depression Inventory (CDI; Kovacs, 2003). The CDI is a well-established measure of depressive symptoms consisting of 27 items rated on a 3-point scale (from 0 to 2); the 1 item about suicidal ideation was omitted. Symptom subscales include Anhedonia, Negative Mood, Negative Self-Esteem, Ineffectiveness, and Interpersonal Problems. Total scores are computed by summing the items (0–52); higher scores indicate greater depressive symptom severity. Cronbach’s α = .90 for the Total score within the study sample.
Pediatric Gastroenterologist Completed Measures
Patients’ treating gastroenterologist completed an index of disease activity for study purposes during the patient recruitment visit.
Disease Activity
Disease activity was assessed via the Pediatric Crohn’s Disease Activity Index (PCDAI; Hyams et al., 1991) for children with Crohn’s disease and via the Pediatric Ulcerative Colitis Activity Index (PUCAI; Turner et al., 2007) for children with ulcerative colitis. The PCDAI is a rating of clinical disease activity that incorporates patient report, laboratory values, physician examination results, and growth parameters. PCDAI scores range from 0 to 100. Scores < 10 reflect disease remission, scores of 11–30 reflect mild disease activity, and scores > 30 reflect moderate-to-severe disease activity. The PUCAI is a 6-item validated measure of disease activity based on patient report of symptoms. The PUCAI is completed by pediatric gastroenterologists and scores range from 0 to 85. Scores < 10 reflect remission, scores of 10–34 reflect mild disease activity, scores of 35–64 reflect moderate disease activity, and scores of 65 or higher reflect severe disease activity. For the purposes of this study, participants were categorized as experiencing remission/no disease activity or mild/moderate/severe disease activity.
Analyses
Descriptive statistics and correlation analyses were used to describe the sample in terms of demographics and study variables. Mediation analysis was performed using Hayes’ PROCESS macro, a regression-based path analytic technique (Hayes, 2013). Using an ordinary least squares framework, PROCESS estimates direct and indirect effects in multiple mediator models. To test mediation hypotheses, PROCESS uses bootstrapping to construct confidence intervals (CIs) for indirect effects through repeated sampling of the data set. Findings are based on 5,000 bias-corrected bootstrapped samples. In the event that 0 does not lie within the 95% CI for the bootstrapped results for indirect effects, we can conclude that the indirect effect is significantly different from 0 and that mediation is demonstrated (Preacher & Hayes, 2004). Analyses were conducted using IBM Statistical Package for the Social Sciences 24.0.
Results
Descriptive Results
Descriptive data for the sample are provided in Table I. The majority of the sample was classified as having inactive disease based on clinical disease activity indices (63%), and the remaining were classified as experiencing active disease at the time of data collection (37%). Correlations among study variables are provided in Table II.
Table I.
Sample Characteristics (n = 183 dyads)
| Characteristic | Parent | Child |
|---|---|---|
| Age, M (SD) | 44.38 (6.87) | 13.75 (2.70) |
| Age, range | 27–67 | 8–18 |
| Gender, n (%) female | 165 (90.2) | 87 (47.5) |
| Ethnicity, n (%) Hispanic | 3 (1.6) | 8 (4.4) |
| Race, n (%) Caucasian | 170 (92.9) | 161 (88.0) |
| Education, n (%) 4-year college degree or higher | 89 (48.6) | – |
| Employment status, n (%) employed full-time | 81 (44.3) | – |
| Disease, n (%) | ||
| Crohn’s disease | – | 125 (68.3) |
| Ulcerative colitis | – | 58 (31.7) |
| Time since diagnosis in years, M (SD) | – | 2.30 (2.41) |
| Disease activity, n (%) | ||
| Quiescent | – | 114 (63) |
| Mild/Moderate/Severe | – | 68 (37) |
Table II.
Correlations Among Study Variables and Descriptive Statistics (n = 183)
| First Column Head Table II:Study Variables | 1 | 2 | 3 | 4 | 5 | 6 | M (SD) | Scale |
|---|---|---|---|---|---|---|---|---|
| 1. Child gender (M1, F2) | 1.00 | .02 | −.02 | .04 | .21** | .21** | NA | NA |
| 2. Child age | 1.00 | .12 | .12 | .13 | −.001 | 13.75 (2.70) | NA | |
| 3. Family Stress | 1.00 | .27** | .24** | .28** | 7.29 (5.04) | 0–25 | ||
| 4. Parent-reported passive coping | 1.00 | .31** | .61** | 1.12 (.66) | 0–3.07 | |||
| 5. Child-reported depressive symptoms | 1.00 | .35** | 8.26 (7.38) | 0–36 | ||||
| 6. Observed pain-related distress | 1.00 | 1.58 (.88) | 0–3.75 |
Note. *p < .05; **p < .01.
The 10 most frequently endorsed family stressors in the past year as described on the FILE included (1) increased strain on family “money” for medical/dental expenses (42% of the sample); (2) increased strain on family “money” for food, clothing, energy, and home care (33%); (3) a child/adolescent member changed to a new school/started a new school in fall (31%); (4) increase in the number of tasks or chores that don’t get done (30%); (5) increase in arguments between parent(s) and child(ren) (25%); (6) change in conditions which hurt family investments and/or income (22%); (7) increase in number of “outside activities” in which the child(ren) are involved (21%); (8) increased difficulty in managing teenage child(ren) (20%); (9) increased strain on family “money” for child(ren)’s education (20%); and (10) a child became seriously ill or injured (20%).
Multiple Mediator Model
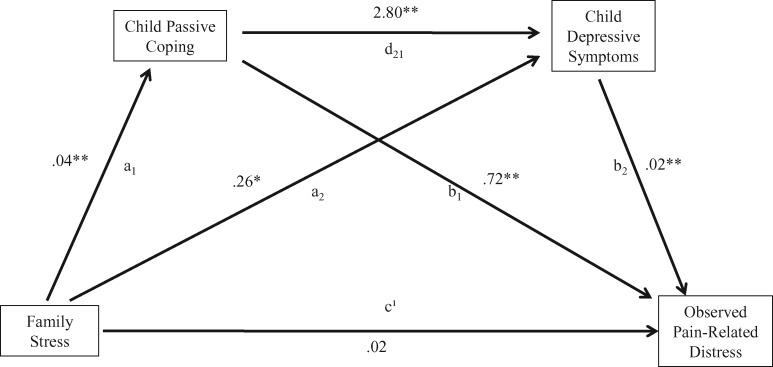
The full model tested the effect of having experienced family stressors on parent-reported pain-related distress in children diagnosed with IBD in relation to children’s use of passive coping strategies as reported by parents and child-reported depressive symptoms. Figure 1 displays the full model with unstandardized B weights for the path coefficients. Child gender was included as a covariate given its significant relationship with depressive symptoms and expressed pain-related distress (Table II). Mediation analysis conducted using ordinary least squares path analysis found that life stress indirectly influenced expressed pain-related distress through its effects on passive coping strategies and depressive symptoms. In the total effect model before inclusion of proposed mediators, having experienced a greater number of family stressors within the past 12 months was positively associated with expressed pain-related distress (c = .05, p < .001).
Figure 1.
Mediation model.
Note. a1b1: Significant indirect effect; a1d21b2: significant indirect effect; a2b2: significant indirect effect. *p < .05, **p < .01
In the full model with proposed mediators, greater experience of family stressors was positively associated with use of passive coping strategies (a1 = .04, p < .001). Passive coping was positively associated with depressive symptoms (d21 = 2.80, p < .001) and pain-related distress (b1 = .72, p < .001). Depressive symptoms were also positively associated with pain-related distress (b2 = .01, p < .05). Mediated effects were demonstrated by significant indirect effects in the relationship between family stress and expressed pain-related distress through passive coping (path a1b1 point estimate = .03, standard error [SE] = .01, 95% CI = .01 to.04), depressive symptoms (path a2b2 point estimate = .001, SE = .001, 95% CI = .0003 to .004), and both passive coping and depressive symptoms sequentially (path a1d21b2 point estimate = .004, SE = .003, 95% CI = .00 to .01). Thus, expressed pain-related distress on the PBCL would be expected to increase by .03 units and .001 for every 1 unit increase in passive coping and depressive symptoms, respectively. The full model including mediators accounted for 44% of the variance in expressed pain-related distress; family stress alone accounted for 13% of the variance in expressed pain-related distress.
Discussion
Psychosocial factors related to pain-related distress in youth with IBD is an understudied yet important area given the lifelong risk for experiencing pain, frequently even during times of disease remission (Zimmerman et al., 2013). Data on the psychosocial factors associated with pain-related distress may allow for the identification of patients at particular risk and targeted treatment. Our study identified several potentially modifiable psychosocial factors (i.e., passive coping and depressive symptoms) associated with greater expression of pain-related distress. Further, family stress was shown to be associated with poorer psychosocial functioning in youth with IBD across all domains studied.
Children whose families had experienced a greater number of stressful life events in the past 12 months demonstrated higher ratings of pain-related distress as reported by parents, including symptoms of irritability, anger, appearing upset, and remarking on the unfairness of their pain. Notably, results demonstrate how commonly endured family stressors (e.g., financial difficulties, changing schools, greater arguing at home) experienced by youth with and without chronic illness are associated with illness-relevant outcomes in youth diagnosed with IBD (i.e., expressed pain-related distress). Though interesting in and of itself, the full mediation model identified several potentially modifiable psychosocial variables accounting for the relationship between family stress and overt indicators of pain-related distress, paving the way for targeted intervention. Specifically, we found that greater family stress was associated with greater passive coping and depressive symptoms, which each independently and sequentially was associated with expressed pain-related distress. Biopsychosocial medicine is advancing how we conceptualize clinically meaningful treatment outcomes for youth with chronic GI disorders, including IBD. Rather than solely focusing on objective indicators of disease or inflammation as relevant treatment outcomes, healthcare providers are increasingly addressing the full patient experience, including physical, emotional, and behavioral health outcomes (Reed-Knight et al., 2017). As this broader range of outcomes are considered relevant and appropriate, more nuanced models of overall health and distress are necessary. Rather than focusing solely on coping or depressive symptoms, more sophisticated models may lead us to address multiple points along the pathway between more universal and common factors (e.g., family stress) and disease-specific morbidity.
Targeted treatment to address passive coping could first guide patients in building awareness of how they typically cope with stressors and then develop more adaptive alternatives to passive strategies, including catastrophizing, disengaging from activities, and socially isolating. For example, patients could be trained in cognitive restructuring methods standard in cognitive-behavioral therapy to address catastrophic thinking and could be actively encouraged to set goals around staying engaged in school, extracurricular activities, and visiting with friends. Patients may need explicit guidance regarding the importance of relying on active, adaptive coping as primary strategies for managing IBD outcomes like pain-related distress and not simply “extras” to adopt once IBD is managed or extra time allows. Each of these strategies would likely be standard when working with a psychologist or behavioral healthcare provider but could also be targets of biopsychosocial or IBD care aimed at incorporating strategies for adaptive coping as part of regular medical care. Further, patients experiencing depressive symptoms may benefit from full evaluation of symptoms with a mental health provider and referral for treatment if indicated, such as cognitive-behavioral therapy and/or antidepressant medication. Finally, findings support treatment directed at the entire family with a focus on generalized family stress prevention and coping. A handful of illness-specific family and parenting stress interventions have been developed and tested (Golfenshtein, Srulovici, & Deatrick, 2016; Monaghan, Hilliard, Cogen, & Streisand, 2011), though our findings suggest that patients and families may also need treatment for managing common, non-illness-related family stressors. Such interventions may help families of youth diagnosed with IBD build awareness of how family stressors affect the child with IBD and disease-related distress. With awareness of how generic family stressors can affect pain-related distress in youth with IBD, families may be increasingly motivated to learn adaptive coping strategies.
Past research on psychosocial factors associated with pain perception and pain-related distress in children with abdominal pain has primarily been conducted with children diagnosed with functional abdominal pain or recurrent abdominal pain (Campo et al., 2004; Walker & Greene, 1989; Walker et al., 2005), with comparably less focus on children diagnosed with organic diseases like IBD. Pain is increasingly being recognized as a subjective experience regardless of etiology, however, influenced by emotional, cognitive, biological, and environmental factors (Srinath et al., 2014). Advances in our understanding of the pain experience are promising for patients with IBD, as they highlight the importance of assessing factors contributing to pain and pain-related distress beyond disease activity alone. Effect sizes in the current study are small. However, the complexity of pain-related distress in youth with IBD and the distance of the relationships between generic family stressors, pain-related distress, and psychological mediators signify that even small effects may be meaningful opportunities for change and clinical impact. Taking a holistic approach to addressing pain and associated distress in patients with IBD through psychosocial factors gives us an advantage for addressing poor quality of life (Karwowski, 2009) and risk for serious disease-related morbidity, including opioid dependence (Buckley, Cook, Allen, & Kappelman, 2015).
This study has several limitations worth noting. First, use of cross-sectional and observational data precludes us from making conclusions regarding causation. Without longitudinal data, we cannot speculate how family stress, depression, and coping are temporally related to the development of pain-related distress and with regards to one another over time. Second, analyses are based on parent report of child behaviors indicative of pain-related distress and coping, which may reflect parent’s own biases and psychological states, including anxiety or tendencies to catastrophize. Parent perceptions may or may not correspond to subjective child experience, and therefore, findings may be limited with regards to generalizing to child-perceived distress and coping. Future research including children’s reports of their own coping and pain-related distress will be an important next step. In addition, research on agreement between parents and children with regards to pain-related distress and coping may clarify how families make decisions to seek medical care or limit activities based on perceived distress and coping. However, parent report confers valuable information regarding behaviors of which children may not be aware, allows us to access a source of data aside from child report, and thus may be important when considering outcomes such as healthcare utilization that are typically parent-initiated. That being said, future research will benefit from inclusion of child report as well as more objective data on child coping and pain-related distress. Third, the majority of children in our sample were experiencing inactive disease, and results may not generalize to children with active disease. Telephone administration of child study questionnaires as opposed to self-administration may have impacted responses if children underestimated symptoms because of social desirability or embarrassment. However, past research has demonstrated comparability between phone interviews and in-person interviews for emotional symptoms (Rohde, Lewinsohn, & Seeley, 1997). Additionally, we are unfortunately unable to report data on recruitment rates or potential differences between participants and those who were approached for participation but declined, as these data points were not collected. Although similar to previously published research samples in IBD (Kunz, Hommel, & Greenley, 2010), the present sample was primarily Caucasian and well-educated, which may limit generalizability to youth with IBD from different ethnicities and education levels. Future research should examine how changes in family stress and psychosocial functioning, either because of direct intervention or time alone, affect pain-related distress.
This study provides initial evidence for the importance of taking a holistic, biopsychosocial approach for understanding the pain experience in youth with IBD and suggests that family factors (i.e., family stress) can place children at risk for greater expressed pain-related distress through effects on coping and depressive symptoms. As medical advances allow for increasingly effective and aggressive treatment of IBD, the importance of addressing comorbid psychosocial difficulties including maladaptive coping and depressive symptoms will likely become increasingly apparent for helping to close the gap between disability and health.
Funding
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (award number R01HD050345 to R. L. Levy) and (award number 1 UL1 RR025014 to Children's Hospital and Regional Medical Center).
Conflicts of interest: None declared.
References
- Bernstein C. N., Singh S., Graff L. A., Walker J. R., Miller N., Cheang M. (2010). A prospective population-based study of triggers of symptomatic flares in IBD. American Journal of Gastroenterology, 105, 1994–2002. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K., Davis B., Binion D. G. (2009). Pain and inflammatory bowel disease. Inflammatory Bowel Diseases, 15, 778–788. doi:10.1002/ibd.20848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton A., Dobkin P. L., Edwardes M. D., Sewitch M. J., Meddings J. B., Rawal S., Cohen A., Vermeire S., Dufresne L., Franchimont D., Wild G. E. (2008). Predicting relapse in Crohn's disease: A biopsychosocial model. Gut, 57, 1386–1392. doi:10.1136/gut.2007.134817 [DOI] [PubMed] [Google Scholar]
- Buckley J. P., Cook S. F., Allen J. K., Kappelman M. D. (2015). Prevalence of chronic narcotic use among children with inflammatory bowel disease. Clinical Gastroenterology and Hepatology, 13, 310–315.e2. doi:10.1016/j.cgh.2014.07.057 [DOI] [PubMed] [Google Scholar]
- Campo J. V., Bridge J., Ehmann M., Altman S., Lucas A., Birmaher B., Di Lorenzo C., Iyengar S., Brent D. A. (2004). Recurrent abdominal pain, anxiety, and depression in primary care. The Journal of Pediatrics, 113, 817–824. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Wilson K. T., Binion D. G. (2005). Narcotic use in patients with Crohn's disease. The American Journal of Gastroenterology, 100, 2225–2229. doi:10.1111/j.1572-0241.2005.00256.x [DOI] [PubMed] [Google Scholar]
- Drossman D. A. (2005). Brain imaging and its implications for studying centrally targeted treatments in irritable bowel syndrome: A primer for gastroenterologists. Gut, 54, 569–573. doi:10.1136/gut.2004.058446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. T., Radford‐Smith G. L., Florin T. H. (2001). Chronic narcotic use in inflammatory bowel disease patients: Prevalence and clinical characteristics. Journal of Gastroenterology and Hepatology, 16, 1235–1238. doi:10.1046/j.1440-1746.2001.02468.x [DOI] [PubMed] [Google Scholar]
- Edwards V. J., Holden G. W., Felitti V. J., Anda R. F. (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry, 160, 1453–1460. doi:10.1176/appi.ajp.160.8.1453 [DOI] [PubMed] [Google Scholar]
- Golfenshtein N., Srulovici E., Deatrick J. A. (2016). Interventions for reducing parenting stress in families with pediatric conditions: An integrative review. Journal of Family Nursing, 22, 460–492. doi:10.1177/1074840716676083 [DOI] [PubMed] [Google Scholar]
- Gracie D. J., Williams C. J., Sood R., Mumtaz S., Bholah M. H., Hamlin P. J., Ford A. C. (2017). Negative effects on psychological health and quality of life of genuine irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease. Clinical Gastroenterology and Hepatology, 15, 376–384. e375. doi:10.1016/j.cgh.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Hayes A. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (1st ed.). New York, NY: The Guilford Press. [Google Scholar]
- Hyams J. S., Ferry G. D., Mandel F. S., Gryboski J. D., Kibort P. M., Kirschner B. S., Griffiths A. M., Katz A. J., Grand R. J., Boyle J. T., Michener W. M., Levy J. S., Lesser M. L. (1991). Development and validation of a pediatric Crohn's disease activity index. Journal of Pediatric Gastroenterology and Nutrition, 12, 439–447. [PubMed] [Google Scholar]
- Jones M. P., Wessinger S., Crowell M. D. (2006). Coping strategies and interpersonal support in patients with irritable bowel syndrome and inflammatory bowel disease. Clinical Gastroenterology and Hepatology, 4, 474–481. doi:http://dx.doi.org/10.1016/j.cgh.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Karwowski C. A. (2009). Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflammatory Bowel Diseases, 15, 1755. doi:10.1002/ibd.20919 [DOI] [PubMed] [Google Scholar]
- Kerns R. D., Haythornthwaite J., Rosenberg R., Southwick S., Giller E. L., Jacob M. C. (1991). The Pain Behavior Check List (PBCL): Factor structure and psychometric properties. Journal of Behavioral Medicine, 14, 155–167. [DOI] [PubMed] [Google Scholar]
- Kovacs M. (2003). Children's Depression Inventory (CDI): Technical manual update. North Tonawanda, NY: Multi-Health Systemc, Inc. [Google Scholar]
- Kunz J. H., Hommel K. A., Greenley R. N. (2010). Health-related quality of life of youth with inflammatory bowel disease: A comparison with published data using the PedsQL 4.0 generic core scales. Inflammatory Bowel Diseases, 16, 939–946. doi:10.1002/ibd.21128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. L., Romano J. M., Mancl L., Levy R. L. (2014). Parental Catastrophizing Partially Mediates the Association between Parent-Reported Child Pain Behavior and Parental Protective Responses. Pain Res Treat, 2014, 751097. doi:10.1155/2014/751097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst J., Hofstetter A., Wolfe F., Hauser W. (2013). Short-term stress, but not mucosal healing nor depression was predictive for the risk of relapse in patients with ulcerative colitis: A prospective 12-month follow-up study. Inflammatory Bowel Diseases, 19, 2380–2386. doi:10.1097/MIB.0b013e3182a192ba [DOI] [PubMed] [Google Scholar]
- Lazarus R. S., Folkman S. (1984). Stress, appraisal, and coping. New York, NY: Springer. [Google Scholar]
- Leitenberg H., Gibson L. E., Novy P. L. (2004). Individual differences among undergraduate women in methods of coping with stressful events: The impact of cumulative childhood stressors and abuse. Child Abuse and Neglect, 28, 181–192. doi:10.1016/j.chiabu.2003.08.005 [DOI] [PubMed] [Google Scholar]
- Levy R. L., van Tilburg M. A., Langer S. L., Romano J. M., Walker L. S., Mancl L. A., Whitehead W. E. (2016). Effects of a Cognitive Behavioral Therapy Intervention Trial to Improve Disease Outcomes in Children with Inflammatory Bowel Disease. Inflammatory Bowel Diseases, 229, 2134–2418. doi:10.1097/MIB.0000000000000881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackner L. M., Crandall W. V. (2005). Long-term psychosocial outcomes reported by children and adolescents with inflammatory bowel disease. American Journal of Gastroenterology, 100, 1386–1392. doi:10.1111/j.1572-0241.2005.41428.x [DOI] [PubMed] [Google Scholar]
- Mackner L. M., Crandall W. V. (2006). Brief report: Psychosocial adjustment in adolescents with inflammatory bowel disease. Journal of Pediatric Psychology, 31, 281–285. doi:10.1093/jpepsy/jsj023 [DOI] [PubMed] [Google Scholar]
- Mawdsley J. E., Rampton D. S. (2005). Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut, 54, 1481–1491. doi:10.1136/gut.2005.064261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie A. M., Mulder R. T., Gearry R. B. (2013). How IBD patients cope with IBD: A systematic review. Journal of Crohn's and Colitis, 7, 89–106. doi:10.1016/j.crohns.2012.05.021 [DOI] [PubMed] [Google Scholar]
- McCubbin H. I., Patterson J., Wilson L. D. (1996). Family Inventory of Life Events and Changes (FILE) In McCubbin H. I., Thompson A. I., McCubbin M. A. (Eds.), Family assessment: Resiliency, coping, and adaptation-inventories for research and practice (pp. 103–178). Madison, WI: University of Wisconsin System. [Google Scholar]
- Monaghan M., Hilliard M. E., Cogen F. R., Streisand R. (2011). Supporting parents of very young children with type 1 diabetes: Results from a pilot study. Patient Education and Counseling, 82, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussell M., Bocker U., Nagel N., Singer M. V. (2004). Predictors of disease-related concerns and other aspects of health-related quality of life in outpatients with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology, 16, 1273–1280. [DOI] [PubMed] [Google Scholar]
- Ondersma S. J., Lumley M. A., Corlis M., Tojek T. M. (1997). Adolescents with inflammatory bowel disease: The roles of negative affectivity and hostility in subjective versus objective health. Journal of Pediatric Psychology, 22, 723–738. [DOI] [PubMed] [Google Scholar]
- Preacher K., Hayes A. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments and Computers, 36, 717–731. [DOI] [PubMed] [Google Scholar]
- Reed-Knight B., Maddux M. H., Deacy A. D., Lamparyk K., Stone A. L., Mackner L. (2017). Brain–gut interactions and maintenance factors in pediatric gastroenterological disorders: Recommendations for clinical care. Clinical Practice in Pediatric Psychology, 5, 93–105. doi:10.1037/cpp0000166 [Google Scholar]
- Rohde P., Lewinsohn P. M., Seeley J. R. (1997). Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. American Journal of Psychiatry, 154, 1593–1598. doi:10.1176/ajp.154.11.1593 [DOI] [PubMed] [Google Scholar]
- Schuman S. L., Graef D. M., Janicke D. M., Gray W. N., Hommel K. A. (2013). An exploration of family problem-solving and affective involvement as moderators between disease severity and depressive symptoms in adolescents with inflammatory bowel disease. Journal of Clinical Psychology in Medical Settings, 20, 488–496. doi:10.1007/s10880-013-9368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinath A., Young E., Szigethy E. (2014). Pain management in patients with inflammatory bowel disease: Translational approaches from bench to bedside. Inflammatory Bowel Diseases, 20, 2433–2449. doi:10.1097/mib.0000000000000170 [DOI] [PubMed] [Google Scholar]
- Steinhausen H. C., Kies H. (1982). Comparative studies of ulcerative colitis and Crohn's disease in children and adolescents. Journal of Child Psychology Psychiatry, 23, 33–42. [DOI] [PubMed] [Google Scholar]
- Szigethy E., Levy-Warren A., Whitton S., Bousvaros A., Gauvreau K., Leichtner A. M., Beardslee W. R. (2004). Depressive symptoms and inflammatory bowel disease in children and adolescents: A cross-sectional study. Journal of Pediatric Gastroenterology and Nutrition, 39, 395–403. [DOI] [PubMed] [Google Scholar]
- Targownik L. E., Sexton K. A., Bernstein M. T., Beatie B., Sargent M., Walker J. R., Graff L. A. (2015). The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. American Journal of Gastroenterology, 110, 1001–1012. doi:10.1038/ajg.2015.147 [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Gustafson K. E. (1996). Adaptation to chronic childhood illness. Washington, DC: American Psychological Association. [Google Scholar]
- Treharne G. J., Lyons A. C., Booth D. A., Kitas G. D. (2007). Psychological well-being across 1 year with rheumatoid arthritis: Coping resources as buffers of perceived stress. British Journal of Health Psychology, 12 (Pt 3), 323–345. doi:10.1348/135910706x109288 [DOI] [PubMed] [Google Scholar]
- Turner D., Otley A. R., Mack D., Hyams J., de Bruijne J., Uusoue K., Walters T. D., Zachos M., Mamula P., Beaton D. E., Steinhart A. H., Griffiths A. M. (2007). Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology, 133, 423–432. doi:10.1053/j.gastro.2007.05.029 [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L., Crowell M. D., Drossman D. A., Halpert A. D., Keefer L., Lackner J. M., Murphy T. B., Naliboff B. D., Levy R. L. (2016). Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology, 150, 1355–1367. doi:10.1053/j.gastro.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilburg M. A., Claar R. L., Romano J. M., Langer S. L., Walker L. S., Whitehead W. E., Abdullah B., Christie D. L., Levy R. L. (2015). The role of coping with symptoms in depression and disability: Comparison between inflammatory bowel disease and abdominal pain. Journal of Pediatric Gastroenterology and Nutrition, 61, 431–436. doi:10.1097/MPG.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. S., Greene J. W. (1989). Children with recurrent abdominal pain and their parents: More somatic complaints, anxiety, and depression than other patient families? Journal of Pediatric Psychology, 14, 231–243. [DOI] [PubMed] [Google Scholar]
- Walker L. S., Smith C. A., Garber J., Claar R. L. (2005). Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychology, 24, 364–374. doi:10.1037/0278-6133.24.4.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. S., Smith C. A., Garber J., Van Slyke D. (1997). Development and validation of the pain response inventory for children. Psychological Assessment, 9, 392–405. [Google Scholar]
- Wallander J., Varni J. (1992). Adjustment in children with chronic physical disorders: Programmatic research on a disability-stress-coping model In La Greca A. M., Siegel L. J., Wallander J. L., Walker C. E. (Eds.), Stress and coping in child health (pp. 279–298). New York, NY: The Guilford Press. [Google Scholar]
- Wood B., Watkins J. B., Boyle J. T., Nogueira J., Zimand E., Carroll L. (1987). Psychological functioning in children with Crohn's disease and ulcerative colitis: Implications for models of psychobiological interaction. Journal of the American Academy of Child and Adolescent Psychiatry, 26, 774–781. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck M. J., Skinner E. A. (2011). Review: The development of coping across childhood and adolescence: An integrative review and critique of research. International Journal of Behavioral Development, 35, 1–17. doi:10.1177/0165025410384923 [Google Scholar]
- Zimmerman L. A., Srinath A. I., Goyal A., Bousvaros A., Ducharme P., Szigethy E., Nurko S. (2013). The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflammatory Bowel Diseases, 19, 826–831 doi:10.1097%2FMIB.0b013e3182802a0a [DOI] [PMC free article] [PubMed] [Google Scholar]