Abstract
Purpose
The incidence and severity of Clostridium difficile infection (CDI) have markedly increased over the past decade. However, there is very limited epidemiological data on CDI in China so far, specifically no data in Shandong Province. The aim of this study was to evaluate diagnostic algorithm for CDI and to gain data on molecular epidemiology of CDI in the Shandong Province of China.
Materials and methods
Nonrepetitive unformed fecal specimens (n=504) were investigated by the glutamate dehydrogenase (GDH), C. difficile toxin A&B (CDAB) tests and toxigenic culture. Furthermore, 85 isolates were characterized by toxin gene detection, multilocus sequence typing, ribotyping and antimicrobial susceptibility testing.
Results
The algorithm of combining GDH and CDAB tests could define diagnosis of 54.2% CDI cases and excluded 90% of non-CDI. Further adding the toxigenic culture to the algorithm enhanced the detection sensitivity to 100%. Toxigenic strains comprised 84.7% of isolates, including A+B+CDT− (71.8%, 61/85), A−B+CDT− (11.8%, 10/85) and A+B+CDT+ (1.2%, 1/85) isolates. RT046/ST35 (13.9%, 10/72), RT014/ST2 (12.5%, 9/72) and RT017/ST37 (12.5%, 9/72) were the more common genotypes among toxigenic C. difficile strains. The clinical severity score of A−B+CDT− toxin genes genotype (3.50±0.85) was significantly higher than the A+B+CDT− type (2.59±0.93) (P<0.05). RT046/ST35 isolates were highly prevalent and had high clinical severity scores (3.80±0.92). Variations in resistance from different sequence types (STs) were observed. Toxigenic strains showed higher resistance rates to erythromycin, clindamycin and ciprofloxacin compared to nontoxigenic strains (P<0.05).
Conclusion
The epidemiology of C. difficile in Shandong Province differed from other regions in China. Comprehensive optimized diagnosis strategy and continuous surveillance should be established and applied in order to curb the spread of toxigenic C. difficile strains, especially for hospitalized patients.
Keywords: Clostridium difficile, genotype, antimicrobial resistance, severity score, Shandong Province, China
Introduction
Clostridium difficile, a gram-positive sporulating anaerobic bacillus, is the etiologic pathogen of pseudomembranous colitis and a principal pathogen of antimicrobial-associated diarrhea. Patients with C. difficile infection (CDI) have clinical manifestations ranging from asymptomatic carriage, diarrhea to pseudomembranous colitis, even severe life-threatening toxic megacolon, sepsis and death.1 Generally, TcdA and TcdB toxins (encoded by tcdA and tcdB genes, respectively) are the major virulence factors produced by toxigenic C. difficile strains. However, some strains can also produce C. difficile binary toxin (CDT; encoded by binary genes cdtA and cdtB).2
The increased morbidity and severity of CDI has led to a significant economic burden on the health care systems worldwide, with increased treatment cost and prolonged hospital stay.3,4 CDI is thus regarded an urgent public health threat, and the financial burden is estimated to be $725 million in community settings and $5.4 billion in health care settings in North America.5 Knowledge of the antimicrobial susceptibility profiles and molecular types of C. difficile is important for monitoring spread of this organism. Of the typing methods described for C. difficile, multilocus sequence typing (MLST), which facilitates isolate discrimination by sequencing 7 housekeeping gene fragments, is widely used in studying the population gene structure and global epidemiology of the organism.6,7 However, at the present time, polymerase chain reaction (PCR) ribotyping is the most frequently used typing method because of the high discriminatory power and low costs.8,9 One of the most notable findings achieved by molecular epidemiology studies worldwide has been the detection of the hypervirulent C. difficile clone BI/NAP1/027 (BI: restriction endonuclease analysis group BI; NAP1: North American pulse-field type 1; PCR ribotype 027), which especially occurred in North America and Europe.10,11
In China, there is limited clinical and epidemiologic data on CDIs, with few case reports and studies described in only a few geographical regions, including Beijing, Shanghai, Zhejiang and Guangzhou.7,8,12–15 Shandong Province, the second largest populous province in China, covering an area of 155,800 km2 with a population of around 100 million, has no related report on CDIs to date.
This study, for the first time, evaluated the CDI laboratory diagnostic strategies and explored the molecular epidemiology of C. difficile strains from two hospitals in Shandong Province, aiming to provide local scientific reference data for prevention and control of CDI.
Materials and methods
Ethics
The study was approved by the Human Research Ethics Committee of the Affiliated Hospital of Qingdao University. The written informed consent requirement from patients was waived due to the retrospective nature of the study. Furthermore, all patients’ data was anonymized before the study.
Study design and sample collection
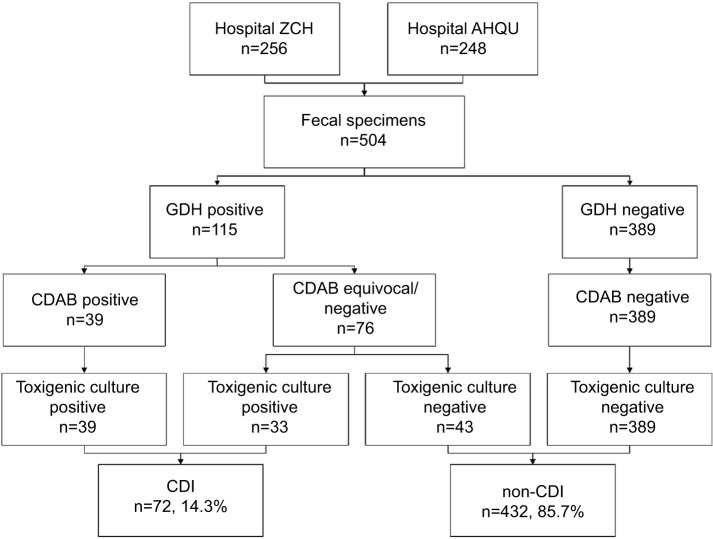
This study was conducted at the Zibo Central Hospital (ZCH) and the Affiliated Hospital of Qingdao University (AHQU), in Shandong Province in Eastern China. Both hospitals are tertiary general hospitals with 2000 beds. The study was conducted from March 2016 to April 2017. A total of 504 nonrepetitive unformed stool specimens were collected from hospitalized patients with suspected CDI symptoms during the study period (Figure 1).
Figure 1.
Flow diagram for the three-step algorithm to confirm toxigenic Clostridium difficile and the diagnosis of CDI.
Abbreviations: ZCH, Zibo Central Hospital; AHQU, the Affiliated Hospital of Qingdao University; GDH, glutamate dehydrogenase; CDAB, C. difficile toxin A&B; CDI, C. difficile infection.
VIDAS glutamate dehydrogenase (GDH) and C. difficile toxin A&B (CDAB) testing
All the fecal specimens were tested by enzyme immunoassay (EIA) methods using commercial VIDAS GDH and CDAB kits (bioMérieux, Marcy l’Etoile, France), following the manufacturer’s instructions.
C. difficile culture and identification
The fecal samples were incubated on ChromID C. difficile agar (CDIF, bioMérieux) at 35°C under anaerobic condition for 48 h. Typical C. difficile colonies were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) with VITEK MS system (bioMérieux).
DNA extraction, toxin gene detection and tcdC sequencing
Genomic DNA was extracted, and a five-plex PCR was performed to simultaneously detect 16S rDNA and toxin genes tcdA (encoding toxin A), tcdB (encoding toxin B), cdtA and cdtB in C. difficile isolates, as previously described.2 Isolates positive for toxin A were further characterized to check for the deletion of the repeating region of tcdA gene by primers NK9 and NKV011.16 The tcdC gene, a negative regulator of tcdA and tcdB, was also sequenced and analyzed as previously described.17
MLST and PCR ribotyping
MLST was performed by using 7 gene loci (adk, atpA, dxr, glyA, recA, sodA and tpi), as previously described.6 PCR products were purified and sequenced at Taihe Biotechnology Company (Beijing, China). DNA sequences were queried against the PubMLST database (http://pubmlst.org/cdifficile/) to obtain the allele numbers, sequence types (STs) and clades. Five novel STs identified in this study were submitted to the database and assigned ST numbers, ST450–ST454.
PCR ribotyping was performed by capillary gel electrophoresis as previously described.18 Gene Marker V2.2.0 (Soft Genetics, America) was used to determine the size of each peak, and ribotypes (RTs) were assigned by presenting the data on the WEBRIBO database (https://webribo.ages.at/) and compared with results reported by Cheng et al.8 Novel RTs observed in this study were named as “SDR” plus two Arabic numbers (e.g., SDR01).
Three reference C. difficile strains, PUCD10 (PUR09/ST81), PUCD301 (RT027/ST1) and PUCD610 (RT017/ST37), were used as internal controls.9
Antimicrobial susceptibility testing
The agar dilution method was used to determine the minimum inhibitory concentrations (MICs) of vancomycin, metronidazole, erythromycin, clindamycin, ciprofloxacin and tetracycline, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines M11-A8.19 The interpretation of breakpoints of metronidazole, clindamycin and tetracycline was based on CLSI M100-S27 criteria.20 In addition, the breakpoints of vancomycin, erythromycin and ciprofloxacin were ≥32, ≥8 and ≥8 mg/L, respectively (Table S1).21 Bacteroides fragilis ATCC 25285 was used for quality control.
Resistance gene detection
The quinolone resistance determining region (QRDR) of gyrA and gyrB genes were amplified and sequenced in 30 selected ciprofloxacin-resistant isolates as previously described by Drudy et al.22
Patient characteristics and severity score
A CDI severity score was determined for each patient based on clinical features, laboratory test findings and clinical impressions of the attending physician, in accordance to the 2010 updated America guidelines.23 The severity of CDI in each patient was assigned a score of 1–6, 1, no clinical CDI; 2, mild; 3, mild to moderate; 4, moderate; 5, moderate to severe; and 6, severe.7,23
Statistical analysis
All data were statistically analyzed by using SPSS software (version 18.0, IBM, New York, USA). Kruskal–Wallis and chi-square tests were used to analyze correlations among STs, RTs and antimicrobial susceptibility patterns of C. difficile strains. A P-value of <0.05 was considered statistically significant.
Results
General clinical information
A total of 504 inpatients with diarrhea from ZCH (n=256) and AHQU (n=248) were included in this study (Figure 1). The average age of the patients, which included 261 males (51.8%) and 243 females (48.2%), was 49.3±18.1 (ranged from 4 to 91). About 24.0% (121/504) of the patients were from hematology and oncology departments, 20.0% (101/504) from gastroenterology department, 16.9% (85/504) from surgery department, 7.9% (40/504) from emergency department, 7.5% (38/504) from intensive care unit, 6.2% (31/504) from pediatric department and 17.5% (88/504) from other departments (i.e., geriatrics, obstetrics and gynecology, cardiovascular, neurology).
Comparison of GDH versus toxigenic culture
Among the 504 fecal specimens tested, 22.8% (115/504) were positive for GDH, and 16.9% (85/504) were C. difficile culture positive. Only one specimen was GDH negative but culture positive (Table 1; Figure 1). Compared to the culture method, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the GDH assay were 98.8%, 92.6%, 73.0% and 99.7%, respectively (Table 2).
Table 1.
Clostridium difficile culture, VIDAS GDH, VIDAS CDAB and toxigenic typing results for 504 fecal samples in the study
| Culture result | GDH | CDAB | Toxigenic type (no. of isolates)
|
Total no. of isolates (%) | |||
|---|---|---|---|---|---|---|---|
| A+B+CDT− | A−B+CDT− | A+B+CDT+ | A−B−CDT− | ||||
| Positive | Positive | Positive | 35 | 3 | 1 | 0 | 39 (7.7) |
| Positive | Positive | Equivocal | 8 | 2 | 0 | 1 | 11 (2.2) |
| Positive | Positive | Negative | 18 | 5 | 0 | 11 | 34 (6.7) |
| Positive | Negative | Negative | 0 | 0 | 0 | 1 | 1 (0.2) |
| Negative | Positive | Equivocal | ND | ND | ND | ND | 9 (1.8) |
| Negative | Positive | Negative | ND | ND | ND | ND | 22 (4.4) |
| Negative | Negative | Negative | ND | ND | ND | ND | 388 (77.0) |
Abbreviations: GDH, glutamate dehydrogenase; CDAB, C. difficile toxin A&B; CDT, C. difficile binary toxin; ND, not done.
Table 2.
Performance of VIDAS GDH and VIDAS CDAB detection for diagnosis of CDI
| Test methods | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
|---|---|---|---|---|
| GDHa | 98.8 (92.7–99.9) | 92.6 (89.6–94.8) | 73.0 (63.8–80.7) | 99.7 (98.3–100.0) |
| CDABb | 54.2 (42.1–65.8) | 100.0 (98.9–100.0) | 100.0 (88.8–100.0) | 92.9 (90.1–95.0) |
Notes:
Compare to culture;
Compare to toxigenic culture.
Abbreviations: GDH, glutamate dehydrogenase; CDAB, C. difficile toxin A&B; PPV, positive predictive value; NPV, negative predictive value.
Detection of toxin genes and comparison with CDAB EIA method
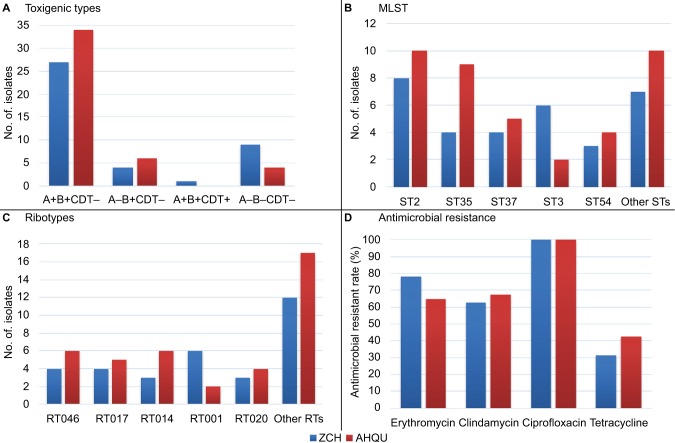
Of the 85 C. difficile strains isolated in this study, 72 (84.7%) were toxin gene positive, among which 61 (71.8%) were tcdA-positive, tcdB-positive and cdtA/cdtB-negative (A+B+CDT−), and 10 (11.8%) were tcdA-negative, tcdB-positive and cdtA/cdtB-negative (A−B+CDT−). Only one strain (CD029) isolated in ZCH was cdt gene positive, and the toxigenic type was tcdA-positive, tcdB-positive and cdtA/cdtB-positive (A+B+CDT+) (Figure 2A; Table 1). The tcdC gene in this isolate had an 18-bp deletion at nucleotide positions 330–347 and a single base pair deletion at nucleotide 117.
Figure 2.
Distribution of toxin genes genotypes among Clostridium difficile isolates (n=85) (A), and MLST STs, PCR ribotypes and antimicrobial resistant rates among toxigenic C. difficile isolates (n=72) (B–D) from 2 hospitals in China.
Abbreviations: CDT, C. difficile binary toxin; MLST, multilocus sequence typing; ST, sequence type; RT, ribotype; ZCH, Zibo Central Hospital; AHQU, the Affiliated Hospital of Qingdao University; PCR, polymerase chain reaction.
Thirty-nine fecal specimens were CDAB positive and toxigenic culture positive (7.7%). However, among 76 CDAB negative/equivocal strains, 33 toxigenic culture positive strains were detected (43.4%) (Figure 1). Therefore, a total of 72 out of 504 patients (14.3%) with diarrhea were defined as CDI according to toxigenic culture results (Figure 1). Compared to toxigenic culture, the sensitivity, specificity, PPV and NPV of CDAB assay were 54.2%, 100.0%, 100.0% and 92.9%, respectively (Table 2).
To overcome the deficiencies of low PPV for GDH and NPV for CDAB methods, we recommended a combined laboratory diagnosis algorithm for CDI based on GDH and CDAB testing and complemented by detection of toxin genes either in toxigenic culture method or directly in stool samples for any discordant results (Figure 1), as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID).24
Genotypes determined by MLST and PCR ribotyping
The 85 C. difficile strains were classified into 23 STs, including 5 STs (1 per isolate) that were novel (Table 3). Among 72 toxigenic strains and 13 nontoxigenic strains, 20 and 4 STs were detected, respectively. Only ST3 comprised both toxigenic (n=8) and non-toxigenic (n=9) strains (Table 3). Among toxigenic C. difficile strains, ST2 (25.0%, 18/72) was the most common, followed by ST35 (18.1%, 13/72), ST37 (12.5%, 9/72), ST3 (11.1%, 8/72) and ST54 (9.7%, 7/72), while ST3 (69.2%, 9/13) was the most common ST among nontoxigenic strains (Table 3; Figure 2B). Nine of 10 A−B+CDT− strains belonged to ST37. The only one A+B+CDT+ strain belonged to ST1 (Table 3).
Table 3.
STs, toxin genotypes and ribotypes of 85 Clostridium difficile clinical isolates
| STs (no. of isolates) | Clade | Toxin genotype (no. of isolates) | Ribotype (no. of isolates) |
|---|---|---|---|
| ST1 (1) | 2 | A+B+CDT+ (1) | 027 (1) |
| ST2 (18) | 1 | A+B+CDT− (18) | 014 (9) |
| 020 (7) | |||
| 006 (1) | |||
| 432 (1) | |||
| ST3 (17) | 1 | A−B−CDT− (9) | 009 (6) |
| 456 (3) | |||
| A+B+CDT− (8) | 001 (8) | ||
| ST4 (1) | 1 | A+B+CDT− (1) | SDR07 (1) |
| ST8 (1) | 1 | A+B+CDT− (1) | SDR06 (1) |
| ST17 (2) | 1 | A+B+CDT− (2) | PUR34 (2) |
| ST27 (1) | 1 | A+B+CDT− (1) | 039 (1) |
| ST33 (1) | 1 | A+B+CDT− (1) | SDR05 (1) |
| ST35 (13) | 1 | A+B+CDT− (13) | 046 (10) |
| SDR09 (3) | |||
| ST37 (9) | 4 | A−B+CDT− (9) | 017 (9) |
| ST42 (1) | 1 | A+B+CDT− (1) | 106 (1) |
| ST54 (7) | 1 | A+B+CDT− (7) | 012 (7) |
| ST81 (1) | 4 | A−B+CDT− (1) | PUR09 (1) |
| ST102 (2) | 1 | A+B+CDT− (2) | PUR02 (2) |
| ST111 (1) | 1 | A+B+CDT− (1) | SDR08 (1) |
| ST129 (1) | 1 | A+B+CDT− (1) | PUR13 (1) |
| ST205 (2) | 1 | A−B−CDT− (2) | SDR04 (2) |
| ST319 (1) | 1 | A+B+CDT− (1) | SDR03 (1) |
| ST450 (1)a | 1 | A−B−CDT− (1) | SDR01 (1) |
| ST451 (1)a | 1 | A+B+CDT− (1) | SDR02 (1) |
| ST452 (1)a | 1 | A−B−CDT− (1) | 010 (1) |
| ST453 (1)a | 1 | A+B+CDT− (1) | 449 (1) |
| ST454 (1)a | 1 | A+B+CDT− (1) | 610 (1) |
Notes:
Novel STs identified in the present study.
Abbreviations: ST, sequence type; CDT, C. difficile binary toxin.
In addition, we found that all isolates of the same ribotypes belonged to the same STs, and none of the ribotypes were shared by different STs. Twenty-nine PCR ribotypes were detected among 72 toxigenic strains. The predominant ribotype was RT046 (13.9%, 10/72), followed by RT014 and RT017 (12.5%, 9/72, each), RT001 (11.1%, 8/72), RT012 and RT020 (9.7%, 7/72, each) (Table 3; Figure 2C). Of note, one C. difficile isolate from ZCH was confirmed to be hypervirulent ribotype 027 (1.4%, 1/72). Among 13 nontoxigenic strains, ribotypes 009 (46.2%, 6/13) and 456 (23.1%, 3/13) dominated, and all isolates of these ribotypes belonged to ST3 (Table 3).
Clinical severity score of CDI patients
Seventy-two CDI patients infected by toxigenic C. difficile strains were evaluated for CDI severity score. No severity score of 6 was found (Table 4). The average (±SD) severity score was 2.97±0.90. There was no difference in severity scores between CDI patients who were CDAB EIA test positive and those who were CDAB EIA test equivocal or negative (P<0.05) (Table 4). However, patients with A−B+CDT− strains had higher severity scores (3.50±0.85) than patients with A+B+CDT− strains (2.59±0.93) (P<0.05). In addition, differences in CDI severity scores were found among patients infected by C. difficile of different ribotypes and STs (Table 4). ST35 strains showed high severity scores, with a score of 3.69±0.85, which was significantly higher than those of ST2, ST3 and ST54 strains (P<0.05), but not significantly different with ST37 (P>0.05, Table 4). In patients with CDI scores of ≥4 (n=20), ribotypes RT046 (35.0%) and RT014 (20.0%) were detected more frequently than RT001 (5.0%) and RT020 (5.0%). PCR ribotype 027 strain isolated from a gastroenterology patient exhibited high severity with a score of 4 (Table 4). There were 4 patients with CDI severity scores of 5, and half of them belonged to the ST35/046 genotype (Table 4).
Table 4.
Correlation between clinical severity, phenotypes and genotypes in 72 toxigenic Clostridium difficile strains
| Phenotype and genotypes | CDI severity score
|
||||
|---|---|---|---|---|---|
| 2 (n=26) | 3 (n=26) | 4 (n=16) | 5 (n=4) | Mean±SD | |
| EIA phenotypea | |||||
| GDH+CDAB+ (n=39) | 15 | 14 | 7 | 3 | 2.95±0.94 |
| GDH+CDAB−/± (n=33) | 11 | 12 | 9 | 1 | 3.00±0.87 |
| Toxigenic type | |||||
| A+B+CDT− (n=61) | 25 | 22 | 11 | 3 | 2.59±0.93 |
| A−B+CDT− (n=10) | 1 | 4 | 4 | 1 | 3.50±0.85 |
| A+B+CDT+ (n=1) | 0 | 0 | 1 | 0 | 4.00 |
| MLST type | |||||
| ST2 (n=18) | 8 | 4 | 5 | 1 | 2.94±0.99 |
| ST35 (n=13) | 1 | 4 | 6 | 2 | 3.69±0.85 |
| ST37 (n=9) | 3 | 3 | 2 | 1 | 3.11±1.05 |
| ST3 (n=8) | 4 | 3 | 1 | 0 | 2.63±0.74 |
| ST54 (n=7) | 3 | 4 | 0 | 0 | 2.57±0.53 |
| PCR ribotype | |||||
| 046 (n=10) | 1 | 2 | 5 | 2 | 3.80±0.92 |
| 014 (n=9) | 3 | 2 | 3 | 1 | 3.22±1.09 |
| 001 (n=8) | 4 | 3 | 1 | 0 | 2.63±0.74 |
| 020 (n=7) | 4 | 2 | 1 | 0 | 2.57±0.79 |
| 027 (n=1) | 0 | 0 | 1 | 0 | 4.00 |
Note:
GDH+CDAB+: toxigenic C. difficile strains with GDH and CDAB EIA tests positive; GDH+CDAB−/±, toxigenic C. difficile strains, GDH test positive but CDAB EIA test equivocal or negative.
Abbreviations: CDI, C. difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; CDAB, C. difficile toxin A&B; CDT, C. difficile binary toxin; MLST, multilocus sequence typing; ST, sequence type; PCR, polymerase chain reaction.
Antimicrobial resistance
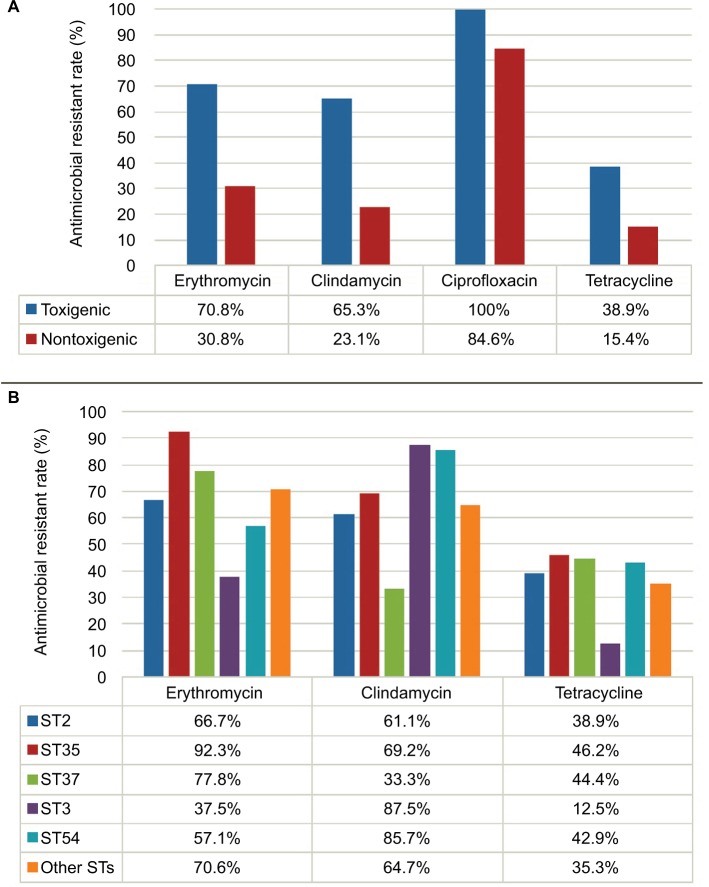
The MICs of 6 antimicrobial agents for 85 C. difficile strains are shown in Table 5. All the isolates were susceptible to vancomycin and metronidazole. Thirty out of 85 isolates (35.3%) were resistant to erythromycin, clindamycin and ciprofloxacin, and 96.7% (29/30) of the co-resistant isolates were toxigenic. In contrast, 64.7%, 58.8%, 97.6% and 35.3% of the 85 isolates were resistant to erythromycin, clindamycin, ciprofloxacin and tetracycline, respectively (Table 5). Toxigenic strains showed higher resistance rates to erythromycin, clindamycin and ciprofloxacin than non-toxigenic strains (P<0.01, Figure 3A; Table 5). Moreover, there were differences in antimicrobial resistance rates among different STs. For instance, ST35 and ST37 exhibited high resistance rates to erythromycin (92.3% and 77.8%, respectively), while ST3 and ST54 showed high resistance rates to clindamycin (87.5% and 85.7%, respectively) (Figure 3B). There was no significant difference in antimicrobial resistance rates of C. difficile strains from the 2 hospitals (Figure 2D).
Table 5.
Antimicrobial resistant rates and MIC ranges for 85 Clostridium difficile clinical isolates
| Antimicrobial agent | All strains (n=85)
|
Toxigenic strains (n=72)
|
Non-toxigenic strains (n=13)
|
P-valuea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | %R | MIC50 (mg/L) | MIC90 (mg/L) | Range (mg/L) | %R | MIC50 (mg/L) | MIC90 mg/L) | Range (mg/L) | %R | ||
| Vancomycin | 0.5 | 1 | 0.125–4 | 0 | 0.5 | 2 | 0.25–4 | 0 | 0.5 | 1 | 0.125–2 | 0 | NS |
| Metronidazole | 0.25 | 0.25 | 0.125–1 | 0 | 0.25 | 0.5 | 0.125–1 | 0 | 0.25 | 0.25 | 0.25–1 | 0 | NS |
| Erythromycin | 128 | >256 | 0.5–>256 | 64.7 | >256 | > 256 | 0.5–>256 | 70.8 | 64 | 128 | 0.5–>256 | 30.8 | 0.005 |
| Clindamycin | 32 | >256 | 0.25–>256 | 58.8 | 64 | > 256 | 0.5–>256 | 65.3 | 8 | 128 | 0.25–>256 | 23.1 | 0.004 |
| Ciprofloxacin | 64 | 128 | 1–256 | 97.6 | 64 | 128 | 8–256 | 100 | 16 | 128 | 1–128 | 84.6 | 0.01 |
| Tetracyclin | 0.5 | 32 | 0.125–64 | 35.3 | 0.5 | 32 | 0.125–64 | 38.9 | 0.25 | 16 | 0.125–32 | 15.4 | NS |
Note:
Statistics for resistant rates of toxigenic strains versus that of nontoxigenic strains.
Abbreviations: MIC, minimum inhibitory concentration; NS, not significant.
Figure 3.
Antimicrobial resistant rates among Clostridium difficile isolates (A) and among different STs of toxigenic C. difficile isolates (B).
Abbreviation: ST, sequence type.
Correlation between fluoroquinolone-resistance and gyrA and gyrB gene mutations
In order to investigate the mechanism responsible for the high ciprofloxacin resistance, 30 ciprofloxacin-resistant isolates were selected for analyzing the gyrA and gyrB gene sequences (Table 6). Only 10 of the 30 isolates (33.3%) had GyrA amino acid substitutions (Thr82→Ile), including 4 with GyrB substitutions (Ser366→Ala and/or Asp426→Val) at the same time (Table 6). The rest 20 (66.7%) of the isolates had wild-type gyrA and gyrB gene sequences (Table 6).
Table 6.
Phenotypic and genotypic characteristics of 30 ciprofloxacin-resistant Clostridium difficile strains
| Isolate | Toxin genotype | MLST | Ribotype | Moxifloxacin
|
Ciprofloxacin
|
Amino acid substitution
|
|||
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) | Criteria | MIC (mg/L) | Criteria | GyrA | GyrB | ||||
| Moxifloxacin-resistant strains | |||||||||
| S43 | A+B+CDT+ | 1 | 027 | 16 | R | 128 | R | Thr82→Ile | WT |
| S25 | A+B+CDT− | 3 | 001 | 8 | R | 64 | R | Thr82→Ile | WT |
| S12 | A+B+CDT− | 3 | 001 | 16 | R | 64 | R | Thr82→Ile | WT |
| S65 | A+B+CDT− | 17 | PUR34 | 32 | R | 64 | R | Thr82→Ile | Ser366→Ala |
| S32 | A+B+CDT− | 35 | 046 | 16 | R | 32 | R | Thr82→Ile | WT |
| S81 | A+B+CDT− | 35 | 046 | 16 | R | 128 | R | Thr82→Ile | WT |
| S74 | A−B+CDT− | 37 | 017 | 64 | R | 128 | R | Thr82→Ile | Ser366→Ala |
| S53 | A−B+CDT− | 37 | 017 | 64 | R | 128 | R | Thr82→Ile | Ser366→Ala |
| S5 | A−B+CDT− | 81 | PUR09 | 64 | R | 128 | R | Thr82→Ile | Ser366→Ala, Asp426→Val |
| S16 | A+B+CDT− | 111 | SDR08 | 16 | R | 128 | R | Thr82→Ile | WT |
| Moxifloxacin-susceptible strains | |||||||||
| S42 | A+B+CDT− | 2 | 014 | 0.25 | S | 32 | R | WT | WT |
| S21 | A+B+CDT− | 2 | 014 | 0.25 | S | 64 | R | WT | WT |
| S2 | A+B+CDT− | 2 | 014 | 0.25 | S | 64 | R | WT | WT |
| S8 | A+B+CDT− | 2 | 020 | 0.5 | S | 64 | R | WT | WT |
| S61 | A+B+CDT− | 2 | 020 | 0.5 | S | 32 | R | WT | WT |
| S83 | A+B+CDT− | 2 | 006 | 1 | S | 128 | R | WT | WT |
| S14 | A+B+CDT− | 2 | 432 | 1 | S | 128 | R | WT | WT |
| S24 | A−B−CDT− | 3 | 009 | 0.25 | S | 64 | R | WT | WT |
| S55 | A−B−CDT− | 3 | 456 | 0.25 | S | 64 | R | WT | WT |
| S47 | A+B+CDT− | 4 | SDR07 | 0.25 | S | 64 | R | WT | WT |
| S71 | A+B+CDT− | 8 | SDR06 | 0.5 | S | 128 | R | WT | WT |
| S9 | A+B+CDT− | 27 | 039 | 0.5 | S | 128 | R | WT | WT |
| S11 | A+B+CDT− | 33 | SDR05 | 2 | S | 64 | R | WT | WT |
| S49 | A+B+CDT− | 35 | 046 | 2 | S | 64 | R | WT | WT |
| S67 | A+B+CDT− | 35 | SDR09 | 0.25 | S | 32 | R | WT | WT |
| S20 | A+B+CDT− | 42 | 106 | 0.25 | S | 32 | R | WT | WT |
| S4 | A+B+CDT− | 54 | 012 | 0.25 | S | 32 | R | WT | WT |
| S73 | A+B+CDT− | 54 | 012 | 0.25 | S | 64 | R | WT | WT |
| S48 | A+B+CDT− | 102 | PUR02 | 1 | S | 32 | R | WT | WT |
| S19 | A+B+CDT− | 129 | PUR13 | 0.5 | S | 64 | R | WT | WT |
Abbreviations: MLST, multilocus sequence typing; MIC, minimum inhibitory concentration; CDT, C. difficile binary toxin; S, susceptible; R, resistant; WT, wild-type.
We further tested moxifloxacin susceptibility among the 30 isolates and found out that moxifloxacin resistance had good correlation with gyrA and gyrB gene mutations; all isolates that had wild-type gyrA and gyrB genes were moxifloxacin susceptible, while isolates with nonsynonymous mutant gyrA +/− gyrB genes were all moxifloxacin resistant. In addition, isolates with mutations in both gyrA and gyrB genes showed high level resistance to moxifloxacin (MICs of ≥32 mg/L) compared to isolates having mutation only in gyrA gene (MICs of 8–16 mg/L) (Table 6).
Discussion
CDI is a significant and increasing public health threat and is regarded as the leading cause of nosocomial diarrhea related to antimicrobial therapy. The morbidity and mortality of CDI have increased substantially in the last decade.25 On account of limited laboratory diagnostic capacity and low clinical awareness, lack of data on CDI in China makes it an underestimated problem.9,26,27 To our best knowledge, this is the first systematic study on the epidemiology of C. difficile from Shandong Province, China.
VIDAS CDAB (bioMérieux) was the first assay approved by China Food and Drug Administration for the laboratory diagnosis of CDI and is to date the most commonly used assay in China. However, our study revealed that 45.8% of the CDI cases would be missed by using CDAB only. GDH assay, in comparison, had notable high NPV (99.7%) but low PPV (73.0%) for diagnosis of CDI. In agreement to previous findings by Cheng et al,26 we also recommend the three-step CDI workflow based on combining GDH and CDAB assays and suggest using molecular detection of toxin genes when any discordant results between GDH and CDAB assays are encountered, and this was described first in the updated ESCMID guidelines in 2016.24
In our study, the majority (84.7%) of the C. difficile strains possessed toxin genes, which is similar to previous findings in China, with toxigenic strains accounting for 70%–90% of the strains.7,21,26,28 The C. difficile isolates from the 2 hospitals exhibited similar epidemic genotype profiles. In addition, the predominant STs in this study, including ST54, ST37, ST3, ST2 and ST35, are also the main epidemic genotypes described in other regions of China (Table 7).7,21,26,28 However, geographic diversity was also observed, e.g., the predominant ST2 clone in the present study (25.0%) was less commonly seen in other regions (up to 13.5%) (Table 7). In addition, previous studies in Beijing reveal a shift in epidemic clones over time. Specifically, ST37 was the most common ST (25.7%) described between the 1980s and 2012 in this locale. However, this ST has become less common as reported in two recent studies (12.2–13.8%). Meanwhile, ST54 has become more prevalent, rising from 5.7% to 16.4–18.9% (Table 7).8,28,29 Moreover, remarkable variations in molecular epidemiology of C. difficile across different countries worldwide have been observed. For example, in Korea and Japan, ST17 is the predominant type (55.7% and 21.5%, respectively), followed by ST2 (8.6% and 10.0%, respectively).30,31 However, in Europe, RT027/ST1 is the most prevalent genotype, especially in Western and Eastern Europe.32
Table 7.
Review of Clostridium difficile studies, ranged by latitude from north to south in mainland China
| No. | Geographic | Year | MLST prevalence
|
RTs prevalence
|
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 1st (%) | 2nd (%) | 3rd (%) | ||||
| 1 | Beijing | 1980s–2012 | ST37 (25.7) | ST35 (18.6) | ST3 (17.1) | 29 | |||
| 2 | Beijing | 2012–2015 | ST54 (16.4) | ST3 (14.7) | ST37 (13.8) | 8 | |||
| 3 | Beijing | 2014–2015 | ST54 (18.9) | ST2 (13.5) | ST37 (12.2) | 28 | |||
| 4 | Hebei | 2013–2014 | ST54 (29.2) | ST3 (25.7) | ST35 (10.6) | 39 | |||
| 5 | Shandong | 2016–2017 | ST2 (25.0) | ST35 (18.1) | ST37 (12.5) | RT046 (13.9) | RT014 (12.5) | RT017 (12.5) | This study |
| 6 | Jiangsu | 2015–2016 | ST54 (32.8) | ST3 (16.4) | ST35 (13.1) | 40 | |||
| 7 | Shanghai | 2012–2013 | ST81 (18.8) | ST54 (14.1) | ST 37 (12.5) | 41 | |||
| 8 | Shanghai | 2012–2013 | RT017 (21.0) | RT012 (17.3) | RTH (16.7) | 14 | |||
| 9 | Sichuan | 2012–2013 | ST3 (16.1) | ST35 (12.9) | ST54 (12.9) | 34 | |||
| 10 | Zhejiang | 2009–2011 | ST54 (23.0) | ST35 (19.3) | ST37 (10.0) | 12 | |||
| 11 | Zhejiang | 2012–2013 | RT006 (55.0) | RT002 (30.0) | RT014 (10.0) | 42 | |||
| 12 | Zhejiang | 2013 | RT017 (50.0) | RT001 (26.8) | RT014 (14.6) | 43 | |||
| 13 | Zhejiang | 2012–2015 | ST37 (16.5) | ST3 (16.3) | ST54 (12.9) | 7 | |||
| 14 | Hunan | 2009–2010 | RT017 (48.0) | RT046 (14.0) | RT012 (14.0) | 44 | |||
Abbreviations: MLST, multilocus sequence typing; RT, ribotype; ST, sequence type.
Of note, RT046/ST35, which has rarely been identified in other countries, but more commonly reported in China,12,33,34 has scarcely been studied in order to understand its clinical pathogenicity. In this study, RT046/ST35 exhibited higher clinical severity (3.80±0.92) than other RTs, with high morbidity and severe complications, including pseudomembranous colitis and toxic megacolon, and high resistance rates to erythromycin (90.0%). These factors suggest that RT046/ST35 strains could be a major threat in Shandong Province of China and need continued monitoring and implementation of appropriate control measures.
Another interesting finding of this study is the detection of hypervirulent RT027/ST1 strain in this region of China. Similar to the majority of RT027 strains identified worldwide, this isolate was also binary toxin gene positive and had an 18-bp deletion in the tcdC gene.9,11 The concerned patient had symptoms of pseudomembranous colitis and was assigned a high-level severity score of 4. To date, C. difficile RT027 cases have only been reported sporadically in China.9,35 However, nosocomial outbreaks of C. difficile RT027 strains have been reported,36 revealing that the threat of RT027 strains might be underestimated, which highlights the need for increasing the laboratory diagnostic capacity for detection of CDI in China and use of molecular typing tools in surveillance programs.37
In our study, all the C. difficile isolates were susceptible to vancomycin and metronidazole, which is in agreement with other studies,8,31 while nearly all (97.6%. 83/85) the isolates studied were resistant to ciprofloxacin, which was also in accordance with a previous report in China by Cheng et al (ciprofloxacin resistant rates 100%).8 However, our further investigations showed that there were significant differences between moxifloxacin and ciprofloxacin activities against C. difficile isolates, and chromosomal mutations in gyrA and gyrB genes were associated with moxifloxacin rather than ciprofloxacin susceptibilities. Moreover, an observational study in England showed that the incidence of CDI declined by about 80% by restricting national fluoroquinolone prescribing and elimination of fluoroquinolone-resistant isolates. This highlights the importance of fluoroquinolone restriction in the control of CDI.38 Therefore, antimicrobial stewardship is a key component in CDI prevention.
Conclusion
The study is the first systematic study on CDI in Shandong Province, China. Our findings highlight the importance of calls for improved efforts in the development of laboratory diagnostic capacity for CDIs in China, including utilizing rational and effective algorithms. Continued regional and national monitoring of CDIs, including molecular epidemiology surveillance, and implementation of comprehensive and systemic control strategies, including antimicrobial steward-ships, are urgently needed in China.
Supplementary material
Table S1.
Antimicrobial resistant breakpoint of six antimicrobial agents used in the study
Acknowledgments
The authors thank Yan Zhao of Zibo Central Hospital for sample collection, Yan Jin and Chun-Hong Shao of the Provincial Hospital Affiliated to Shandong University for the technical support and Fanrong Kong of Westmead Hospital and Ying-Chun Xu of Peking Union Medical College Hospital for critically reviewing the manuscript. This study was financially supported by a Natural Science Foundation of China (grant number 81501807) and a PUMCH Science Fund for Junior Faculty (grant number pumch-2016-1.2).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect. 2008;14(11):1057–1064. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 3.Jones AM, Kuijper EJ, Wilcox MH. Clostridium difficile: A European perspective. J Infect. 2013;66(2):115–128. doi: 10.1016/j.jinf.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Lessa FC, Winston LG, McDonald LC, Emerging Infections Program CdST Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–2370. doi: 10.1056/NEJMc1505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths D, Fawley W, Kachrimanidou M, et al. Multilocus sequence typing of Clostridium difficile. J Clin Microbiol. 2010;48(3):770–778. doi: 10.1128/JCM.01796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin D, Luo Y, Huang C, et al. Molecular epidemiology of Clostridium difficile infection in hospitalized patients in eastern China. J Clin Microbiol. 2017;55(3):801–810. doi: 10.1128/JCM.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JW, Xiao M, Kudinha T, et al. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolates from a university teaching hospital in China. Front Microbiol. 2016;7:1621. doi: 10.3389/fmicb.2016.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng JW, Xiao M, Kudinha T, et al. The first two Clostridium difficile ribotype 027/ST1 isolates identified in Beijing, China—an emerging problem or a neglected threat? Sci Rep. 2016;6:18834. doi: 10.1038/srep18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight DR, Elliott B, Chang BJ, Perkins TT, Riley TV. Diversity and evolution in the genome of Clostridium difficile. Clin Microbiol Rev. 2015;28(3):721–741. doi: 10.1128/CMR.00127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott B, Androga GO, Knight DR, Riley TV. Clostridium difficile infection: evolution, phylogeny and molecular epidemiology. Infect Genet Evol. 2017;49:1–11. doi: 10.1016/j.meegid.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Chen YB, Gu SL, Wei ZQ, et al. Molecular epidemiology of Clostridium difficile in a tertiary hospital of China. J Med Microbiol. 2014;63(Pt 4):562–569. doi: 10.1099/jmm.0.068668-0. [DOI] [PubMed] [Google Scholar]
- 13.Lv Z, Peng GL, Su JR. Factors associated with Clostridium difficile diarrhea in a hospital in Beijing, China. Braz J Med Biol Res. 2014;47(12):1085–1090. doi: 10.1590/1414-431X20143520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, Wu S, Huang H, et al. Toxin profiles, PCR ribotypes and resistance patterns of Clostridium difficile: a multicentre study in China, 2012–2013. Int J Antimicrob Agents. 2016;48(6):736–739. doi: 10.1016/j.ijantimicag.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Lidan C, Linhai L, Yang L, Zhaohui S, Xiaoyan H, Yuling S. Molecular characterization and antimicrobial susceptibility of tcdA-negative Clostridium difficile isolates from Guangzhou, China. Diagn Microbiol Infect Dis. 2016;84(4):361–365. doi: 10.1016/j.diagmicrobio.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Kato N, Katow S, Maegawa T, Nakamura S, Lyerly DM. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol Lett. 1999;175(2):197–203. doi: 10.1111/j.1574-6968.1999.tb13620.x. [DOI] [PubMed] [Google Scholar]
- 17.Curry SR, Marsh JW, Muto CA, O’Leary MM, Pasculle AW, Harrison LH. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol. 2007;45(1):215–221. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indra A, Huhulescu S, Schneeweis M, et al. Characterization of Clostridium difficile isolates using capillary gel electrophoresis-based PCR ribotyping. J Med Microbiol. 2008;57(Pt 11):1377–1382. doi: 10.1099/jmm.0.47714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI) M11-A8. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard—Eighth Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI) M100. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 21.Huang H, Wu S, Wang M, et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents. 2009;33(4):339–342. doi: 10.1016/j.ijantimicag.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Drudy D, Quinn T, O’Mahony R, KynAe L, O’Gaora P, Fanning S. High-level resistance to moxifloxacin and gatifloxacin associated with a novel mutation in gyrB in toxin-A-negative, toxin-B-positive Clostridium difficile. J Antimicrob Chemother. 2006;58(6):1264–1267. doi: 10.1093/jac/dkl398. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 24.Crobach MJ, Planche T, Eckert C, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016;22(Suppl 4):S63–S81. doi: 10.1016/j.cmi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Leffler DA, Lamont JT. Clostridium difficile infection. New Engl J Med. 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 26.Cheng JW, Xiao M, Kudinha T, et al. The role of glutamate dehydrogenase (GDH) testing assay in the diagnosis of Clostridium difficile infections: a high sensitive screening test and an essential step in the proposed laboratory diagnosis workflow for developing countries like China. PloS One. 2015;10(12):e0144604. doi: 10.1371/journal.pone.0144604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galaydick J, Xu Y, Sun L, et al. Seek and you shall find: prevalence of Clostridium difficile in Wuhan, China. Am J Infect Control. 2015;43(3):301–302. doi: 10.1016/j.ajic.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Chen HX, Song LJ, Shen YY, Luo YP. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolated from the Chinese People’s Liberation Army General Hospital in China. Int J Infect Dis. 2018;67:86–91. doi: 10.1016/j.ijid.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Du P, Cao B, Wang J, et al. Sequence variation in tcdA and tcdB of Clostridium difficile: ST37 with truncated tcdA is a potential epidemic strain in China. J Clin Microbiol. 2014;52(9):3264–3270. doi: 10.1128/JCM.03487-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholas A, Kim YK, Lee WK, et al. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolates from two Korean hospitals. PloS One. 2017;12(3):e0174716. doi: 10.1371/journal.pone.0174716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwata Y, Tanimoto S, Sawabe E, et al. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolated from a university teaching hospital in Japan. Eur J Clin Microbiol Infect Dis. 2015;34(4):763–772. doi: 10.1007/s10096-014-2290-9. [DOI] [PubMed] [Google Scholar]
- 32.Davies KA, Ashwin H, Longshaw CM, et al. EUCLID Study Group Diversity of Clostridium difficile PCR ribotypes in Europe: results from the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID), 2012 and 2013. Euro Surveill. 2016;21(29) doi: 10.2807/1560-7917.ES.2016.21.29.30294. [DOI] [PubMed] [Google Scholar]
- 33.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Cai L, Yu R, Huang W, Zong Z. ICU-onset Clostridium difficile infection in a university hospital in China: a prospective cohort study. PloS One. 2014;9(11):e111735. doi: 10.1371/journal.pone.0111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Zhou Y, Wang Z, Xie S. Identification of Clostridium difficile ribotype 027 for the first time in mainland China. Infect Control Hosp Epidemiol. 2014;35(1):95–98. doi: 10.1086/674405. [DOI] [PubMed] [Google Scholar]
- 36.Jia H, Du P, Yang H, et al. Nosocomial transmission of Clostridium difficile ribotype 027 in a Chinese hospital, 2012–2014 traced by whole genome sequencing. BMC Genomics. 2016;17:405. doi: 10.1186/s12864-016-2708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krutova M, Nyc O, Matejkova J, Kuijper EJ, Jalava J, Mentula S. The recognition and characterisation of Finnish Clostridium difficile isolates resembling PCR-ribotype 027. J Microbiol Immunol Infect. 2017 doi: 10.1016/j.jmii.2017.02.002. pii:S1684-1182(17)30051-8. [DOI] [PubMed] [Google Scholar]
- 38.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis. 2017;17(4):411–421. doi: 10.1016/S1473-3099(16)30514-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian TT, Zhao JH, Yang J, et al. Molecular characterization of Clostridium difficile isolates from human subjects and the environment. PloS One. 2016;11(3):e0151964. doi: 10.1371/journal.pone.0151964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan J, Liang J, Lv T, et al. Epidemiology of Clostridium difficile in a county level hospital in China. Jundishapur J Microbiol. 2017;10(6):e14376. [Google Scholar]
- 41.Zhou FF, Wu S, Klena JD, Huang HH. Clinical characteristics of Clostridium difficile infection in hospitalized patients with antibiotic-associated diarrhea in a university hospital in China. Eur J Clin Microbiol Infect Dis. 2014;33(10):1773–1779. doi: 10.1007/s10096-014-2132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu S, Zhang L, Zhang C, Chen X, Chen Q, Li Z. Comparison of polymerase chain reaction ribotyping, toxinotyping and nutritional aspects of toxin production of Clostridium difficile strains. Biomed Rep. 2014;2(4):477–480. doi: 10.3892/br.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang WJ, Jing DZ, Luo Y, et al. Clostridium difficile carriage in hospitalized cancer patients: a prospective investigation in eastern China. BMC Infect Dis. 2014;14:523. doi: 10.1186/1471-2334-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkey PM, Marriott C, Liu WE, et al. Molecular epidemiology of Clostridium difficile infection in a major Chinese hospital: an underrecognized problem in Asia? J Clin Microbiol. 2013;51(10):3308–3313. doi: 10.1128/JCM.00587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Antimicrobial resistant breakpoint of six antimicrobial agents used in the study