Abstract
Pulmonary embolism (PE) is a potentially fatal disease with a broad range of treatment options that spans multiple specialties. The rapid evolution and expansion of novel therapies to treat PE make it a disease process that is well suited to a multidisciplinary approach. In order to facilitate a rapid, robust response to the diagnosis of PE, some hospitals have established multidisciplinary pulmonary embolism response teams (PERTs). The PERT model is based on existing multidisciplinary teams such as heart teams and rapid response teams. A PERT is composed of clinicians from the range of specialties involved in the treatment of PE, including pulmonology critical care, interventional radiology, cardiology, and cardiothoracic surgery among others. A PERT is a 24/7 consult service that is able to provide expert advice on the initial management of PE patients and convene in real time to develop a consensus treatment plan specifically tailored to the needs of a particular patient and consistent with the capabilities of the institution. In this review, we discuss the rationale for establishing a PERT and its potential benefits. We discuss considerations in forming a PERT and present case studies of several PERTs currently in operation at different institutions. We also discuss potential difficulties in forming a PERT and review evidence that has been generated by some of the PERTs that have been in operation the longest.
Keywords: pulmonary embolism, pulmonary embolism response team, thrombosis, thrombolysis, venous thromboembolism
Introduction
Pulmonary embolism (PE) is a complex disease process with high rates of morbidity and mortality.1–3 The management of PE is complicated by a broad range of possible presentations and treatment decisions. Unlike other cardiovascular diseases, management options for PE lack a strong body of supporting evidence and definitive societal guidelines, especially when it comes to the most cutting-edge endovascular options. Thus, at present, the initial care of patients with PE can vary dramatically by the treating specialist.
Patients with PE can present to a wide variety of clinical settings, ranging from the outpatient clinic to the emergency department to de novo on the inpatient wards. Clinical signs and symptoms of PE are often nonspecific, varying from protean symptoms such as dyspnea or syncope to respiratory failure, right ventricular (RV) failure, or life-threatening shock. Diagnostic tools differ in their efficacy. While computed tomography angiography (CTA) has become widely adopted and offers high sensitivity and specificity, its applicability is limited in patients with kidney dysfunction and in patients with hemodynamic instability (who may require bedside testing). There is an ongoing clinical debate regarding what role ventilation/perfusion scanning should play in the diagnosis of PE.4,5 Ventilation/perfusion scans have a high sensitivity and specificity for PE and can be performed in patents with kidney dysfunction.6,7 However, clinicians are becoming less familiar with interpreting results since CTA has become so dominant.8 Echocardiography is also a vital tool for PE risk stratification but as a diagnostic test lacks sensitivity.9–11 Taken together, patients with PE can often present diagnostic dilemmas to frontline clinicians.
Many providers consult specialists at the moment they suspect the diagnosis of PE. Many more consult specialists once the diagnosis is made. However, the referral can be directed to any of pulmonary, cardiology, hematology, critical care, interventional radiology, or vascular surgery services depending on local availability and referral patterns. While each specialty is able to contribute an important expertise and perspective, in isolation treatment biases can become evident. If intervention is appropriate, the consulted specialist will be most familiar with the procedures of their particular specialty; however, a silo mentality may prevent alternative treatment options from being discussed or fully considered. In practice, serial consultations can be encountered, which may consume precious time and also still leave the clinical team without a clear, consensus treatment plan.
Moreover, treatment options have expanded drastically in recent years, with varying degrees of invasiveness and efficacy.12 Current considerations include anticoagulants and systemic intravenous thrombolysis, catheter-directed therapies (CDTs) such as intra-arterial thrombolysis and catheter-directed clot maceration, mechanical- and ultrasound-facilitated fragmentation, percutaneous embolectomy, and more aggressive measures such as surgical embolectomy;13,14 in addition, hemodynamic support strategies like venoarterial extracorporeal membrane oxygenation (VA ECMO) need to be evaluated in select cases.15 For patients who fall in the intermediate or intermediate high-risk categories, multisociety guidelines are generally “class II” level of recommendations (leaving the clinician with guidance of the type “may be considered”).16–18 Without a strong supporting body of primary evidence demonstrating the superiority of any one treatment, therapeutic dilemmas are frequently encountered in complex PE cases. This observation argues that complex multispecialty decisions should be made not by a limited number of clinicians but rather by multidisciplinary consensus benefiting from the expertise and perspective of multiple physicians.14 Moreover, not all specialties may be experienced in all interventional techniques, so having multiple specialties involved in the decision-making can have a positive impact on deciding which interventional treatment techniques should be used for any patient.
In order to improve the rapid recognition of PE and offer customized therapy, some institutions have created multidisciplinary pulmonary embolism response teams (PERTs). A PERT is composed of providers from a broad range of specialties providing diagnostic and therapeutic expertise in PE management. Team members may include representatives from pulmonary, cardiology, critical care, interventional radiology, vascular surgery, cardiac surgery, diagnostic radiology, noninvasive cardiology (such as echocardiography), and hematology. The PERT can be activated by any clinician who encounters a patient with PE and then be rapidly mobilized to create a consensus treatment plan tailored to the specifics of the patient’s case.
The PERT model is similar in concept to several existing models of multidisciplinary response teams where the goal is to rapidly recognize a life-threatening condition and offer a consensus-based treatment plan balancing the risks and benefits of intervention.12 One such example is the heart team concept in cardiology. Heart teams bring a collaborative and multidisciplinary approach to the treatment of extremely complex cardiac patients where current guidelines may not offer clear answers or may not be supported by rigorous data such as randomized controlled trials. This collaborative team-based structure allows multiple specialists to work together to decide on the optimal interventional therapy, for example for complicated cases such as aortic stenosis or complex coronary revascularization.19 Rapid response teams (RRT) provide an urgent and protocolized response to triage and stabilize acutely ill and rapidly decompensating patients within the hospital.12 These teams are required to respond within a set, short time period and must be available 24/7, and have been shown to decrease mortality in certain conditions, such as cardiac arrest.20–22
In recognition of the high morbidity and mortality of stroke and myocardial infarction (MI), it has become routine for centers to have a system for activating the team that is responsible for these emergencies.23–25 There are a vast number of resources that must be swiftly mobilized to properly manage these emergencies. Strokes require a rapid computed tomography (CT) scan, rapid neurologic evaluation, and considerations of systemic intravenous thrombolysis administration versus treatment with interventional neuroradiology. MI also requires rapid recognition and diagnoses via electrocardiogram (ECG) or biomarkers and rapid activation of the cardiac catheterization laboratory. Based on the data generated from these teams, quality standards, such as door-to-balloon time, have been set for these diseases. These standards, by expediting recognition and intervention, have been associated with an improvement in outcomes for patients with MI and stroke. In similar manner, we anticipate that the deployment of PERT will lead to similar quality standards for PE recognition and treatment.
Since similar approaches are already well established for the two leading causes of cardiovascular mortality, namely, MI and stroke, it seems a natural progression to use aspects of this method for the third leading cause of cardiovascular mortality, ie, PE.26 Several aspects of PE make it an ideal target for a combination of both types of multidisciplinary teams. The diagnostic challenges involved and the potential for rapid hemodynamic decompensation demand a rapid and effective evaluation. The PERT can create a protocolized response that fits the capabilities of its particular institution. The therapeutic challenges, with variable options and risk–benefit profiles, and the need for input from a host of diverse specialties demand a customized approach to individual patients. As a disease that spans multiple specialties, a multidisciplinary approach has the potential to benefit PE patients.
Considerations when forming a PERT
A successful PERT requires extensive planning and coordination before the team is ready to consult on its first patient. Importantly, there is no single prescribed method or structure that all PERTs must mirror. While the ideal PERT would include many different specialties, the simplest iteration of a PERT requires only an “afferent limb” and an “efferent limb”.12 The afferent limb receives the consultation and clinically evaluates the patient, while the efferent limb performs the invasive procedure to treat the PE. It is critical that no matter what specialties fill these roles, they must be dedicated to providing consistent and timely service and willing to take the extra time to engage in multidisciplinary decision-making; in many institutions, both of these roles may be filled by the same specialty or even the same clinician. Additional specialties should be integrated into the PERT depending on local availability and interest. In addition, it can be very helpful in the early phases of a new PERT to feature a small number of enthusiastic providers or “champions” from each participating specialty. Over time, representatives from other disciplines open to multidisciplinary collaboration and excited about the PERT concept will be necessary for growth.
Before the first consult, PERT infrastructure must be clearly established. The PERT must decide who will be responsible for patient assessment and subsequent presentation to the team. Depending on the institution, this may be a trainee, a fellow, or an attending physician on a rotating schedule. This designated team member will respond to the bedside, confer with the referring clinician, and evaluate the patient as well as relevant laboratories and imaging. A coordinated timely response is critical to the PERT success, so it is of the utmost importance to create a method of rapidly and reliably activating the PERT. Some centers page the “afferent limb” directly, as pagers are inexpensive and reliable, while others rely on an in-house or outsourced call center to activate the service.14 A consult to the PERT team will trigger a response from the PERT clinician who will assess the patient and may also simultaneously alert all team members of the potential consult if certain criteria are met.
The institution must also establish how the full PERT will convene to discuss the case and form a consensus treatment plan. Some centers prefer to meet in person, but, often due to logistical constraints, it may be more feasible to have the PERT meeting occur in a virtual setting utilizing conference calls or Health Insurance Portability and Accountability Act (HIPAA) compliant software such as GoToMeeting (Log-MeIn Inc., Boston, MA, USA).
Following the PERT meeting, the evaluating clinician will be responsible to relate the consensus plan to the referring clinician and mobilize appropriate resources (such as the interventional suite or the operating room) if intervention is deemed necessary. Ideally all of these events should occur within 90 minutes of receipt of the consult.14
Another critical component of the establishment of the PERT is the informational campaign to accompany its launch. The PERT’s effectiveness depends on identifying and capturing all appropriate consults. As such, the PERT must disperse a list of activation criteria (Box 1) and a protocol for intervention in order to help make providers aware of PERT capabilities and expectations. Posters and pocket cards can be placed in care units throughout the hospital explaining the PERT activation criteria and displaying the activation number (Figure 1). Outreach can also be made during the rollout by speaking at departmental conferences and grand rounds and through nursing education. Special emphasis should be placed on outreach to Emergency Medicine and Intensive Care because a large proportion of PEs are first recognized by these clinicians.
Box 1. NYU Langone Health PERT activation criteria.
| PERT activation criteria |
|---|
| Symptomatic PE with |
| moderate to severe dyspnea |
| hemoptysis |
| moderate to severe chest pain |
| tachyarrhythmia |
| new oxygen requirement |
| serum troponin > upper limits of normal |
| echocardiography with RV dilation and/or hypokinesis |
| CTA with signs of RV volume or pressure overload (rightward shift of |
| intraventricular septum, reflux of contrast into the IVC, RV/LV diameter >1.0) |
Note: Courtesy of NYU Langone Health PERT.
Abbreviations: NYU, New York University; PERT, pulmonary embolism response team; PE, pulmonary embolus; RV, right ventricular; CTA, computed tomography angiography; IVC, inferior vena cava; LV, left ventricular.
Figure 1.
Screensaver slide displayed on computers throughout NYU Langone Medical Center.
Note: Courtesy of NYU Langone Medical Center PERT.
Abbreviations: NYU, New York University; CTA, computed tomography angiography; RV, right ventricle; Tn, troponin; IP, in person; PERT, pulmonary embolism response team.
In creating a PERT, the institution should also consider the value of a follow-up mechanism to ensure the long-term success of a PERT. A follow-up clinic centralizes the posthospitalization care of PE patients and thus can be staffed by interventionalists, pulmonary and cardiology physicians, and hematologists, as appropriate to the specific patient.27 A centralized follow-up augments the ability to study both treatment successes and poor outcomes.
PERT case studies
To be successful as a consult service, the PERT must be easily activated and appropriately utilized. Existing models at the Massachusetts General Hospital (MGH), New York University (NYU) Langone Health, Cedars-Sinai Medical Center, Vanderbilt University Medical Center, and Ellis Hospital highlight how a PERT can operate effectively with a range of team members and methods.
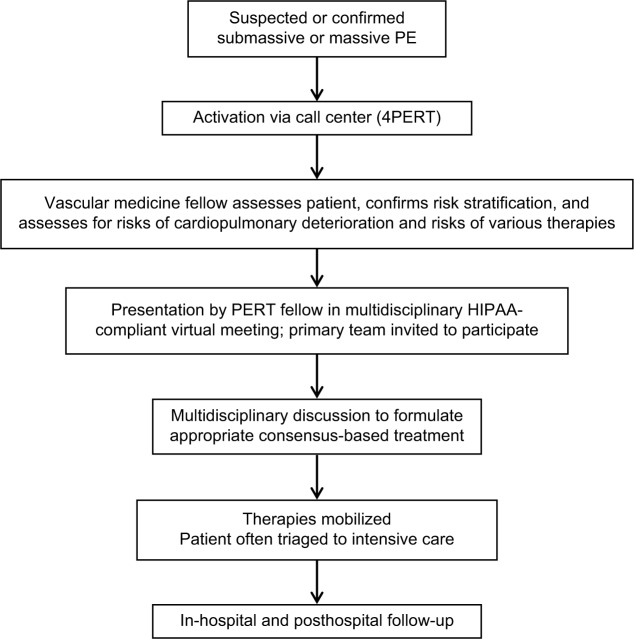
The MGH PERT was launched in October 2012 and leverages the expertise of the Vascular Medicine and Intervention clinicians and fellows.28 Vascular medicine and intervention fellows, who form the backbone of the MGH PERT, bring a unique and highly specialized diagnostic and technical expertise to the frontline of PE care. These fellows are generally Post Graduate Year - 8 trainees, having completed training in cardiovascular medicine and interventional cardiology. MGH PERT has accepted consults for suspected PE (eg, patients presenting with protean cardiopulmonary symptoms or undifferentiated shock) or PE of any severity; thus, one of the fellows’ key roles is to confirm the diagnosis of PE and confirm PE-risk stratification (into low risk, intermediate risk, or high risk; Figure 2). The PERT fellow also performs targeted evaluation of bleeding risks, candidacy for interventional therapies, and patient preferences and wishes. Low-risk PE patients for whom the treatment will be anticoagulation are not always presented to the entire PERT, but most intermediate-risk and high-risk PE patient cases are presented to the PERT via a conference call in a closed, HIPAA-compliant meeting environment (GoToMeeting). The primary clinical team and referring clinician are invited to participate in this discussion and call. PERT members are invited to this call by a group page from the call center. During this discussion, the patient’s case is presented and via online software laboratory values, ECG, CT, and echocardiography images can be visualized by all members and discussed. The PERT reviews treatment options and achieves consensus and then actualizes the treatment program. For many of the potential interventions (eg, CDTs), the vascular medicine and intervention fellow who evaluated the patient will be also the physician performing the treatment. The PERT follows the patient throughout the hospitalization.
Figure 2.
Activation flow chart of MGH’s PERT.
Note: Courtesy of MGH.
Abbreviations: MGH, Massachusetts General Hospital; PERT, pulmonary embolism response team; PE, pulmonary embolus; HIPAA, Health Insurance Portability and Accountability Act.
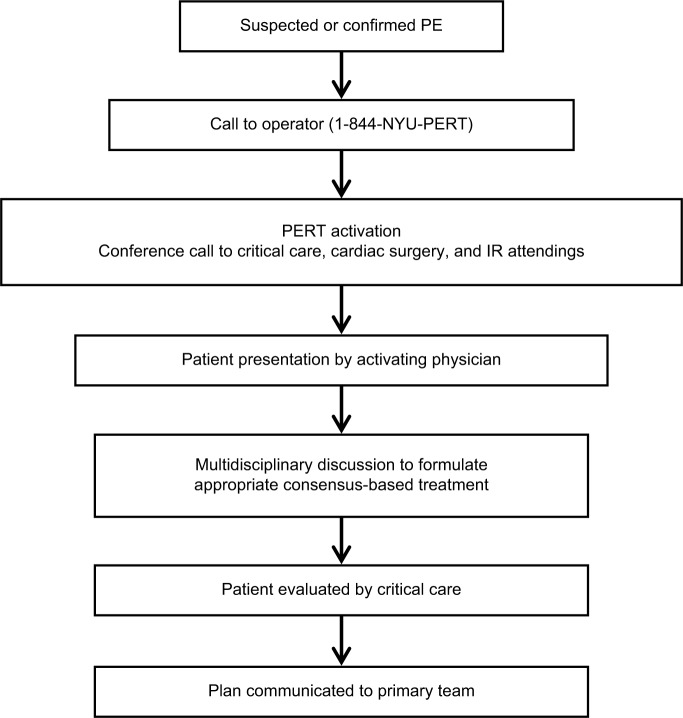
The NYU PERT has uniquely focused on early activation of the entire team for all consults. Referring clinicians call the 24/7 call center at 4PERT (or 844-NYU PERT), and an operator uses an application called Send Word Now (SWN Communications Inc., Secaucus, NJ, USA) that automatically text messages and calls the interventional radiology (IR) attending, critical care attending, and cardiac surgery attending on call. This allows the referring clinician to present the case directly to all the specialists and get rapid initial feedback. The critical care intensivist then assesses the patient in person and convenes again with the multidisciplinary team when necessary (Figure 3).
Figure 3.
Activation flow chart of NYU’s PERT.
Note: Courtesy of NYU’s PERT.
Abbreviations: NYU, New York University; PERT, pulmonary embolism response team; PE, pulmonary embolus; IR, interventional radiology.
Cedars-Sinai Medical Center’s PERT members include clinicians from pulmonary and critical care, interventional cardiology, interventional radiology, and cardiac surgery. The PERT is activated by dialing 3CLOT from any hospital phone. The provider is immediately put in contact with the pulmonary critical care fellow. The patient is then seen by a pulmonary critical care fellow and an attending. If a decision is made that the patient is likely to need an intervention, an interventionist or surgeon is notified. The PERT surgeon is skilled in endovascular procedures, ECMO, and mechanical circulatory support. This allows a broad spectrum of aggressive treatment options with clot retrieval devices including FlowTriever® (Inari Medical Inc, Irvine, CA, USA) and AngioVac® (Vortex Medical Inc, Marlborough, MA, USA) as well as CDT even for high-risk patients. Cedars-Sinai Medical Center has a robust and active mechanical circulatory support program and specifically rapid access to ECMO. This allows their PERT to readily deploy ECMO as a bridge to intervention or as backup if a patient decompensates.
In order to educate colleagues about the PERT’s capabilities and encourage them to utilize the PERT as a resource, the Cedars-Sinai Medical Center’s PERT has been conducting grand rounds and lectures around the hospital and is soon to launch a hospital wide screen saver reminding providers to activate for high-risk PE and complex PE-related decisions. The team is also soon launching a continuing medical education accredited multidisciplinary conference, which will be a forum to discuss complex cases, review recent literature, and educate trainees.
Vanderbilt University operates a multidisciplinary PERT at their institution as the keystone of an Acute PE Network. The Vanderbilt University’s PERT is composed of providers from the fields of cardiac surgery, interventional cardiology, radiology, vascular medicine, and critical care anesthesia. A provider within the hospital who suspects a patient has a PE can place a PERT consult via the electronic medical record system. This will then trigger a page to either the on-call cardiology fellow or the fellow of cardiovascular intensive care unit (CVICU), both of whom are in-house 24/7. The fellow will respond to the bedside, assess the patient, order the appropriate laboratory tests and imaging studies and then contact the on-call PERT leader, either an attending cardiac surgeon or interventional cardiologist. After all the relevant data are reviewed and discussed, the treatment best suited to the particular patient’s needs is provided (Figure 4).29
Figure 4.
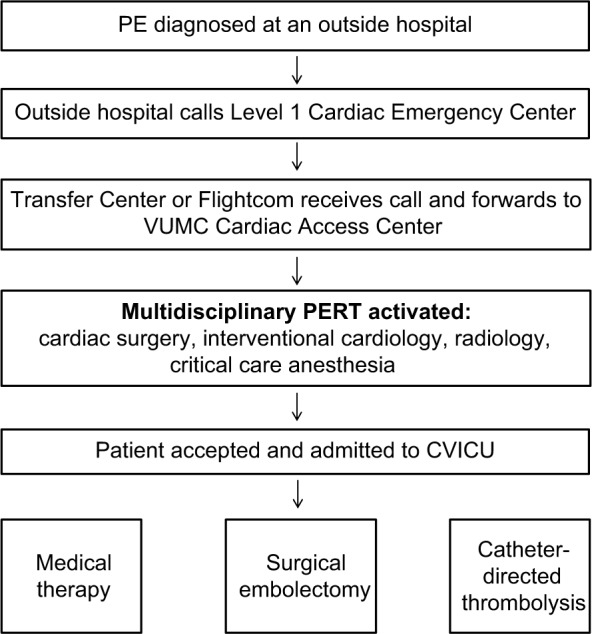
Activation flow chart of Vanderbilt University’s PERT.
Abbreviations: PERT, pulmonary embolism response team; PE, pulmonary embolism; VUMC, Vanderbilt University Medical Center; CVICU, Cardiovascular Intensive Care Unit.
One interesting aspect of Vanderbilt University’s PERT model is the way they have extended the reach of their PERT beyond the doors of their own institution through the establishment of an Acute PE Network. In 2012, Vanderbilt University established the Acute PE Network utilizing the same referral infrastructure that the institution already had in place for ST elevation myocardial infarction (STEMI) referrals, the Vanderbilt Level 1 Emergency Cardiac System. With a single phone call, a referring physician at an outside hospital can mobilize Vanderbilt University’s PERT and Vanderbilt University’s emergency medical services (EMS) to rapidly retrieve and transport the patient back to Vanderbilt University for PERT evaluation and definitive treatment. The Vanderbilt University’s network emphasizes early mobilization and rapid transfer of the patient, so that valuable time is not lost determining the patient’s PE risk class prior to transfer. After activation, the Vanderbilt University’s PERT leader will obtain an initial history from the referring physician and provide guidance on the initial management prior to the transfer.29 As telemedicine technology has improved and the PERT model has become more common, it is expected that interfacility PERT consultations and referrals will become more common as well.28
Although the PERTs detailed earlier all operate at large academic centers, there are currently PERTs in operation at smaller, nonacademic centers as well. The PERT at Ellis Hospital in Schenectady, NY, is an example of such a team. Their PERT operates in a manner very similar to PERTs at major academic medical centers, with participants from pulmonary critical care, emergency medicine, noninvasive cardiology, interventional cardiology, and cardiothoracic surgery. The team is activated when any clinician pages a PERT consult via the hospital switchboard. Pulmonary/critical care takes the initial call and evaluates that patient before presenting the case to the rest of the team for the formation of a consensus treatment plan.
Potential difficulties in forming and maintaining a PERT
There are several potential difficulties that should be anticipated when a PERT is being formed. While there are possible benefits, clinical evidence in support of routine PERT implementation is still in its infancy. Data proving the PERT model’s efficacy in terms of optimized outcomes, as well as cost-effectiveness data in terms of better outcomes despite a possible higher utilization of testing and novel diagnostics, will hopefully be forthcoming in the near future. As such, garnering institutional support may pose a barrier, and it may be a useful strategy to focus on the potential process, quality improvement, and safety aspects of PERTs.
As with any new endeavor, it is important to recognize that there may be “naysayers” resistant to the idea of changes in practice. It is essential to frame the establishment of a PERT in terms of the benefit to both the patient and the providers. The patient benefits from receiving an individually tailored, expedited, evidenced-based treatment plan formed by a consensus of expert opinion. The providers benefit through the acquisition of expertise gained through interdisciplinary collaboration and the increased exposure to PE as a disease process. Although there is not yet a robust body of evidence for the PERT model, the early results of the experience of some of the longer-standing teams are very promising.17,30 This early data may be helpful in persuading colleagues about the benefits of the PERT model.
Physicians joining a PERT will have to adjust to a new collaborative model of care delivery. Specialists are accustomed to deciding on a treatment plan within their discipline and proceeding down that pathway. For example, a recently published survey of treatment preferences demonstrated that endovascular physicians are more likely to use CDT than their medical colleagues.31 These biases may cause some providers to worry that establishing a PERT will lead to overuse of novel or invasive therapies; however, data published by the PERT at MGH show that of their first 350 consults, only 9.6% underwent advances in percutaneous therapy and 4.1% underwent surgical pulmonary embolectomy.12 In the collaborative model of a PERT, there will be times where the team reaches a consensus that may differ from a particular specialist’s recommended course of treatment. Such cases still optimally require a unified team approach and management strategy to present a consensus opinion to the referring clinician.
It should also be noted that because PE can arise at any time, PERT activation can occur anytime, including nights and weekends. As such, physicians recruited to join a PERT commit to some additional 24-hour call responsibilities. Reimbursement for such time may also need to be addressed locally. Moreover, the multidisciplinary nature of the PERT model negates the need for serial consultations by specialists. This means that in a fee-for-service model, some specialists may be unable to bill for their consultation, potentially leading to uncompensated effort for PERT physicians. Reimbursement models for heart teams are evolving in general; however, the PERT model will fit well into a capitated payment system, especially if evidence reveals that PERTs reduce morbidity and mortality and lead to shorter lengths of stay.12
Once a PERT is operational, steps should be taken to demonstrate the service’s value and to ensure its longevity. Like much of PE care, the evidence base for PERTs is still lacking. PERTs can help justify their mission by evaluating PE patients while collecting data on demographics, diagnostics, treatments, and outcomes in an effort to address the gap in clinical experience in modern PE therapeutics. Forming an institutional registry of PERT consultations can yield valuable data for both clinical research and institutional quality improvement efforts.12,29
PERTs in operation
Although the PERT model is relatively new, programs that have been in operation the longest are starting to publish promising data on the benefits to patients. The PERT program at MGH reported on the first 30 months that their team was in operation.17 The MGH PERT was activated 394 times during this time period, 314 of which were for a confirmed PE, of which 46% were intermediate risk (submassive) and 26% were high risk (massive). PERT activations at MGH increased by 16% every 6 months in this period, and in its fifth year, MGH PERT has treated more than 800 patients (unpublished data). The MGH 30-month experience showed that as time progressed, while the PERT was activated more frequently for both low-risk and intermediate-risk PE patients, the team was also consulted for complex and unstable patients without confirmed PE, suggesting that referring physicians perceived a benefit from an expert consultant’s input and/or procedural expertise. The most common treatment administered was anti-coagulation alone (~70%), though ~9% of patients received a CDT. Bleeding event rates were similar between patients treated with either anticoagulation or catheter-directed thrombolysis (4% at 7 days, 10–11% at 30 days). All-cause 30-day mortality was 12% among all patients with confirmed PE and 25% among those patients with the highest-risk PE.
The Weill Cornell Medicine’s PERT recently described its first 20 months of experience treating submassive and massive PE. In all, 87 patients were identified, and activations occurred twice as commonly in the last 10 months compared with the first 10 months. A total of 25 patients received CDT, six received systemic thrombolysis (ST), and the rest received anti-coagulation alone. The in-hospital mortality in this cohort was 13.7%, mostly in patients with metastatic cancer. BNP level >100 pg/mL was the only variable that was associated with an increased length of stay; CDT, hemodynamic factors, and the simplified Pulmonary Embolism Severity Index (sPESI) score were not associated with an increased length of stay.30
More prospective data are needed to quantitatively determine whether PERTs add value to the current standard of care for PE patients.
PERT Consortium
The PERT Consortium32 is an organization dedicated to advancing the clinical, educational, and research infrastructure for PE care. The PERT Consortium membership includes physicians, nurses, and pharmacists from >80 different institutions across the US and globally, who have active PERTs. Its goals are to help promote the creation of additional PERTs across the world and improve PE clinical care. The PERT Consortium also aims to expand the current body of literature available on PE by creating a national registry and sponsoring clinical trials. Finally, the PERT Consortium aims to educate the general public to help increase PE awareness. The PERT Consortium holds an annual conference where research on PE treatment and PERT cases are presented and serves as a resource for physicians looking to establish a PERT at their own institution.
Conclusion
The PERT model has emerged as an innovative and collaborative model for providing better and more coordinated care to PE patients. The establishment of an institutional PERT requires collaborators from the range of disciplines involved in the treatment of PE. Considerable investments to educate referring clinicians about this new resource are necessary prior to launch at an institution. Successful PERT operation also depends on the continuous motivation and cross-disciplinary education of PERT members, as well as the collection of data to evaluate the value of the multidisciplinary team as a consult service.
Acknowledgments
The authors wish to acknowledge Tyler L Bloomer, MD, and Pete P Fong, MD, of Vanderbilt University Medical Center for granting us permission to use images relating to the Vanderbilt University’s PERT and Acute PE Network and for providing information on their PERT. The authors also wish to acknowledge Dr Saddam Abisse, MD, for providing information on Ellis Hospital’s PERT.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358(10):1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 3.Alikhan R, Peters F, Wilmott R, Cohen AT. Fatal pulmonary embolism in hospitalised patients: a necropsy review. J Clin Pathol. 2004;57(12):1254. doi: 10.1136/jcp.2003.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Beek EJR. Should lung scan be abandoned for pulmonary embolism diagnosis in the age of multislice spiral CT? Yes. Intern Emerg Med. 2009;4(3):189–191. doi: 10.1007/s11739-009-0252-5. [DOI] [PubMed] [Google Scholar]
- 5.Miniati M, Monti S. Should lung scan be abandoned for pulmonary embolism diagnosis in the age of multislice spiral CT? No. Intern Emerg Med. 2009;4(3):193–194. doi: 10.1007/s11739-009-0251-6. [DOI] [PubMed] [Google Scholar]
- 6.Habib G, Nashashibi M, Gips S. The predictive value of ventilation-perfusion scanning for the diagnosis of pulmonary embolism in patients with impaired renal function. Isr Med Assoc J. 2014;16(4):239–243. [PubMed] [Google Scholar]
- 7.Miniati M, Sostman HD, Gottschalk A, Monti S, Pistolesi M. Perfusion lung scintigraphy for the diagnosis of pulmonary embolism: a reappraisal and review of the prospective investigative study of acute pulmonary embolism diagnosis methods. Semin Nucl Med. 2008;38(6):450–461. doi: 10.1053/j.semnuclmed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Mroczenski AA, Berent SM, Hall AA, Hung JC, Herold TJ, Mullan BP. Trends of radiopharmaceutical use at Mayo Clinic Rochester. J Nucl Med Technol. 2007;35(3):154–158. doi: 10.2967/jnmt.106.038992. [DOI] [PubMed] [Google Scholar]
- 9.Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017;10(2):e004345. doi: 10.1161/CIRCINTERVENTIONS.116.004345. [DOI] [PubMed] [Google Scholar]
- 10.Vaid U, Singer E, Marhefka GD, Kraft W, Baram M. Poor positive predictive value of McConnell’s Sign on transthoracic echocardiography for the diagnosis of acute pulmonary embolism. Hosp Pract (1995) 2013;41(3):23–27. doi: 10.3810/hp.2013.08.1065. [DOI] [PubMed] [Google Scholar]
- 11.Dresden S, Mitchell P, Rahimi L, et al. Right ventricular dilatation on bedside echocardiography performed by emergency physicians aids in the diagnosis of pulmonary embolism. Ann Emerg Med. 2014;63(1):16–24. doi: 10.1016/j.annemergmed.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation. 2016;133(1):98–103. doi: 10.1161/CIRCULATIONAHA.115.015086. [DOI] [PubMed] [Google Scholar]
- 13.Walter RJ, Moores LK, Jiménez D. Pulmonary embolism: current and new treatment options. Curr Med Res Opin. 2014;30(10):1975–1989. doi: 10.1185/03007995.2014.936931. [DOI] [PubMed] [Google Scholar]
- 14.Dudzinski DM, Horowitz JM. Start-up, organization and performance of a multidisciplinary pulmonary embolism response team for the diagnosis and treatment of acute pulmonary embolism. Rev Esp Cardiol (Engl Ed) 2017;70(1):9–13. doi: 10.1016/j.rec.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Friedman O, Horowitz JM, Ramzy D. Advanced cardiopulmonary support for pulmonary embolism. Tech Vasc Interv Radiol. 2017;20(3):179–184. doi: 10.1053/j.tvir.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 17.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team. Chest. 2016;150(2):384–393. doi: 10.1016/j.chest.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Zamorano JL, Achenbach S, Baumgartner H, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) endorsed by the European Respiratory Society (ERS) Eur Heart J. 2014;35(43):3033–3073. [Google Scholar]
- 19.Holmes DR, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61(9):903–907. doi: 10.1016/j.jacc.2012.08.1034. [DOI] [PubMed] [Google Scholar]
- 20.Al-Qahtani S, Al-Dorzi HM, Tamim HM, et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41(2):506–517. doi: 10.1097/CCM.0b013e318271440b. [DOI] [PubMed] [Google Scholar]
- 21.Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170(1):18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 22.Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19(1):254. doi: 10.1186/s13054-015-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberts MJ, Chaturvedi S, Graham G, et al. Acute stroke teams. Stroke. 1998;29(11):2318. doi: 10.1161/01.str.29.11.2318. [DOI] [PubMed] [Google Scholar]
- 24.Coons JC, Fera T. Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure. Am J Health Syst Pharm. 2007;64(12):1274. doi: 10.2146/ajhp060281. [DOI] [PubMed] [Google Scholar]
- 25.Yaylali YT. Door-to-needle times in acute myocardial infarction. Asian Cardiovasc Thorac Ann. 2010;18(2):122–126. doi: 10.1177/0218492309338130. [DOI] [PubMed] [Google Scholar]
- 26.Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- 27.Reza N, Dudzinski DM. Pulmonary embolism response teams. Curr Treat Options Cardiovasc Med. 2015;17(6):27. doi: 10.1007/s11936-015-0387-9. [DOI] [PubMed] [Google Scholar]
- 28.Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract (1995) 2014;42(1):31–37. doi: 10.3810/hp.2014.02.1089. [DOI] [PubMed] [Google Scholar]
- 29.Bloomer TL, Thomassee EJ, Fong PP. Acute pulmonary embolism network and multidisciplinary response team approach to treatment. Crit Pathw Cardiol. 2015;14(3):90–96. doi: 10.1097/HPC.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 30.Sista AK, Friedman OA, Dou E, et al. A Pulmonary Embolism, Response Team’s initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med. 2017 Sep;:1. doi: 10.1177/1358863X17730430. Epub. [DOI] [PubMed] [Google Scholar]
- 31.Taslakian B, Chawala D, Sista AK. A survey of submassive pulmonary embolism treatment preferences among medical and endovascular physicians. J Vasc Interv Radiol. 2017;28(12):1693–1699.e2. doi: 10.1016/j.jvir.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Pert Consortium [homepage] [Accessed January 24, 2018]. Available at: http://www.pertconsortium.org.