Abstract
Background
Norquetiapine (N-desalkyl quetiapine, NQ) is an active metabolite of quetiapine with stable pharmacokinetic and pharmacological properties. However, its short half-life is a drawback for clinical applications, and long-acting formulations are required.
Purpose
The objectives of this study were to prepare improved entrapment efficiency NQ freebase microspheres by the solvent evaporation method with poly(d,l-lactic-co-glycolic acid) (PLGA) as a release modulator and to evaluate their physicochemical and in vitro/in vivo release properties.
Methods
NQ freebase PLGA (1:5 w/w) formulations were prepared by the oil-in-water (o/w) emulsion–solvent evaporation method. A solution of the drug and PLGA in 9:1 v/v dichloromethane:ethanol was mixed with 0.2% polyvinyl alcohol and homogenized at 2,800 rpm. The emulsion was stirred for 3 h to dilute and evaporate the solvent. After that, the resulting product was freeze-dried. Drug-loading capacity was measured by the validated RP-HPLC method. The surface morphology of the microspheres was observed by scanning electron microscopy (SEM), and the physicochemical properties were evaluated by differential scanning calorimetry, powder X-ray diffraction, and Fourier-transform infrared spectroscopy particle size distribution. The in vitro dissolution test was performed using a rotary shaking bath at 37°C, with constant shaking at 50 rpm in sink condition.
Results
The NQ freebase microspheres prepared by o/w emulsion-solvent evaporation showed over 30% efficiency. NQ was confirmed to be amorphous in the microspheres by powder X-ray diffraction and differential scanning calorimetry. Special chemical interaction in the microspheres was not observed by FT-IR. The in vitro dissolution test demonstrated that the prepared microspheres’ release properties were maintained for more than 20 days. The in vivo test also confirmed that the particles’ long acting properties were maintained. Therefore, good in vitro–in vivo correlation was established.
Conclusion
In this study, NQ freebase-PLGA microspheres showed potential for the treatment of schizophrenia for long-periods.
Keywords: schizophrenia, freebase, o/w emulsion-solvent evaporation, freeze dry, active metabolite, IVIVC
Introduction
Schizophrenia is defined by the National Institutes of Health (NIH) as a chronic and severe mental disorder that affects how a person thinks, feels, and behaves. Treatment is long term and often subjected to inconsistent dosing regimens, which increases the risk of relapse. High doses and fluctuating drug plasma concentrations associated with ordinary daily treatment may also cause tardive dyskinesia.1 Schizophrenia is commonly treated with oral antipsychotics, due to ease of administration and noninvasiveness of therapy; however, the frequency of medication results in low patient compliance, which limits the scope of schizophrenia treatment.2 A long-acting formulation that maintains consistent drug concentration in plasma and that delivers the lowest effective dose could both prevent the recurrences of psychosis and lower the risk of side effects such as tardive dyskinesia.3
N-desalkyl quetiapine (or norquetiapine, NQ) is a major active metabolite of quetiapine in humans, produced by the isoenzyme CYP3A4 in cytochrome P450.20 Quetiapine was originally developed as a second-generation antipsychotic agent for the treatment of schizophrenia.4 The body recognizes quetiapine as a xenobiotic or a foreign agent and metabolizes it into several compounds, including active metabolite. An active metabolite is a substance that has therapeutic activity.5 Quetiapine has been reported to have pharmacokinetic variability among older adults and in patients using concomitant medications.6 Inductors such as carbamazepine and phenytoin enhance the activity of quetiapine, leading to the formation of NQ, whereas inhibitors such as ketoconazole, itraconazole, erythromycin, and fluvoxamine suppress the activity of que-tiapine.7 Norquetiapine, an active metabolite of quetiapine, is less affected by differences in the degree of metabolism among individuals, such as age, and by other drugs, and it has therapeutic effect. Norquetiapine inhibits the noradrenaline transporter and has partial agonistic activity at 5-HT1A receptors.8 Activity at both these targets is hypothesized to contribute to antidepressant activity. Norquetiapine has a 9–12 h half-life in humans.9 For maintaining constant blood concentration for better schizophrenia treatment, a long-acting formulation of norquetiapine is required. Many antipsychotics have been studied as injectable long-acting formulations. The first injectable long-acting antipsychotic formulation was developed in the 1960s. Currently, some antipsychotic drugs are being developed in a similar manner, such as Abilify Maintena (aripiprazole monohydrate), Zyprexa Relprevv (olanzapine pamoate), and Invega Trinza (paliperidone palmitate), which are available in the US and in the UK.10 Long-acting antipsychotic formulations can avoid the first-pass metabolism and there is certainty of delivery of the therapeutic agents. Further, the long-acting effect is expected to mitigate non-adherence and reduce the risk of relapse in comparison to the oral administration that has low patient compliance.11 The number of days of hospitalization for patients can be reduced. Poly(d,l-lactic-co-glycolic acid) (PLGA) polymers are widely used for controlled delivery of drugs via parenteral route. They have been approved as safe for the human body by the US Food and Drug Administration (FDA) with good biodegradability and biocompatibility.12 Many studies on the PLGA formulation have been conducted, ensuring sustained release of therapeutic agents, ranging from a few weeks to several months. PLGA microspheres with atypical antipsychotics reduced the dosing frequency, leading to measurable increase in adherence to treatment regimens in a schizophrenic patient population.13 The NQ freebase PLGA 502H formulation was chosen to investigate the physiochemical characteristics, in vitro dissolution, in vivo release, and in vitro–in vivo correlation (IVIVC). The aims of this study were to develop long-acting microspheres using PLGA with the advantages of norquetiapine as an active metabolite and to evaluate their long-term release properties for better treatment, continuous management of disease, and patient compliance.
Materials and methods
Materials
Norquetiapine dihydrochloride (NQ⋅2HCl) was purchased from Toronto Research Chemicals, Ltd. (Toronto, Canada) and used for the synthesis of norquetiapine freebase (NQ freebase). Poly(d,l-lactic-co-glycolic acid) and PLGA 502, 502H, 503, and 503H were supplied by Evonik, Ltd. (Essen, Germany). Polyvinyl alcohol 500 (PVA 500) was obtained from OCI Company, Ltd. (Seoul, Republic of Korea). Methanol, dichloromethane, and acetonitrile were of high-performance liquid chromatography (HPLC) grade and were purchased from Honeywell Burdick & Jackson Ltd. (Muskegon, MI, USA). Anhydrous dibasic sodium phosphate was purchased from Georgiachem (Suwanee, GA, USA). Potassium phosphate monobasic was purchased from Daejung Ltd. (Siheung, Republic of Korea). All experiments were carried out using Milli-Q® distilled water.
Preparation of NQ freebase
One gram of NQ⋅2HCl was dissolved completely in 5 mL of 1 N sodium carbonate solution and stirred at 24°C–27°C for 30 min. The synthetic product obtained was extracted using methylene chloride. Then, the product was filtered and dried. A total of 0.89 g of NQ freebase was obtained.
Preparation of PLGA microspheres with NQ freebase
NQ PLGA microspheres were prepared by oil-in-water (o/w) emulsion–solvent evaporation.14 For each PLGA formulation (PLGA 502, PLGA 502H, PLGA 503, and PLGA 503H), 300 mg was dissolved along with 60 mg NQ freebase in 15 mL dichloromethane:methanol solution (9:1 v/v) and injected into 300 mL 0.2% polyvinyl alcohol at 1 mL/s with a high-speed dispersion homogenizer (S18N-19G; IKA, Staufen, Germany) at 2,800 rpm for 1 min to form an o/w emulsion.15 The emulsion was stirred for 3 h at 24°C–27°C for dilution and evaporation of the solvent. The microspheres were centrifuged at 1,500 rpm for 3 min and washed three times with distilled water before being collected using a 0.45 μm filter and distributed with 5 mL distilled water. Finally, the microspheres were pretreated and lyophilized for two days at −75°C.
Morphology and particle size
The scanning electron microscopic (SEM) images of NQ freebase PLGA 502H microspheres were obtained using GEMINI LEO 1530® (Zeiss Ltd., Jena, Germany), as the dissolution patterns were appropriate for the desired long-lasting effect. Samples were mounted on an aluminum plate using a carbon tape, then placed inside a Hummer VI sputtering device (Minneapolis, MN, USA), and coated with platinum to discharge the particles. The particle size of the NQ freebase PLGA 502H microspheres was measured with image analysis software (NIH, ImageJ).
Determination of drug-loading efficiency and encapsulation efficiency
The entrapped amount of drug in the NQ freebase PLGA 502H microspheres was measured by HPLC. Five milligram of NQ freebase PLGA 502H microspheres was dissolved in 50 mL of 60% acetonitrile solution using Branson Model 8510® Ultrasonic Cleaner (BRANSON Ltd., St Louis, MO, USA) for 30 min. Analysis was performed using Ultimate 3000® HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) and 5 μm C8 column (100A 250×4.6 mm) from Phenomenex Ltd. (Torrance, CA, USA). The mobile phase was delivered at a flow rate of 1.5 mL/min, the detection wavelength was set at 225 nm, and the injection volume was 20 μL. The drug-loading efficiency (%) and entrapment efficiency (%) were calculated using the following equations (Equations 1 and 2), respectively. All measurements were conducted in triplicate.
| (1) |
| (2) |
Differential scanning calorimetry (DSC)
DSC was performed using Q2000® (TA Instruments Ltd., New Castle, DE, USA). One milligram of the prepared microspheres was placed on aluminum pan and hermetically sealed. The samples were heated from 0°C to 300°C at a rate of 20°C/min under a nitrogen flow of 50 mL/min.
Powder X-ray diffraction (PXRD)
PXRD patterns were obtained using X’Pert PRO MRD® (PANalytical Ltd., Almelo, the Netherlands) with Cu Kα radiation generated at 200 mA and 45 kV. The samples were placed on a silicon plate at room temperature and 2θ scans were collected from 5° to 60°.
Fourier-transform infrared spectroscopy (FT-IR)
Infrared spectroscopy was performed on the prepared micro-spheres in the range of 600 to 4,000 cm−1, using FT/IR-4100 type A (Jasco Corporation, Tokyo, Japan).
In vitro release study
A total of 10 mg of NQ freebase PLGA microspheres was suspended in 10 mL of phosphate-buffered saline (PBS, pH =7.4) and incubated at 37°C±0.5°C in a rotary shaking incubator (C-SKI-2; Chang-Sing Science, Seoul, Republic of Korea) at 50 rpm for 20 days. At each predetermined sampling point, the supernatant was separated for a validated RP-HPLC assay, and fresh medium of equal volume was added. All assays were performed in triplicate.16
In vivo pharmacokinetic study
Animals were treated and maintained in accordance with the principles of laboratory animal care and approved by the Committee for Animal Experiments of Chungbuk National University (Cheongju, Republic of Korea). Sprague Dawley rats (male, 400–460 g) were purchased from Daehan Biolink Co. (Eumseong, Republic of Korea) and fed ad libitum. The rats were randomly divided into two groups. One group (n=6) received intramuscular (IM) injections of NQ freebase 30% ethanol solution (5.4 μg/0.2 mL), chosen due to the poor solubility of the NQ freebase in water. The other group (n=6) was administered NQ freebase PLGA 502H microspheres (corresponding to 162 μg NQ/0.2 mL) intramuscularly. At each predetermined sampling point, blood samples were collected for liquid chromatography–mass spectrometry assay.
IVIVC
IVIVC is a predictive model that is used to describe the relationship between the in vitro behavior of formulation and relevant in vivo profiles.17 Level A correlation which is the most informative and recommended by the FDA is a point-to-point relationship in vitro and in vivo. That is a point-to-point relationship in vitro and in vivo. For applying level A correlation, same time points were chosen. Fraction of absorption was obtained by deconvolution using the Wagner–Nelson method.18
Results and discussion
Preparation of NQ freebase PLGA 502H microspheres
NQ freebase was synthesized from NQ⋅2HCl. The NQ freebase PLGA microspheres were prepared by o/w emulsion, which results in high yield and uniform particles.19 In preliminary experiments, PLGA 503 microspheres containing NQ⋅2HCl were prepared using o/w emulsion solvent evaporation (data not shown). The entrapment efficiency was observed to be less than 0.1% in the prepared particles, meaning that when the oil phase was mixed with the water phase, the salt form of NQ was almost completely dissolved in the water phase. NQ freebase was synthesized to increase the entrapment efficiency of the drug in the microspheres.20 Table 1 shows the pKa, molecular weight, and solubility values of NQ⋅2HCl and NQ freebase as analyzed by using the T3 equipment (Lakewood, NJ, USA). According to the solubility values obtained, NQ⋅2HCl had a solubility of 325.78 μg/mL and NQ freebase 6.82 μg/mL. The salt form showed about 50 times higher solubility than the drug freebase. NQ freebase microspheres prepared by o/w emulsion solvent evaporation were confirmed to have much higher entrapment efficiency than that of NQ⋅2HCl in PLGA 503. NQ freebase PLGA microspheres using different PLGA weights (PLGA 502, PLGA 502H, PLGA 503, PLGA 503H) were prepared to identify the correlation between entrapment efficiency and both molecular weight and the presence of acid terminals. The entrapment efficiency and amount of drug loaded in the prepared microspheres are shown in Table 2. The larger molecular weight showed an increase of about 2%. Acid-terminated non-end-capped PLGA (PLGA 502H and 503H) showed about 10% higher drug efficiency than the same molecular weight PLGA without the acid terminus. This suggests that the acid-terminated part of the polymer interacts with the amine group of the NQ.16
Table 1.
Solubility of norquetiapine dihydrochloride and norquetiapine freebase
| Property | NQ⋅2HCl | NQ freebase |
|---|---|---|
| pKa | 3.93, 8.49 | 3.90, 8.55 |
| MW (g/mol) | 368.32 | 295.40 |
| Solubility (μg/mL) | 325.78 | 6.82 |
| Log P | 3.12 | 3.18 |
Abbreviations: MW, molecular weight; NQ, N-desalkyl quetiapine.
Table 2.
Drug efficiency and drug-loading capacity of NQ freebase PLGA 502, PLGA 502H, PLGA 503, and PLGA 503H microspheres
| Property | NQ freebase PLGA 502 MSs | NQ freebase PLGA 502H MSs | NQ freebase PLGA 503 MSs | NQ freebase PLGA 503H MSs |
|---|---|---|---|---|
| Efficiency (%) | 30.33±0.51 | 46.19±0.33 | 32.69±1.28 | 48.30±3.04 |
| Drug-loading capacity (%) | 5.0±0.08 | 7.4±0.06 | 5.48±0.19 | 8.21±0.52 |
Abbreviations: NQ, N-desalkyl quetiapine; MSs, microspheres; PLGA, poly(d,l-lactic-co-glycolic acid).
Physiochemical characteristics of PLGA 502H microspheres
SEM and particle size distribution
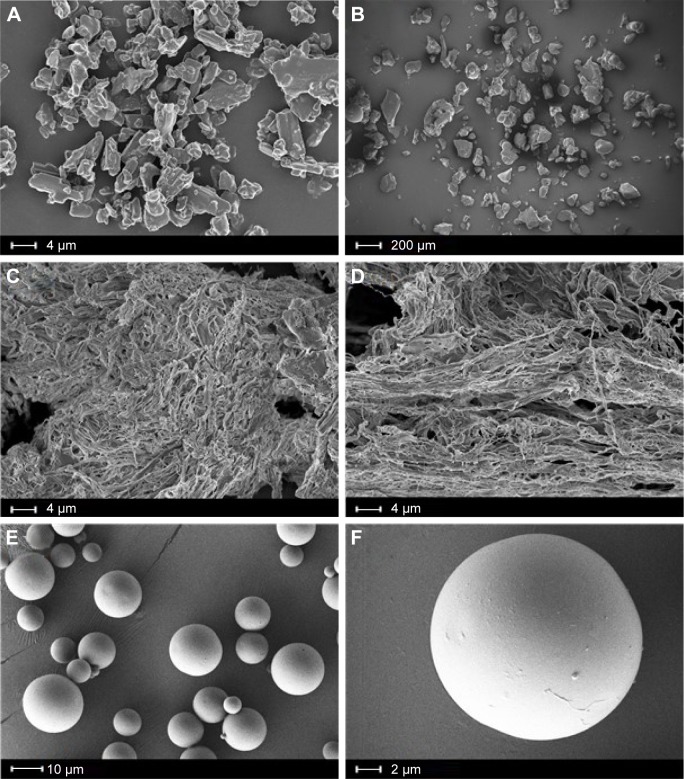
The SEM images of NQ⋅2HCl, NQ freebase, PLGA 502, PLGA 502H, and NQ freebase PLGA 502H microspheres are shown in Figure 1. NQ⋅2HCl was shown to have an angular, crystalline form (Figure 1A). NQ freebase microspheres (Figure 1B) grew larger and developed a round shape during synthesis. PLGA 502 and PLGA 502H (Figure 1C and D, respectively) both showed a fibrous morphology and no difference in the SEM images. The prepared microspheres did not have the original shape of the drug, and the fibrous nature of the surface polymer was lost. Figure 1E and F shows that the microspheres have a non-porous structure, round shape, and a smooth surface with no drug crystals. Large microspheres can cause severe pain when administered intramuscularly. The prepared PLGA 502H microspheres were measured using an image analysis program (NIH, imageJ); they had a diameter of 8.86±3.11 μm that is acceptable for IM injection system.21
Figure 1.
SEM micrographs of NQ freebase PLGA 502H MSs: (A) NQ⋅2HCl (5,000×), (B) NQ freebase (100×), (C) PLGA 502 (5,000×), (D) PLGA 502H (5,000×), and (E and F) NQ freebase PLGA 502H MSs (2,500× and 10,000× respectively).
Abbreviations: SEM, scanning electron microscopy; NQ, N-desalkyl quetiapine; MS, microsphere; PLGA, poly(d,l-lactic-co-glycolic acid).
DSC
The DSC thermograms of NQ⋅2HCl, NQ freebase, PLGA 502H, and NQ freebase PLGA 502H microspheres, as well as a physical mixture of the microspheres, were obtained and the results are shown in Figure 2. The DSC result was obtained by raising the temperature to 20°C/min. The Tm of NQ⋅2HCl was found to be around 180°C–210°C, whereas the NQ freebase was found to have a Tg between 50°C and 65°C. The Tm of the NQ freebase was not observed. It is likely that the temperature difference was caused by the crystal form changing into the amorphous form upon the removal of the base from NQ⋅2HCl. A clear Tm peak in the NQ freebase was not observed. In the case of PLGA 502H, the Tm peak was not observed and the Tg was observed at about 50°C. The physical mixture of PLGA 502H and NQ freebase PLGA 502H microspheres showed similar results. When the PLGA and the NQ freebase were present together, the Tg regions were similar, and the difference in the PLGA peaks appeared to be due to the difference in the peak size and ratio.
Figure 2.
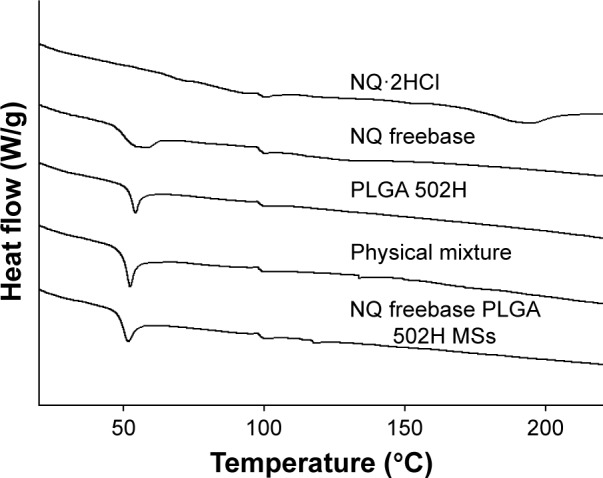
DSC thermograms of NQ freebase PLGA 502H MSs.
Abbreviations: DSC, differential scanning calorimetry; MS, microsphere; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid).
PXRD
The PXRD results are shown in Figure 3. A sharp peak was observed at around 10°, 15°, 20°, and 25° for NQ⋅2HCl, which means that it has the crystallinity of NQ⋅2HCl. These crystal peaks were not observed for NQ freebase, meaning that the NQ freebase has assumed an amorphous state due to the removal of the base. This implies that the hydrochloride is involved in the crystal structure.22 The PLGA 502H, the physical mixture, and the prepared NQ freebase PLGA 502H microspheres were found to be amorphous, which appeared to follow the peak of PLGA 502H. Although the drug is present in the particles and mixture, the peak intensity of the drug is not seen and appears that PLGA 502H peak is dominated.
Figure 3.

PXRD patterns of NQ freebase PLGA 502H MSs.
Abbreviations: PXRD, powder X-ray diffraction; MS, microsphere; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid).
FT-IR
The FT-IR spectra of NQ⋅2HCl, NQ freebase, PLGA 502H, NQ freebase PLGA 502H microspheres, and their physical mixture, were obtained and the results are shown in Figure 4. NQ⋅2HCl and NQ freebase showed similar intensity patterns. They had peaks at 1,608 cm−1 (N−H bond), 928 cm−1 (C−H bond), and 765 cm−1 (C−H bond). The peak around 920 cm−1 was only observed for NQ⋅2HCl. This disappeared for NQ freebase due to the disappearance of the hydrochloride salt as it turned into freebase. The peak of 1,530–1,630 cm−1 for NQ freebase was observed at the peak of the physical mixture, NQ freebase PLGA 502H microspheres. But, they seemed to follow the PLGA 502H peak as a whole. The peak tendency for the NQ freebase PLGA 502H microspheres was similar to that of the physical mixture, indicating a tendency dependent upon the ratio between the drug and the polymer. There was no newly formed functional group in the NQ freebase PLGA 502H microspheres.
Figure 4.
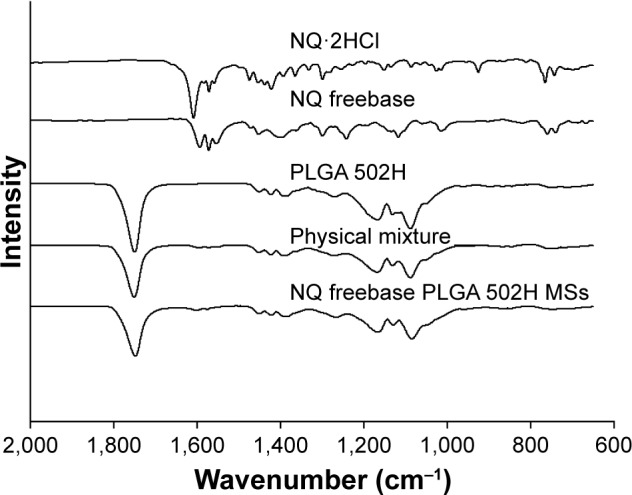
FT-IR spectra of NQ freebase PLGA 502H MSs.
Abbreviations: FT-IR, Fourier-transform infrared spectroscopy; MS, microsphere; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid).
In vitro release study
The dissolution profiles of NQ freebase PLGA 502 micro-spheres, NQ freebase PLGA 502H microspheres, NQ freebase PLGA 503 microspheres, and NQ freebase PLGA 503H microspheres were obtained, and the results are shown in Figure 5. A typical biphasic release pattern was observed for all formulations, with an initial burst release followed by a more controlled secondary phase.23 The rapid release could represent the release of poorly entrapped and surface-associated NQ freebase. The drug release during the slower release phase may result from polymer degradation, from drug diffusion through the polymer matrix, or from both. NQ freebase PLGA 502H microspheres released about 20% of the NQ freebase during the burst-release phase. PLGA 502, PLGA 503, and PLGA 503H released about 12%, 27%, and 29%, respectively, of NQ freebase during the burst release. For 5 days after burst release, 60%–70% of the drug was released. After 5 days, slower dissolution patterns in all formulations were observed. After 20 days, NQ freebase PLGA 502H formulation showed 82.1% release and other formulations released about 70% of the drug. The dissolution pattern and entrapment efficiency of the PLGA 502H formulation were suited for the desired 20-day long-lasting formulation. The difference in dissolution patterns would be due to the molecular weight and different terminal group of PLGA, as an increase in molecular weight decreases the dissolution rate and the carboxylic acid terminal catalyzes the hydrolysis of PLGA. Therefore, PLGA 502H microspheres, which have low molecular weight and possess a carboxylic acid terminal group, are shown to have the fastest dissolution pattern.24 The release profiles were fitted to various mathematical models to analyze the mechanism of the drug release. The highest correlation coefficient was estimated using the Higuchi matrix kinetics, as shown in Table 3. When plotted in accordance with the Korsmeyer–Peppas method, the n-value was 0.5<n<1.0, suggesting non-Fickian transport. Drug diffusion was assumed to be via the dominant mechanism throughout the release period.25
Figure 5.

The dissolution patterns of NQ freebase PLGA 502 MSs, NQ freebase PLGA 502H MSs, NQ freebase PLGA 503 MSs, and NQ freebase PLGA 503H MSs (mean ± SD, n=3).
Abbreviations: MS, microsphere; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid); SD, standard deviation.
Table 3.
Release kinetic profiles of NQ freebase PLGA 502H microspheres
| Model | Release constant (k) | Correlation coefficient (R2) |
|---|---|---|
| Zero order | 0.7525 | 0.7525 |
| First order | 0.8874 | 0.8874 |
| Higuchi | 4.0433 | 0.9404 |
| Korsmeyer–Peppas | 4.6014 | 0.9681 |
| (n-value =0.6083) |
Abbreviations: NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid).
In vivo pharmacokinetic study
Pharmacokinetic profile of NQ freebase solution and NQ freebase PLGA 502H microspheres following IM administration
During the in vivo test, both groups administered with NQ solution and prepared formulation did not exhibit observable side effects such as rashes, irritation, and abnormal behaviors.
The mean plasma concentration–time profiles of NQ after IM administration are shown in Figure 6. The calculated pharmacokinetic parameters are shown in Table 4. After administration of the NQ solution, Cmax was observed to be 25.08±10.41 ng/mL at time point 0.25 h (0.01 day). Tmax was observed within 0.25 h after IM injection. It is presumed that the drug is easily transferred to plasma from muscles. The area under the curve (AUC) was 33.17±9.33 ng⋅h/mL and t1/2 was 5.02±1.28 h. NQ was not observed in plasma after 12 h of administration. On the other hand, the Cmax of the prepared formulation was observed to be 22.48±4.46 ng/mL, lower than that of the NQ freebase even though 30 times the amount of drug was administered. t1/2 was 172.79±23.79 h, which is longer than the half-life of the NQ freebase (5.05±1.28 h). The AUC was 916.46±186.57 ng⋅h/ mL. The prepared formulation showed <12 days of drug release in vivo compared to over 20 days of drug release in vitro. The faster release kinetics in vivo was suggested to be a result of enhanced PLGA degradation, due to the presence of enzymes, limited muscle mass,26 and other in vivo factors. Similar increased enzymatic polymer degradation in vivo has been reported by other researchers.27,28
Figure 6.
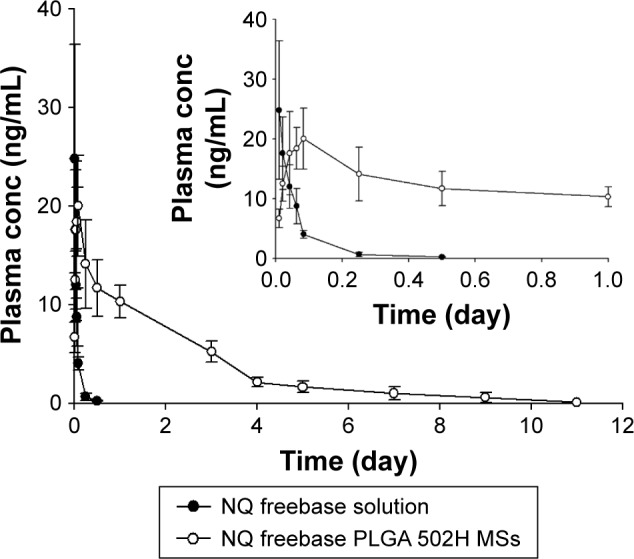
Plasma concentration profile of NQ after intramuscular injection of NQ freebase solution and NQ freebase PLGA 502H MSs (mean ± SD, n=6).
Abbreviations: conc, concentration; MS, microsphere; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid); SD, standard deviation.
Table 4.
Pharmacokinetic parameters of norquetiapine in rat (n=6) administered with NQ freebase solution and NQ freebase PLGA 502H microspheres
| Parameter | NQ freebase solution | NQ freebase PLGA 502H microspheres |
|---|---|---|
| Cmax (ng/mL) | 25.08±11.41 | 22.48±4.46 |
| Tmax (h) | 0.29±0.10 | 1.50±0.45 |
| t1/2 (h) | 5.05±1.40 | 172.79±23.79 |
| AUC (ng⋅h/mL) | 33.17±9.33 | 916.46±186.57 |
| AUMC (ng⋅h2/mL) | 43.40±9.80 | 45,421.86±14,766.68 |
Note: Data presented as mean ± SD.
Abbreviations: AUC, area under the curve; AUMC, area under the moment curve; NQ, N-desalkyl quetiapine; PLGA, poly(d,l-lactic-co-glycolic acid).
Correlation of in vitro release and in vivo performance
The IVIVC allows in vitro study to be used as a surrogate for in vivo study from conventional or long-acting dosage forms.29 Level A correlation is the highest level of IVIVC. That represents a point-to-point relationship between in vitro dissolution and in vivo input rate. The same time points (1, 6, 12, 24, 72, 120, 216 h) were chosen, and the fraction of dissolution and fraction of absorption calculated by Wagner– Nelson method at that point were plotted (Figure 7). The correlation coefficient (r2) was 0.9893, indicating a good IVIVC correlation. Although in vivo pharmacokinetic profile was obtained faster than in vitro dissolution profile, it could gain successful correlation in IVIVC. This shows that in vitro drug release test results can be used to predict in vivo performance on mouse models.
Figure 7.
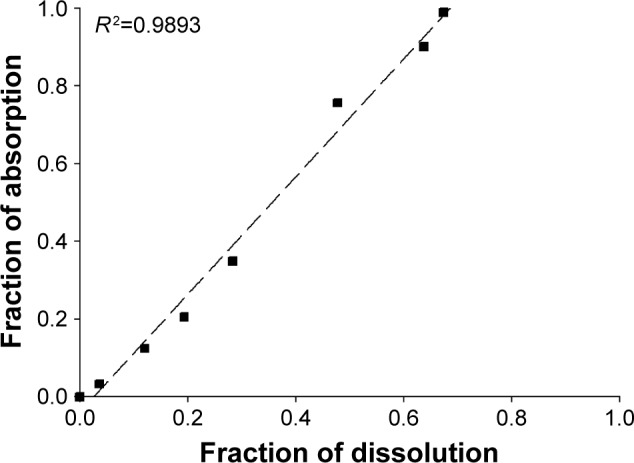
Correlation of the fraction of NQ absorption in vivo versus the fraction of NQ dissolved in vitro.
Abbreviation: NQ, N-desalkyl quetiapine.
Conclusion
In this study, a sustained release of NQ freebase PLGA microspheres was successfully prepared by o/w emulsion– solvent evaporation method. The physiochemical properties of the synthesized NQ freebase play an important role in the amount of drug loaded in the microspheres. The drug was released from microspheres in PBS medium, following biphasic kinetics. The main mechanism of release was confirmed by the mathematical model to be diffusion and it lasted over 20 days in vitro. The release of the NQ profile of the formulation in vivo was observed to be faster than dissolution, due to various in vivo factors. The long-acting release of the prepared NQ freebase PLGA 502H micro-spheres compared to that of the NQ freebase solution was shown. These results suggest the potential use of NQ freebase PLGA microspheres for the treatment of schizophrenia over long periods.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Education) (NRF-2015R1D1A1A01059389, NRF-2015R1C1A1A02036702).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Higashi K, Medic G, Littlewood KJ, Diez T, Granström O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–218. doi: 10.1177/2045125312474019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glazer WM. Review of incidence studies of tardive dyskinesia associated with typical antipsychotics. J Clin Psychiatry. 2000;61(Suppl 4):15–20. [PubMed] [Google Scholar]
- 3.López-Muñoz F, Álamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;4:102. doi: 10.3389/fpsyt.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 5.Fura A. Role of pharmacologically active metabolites in drug discovery and development. Drug Discov Today. 2006;11(3):133–142. doi: 10.1016/S1359-6446(05)03681-0. [DOI] [PubMed] [Google Scholar]
- 6.Bakken GV, Rudberg I, Molden E, Refsum H, Hermann M. Pharmacokinetic variability of quetiapine and the active metabolite N-desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2011;33(2):222–226. doi: 10.1097/FTD.0b013e31821160c4. [DOI] [PubMed] [Google Scholar]
- 7.Winter HR, Earley WR, Hamer-Maansson JE, Davis PC, Smith MA. Steady-state pharmacokinetic, safety, and tolerability profiles of quetiapine, norquetiapine, and other quetiapine metabolites in pediatric and adult patients with psychotic disorders. J Child Adolesc Psychopharmacol. 2008;18(1):81–98. doi: 10.1089/cap.2007.0084. [DOI] [PubMed] [Google Scholar]
- 8.Kim D-W, Weon K-Y, Hong E-P, Chung EK, Lee K-T. Comparative physicochemical and pharmacokinetic properties of quetiapine and its active metabolite norquetiapine. Chem Pharm Bull. 2016;64(11):1546–1554. doi: 10.1248/cpb.c16-00223. [DOI] [PubMed] [Google Scholar]
- 9.Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man. Vol. 8. Seal Beach: Biomedical publications; 2011. [Google Scholar]
- 10.Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Mental Health. 2015;18(2):36–39. doi: 10.1136/eb-2015-102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane JM, Detke HC, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2009;167(2):181–189. doi: 10.1176/appi.ajp.2009.07081221. [DOI] [PubMed] [Google Scholar]
- 12.Edlund U, Albertsson AC. Degradable polymer microspheres for controlled drug delivery. In: Albertsson A-C, editor. Degradable Aliphatic Polyesters. Berlin Heidelberg: Springer-Verlag; 2002. pp. 67–112. [Google Scholar]
- 13.Burgess DJ, Hickey AJ. Micro-sphere technology and applications. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker Inc; 1994. pp. 1–29. [Google Scholar]
- 14.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 15.Hickey T, Kreutzer D, Burgess D, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, Quan P, Fang L, Cun D, Yang M. Sustained release donepezil loaded PLGA microspheres for injection: preparation, in vitro and in vivo study. Asian J Pharm Sci. 2015;10(5):405–414. [Google Scholar]
- 17.Jun H, Lee H-J, Shin B-S, Park C-W. Preparation and in vivo characterization of dual release tablet containing sarpogrelate hydrochloride. J Pharm Invest. 2017;7:1–10. [Google Scholar]
- 18.Xu H, Shi Y, Vela S, Marroum P, Gao P. Developing quantitative in vitro–in vivo correlation for fenofibrate immediate-release formulations with the biphasic dissolution-partition test method. J Pharm Sci. 2018;107(1):476–487. doi: 10.1016/j.xphs.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell PB, McGinity JW. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28(1):25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 20.Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5(6):615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P, Johnson H, Allexon C. In vitro and in vivo evaluation of clarithromycin/poly (lactic acid) microspheres for intramuscular drug delivery. J Control Release. 1993;26(3):229–238. [Google Scholar]
- 22.Newman A, Wenslow R. Solid form changes during drug development: good, bad, and ugly case studies. AAPS Open. 2016;2(1):2. [Google Scholar]
- 23.Tamaddon L, Mostafavi SA, Karkhane R, Riazi-Esfahani M, Dorkoosh FA, Rafiee-Tehrani M. Design and development of intraocular polymeric implant systems for long-term controlled-release of clindamycin phosphate for toxoplasmic retinochoroiditis. Adv Biomed Res. 2015;4:32. doi: 10.4103/2277-9175.150426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Shavi GV, Nayak UY, Reddy MS, et al. A novel long-acting biodegradable depot formulation of anastrozole for breast cancer therapy. Mater Sci Eng C Mater Biol Appl. 2017;75:535–544. doi: 10.1016/j.msec.2017.02.063. [DOI] [PubMed] [Google Scholar]
- 26.Nowland MH. Guidelines on Adjuvant Use in Rabbits Rats and Mice 2016. [Accessed February 28, 2018]. Available from: https://wiki.med.umich.edu/display/ULAMGSOP/Guidelines+on+Adjuvant+Use+in+Rabbits+Rats+and+Mice.
- 27.Zolnik BS, Burgess DJ. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. J Control Release. 2008;127(2):137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Sandor M, Harris J, Mathiowitz E. A novel polyethylene depot device for the study of PLGA and P(FASA) microspheres in vitro and in vivo. Biomaterials. 2002;23(22):4413–4423. doi: 10.1016/s0142-9612(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 29.D’souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23(3):460–474. doi: 10.1007/s11095-005-9397-8. [DOI] [PubMed] [Google Scholar]