With >2 years of follow-up, Japanese patients from the international phase III CheckMate 025 study had a higher response rate with nivolumab versus everolimus and a favorable safety profile.
Keywords: everolimus, immune checkpoint inhibitor, Japanese, nivolumab, renal cell carcinoma
Abstract
Background
Nivolumab improved overall survival (OS) and objective response rate (ORR) versus everolimus in previously treated patients with advanced renal cell carcinoma in the phase III CheckMate 025 study (minimum follow-up: 14 months). We report efficacy and safety in the global and Japanese populations (minimum follow-up: 26 months).
Methods
Patients were randomized 1:1 to receive nivolumab 3 mg/kg intravenously every 2 weeks or everolimus 10-mg tablet orally once daily. Primary endpoint: OS, key secondary endpoints: ORR, progression-free survival and safety.
Results
Of 410 (nivolumab) and 411 (everolimus) patients, 37 (9%) and 26 (6%), respectively, were Japanese. Median OS for the global population was 26.0 months (nivolumab) and 19.7 months (everolimus; hazard ratio 0.73 [95% confidence interval [CI]: 0.61–0.88]; P = 0.0006), with medians not reached for Japanese patients. ORR for the global population was 26% (nivolumab) versus 5% (everolimus; odds ratio 6.13; 95% CI: 3.77–9.95); ORR for Japanese patients: 43% versus 8% (odds ratio 9.14; 95% CI: 1.76–88.33). In Japanese patients, any-grade treatment-related adverse events (AEs) occurred in 78% (Grade 3–4, 19%; most common, anemia [5%]) treated with nivolumab and 100% (Grade 3–4, 58%; most common, hypertriglyceridemia [12%]) treated with everolimus; the most common with nivolumab was diarrhea (19%) and with everolimus was stomatitis (77%). Quality of life was stable in the nivolumab arm.
Conclusions
With >2 years of follow-up, Japanese patients had a higher response rate with nivolumab versus everolimus that was more pronounced yet consistent with the global population, with median OS not reached, and a favorable safety profile.
Introduction
In 2015, there were six targeted therapies approved in Japan for treatment of advanced renal cell carcinoma (aRCC), including the tyrosine kinase inhibitors (TKIs) sorafenib, sunitinib, axitinib and pazopanib, and the mammalian target of rapamycin (mTOR) inhibitors everolimus and temsirolimus (1). In 2016, the seventh targeted therapy, the programmed death-1 (PD-1) immune checkpoint inhibitor nivolumab, was approved in Japan for patients with previously treated unresectable or metastatic RCC based on results from the international Phase III CheckMate 025 study (2). In that report, nivolumab improved overall survival (OS) and objective response rate (ORR) versus everolimus in previously treated patients with aRCC (2). Median OS was 25.0 months (95% confidence interval [CI], 21.8–not reached) for nivolumab versus 19.6 months (95% CI: 17.6–23.1) for everolimus, with a minimum follow-up of 14 months; ORR was 25% for nivolumab and 5% for everolimus (odds ratio, 5.98 [95% CI: 3.68–9.72]; P < 0.001) (2). Nivolumab was associated with fewer Grade 3 or 4 treatment-related adverse events (AEs) and fewer treatment-related AEs leading to discontinuation than was everolimus (2).
Observed differences in efficacy and safety of therapies for aRCC in Asian patients may be the result of environmental and/or genetic differences that necessitate specific investigation of agents in this population (3–6). Additionally, treatment patterns in Asian countries differ from those in Western countries; for example, cytokine therapy is still widely used for first-line treatment in Japan (6,7). These different treatment patterns potentially add a confounding factor in clinical trials of second-line agents. Here, we present efficacy and safety data from the global population as well as the Japanese subgroup of patients treated with nivolumab or everolimus from CheckMate 025, with a minimum follow-up of at least 26 months.
Patients and methods
Study design and treatment
This was a Phase III, randomized open-label study of nivolumab versus everolimus. The detailed study design was described previously (2). Patients were randomized 1:1 to receive nivolumab 3 mg/kg intravenously over 60 min every 2 weeks or everolimus 10-mg tablet orally once daily. Randomization was stratified according to region (United States or Canada, Western Europe and the rest of the world), Memorial Sloan Kettering Cancer Center prognostic risk group and number of prior anti-angiogenic therapies (one or two) for aRCC. Japanese patients were included as part of the ‘rest of the world’ stratification group.
Patients
Adults with histological confirmation of aRCC with a clear-cell component were eligible. Patients had to have received one or two prior anti-angiogenic therapies and had to have progression within 6 months before study enrollment and Karnofsky performance status (KPS) of at least 70 at study entry. Additional eligibility criteria were reported previously (2). Analyses are based on data collected with the use of a case report form.
Endpoints and assessments
The primary endpoint was OS, defined as time from randomization to death. The key secondary endpoints were investigator-assessed ORR, defined as the number of patients with complete response or partial response divided by the number of randomized patients, and progression-free survival (PFS). Disease assessments (per Response Evaluation Criteria in Solid Tumors [RECIST] v1.1) (8) were performed using computed tomography or magnetic resonance imaging at baseline and every 8 weeks after randomization for the first year, then every 12 weeks until progression or treatment discontinuation. Safety was assessed at each clinic visit. Quality of life was assessed using the Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease-Related Symptoms (FKSI-DRS) scoring algorithm (9). The questionnaire consisted of nine symptom-specific questions, as previously reported (10). The summary score ranged from 0 to 36, with 36 as the best possible score (9,10). A change of at least 2 points was considered a clinically meaningful change.
Study oversight
This study was approved by the institutional review board or independent ethics committee at each center and conducted in accordance with Good Clinical Practice guidelines defined by the International Conference on Harmonisation. All patients provided written informed consent to participate based on the principles of the Declaration of Helsinki.
Statistical analyses
OS, PFS and duration of response were estimated using Kaplan–Meier methodology. OS medians and corresponding 95% CIs were determined using Brookmeyer and Crowley methodology (11). 95% CIs were constructed using log–log transformation. A stratified log-rank test was performed for the global population only. Hazard ratios and CIs were obtained for OS and PFS for nivolumab versus everolimus by fitting an unstratified Cox model (stratified for the global population with the group variable as a single covariate). ORRs and the corresponding 95% CIs were based on the Clopper and Pearson method (12).
Results
Patients
Of the 410 and 411 patients who were randomized to nivolumab and everolimus, respectively (hereafter referred to as the global population), 96 and 98, respectively, were stratified by the ‘rest of the world’ region, which included Japan. Thirty-seven of 410 patients (9%) and 26 of 411 patients (6%), respectively, were Japanese (hereafter referred to as the Japanese population). All Japanese patients who were randomized received treatment. Demographic and baseline characteristics of the global and Japanese populations were generally similar, except that a higher proportion of Japanese patients overall had baseline KPS of 100, and lower proportions of Japanese patients in the everolimus arm had ≥2 sites of metastases, liver metastases, and PD-1 ligand 1 (PD-L1) expression ≥1% (Table 1). The distribution of prior treatment regimens in the metastatic setting differed between the global and Japanese populations. Higher proportions of Japanese patients versus the global population had prior treatment with axitinib (20/63 [32%] versus 101/821 [12%], respectively), interferon-α (IFN-α; 17/63 [27%] versus 18/821 [2%]) and sorafenib (22/63 [35%] versus 57/821 [7%]), and lower proportions of Japanese patients were treated with pazopanib (2/63 [3%] versus 250/821 [30%]) and sunitinib (28/63 [44%] versus 488/821 [59%]). At a minimum of 26 and 28 months of follow-up for the global and Japanese populations, respectively (median follow-up: 33.6 and 33.2 months), 11% of the global population and 16% of the Japanese population continued to receive treatment with nivolumab (2% and 4% in the everolimus arm, respectively). The primary reason for discontinuation was disease progression with nivolumab or everolimus in both the global (74% versus 72%, respectively) and Japanese populations (62% versus 65%, respectively).
Table 1.
Patient demographic and baseline characteristics
| Characteristic | Global population | Japanese population | ||
|---|---|---|---|---|
| Nivolumab N = 410 | Everolimus N = 411 | Nivolumab N = 37 | Everolimus N = 26 | |
| Median age (range), years | 62 (23–88) | 62 (18–86) | 65 (39–81) | 67 (43–81) |
| Male sex, n (%) | 315 (77) | 304 (74) | 26 (70) | 21 (81) |
| KPS,an (%) | ||||
| 100 | 126 (31) | 134 (33) | 22 (59) | 15 (58) |
| 90 | 150 (37) | 130 (32) | 10 (27) | 9 (35) |
| 80 | 110 (27) | 116 (28) | 5 (14) | 2 (8) |
| MSKCC risk score, n (%) | ||||
| Favorable | 145 (35) | 148 (36) | 16 (43) | 13 (50) |
| Intermediate | 201 (49) | 203 (49) | 18 (49) | 12 (46) |
| Poor | 64 (16) | 60 (15) | 3 (8) | 1 (4) |
| Not reported | 1 (< 1) | 0 (0) | 0 (0) | 0 (0) |
| Prior anti-angiogenic therapiesb, n (%) | ||||
| 1 | 317 (77) | 312 (76) | 28 (76) | 21 (81) |
| 2 | 90 (22) | 99 (24) | 9 (24) | 5 (19) |
| Prior nephrectomy, n (%) | 364 (89) | 359 (87) | 33 (89) | 26 (100) |
| ≥2 sites of metastases, n (%) | 341 (83) | 338 (82) | 31 (84) | 19 (73) |
| Liver metastases, n (%) | 100 (24) | 87 (21) | 8 (22) | 2 (8) |
| PD-L1 expression, n/N (%) | ||||
| ≥1% | 94/370 (25) | 87/386 (23) | 10 (27) | 2 (8) |
| <1% | 276/370 (75) | 299/386 (77) | 27 (73) | 24 (92) |
KPS, Karnofsky performance status; MSKCC, Memorial Sloan Kettering Cancer Center; PD-L1, programmed death-1 ligand 1.
aTwenty-four patients (6%) in the nivolumab arm and 31 patients (8%) in the everolimus arm in the global population had KPS ≤70 at randomization.
bThree patients in the nivolumab arm from the global population had >2 prior anti-angiogenic therapies.
Efficacy
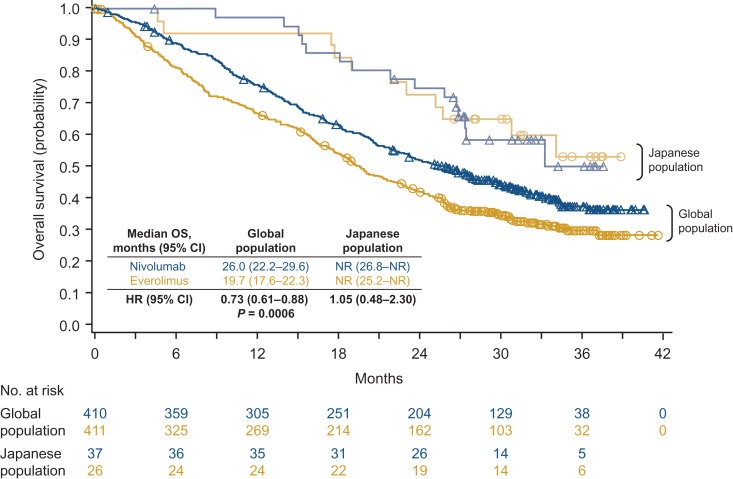
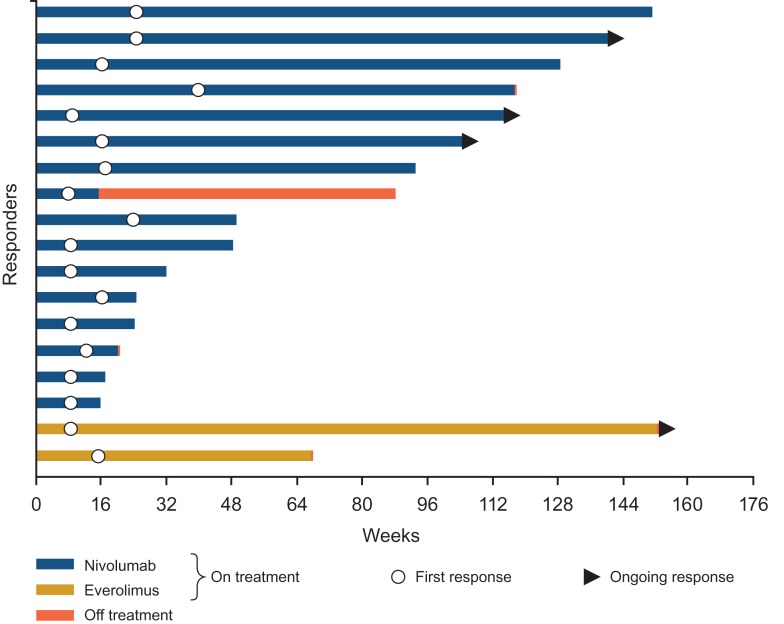
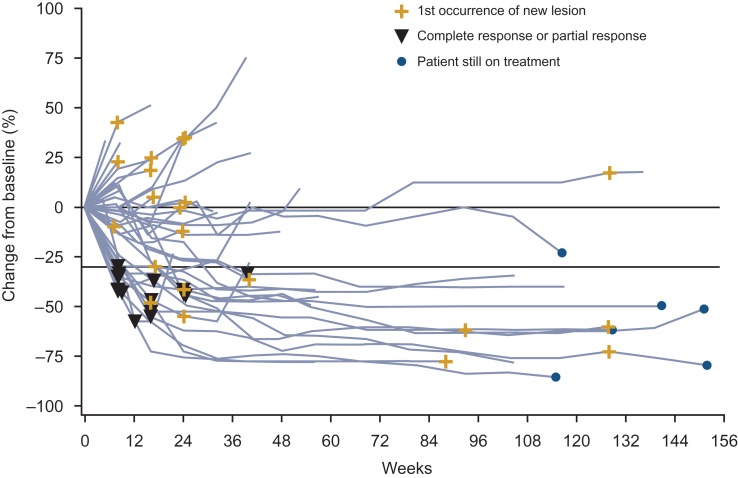
Median OS for the global population was 26.0 months with nivolumab and 19.7 months with everolimus (hazard ratio 0.73 [95% CI: 0.61–0.88]; P = 0.0006; Fig. 1). Median OS for the Japanese population was not reached for both arms (hazard ratio 1.05 [95% CI: 0.48–2.30]; Fig. 1). ORR was higher with nivolumab than with everolimus for both the global and Japanese populations (global: 26% versus 5%, respectively; odds ratio 6.13; 95% CI: 3.77–9.95; Japanese: 43% versus 8%; odds ratio 9.14; 95% CI: 1.76–88.33; Table 2). Lower proportions of patients in both arms in the Japanese population had a best response of progressive disease compared with the global population (Table 2). Median time to response in the global population was 3.5 months (range, 1.4–24.8) with nivolumab and 3.7 months (range, 1.5–11.2) with everolimus (2). Median time to response in Japanese patients was 3.3 months (range, 1.9–9.2) with nivolumab and 2.7 months (range, 1.9–3.5) with everolimus (Fig. 2). Median duration of response for the global population was 12.0 months (95% CI: 9.1–18.2) with nivolumab and 12.0 months with everolimus (95% CI: 6.4–21.7). Median duration of response in Japanese patients was 13.4 months (95% CI: 2.2–25.8) with nivolumab and was not reached for everolimus (95% CI: 12.0–not reached) (Fig. 2). Among patients from the global population who responded to treatment, 30 of 105 (29%) in the nivolumab arm and 3 of 22 (14%) in the everolimus arm had an ongoing response. In Japanese patients, 3 of 16 (19%) and 1 of 2 (50%) patients, respectively, had an ongoing response. Responses were durable with nivolumab in Japanese patients, as shown in Fig. 3. Median PFS for the global population was 4.6 months with nivolumab and 4.4 months with everolimus (hazard ratio 0.88 [95% CI: 0.75–1.03]; 14-month minimum follow-up), as previously published (2). Median PFS was 5.6 and 9.4 months, respectively, for the Japanese population (hazard ratio 1.04 [95% CI: 0.59–1.82]; current analysis with 28-month minimum follow-up).
Figure 1.
Overall survival with 2 years of follow-up.
Table 2.
Antitumor activity
| Global population | Japanese population | |||
|---|---|---|---|---|
| Nivolumab | Everolimus | Nivolumab | Everolimus | |
| N = 410 | N = 411 | N = 37 | N = 26 | |
| ORR, n (%) | 105 (26) | 22 (5) | 16 (43) | 2 (8) |
| P value | <0.0001 | Not applicable | ||
| Best overall response, n (%) | ||||
| Complete response | 3 (1) | 2 (1) | 0 (0) | 1 (4) |
| Partial response | 102 (25) | 20 (5) | 16 (43) | 1 (4) |
| Stable disease | 138 (34) | 230 (56) | 15 (40) | 20 (77) |
| Progressive disease | 143 (35) | 113 (28) | 6 (16) | 4 (15) |
| Unable to determine | 24 (6) | 46 (11) | 0 (0) | 0 (0) |
| Median duration of response, months (95% CI) | 12.0 (9.1–18.2) | 12.0 (6.4–21.7) | 13.4 (2.2–25.8) | NR (12.0–NR) |
CI, confidence interval; NR, not reached; ORR, objective response rate.
Figure 2.
Time to and duration of response in Japanese patients. Bar indicates progression-free survival.
Figure 3.
Change from baseline in tumor burden over time in Japanese patients treated with nivolumab.
Safety
In the Japanese population, any-grade treatment-related AEs occurred in 78% of patients treated with nivolumab and 100% of patients treated with everolimus (Table 3). Results were similar in the global population (79% versus 88%, respectively). The most common treatment-related AEs in Japanese patients treated with nivolumab was diarrhea (19%) and the most common with everolimus was stomatitis (77%) (Table 3). The most common treatment-related AE in the global population was fatigue for both nivolumab and everolimus (34% versus 34%). Grade 3 or 4 treatment-related AEs occurred in 19% of Japanese patients treated with nivolumab and 58% of Japanese patients treated with everolimus (Table 3). The most common Grade 3 or 4 treatment-related AEs in Japanese patients treated with nivolumab was anemia (5%) and with everolimus was hypertriglyceridemia (12%). Grade 3 or 4 treatment-related AEs were experienced by 20% versus 37% of patients in the global population, respectively. The most common in the global population were fatigue (2%) and anemia (2%) with nivolumab and anemia (9%) with everolimus. Any-grade treatment-related AEs leading to discontinuation were observed in 16% (Grade 3 or 4, 3%) and 23% (Grade 3 or 4, 12%) of Japanese patients in the nivolumab and everolimus arms, respectively. Among Japanese patients, 15 (41%) and 11 (42%) died in the nivolumab and everolimus arms, respectively; no treatment-related deaths occurred in either arm. Among the global population, no treatment-related deaths were reported with nivolumab and two deaths were reported with everolimus. Treatment-related select AEs with immune etiology for nivolumab versus everolimus in Japanese patients were observed as follows: endocrine (5% versus 0%), hepatic (22% versus 31%), skin (22% versus 69%), gastrointestinal (19% versus 12%), renal (3% versus 15%) and pulmonary (5% versus 46%). While on nivolumab and everolimus, 51% and 81% of Japanese patients, respectively, received immune-modulating therapy. Dermatological corticosteroids were administered to 46% and 73% of Japanese patients in the nivolumab and everolimus arms, respectively; systemic corticosteroids were administered to 19% and 35% of Japanese patients.
Table 3.
Treatment-related AEs occurring in >15% of Japanese patients in either arm
| Nivolumab N = 37 | Everolimus N = 26 | |||
|---|---|---|---|---|
| Any gradea | Grade 3–4 | Any gradea | Grade 3–4 | |
| Treatment-related AEs, n (%) | 29 (78) | 7 (19) | 26 (100) | 15 (58) |
| Diarrhea | 7 (19) | 1 (3) | 2 (8) | 0 (0) |
| Anemia | 6 (16) | 2 (5) | 12 (46) | 2 (8) |
| Fatigue | 6 (16) | 0 (0) | 5 (19) | 1 (4) |
| Pyrexia | 5 (14) | 0 (0) | 6 (23) | 0 (0) |
| Pruritus | 3 (8) | 0 (0) | 6 (23) | 0 (0) |
| Rash | 3 (8) | 0 (0) | 6 (23) | 0 (0) |
| Hypertriglyceridemia | 2 (5) | 0 (0) | 7 (27) | 3 (12) |
| Interstitial lung disease | 2 (5) | 0 (0) | 7 (27) | 2 (8) |
| Thrombocytopenia | 1 (3) | 1 (3) | 13 (50) | 1 (4) |
| Stomatitis | 1 (3) | 0 (0) | 20 (77) | 2 (8) |
| Hyperglycemia | 1 (3) | 0 (0) | 7 (27) | 2 (8) |
| Blood cholesterol increased | 0 (0) | 0 (0) | 5 (19) | 0 (0) |
| Palmar-plantar erythrodysesthesia | 0 (0) | 0 (0) | 5 (19) | 0 (0) |
| Pneumonitis | 0 (0) | 0 (0) | 5 (19) | 0 (0) |
| Proteinuria | 0 (0) | 0 (0) | 5 (19) | 2 (8) |
AEs, adverse events.
aNo Grade 5 events occurred.
Quality of life
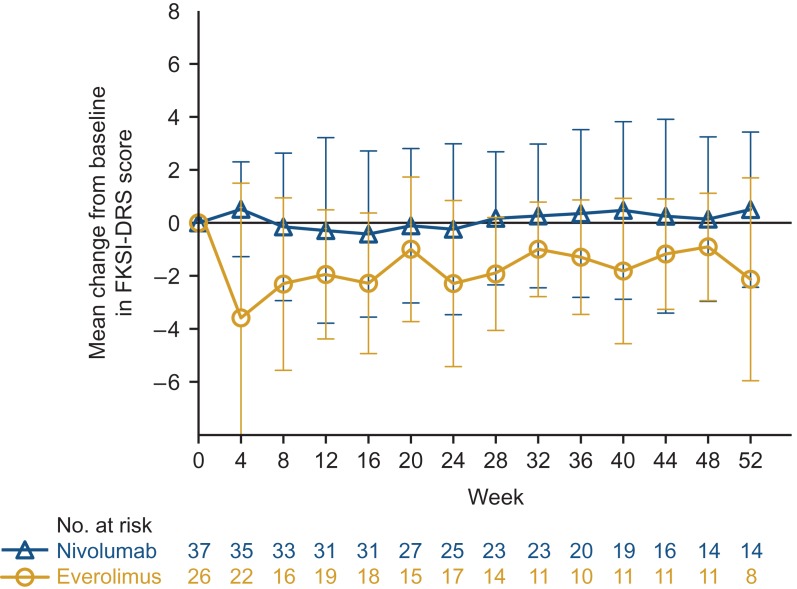
Among Japanese patients, the FKSI-DRS quality-of-life survey completion rate exceeded 90% through 1 year of the study, with exception of weeks 8 (76%) and 36 (83%) in the everolimus arm (similar to the global population completion rate, as previously reported (2)). The median FKSI-DRS score at baseline was 34.0 in the nivolumab arm and 33.5 in the everolimus arm, higher than the median for the global population (31.0 for both arms, as previously reported (2)). The mean change in scores for the FKSI-DRS, assessed every 4 weeks, were generally equal to or slightly exceeded baseline values at every assessment in the nivolumab arm (Fig. 4). Scores in the everolimus arm were lower at every assessment with 5 assessments at least 2 points below baseline, considered a meaningful change in score (Fig. 4). These results are generally consistent with results at 1 year for the global population, as previously reported (2), except in the global population the decrease in scores with everolimus were not as striking (2,10).
Figure 4.
Mean change from baseline in FKSI-DRS score through 1 year in Japanese patients. Bars show standard error. FKSI-DRS, Functional Assessment of Cancer Therapy Kidney Symptom Index–Disease-Related Symptoms.
Subsequent therapy
In all, 25 (68%) and 21 (81%) Japanese patients received subsequent systemic therapy after nivolumab and everolimus, respectively. The most common subsequent systemic therapy was axitinib for both arms (15/37 [41%] in the nivolumab arm; 13/26 [50%] in the everolimus arm). The median time from randomization to subsequent therapy in Japanese patients was 13.6 months (95% CI: 9.8–30.2) and 11.3 months (95% CI: 6.0–16.2) in the nivolumab and everolimus arms, respectively.
In the global population, 251 (61%) and 274 (67%) patients received subsequent systemic therapy after nivolumab and everolimus, respectively. The most common subsequent systemic therapy in the nivolumab arm was everolimus (129/410 [31%]) and in the everolimus arm was axitinib (162/411 [39%]). The median time from randomization to subsequent therapy was 12.4 months (95% CI: 10.3–13.2) and 8.0 months (95% CI: 7.0–9.6) in the nivolumab and everolimus arms, respectively.
Discussion
CheckMate 025 continued to demonstrate superior OS and higher ORR with nivolumab versus everolimus in the global study population, with more than 2 years of follow-up. OS was higher with nivolumab in the Japanese population than in the global population and was similar between nivolumab and everolimus in Japanese patients, with medians not reached. The higher KPS and differences in prior and potentially subsequent therapies in Japanese patients compared with the global population may have contributed to this result. Importantly, the small sample size in the Japanese population limits interpretation of OS. Additionally, the imbalance in prognostic factors at baseline between nivolumab and everolimus arms in Japanese patients, such as fewer patients with ≥1% PD-L1 expression, >2 sites of metastases and liver metastases in the everolimus arm, may have contributed to the similar OS noted in both arms.
Consistent with the global study findings at 14 months, ORR was higher for nivolumab versus everolimus in the Japanese population. ORR was substantially higher for nivolumab in the Japanese population than for the global population and the difference between arms was more notable in the Japanese population. Differences in prior therapies and the higher baseline KPS in Japanese patients versus the global population may have contributed to this result as well.
In previous studies of targeted therapies, the safety profile has in some cases differed in Japanese patients compared with Western patients (13–15). An understanding of whether differences are observed in Japanese patients – a historically under-represented population in clinical trials – and what these differences are may improve management of AEs, which in turn may improve overall outcomes in these patients. In the current study, safety in Japanese patients was generally consistent with the global population, with the exception of decreased incidence of Grade 3 or 4 treatment-related AEs with nivolumab in Japanese patients and increased incidence of Grade 3 or 4 treatment-related AEs with everolimus. Consistent with prior reports, the incidence of treatment-related AEs with nivolumab was lower than with everolimus, including treatment-related select AEs with immune-mediated etiology, except for endocrine and gastrointestinal AEs. Incidence of stomatitis was higher in Japanese patients in both arms compared with previous reports from the global population in this study (2) and consistent with an independent study of everolimus in Japanese and non-Japanese patients (16). In the everolimus arm but not the nivolumab arm, incidence of rash, thrombocytopenia and proteinuria was high in Japanese patients, a result also observed in studies of axitinib and everolimus (16,17). Quality of life among Japanese patients was assessed only through 1 year, given the small sample size in the second year of the study. Compared with the global population, Japanese patients overall had higher baseline FKSI-DRS quality of life scores and modest improvement with nivolumab, not surprising given that the baseline score was only 2 points lower than the best possible score. Conversely, the decrease in quality of life over time with everolimus was more pronounced compared with the global population (2,10).
To our knowledge, this is the first report of an analysis of Japanese patients treated with nivolumab for RCC. A number of large global trials in recent years have performed subgroup analyses of efficacy and/or safety of TKIs and mTOR inhibitors in Japanese patients (16,18–21). Notably, improved efficacy in Japanese patients compared with the global population was seen in most studies, although Japanese patients generally had more favorable baseline disease characteristics, as was the case in this study (16,18–21). Randomization and stratification of Japanese patients specifically, not as part of a larger group, may help to circumvent this issue. In Japanese patients with metastatic RCC treated with sunitinib, there was a trend in greater antitumor activity and higher incidence of hematological AEs compared with historical results observed in Caucasian patients (20). In a Phase III study that compared pazopanib and sunitinib in treatment-naïve patients, PFS was similar among Asian, North American and European populations, with varying incidence of some AEs across groups (21). In a small Japanese subgroup analysis from the Phase III RECORD-1 study of everolimus versus placebo in previously treated patients with metastatic RCC, Japanese patients experienced similar or better efficacy than the overall study population with similar types and higher incidences of AEs (16). In Phase II and III studies of axitinib in patients with RCC, efficacy with axitinib, particularly in patients with prior cytokine therapy, was higher in Japanese patients compared with the global population, though Japanese patients had more favorable baseline disease characteristics than non-Japanese patients and much higher rates of cytokine pretreatment (18,19).
There are several limitations to this analysis. The small sample size of Japanese patients and the different sample size between arms due to stratification as part of a larger regional group (that included non-Japanese patients) may have affected outcomes. Additionally, there is an unknown effect of prior therapies on the efficacy of nivolumab. Japanese patients are often treated with different therapies than are Western patients, so comparisons with the global CheckMate 025 population should be made with caution. In a retrospective analysis of 110 Japanese patients treated with sorafenib, those who had previous cytokine treatment had significantly higher OS (P = 0.002) and PFS (P = 0.017) than did patients without prior cytokine treatment (22).
The results from this study support the recent approval of nivolumab for previously treated patients in Japan. A multinational study examining the combination of nivolumab with ipilimumab in first-line RCC is ongoing and includes Japanese patients (CheckMate 214). Given that Asian patients with RCC have, in some cases, had different outcomes than patients in Western countries, future global studies should include additional Asian patients and include univariate and multivariate analyses of potential predictive factors to better examine the efficacy and safety of novel therapies in this patient population.
Authors' contributions
Yoshihiko Tomita had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tomita, Uemura, McHenry, Berghorn, Ozono.
Provision of study materials or patients: Tomita, Fukasawa, Shinohara, Kitamura, Oya, Eto, Tanabe, Kimura, Yonese, Yao, Motzer, Uemura, Ozono.
Collection and assembly of data: McHenry, Berghorn.
Data analysis and interpretation: All authors.
Drafting of the manuscript: Tomita.
Critical revision of the manuscript for important intellectual content: All authors.
Final approval of manuscript: All authors.
Funding
This work was sponsored by Bristol-Myers Squibb and Ono Pharmaceutical Company Limited. Authors received no financial support or compensation for publication of this manuscript. The funders contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript in collaboration with the investigators and authors of this report.
Conflict of interest statement
Yoshihiko Tomita has received consultancy/advisory fees from Novartis and Ono Pharmaceutical Co.; honoraria from Astellas, Novartis, Ono Pharmaceutical Co., Pfizer, and Sanofi Aventis; and research funding from Astellas, AstraZeneca and Pfizer.
Satoshi Fukasawa, Hiroshi Kitamura, Kazunari Tanabe, Junji Yonese, and Masahiro Yao do not have any conflicts of interest to disclose.
Nobuo Shinohara has received consultancy/advisory fees from Ono Pharmaceutical Co. and Takeda Pharmaceuticals, and honoraria from Bayer, GlaxoSmithKline, Novartis, and Pfizer.
Mototsugu Oya has received honoraria from Bayer, Bristol-Myers Squibb, Novartis, Ono Pharmaceutical Co., and Pfizer.
Masatoshi Eto has received consultancy fees from Shionogi, Novartis, Pfizer, GlaxoSmithKline, Sanofi and Takeda; and had advisory roles from Novartis, Pfizer and Bristol-Myers Squibb.
Go Kimura has received consultancy fees/honoraria from Bristol-Myers Squibb and Ono Pharmaceutical Co.
Robert J. Motzer has received consultancy/advisory fees from Eisai Pharmaceuticals, Exelixis, Novartis, and Pfizer, and research funding from Bristol-Myers Squibb, Eisai Pharmaceuticals, Exelixis, Genentech/Roche, Novartis and Pfizer.
Hirotsugu Uemura has received consultancy/advisory fees and honoraria from Bristol-Myers Squibb and Ono Pharmaceutical Co.
M. Brent McHenry and Elmer Berghorn are employees of and hold stock options in Bristol-Myers Squibb.
Seiichiro Ozono has received consultancy/advisory fees from Bayer Yakuhin Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., and Pfizer.
Acknowledgments
We thank the patients and their families, as well as the investigators and participating study teams, for making this study possible, and Justin Doan for providing quality-of-life analyses and interpretation. This study in Japan was conducted at the following institutions: Hokkaido University Hospital, Sapporo Medical University Hospital, Akita University Hospital, Iwate Medical University Hospital, Yamagata University Hospital, Chiba Cancer Center, Cancer Institute Hospital, Keio University Hospital, Teikyo University Hospital, Tokyo Women's Medical University Hospital, The University of Tokyo Hospital, Nippon Medical School Hospital, Yokohama City University Hospital, Hamamatsu University Hospital, University Hospital Kyoto Prefectural University of Medicine, Osaka University Hospital, Kindai University Hospital, Tokushima University Hospital, Kyushu University Hospital, and Kumamoto University Hospital. All authors contributed and approved the manuscript; medical writing assistance was provided by Jennifer Granit, Ph.D., of PPSI (a PAREXEL company), funded by Bristol-Myers Squibb.
References
- 1. Yoshimura K, Uemura H. Pharmacotherapies for renal cell carcinoma in Japan. Int J Urol 2016;23:194–202. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naito S, Tomita Y, Rha SY, et al. Kidney Cancer Working Group report. Jpn J Clin Oncol 2010;40:i51–i6. [DOI] [PubMed] [Google Scholar]
- 4. Oh WK, McDermott D, Porta C, et al. Angiogenesis inhibitor therapies for advanced renal cell carcinoma: toxicity and treatment patterns in clinical practice from a global medical chart review. Int J Oncol 2014;44:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuchiya N, Narita S, Inoue T, et al. Risk factors for sorafenib-induced high-grade skin rash in Japanese patients with advanced renal cell carcinoma. Anticancer Drugs 2013;24:310–4. [DOI] [PubMed] [Google Scholar]
- 6. Ye D, Eto M, Chung JS, et al. Use of targeted therapies for advanced renal cell carcinoma in the Asia-Pacific region: opinion statement from China, Japan, Taiwan, Korea, and Australia. Clin Genitourin Cancer 2014;12:225–33. [DOI] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN Guidelines®): kidney cancer. Version 3. 2016. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. (1 November 2016, date last accessed).
- 8. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 9. Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health 2007;10:285–93. [DOI] [PubMed] [Google Scholar]
- 10. Cella D, Grunwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29–41. [Google Scholar]
- 12. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–14. [Google Scholar]
- 13. Iijima M, Fukino K, Adachi M, et al. Sorafenib-associated hand-foot syndrome in Japanese patients. J Dermatol 2011;38:261–6. [DOI] [PubMed] [Google Scholar]
- 14. Nozawa M, Nonomura N, Ueda T, et al. Adverse event profile and dose modification of everolimus for advanced renal cell carcinoma in real-world Japanese clinical practice. Jpn J Clin Oncol 2013;43:1132–8. [DOI] [PubMed] [Google Scholar]
- 15. Tomita Y, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010;40:1166–72. [DOI] [PubMed] [Google Scholar]
- 16. Tsukamoto T, Shinohara N, Tsuchiya N, et al. Phase III trial of everolimus in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from RECORD-1. Jpn J Clin Oncol 2011;41:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer 2011;47:2592–602. [DOI] [PubMed] [Google Scholar]
- 18. Tomita Y, Fukasawa S, Oya M, et al. Key predictive factors for efficacy of axitinib in first-line metastatic renal cell carcinoma: subgroup analysis in Japanese patients from a randomized, double-blind phase II study. Jpn J Clin Oncol 2016;46:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ueda T, Uemura H, Tomita Y, et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized phase 3 AXIS trial. Jpn J Clin Oncol 2013;43:616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 2010;40:194–202. [DOI] [PubMed] [Google Scholar]
- 21. Guo J, Jin J, Huang Y, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). J Clin Oncol 2013;31(Suppl 6; abstr 366). [Google Scholar]
- 22. Suzuki H, Suzuki T, Ishizuka O, Nishizawa O, Ueno M. Efficacy and safety of advanced renal cell carcinoma patients treated with sorafenib: roles of cytokine pretreatment. Int J Clin Oncol 2014;19:686–92. [DOI] [PubMed] [Google Scholar]