Abstract
Background
This study aimed to investigate the therapeutic effect of low, medium, and high concentrations of medical ozone on trauma-induced lumbar disc herniation.
Material/Methods
A total of 80 patients were included and were grouped into a control group, a low medical ozone (20 μg/ml) group, a medium medical ozone (40 μg/ml) group, and a high medical ozone (60 μg/ml) group. The CT scan and enzyme-linked immunosorbent assay (ELISA) were used to detect IL-6 level, SOD activity, IgM, and IgG levels upon admission and at 6 and 12 months after follow-up. The area under the ROC curve (AUC) was calculated for visual analogue scale (VAS) and efficiency rate.
Results
All patients showed disc retraction at 6- and 12-month follow-up; while patients in the medium medical ozone (40 μg/ml) group showed the greatest disc retraction rate. The IL-6, IgM, IgG, and VAS levels significantly decreased while SOD activity increased among all groups over time (p<0.05). The AUCIL-6, AUCIgG, AUCIgM, and AUCSOD was closest to 1 in the medium medical ozone (40 μg/ml) group compared with other groups (p<0.01), with the highest efficacy at 6 (35%) and 12 (85%) months during follow-up.
Conclusions
Low concentrations of medical ozone (20 μg/ml and 40 μg/ml) reduced the serum IL-6, IgG, and IgM expression, presenting as analgesic and anti-inflammatory effects, while high concentrations of medical ozone (60 μg/ml) increased the serum IL-6, IgG, IgM expression, presenting as pain and pro-inflammatory effects.
The medical ozone concentration of 40 μg/ml showed the optimal treatment efficacy.
MeSH Keywords: Lumbar Vertebrae, Ozone, Treatment Outcome
Background
Lumbar disc herniation (LDH) is one of the bone diseases with highest prevalence due to lumbar disc degeneration, external injury, humid and cold environments, and genetic factors [1]. LDH mainly manifests as lumbar and leg pain, limited mobility, and decreased muscle strength, greatly reducing patient quality of life [2]. The occurrence of LDH is closely associated with age, disc herniation, degeneration, and trauma [3–5]. In LDH, the nucleus breaks through the ruptured annulus and protrudes into the spinal canal, most commonly affecting patients age 40–45 years [6]. Currently, the pain is considered to result from the compression, irritation, and chemical inflammation of peripheral nerves surrounding intervertebral discs [7–9]. The nerve root disease results from spinal nerve axons or nerve root conduction damage with abnormal sensory and motor function [10,11]. Free radical damage is shown to be involved in the development of many diseases; for example, the progressive and cumulative injuries are associated with normal or abnormal metabolic process of free radicals [12]. Superoxide dismutase (SOD) is the main superoxide anion radical scavenging enzyme [13], whose activity reflects the body’s antioxidant capacity and oxidation balance [14]. This enzyme catalyzes the conversion of superoxide radicals to oxygen and hydrogen peroxide and scavenges superoxide anion radicals, protecting against cellular immune injury. SOD content is an important indicator of the capacity to remove free radicals in peripheral nerve tissues, and SOD activity is closely associated with inflammation and autoimmune diseases [12]. The lumbar disc herniation treatments include physical therapy, drug therapy, massage traction, minimally invasive therapy, and surgical therapy [15–17].
In this study, we investigated the effects of low, medium, and high concentrations of medical ozone (20, 40, and 60 μg/ml) on IL-6, IgM, IgG, SOD activity, visual analogue scale (VAS) score, and effective rate in patients with LDH to explore the treatment efficacy of medical ozone.
Material and Methods
Subjects
All patients diagnosed as LDH at Jining First People’s Hospital from 2015 to 2016 were included. The control group (Group A) received conventional drug treatment without any other intervention. The medical ozone injection (20 μg/ml in Group B, 40 μg/ml in Group C, and 60 μg/ml in Group D) was performed by CT-guided puncture of the intervertebral discs. The study was approved by the Medical Ethics Board of Jining First People’s Hospital. Informed consent was obtained from all patients or their families.
ELISA
The serum levels of IL-6, IgM, and IgG, as well as VAS, SOD activity, and treatment efficiency, were measured by enzyme-linked immunosorbent assay (ELISA). The OD values of each sample were determined at 450 nm wavelength.
VAS score
VAS scores were used for pain assessment, using a ruler with a length of 10 cm marked with a scale of 10 points. Zero points indicated no pain, while 10 points indicated the most severe pain: patients with 1–3 points showed mild pain; patients with 4–6 points showed moderate pain that affected sleep; and patients with 7–10 points showed severe pain that affected eating and sleep.
Statistical analysis
The SPSS (version 19.0, IBM, USA) were used for statistical analysis. The measurement data are expressed as mean ± standard deviation (mean ±SD). One-way analysis of variance (ANOVA) was used to compare the data among groups; Fisher’s least significant difference (LSD) and t test were used among groups. Friedman M test and q test were used for nonparametric testing. P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 80 patients were recruited. They were randomly divided into 4 groups, with 20 in each group. There were 49 males and 31 females, with average age of 48 years (range, 24–76 years). All patients had LDH symptoms and were diagnosed by MR and CT. The 4 groups were matched for symptoms, physical examinations, and MR/CT presentations. There were 5 cases of L3–4 LDH, 60 cases of L4–5 (5 cases of protrusion), and 15 cases of L5–S1 (3 cases of protrusion).
CT comparisons
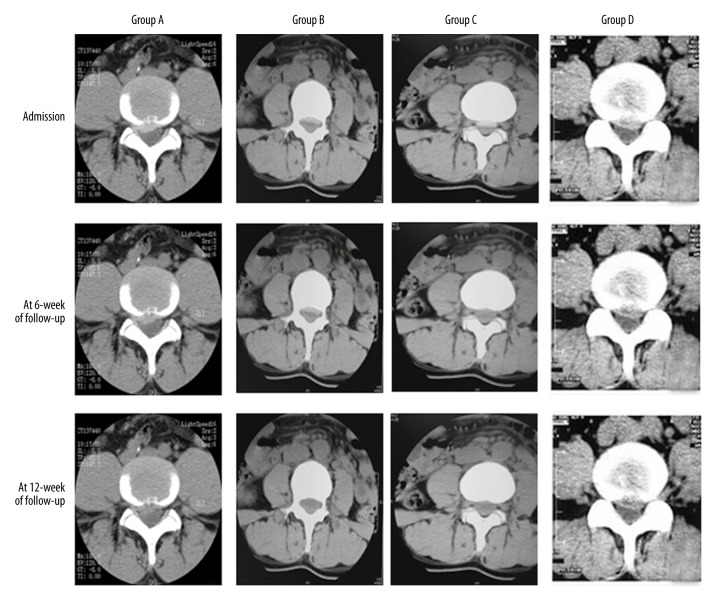
To determine the ozone treatment efficacy, their CT scans were analyzed. After treatment, the LDH retraction rate significantly increased at 6 and 12 months post-treatment in all groups. Group C showed the greatest retraction rate among all groups (Figure 1), indicating that 40 ug/ml medical ozone most effectively treated LDH compared with other ozone levels.
Figure 1.
CT images of Group A, Group B, Group C, and Group D. Upon admission, the intervertebral discs protruded; the intervertebral disc significantly retracted at 6-month follow-up; the intervertebral disc significantly retracted at 12-month follow-up.
IL-6 levels
To determine the effects of ozone on IL-6 levels, the IL-6 levels upon admission and at 6- and 12-month follow-up were analyzed. Compared with the control group (Group A), the levels of IL-6 in groups B and C decreased, but they increased in group D (P<0.05). The serum IL-6 levels gradually decreased (P<0.05) over time among all groups (P<0.05), as shown in Table 1. These results show that medical ozone significantly decreased the serum IL-6 as anti-inflammatory effects, and high ozone level (60 ug/ml) increased the serum IL-6 as pro-inflammatory effects.
Table 1.
The IL-6 levels in all groups (pg/ml, n=20).
| Upon admission | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Group A | 224.7±3.5 | 191.3±3.4* | 172.8±2.6* |
| Group B | 225.8±3.2# | 172.8±2.5#* | 160.7±3.2#* |
| Group C | 229.6±2.7# | 163.7±3.1#* | 135.9±2.4#* |
| Group D | 228.7±3.1# | 200.7±2.5#* | 186.7±2.8#* |
Compared with Group A,
P<0.05; compared with that upon admission,
P<0.05.
IgM levels
To determine the effects of ozone on IgM levels, the IgM levels at admission and at 6- and 12-month follow-ups were analyzed. Compared with Group A, the levels of IgM in Groups B and C decreased, but they increased in Group D (P<0.05). The serum IgM levels gradually decreased (P<0.05) over time among all groups (P<0.05), as shown in Table 2. These results show that ozone suppressed the inflammation and its development, presenting as decreased serum IgM. Ozone at 40 ug/ml showed the highest suppression effect.
Table 2.
The IgM levels in all groups (mg/ml, n=20).
| Upon admission | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Group A | 2.73±1.25 | 2.05±1.41* | 1.86±1.61* |
| Group B | 2.65±1.22# | 1.89±1.21#* | 1.71±1.11#* |
| Group C | 2.62±1.12# | 1.34±1.21#* | 1.17±1.04#* |
| Group D | 2.71±1.31# | 2.27±1.21#* | 1.93±1.38#* |
Compared with Group A,
P<0.05; compared with that upon admission,
P<0.05.
IgG levels
To determine the effects of ozone on IgG levels, the IgG levels at admission and at 6- and 12-month follow-ups were analyzed. Compared with Group A, the levels of IgG in Groups B and C decreased, but they increased in Group D (P<0.05). The serum IgG levels gradually decreased (P<0.05) over time among all groups (P<0.05), as shown in Table 3. These results indicate that ozone significantly suppressed the inflammation, presenting as decreased IgG, and ozone at 40 ug/ml showed the highest suppression effect.
Table 3.
The IgG levels in all groups (g/L, n=20).
| Upon admission | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Group A | 27.73±1.25 | 22.05±1.31* | 18.76±1.21* |
| Group B | 27.43±1.12 | 19.79±1.11#* | 17.61±1.41#* |
| Group C | 27.12±1.32 | 17.34±1.13#* | 15.17±1.24#* |
| Group D | 27.38±1.21 | 24.27±1.11#* | 19.93±1.18#* |
Compared with Group A,
P<0.05; compared with that upon admission,
P<0.05.
SOD activity
To determine the effects of ozone on SOD activity, the SOD activity at admission and at 6- and 12-month follow-ups were analyzed. Compared with Group A, the levels of SOD activity in Groups B and C decreased, but they increased in group D (P<0.05). The SOD activity gradually decreased (P<0.05) over time among all groups (P<0.05), as shown in Table 4. These results indicate that ozone increased the SOD activity in LDH patients, with the best treatment efficacy at 40 ug/ml.
Table 4.
The SOD activity in all groups (g/L, n=20).
| Upon admission | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Group A | 97.73±1.15 | 132.05±1.31* | 156.76±1.21* |
| Group B | 99.43±1.21 | 152.29±1.11#* | 170.61±1.41#* |
| Group C | 98.12±1.12 | 162.14±1.13#* | 192.17±1.24#* |
| Group D | 99.38±1.21 | 110.27±1.11#* | 149.93±1.18#* |
Compared with Group A,
P<0.05; compared with that upon admission,
P<0.05.
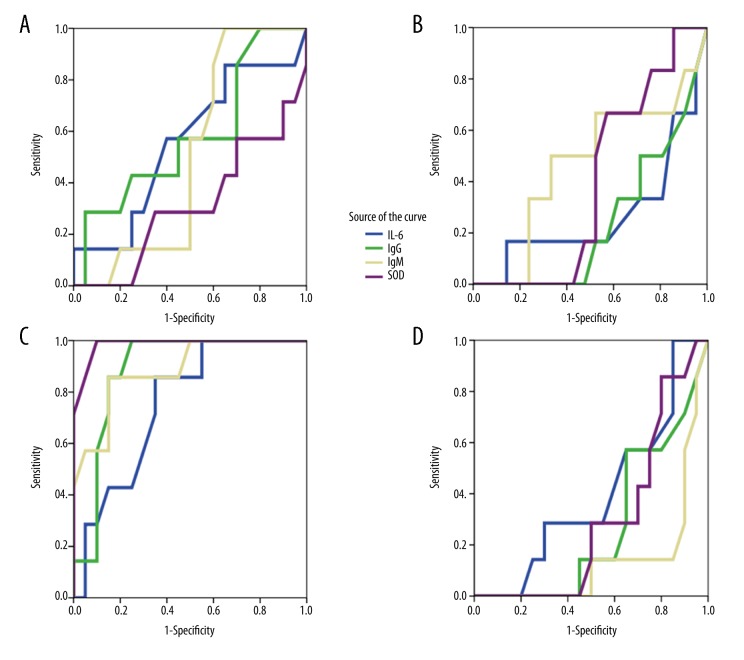
The ROC and area under the ROC curve (AUC) of IL-6, IgM, IgG, and SOD activity
To determine the efficacy of ozone, the ROC and AUC of IL-6, IgM, IgG, and SOD activity were analyzed. The AUCIL-6, AUCIgG, AUCIgM, and AUCSOD was closest to 1 in Group B compared with other groups (p<0.01), indicating the highest efficacy (Figure 2).
Figure 2.
ROC curve analysis. (A) ROC curve of Group A. AUCIL-6: 0.557; AUCIgG: 0.582; AUCIgM: 0.504; AUCSOD: 0.314. (B) ROC curve of Group B. AUCIL-6: 0.396; AUCIgG: 0.268; AUCIgM: 0.139; AUCSOD: 0.296. (C) ROC curve of Group C. AUCIL-6: 0.754; AUCIgG: 0.886; AUCIgM: 0.878; AUCSOD: 0.986. (D) ROC curve of Group D. AUCIL-6: 0.270; AUC IgG: 0.238; AUC IgM: 0.468; AUCSOD: 0.393.
The VAS score
To determine the efficacy of ozone, the VAS score was analyzed. Compared with Group A, the levels of VAS score in Groups B and C decreased, but they increased in group D (P<0.05). The VAS cores gradually decreased (P<0.05) over time among all groups (P<0.05), as shown in Table 5. These results indicate that VAS scores decreased with increasing treatment duration. VAS scores decreased the most over time at an ozone dose of 40 ug/ml.
Table 5.
The VAS scores in all groups (points, n=20).
| Upon admission | 6-month follow-up | 12-month follow-up | |
|---|---|---|---|
| Group A | 8.76±1.23 | 6.05±1.21* | 4.66±1.22* |
| Group B | 8.63±1.24# | 4.79±1.11#* | 3.31±1.41#* |
| Group C | 8.72±1.42# | 3.34±1.14#* | 2.17±1.24#* |
| Group D | 8.58±1.31# | 5.37±1.21#* | 3.93±1.16#* |
Compared with Group A,
P<0.05; compared with that upon admission,
P<0.05.
Treatment efficacy
To determine the efficacy of ozone, the treatment efficiency of each group was compared. The treatment efficiency of Group A was 15% (3/20) at 6-month follow-up and 60% (12/20) at 12-month follow-up; the treatment efficiency of Group B was 20% (4/20) at 6-month follow-up and 70% (14/20) at 12-month follow-up; the treatment efficiency of Group C was 35% (7/20) at 6-month follow-up and 85% (17/20) at 12-month follow-up; the treatment efficiency of Group D was 10% (2/20) at 6-month follow-up and 55% (11/20) at 12-month follow-up. These results indicate that Group C (40 ug/ml) had the highest treatment efficacy.
Discussion
The vertebral disc is mainly made of 3 parts: the nucleus pulposus, the annulus fibrosus, and the cartilaginous endplates [18]. The nucleus pulposus is in the central disc, with gelatinous water content as high as 75–80% [19,20]. The matrix is principally composed of water, proteoglycan, and type II collagen fibers in network structures and elastin in radial structure [21]. Cells are mainly arranged in cartilage-like dispersion [20]. The vertebral disc has no blood supply, and its nutritional supply and material metabolism are mainly provided by diffusion through the cartilaginous endplates [22]. The absence of vascular supply and nerves make it difficult to repair trauma injuries and degeneration; therefore, the integrity of intervertebral disc matrix is essential for maintaining the function of the intervertebral disc [23].
A variety of factors, including redox imbalance, inflammation, and immune imbalance, as well as local factors, are involved in the pathogenesis of LDH, which causes lower back pain [24,25].
The pathogenesis of LDH is not yet fully elucidated. The nerve compression and inflammation are suggested to cause the numbness and pain [26]. Infiltration of neutrophils and macrophages often cause inflammation; IL-6 and TNF-α can further strengthen the response [27–29]. Pain would disappear if the inflammatory response disappeared, even with presence of protrusion [27–29].
Production of prostaglandins, phospholipase A2, leukotrienes, thromboxanes, and nitric oxide is increased in prominent intervertebral disc tissue [30,31], and the expression of pro-inflammatory cytokines (TNF-α and IL-6) also increase in patients with LDH. The increase of IL-6 expression suggests Thl cell activation and LDH development [32]. The increased in IL-6 may stimulate the synthesis of PGE2 [33–35], inducing the expression of TNF-α and the apoptosis of dorsal root ganglion neurons [36,37], leading to the development of pain and hyperalgesia.
In the present study, the serum IL-6 level decreased in all groups over time (P<0.05), indicating that the longer treatment was associated with weaker inflammatory response. Compared with group A, the levels of IL-6 in Groups B and C were significantly decreased and those in Group D were significantly increased (P<0.05). In addition, the AUC of IL-6 levels was the highest in Group C. The above results indicate that low ozone (20 μg/ml or 40 μg/ml) can reduce the serum IL-6 levels as an anti-inflammatory effect, while the high ozone (60 μg/ml) level can increase serum IL-6 levels as a pro-inflammatory effect.
The pain induced by inflammatory response may be due to direct local chemical stimulation and/or autoantigen autoimmune responses caused by the prominent nucleus pulposus [38]. LDH patients often have abnormal immunity, presenting as increased IgG and IgM in serum, cerebrospinal fluid, and intervertebral disc tissue [27,28]. It has been proposed that LDH patients have abnormal autoimmune status, showing as abnormal increase in peripheral blood immunoglobulin, mainly IgG and IgM [39,40]. In the present study, serum IgG and IgM were selected as the efficacy indicators for LDH treatment. It shows that IgG and IgM can form antigen-antibody complexes when intervertebral disc antigens are exposed to the body’s immune system [41], causing autoimmune inflammation [42] and low back pain and sciatica [43]. When more antigens are exposed, there are higher immunoglobulin levels, as well as more severe autoimmune inflammation and clinical symptoms [44]. Compared with Group A, the levels of IgM and IgG in Groups B and C decreased, while those in Group D increased (P<0.05). Serum IgM and IgG decreased gradually over time in all groups (P<0.05), indicating the abnormal immunity in LDH patients and possible autoantibody of the intervertebral disc tissue as IgG and IgM.
It was reported that a certain concentration of ozone may stimulate the production of a variety of immunoregulatory cytokines and inhibit the proliferation of human lymphocytes, and can be used as a treatment for ulcerative colitis, Crohn’s disease, and other autoimmune diseases [45–48]. We believe that the mechanism of ozone treatment for LDH lies in the suppression of humoral immunity. Our results showed that the levels of IgG and IgM, as well as VAS scores, in LDH patients decreased after treatment (P<0.05), indicating that 20 μg/ml and 40 μg/ml medical ozone can inhibit the occurrence and development of autoimmune inflammatory responses and alleviate the clinical symptoms. Medical ozone at 40 μg/ml showed the optimal treatment efficacy.
SOD is an enzyme that catalyzes superoxide radical into either ordinary molecular oxygen or hydrogen peroxide, acting as an antioxidant defense in nearly all living cells exposed to oxygen [12]. SOD and CAT participate in the pathogenesis of LDH [49], and clinical symptoms improved as SOD and CAT activity increased [50]. In patients with normal immunity, the free radical scavenging system can keep the free radicals at a harmless low level [51]. However, in the degeneration, chronic injury, and disturbed microcirculation, SOD activity decreased, cell permeability increased, and cytokines increased, leading to tissue inflammation and pain [52]. Increased free radicals and tissue damage can cause increased (compensatory mechanism) SOD activity to remove excess free radicals and protect the body [53].
The present study is limited by its short follow-up time (12 months). Long-term efficacy needs further study.
Conclusions
In this study, we show that low concentrations of medical ozone (20 μg/ml and 40 μg/ml) can reduce the serum IL-6, IgG, and IgM expression, presenting as analgesic and anti-inflammatory effects; while high concentrations of medical ozone (60 μg/ml) increase the serum IL-6, IgG, and IgM expression, presenting as pain and pro-inflammatory effects. The medical ozone concentration of 40 μg/ml showed the optimal treatment efficacy.
Acknowledgements
We thank Professor Baohua Xing, Professor Jishou Lu, Professor Yanchun Wei, and Professor Hongtu Wei for their valuable suggestions.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Tang S, Rui B, Xu W. [Study on clinical application of medical ozone to the treatment of lumbar intervertebral disc protrusion]. Anhui Medical Journal. 2010;31:1305–7. [in Chinese] [Google Scholar]
- 2.Jia F, Wang F, Xu C. [Clinical analysis of medical ozone interventional in the treatment of lumbar disc herniation]. Chinese Journal of the Frontiers of Medical Science (Electronic Version) 2014;6:68–70. [in Chinese] [Google Scholar]
- 3.Cheung KMC, Karppinen J, Chart D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34:934–40. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 4.Karamouzian S, Eskandary H, Faramarzee M, et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35:881–86. doi: 10.1097/BRS.0b013e3181b9c986. [DOI] [PubMed] [Google Scholar]
- 5.Weber H. The natural history of disc herniation and the influence of intervention. Spine. 1994;19:2234–38. doi: 10.1097/00007632-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–8. [PubMed] [Google Scholar]
- 7.Miyoshi S, Sekiguchi M, Konno SI, et al. Increased expression of vascular endothelial growth factor protein in dorsal root ganglion exposed to nucleus pulposus on the nerve root in rats. Spine. 2011;36:El–6. [PubMed] [Google Scholar]
- 8.McCarron RF, Wimpee MW, Hudkins PG, Laros GS. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine. 1987;12:760–64. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinou K, Duma KM. Sciatica: Review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464–72. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 10.Merskey H, Bogduk N. Classification of chronic pain Descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 11.Murphy DR, Hurwitz EL, Gerrard JK, Clary R. Pain patterns and descriptions in patients with radicular pain: Does the pain necessarily follow a specific dermatome? Chiropr Osteopat. 2009;17:9. doi: 10.1186/1746-1340-17-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng W, Hou J, Chen W, et al. [Effect of zhouluotong on the function of sciatic nerve, activity of aldose reductase and ability of aldose reductase against free radicals in the rats with diabetes]. Journal of Beijing University of Traditional Chinese Medicine. 2004;27:45–48. [in Chinese] [Google Scholar]
- 13.Zhang X, Ma Y, Yang J. [Association between cervical spondylosis, lumbar disc herniation]. Journal of Neck and Back Pain. 1995;16:228. [in Chinese] [Google Scholar]
- 14.Yang W, Wang R, Zhang Y. [Determination of superoxide dismutase in patients with psoriasis]. Chinese Journal of Dermatology. 1992;25:921. [in Chinese] [Google Scholar]
- 15.Jones KL, Bryan TW, Jinkins PA, et al. Superoxide released from neutrophils causes reduction in nitric oxide gas. Am J Physiol. 1998;279:1120–26. doi: 10.1152/ajplung.1998.275.6.L1120. [DOI] [PubMed] [Google Scholar]
- 16.Gibson JNA, Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev. 2009;1:CD001350. doi: 10.1002/14651858.CD001350.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Manchikanti L, Falco FJE, Benyamin RM, et al. An update of the systematic assessment of mechanical lumbar disc decompression with nucleoplasty. Pain Physician. 2013;16(2 Suppl):SE25–54. [PubMed] [Google Scholar]
- 18.Yu Z, He X, He S, et al. [The treatment of lumbar disc herniation: Intradiscal ozone distribution and effect]. Journal of Clinical Radiology. 2003;22:869–72. [in Chinese] [Google Scholar]
- 19.Muto M, Aveik F. Percutaneous treatment of herniated lumbar disc by intradiscal oxygen-ozone injection. Interv Neuroradiol. 1998;4(4):279–86. doi: 10.1177/159101999800400403. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y. Lumbar intervertebral disc herniation. 2nd ed. Beijing: People’s medical publishing house; 1995. p. 72. [Google Scholar]
- 21.Liang C, Xu Y, Zhang X. [Risk factors and prevention for postoperative spondylodiscitis after lumbar intervertebral disc protrusion]. Medical Innovation of China. 2012;9:6–8. [in Chines] [Google Scholar]
- 22.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976) 2000;25:853–57. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 23.Xing J, Guo X, Cui Z, et al. [Clinical observation of percutaneous discectomy combined with ozone injection and ozone injection in the treatment of lumbar disc herniation]. Jilin Medical Journal. 2012;33:6962–64. [in Chinese] [Google Scholar]
- 24.He X, Yu Z, Xi F, et al. [Treatment of lumbar disc herniation by using percutaneous intradiscal and paraspinal space injection of O2–O3 mixture]. Chinese Journal of Radiology. 2003;37:827–30. [in Chinese] [Google Scholar]
- 25.Jin J, Zhao Z, Weng X, et al. [Reoperative treatment of lumbar disc herniation]. Acta Academiae Medicinae Sinicae. 2005;27:160–64. [in Chinese] [PubMed] [Google Scholar]
- 26.Bai Y, Yang J, Ou Y, et al. [Long-term outcome after lumbar disc herniation]. Chinese Journal of Orthopaedic Surgery. 2003;11:832. [in Chinese] [Google Scholar]
- 27.Hirsch JA, Singh V, Falco FJE, et al. Automated percutaneous lumbar discectomy for the contained herniated lumbar disc: A systematic assessment of evidence. Pain Physician. 2009;12:601–20. [PubMed] [Google Scholar]
- 28.Spiliopoulou I, Korovessis P, Konstantinou D, et al. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine. 1994;19:1320–23. doi: 10.1097/00007632-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Satoh K, Konno S, Nishiyama K, et al. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine. 1999;24:1980–84. doi: 10.1097/00007632-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell JL, O’Donnell AL. Prostaglandin E2 content in herniated lumbar disc disease. Spine. 1996;21:1653–55. doi: 10.1097/00007632-199607150-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, Stefanovic-Racic M, Me Intyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–73. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Shamji M, Setton LA, Jarvis W. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–82. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Rand N, Reichert F, Floman Y, et al. Murine nucleus pulposus-derived cells secrete interleukins-1-β, -6, and-10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–601. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 35.Torri G, Della Grazia A, Casadei C. Clinical experience in the treatment of lumbar disk disease, with a cycle of lumbar muscle injections of an oxygen-ozone mixture. Int J Med Biol Environ. 1999;27:177–83. [Google Scholar]
- 36.Murata Y, Rydevik B, Nannmark U, et al. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-α in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine. 2001;36:926–32. doi: 10.1097/BRS.0b013e3181e7f4a9. [DOI] [PubMed] [Google Scholar]
- 37.Murata Y, Nannmark U, Rydevik B, et al. The role of tumor necrosis factor-α inapoptosis of dorsal root ganglion cells induced by herniated nucleus pulposus in rats. Spine. 2008;33:155–62. doi: 10.1097/BRS.0b013e3181605518. [DOI] [PubMed] [Google Scholar]
- 38.Spilipoulou I, Korovessis P, Konsraninou D, et al. IgG and IgM concentration in the prolapsed human intravertebral disc and sciatica etiology. Spine. 1994;19:1320–24. doi: 10.1097/00007632-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Naylor A. Enzymic and immunological activity in the intervertebral disc. Orthop Clin N Am. 1975;6:51–53. [PubMed] [Google Scholar]
- 40.Wang K, Hu Y. [Autoimmune status of lumbar disc herniation]. Chinese Journal of Orthopaedics. 1994;14:258–62. [in Chinese] [Google Scholar]
- 41.Naylar A. Enzymic and immunological activity in the intervertebral disc. Orthop Clin North N Am. 1975;6:51–58. [PubMed] [Google Scholar]
- 42.Goidie L. Granulation tissue in the ruptured intervertebral disc. Acta Orthop Stand. 1959;22:302–3. doi: 10.1111/j.1699-0463.1958.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 43.Macnab I. The mechanism of spondylogenic pain in cervical pain. London: Oxford Pergamon Press; 1971. pp. 89–95. [Google Scholar]
- 44.Chen Q, Wang D, Liu L, et al. [Correlation study of serum immunoglobulin after intervertebral disc collagenase dissolution]. Journal of Shandong University (Health Sciences) 2008;46:153. [in Chines] [Google Scholar]
- 45.Bocci V. Biological and clinical effects of ozone: Has ozone therapy a future in medicine. Br J Biomed Sci. 1999;56:270–79. [PubMed] [Google Scholar]
- 46.Bocci V. Does ozone therapy normalize the cellular redox balance? Implications for the therapy of human immunodeficiency vires infection and several other diseases medical hypotheses. Br J of Biomed Sei. 1996;46:150–4. doi: 10.1016/s0306-9877(96)90016-x. [DOI] [PubMed] [Google Scholar]
- 47.Tafil-Klawe M, Woźniak A, Drewa T, et al. Ozone therapy and the activity of selected lysosomal enzymes in blood serum of patients with lower limb ischaemia associated with obliterative atheromatosis. Med Sci Monit. 2002;8(7):CR520–25. [PubMed] [Google Scholar]
- 48.Biçer Ş, Sayar İ, Gürsul C, et al. Use of ozone to treat ileostomy dermatitis in an experimental rat model. Med Sci Monit. 2016;22:757–65. doi: 10.12659/MSM.897696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Wang H, Le S, et al. [The significance of detection of disc herniation of the nucleus pulposus of nitric oxide and interleukin −6 in lumbar]. Journal of Neck and Back Pain. 1999;20:324. [in Chinese] [Google Scholar]
- 50.Wen Z, Liu W, Xu X. [Comprehensive treatment of lumbar intervertebral disk hernia by TCD and traction and its influence on serum SOD activity]. The Journal of Traditional Chinese Orthopedics and Traumatology. 2005;17:10–11. [in Chinese] [Google Scholar]
- 51.Xu P, Jianfeng E, Cai Q, et al. [Analysis of the illness severity and the level of free radicals in patients with osteoarthritis]. The Orthopedic Journal of China. 2001;8:470. [in Chinese] [Google Scholar]
- 52.Song B, Guo Z, Chen Z, et al. [Oxygen free radical as a newly discovered pain mediator]. Chinese Journal of Pharmacology. 1996;13:43. [in Chinese] [Google Scholar]
- 53.Feng D, Huang D. Relationship between nitric oxide, SOD and lumbar disc herniation. Modern Rehabilitation. 1998;2:1098–99. [Google Scholar]