This review presents an overview of the current treatment approaches, problems with overdiagnosis and potential future management strategies for ductal carcinoma in situ of the breast.
Keywords: breast cancer, ductal carcinoma in situ, low grade DCIS, overtreatment, clinical trial
Abstract
Ductal carcinoma in situ (DCIS) has a good prognosis with the current treatment approach, with a 10-year breast cancer-specific survival rate of 97–98%. In ductal carcinoma in situ without micrometastasis, surgery and postoperative adjuvant therapy significantly improve local control, however it has been reported that the selection of the surgical procedure and adjuvant therapy does not influence breast cancer death. On the other hand, owing to widespread mammography screening, the frequency of early breast cancer detection has increased. In early breast cancer, increased incidence of DCIS is remarkable. However, there is not enough reduction of advanced cancer to match it. Problems with overdiagnosis are now being discussed all over the world. It has been reported that surgery for low-grade ductal carcinoma in situ does not contribute to breast cancer-specific survival. However, it is currently impossible to reliably identify a population that does not progress to invasive cancer even without treatment. Recently, a non-surgery clinical trial for low-risk ductal carcinoma in situ was started. There is a possibility of achieving individualized treatment for ductal carcinoma in situ with less treatment intervention, without compromising the good prognosis obtained with the current treatment approach. This review presents an overview of the current treatment approaches, problems with overdiagnosis and potential future management strategies for ductal carcinoma in situ of the breast.
Introduction
The prognosis of ductal carcinoma in situ (DCIS) is extremely good, and the 10-year cumulative breast cancer death rate has been reported to be 1.4–2.8% (National Surgical Adjuvant Breast and Bowel Project [NSABP] B-17, B-24) (1). DCIS currently accounts for about 20–25% in all new diagnosed breast cancer cases in the United States and for 17–34% in mammography (MMG)-detected cases (2). On the other hand, in Japan, A number of new primary breast cancer cases was estimated to be 90 000 according to the prediction of cancer incidence in 2016 and among these cases, DCIS was estimated to represent around 13 000 cases (3).
The basis of breast cancer treatment is local treatment with surgical therapy and radiotherapy, and systemic treatment with drug therapy for eradication of micrometastasis. In DCIS without micrometastasis, surgery and postoperative adjuvant therapy significantly improve local control; however, it has been reported that the selection of the surgical procedure (mastectomy vs breast-conserving therapy) and adjuvant therapy does not influence breast cancer death (4). Recently, it was reported that some DCIS does not become invasive cancer even without treatment (5); however, presently, such a population has not been reliably identified.
This review presents an overview of the current treatment approaches, problems with overdiagnosis and potential future management strategies for DCIS of the breast.
Standard treatment for DCIS
Surgery for DCIS
There are many meta-analyses and reviews on breast-conserving therapy for DCIS, and it has been shown that breast-conserving therapy is possible for DCIS (6–9). The indication is considered similar to that for invasive cancer. Whether to perform partial resection of the breast or mastectomy in routine practice is determined based on the localization and extent of the lesion and the possibility of mammary irradiation. If the lesion is completely resectable and cosmetic compatibility can be maintained, partial resection of the breast is selected. On the other hand, mastectomy is selected if the lesion is relatively extensive or suspected to involve multiple lesions. Additionally, although some patients are indicated for partial resection of the breast, they might prefer mastectomy and breast reconstruction rather than partial resection as partial resection might result in deformed breasts.
Because axillary lymph node metastasis is extremely rare in DCIS, axillary lymph node dissection is generally not performed. Sentinel lymph node biopsy is a minor surgery, but complications, such as numbness and edema of the upper arm, may occur. Therefore, disadvantages are considered greater for patients who have an extremely low probability of lymph node metastasis with partial resection of the breast. On the other hand, sentinel lymph node biopsy is performed when there is a high risk of mixed invasive cancer, such as a case with a wide range of lesions. In addition, sentinel lymph node biopsy is performed simultaneously with mastectomy. If the lesion is diagnosed as invasive cancer according to the pathological result after the operation, it is impossible to administer the reagent for sentinel lymph node biopsy after the mastectomy. The frequency of positive lymph node metastasis in DCIS has been reported to be 0.98–13%, but most of the cases were micrometastases or isolated tumor cells (ITCs) (10–12). It is presumed that microinvasive cancer exists, but its clinical significance is minor. Also, a retrospective cohort study on sentinel lymph node biopsy for DCIS (OSNA: one-step nucleic acid amplification assay and FS: frozen-section histology) has been reported from Japan. The OSNA assay detected more cases of sentinel lymph node metastases than FS histology (4.2% vs 0.3%). Like overseas reports, most of the metastases were micrometastases (13).
In the selection of the surgical method, awareness of the underestimation for invasive cancer on needle biopsy is essential. It has been reported that 10–38% of cases diagnosed as DCIS by needle biopsy are underestimated and wide tumor area, palpable mass, high nuclear grade, tumor findings on MMG are listed as risk factors with mixed invasive cancer (14–23). On the other hand, in a meta-analysis of the risk factors with mixed invasive cancer after DCIS diagnosis, tumor shadow on ultrasonography (US) was reported more frequently in DCIS and it was not a risk factor with mixed invasive cancer (24). In Japan Association of Breast and Thyroid Sonology (JABTS) BC-02 study, US image classification for DCIS was reported. The most frequent findings were hypo-echoic areas in the mammary gland (48.6%), followed by solid masses (28.0%) and duct abnormalities (10.2%) or mixed masses (8.1%) (25). Specific findings of US to predict mixed invasive cancer are halo-positive masses and interruption of the interface between the adipose tissue and gland (26). A summary of the risk factors with mixed invasive cancer is presented in Table 1.
Table 1.
Predictors of invasive components in patients with preoperatively diagnosed ductal carcinoma in situ
| Total no. of patients | No. of underestimates of invasive cancer (%) | Predictors of invasive component | |
|---|---|---|---|
| Jackman et al. (14) | 1 326 | 183 (14%) |
|
| Renshaw et al. (15) | 91 | 17 (19%) | Comedo DCIS with cribriform/papillary pattern (OR: not shown , P = 0.002) |
| Hoorntje et al. (16) | 255 | 41 (16%) | NG 3 DCIS (OR = 2.9, 95% CI 1.0–7.8) Periductal inflammation in core biopsies (OR = 3.3, 95% CI 1.3–8.7) |
| Yen et al. (17) | 398 | 80 (20%) |
|
| Mittendorf et al. (18) | 85 | 7 (20%) | Diagnosis by core-needle biopsy (Comparison with open biopsy, OR: not shown) |
| Wilkie et al. (19) | 675 | 66 (10%) |
|
| Goyal et al. (20) | 587 | 220 (38%) |
|
| Huo et al. (21) | 200 | 41 (20.5%) |
|
| Miyake et al. (22) | 103 | 37 (35.9%) |
|
| Park et al. (23) | 86 | 27 (31.4%) |
|
DCIS, ductal carcinoma in situ; VAB, vacuum-assisted biopsy; CNB, core needle biopsy.
Radiotherapy for DCIS
There are four randomized controlled trials (RCTs) about radiotherapy for breast cancer with DCIS after partial dissection: the NSABP B-17 trial (1) European Organization for Research and Treatment of Cancer (EORTC) 10 853 trial (27), UK, Australia, and New Zealand (UK/ANZ) DCIS trial (28) and Swedish DCIS (SweDCIS) trial (29). In all these trials, radiotherapy reduced ipsilateral breast recurrence.
In the meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), radiotherapy reduced the absolute 10-year risk of any ipsilateral breast recurrence (either recurrent DCIS or invasive cancer) by 15.2% (SE 1.6%, 12.9% vs 28.1%, 2P < 0.00001) (30). However, there was no significant effect on breast cancer mortality, mortality from causes other than breast cancer, or all-cause mortality after 10 years of follow-up. Furthermore, detailed results of the NSABP B-17 (1) and B-24 trial (1,31), which involved validation of tamoxifen (described later), are presented in Table 2a–c.
Table 2b.
Cumulative incidence of breast cancer death (%)
| 5 years | 10 years | 15 years | 20 years | |
|---|---|---|---|---|
| Bp (B-17) | 0.8 | 1.8 | 3.1 | 3.6 |
| Bp+RT (B-17) | 1.5 | 2.8 | 4.7 | 5 |
| Bp+RT+placebo (B-24) | 0.7 | 1.7 | 2.7 | – |
| Bp+RT+TAM (B-24) | 0.3 | 1.4 | 2.3 | – |
Table 2c.
Cumulative incidence of invasive contralateral breast cancer (%)
| 5 years | 10 years | 15 years | 20 years | |
|---|---|---|---|---|
| Bp (B-17) | 3.3 | 7.2 | 10.3 | 13.4 |
| Bp+RT (B-17) | 3.2 | 7.9 | 10.2 | 11.8 |
| Bp+RT+placebo (B-24) | 3.6 | 6.9 | 10.8 | – |
| Bp+RT+TAM (B-24) | 2.4 | 4.7 | 7.3 | – |
Table 2a.
Cumulative incidence of invasive ipsilateral breast tumor recurrence (%)
| 5 years | 10 years | 15 years | 20 years | Annual failure rate | |
|---|---|---|---|---|---|
| Bp (B-17) | 10.2 | 16.4 | 19.4 | 20.4 | 1.88 |
| Bp+RT (B-17) | 3.2 | 5.5 | 8.9 | 13.5 | 0.9 |
| Bp+RT+placebo (B-24) | 3.9 | 7.3 | 10 | – | 0.88 |
| Involved margins | – | – | 17.4 | – | – |
| Free margins | – | – | 7.4 | – | – |
| Bp+RT+TAM (B-24) | 2 | 4.6 | 8.5 | – | 0.6 |
| Involved margins | – | – | 11.5 | – | – |
| Free margins | – | – | 7.5 | – | – |
Bp, partial resection of the breast; RT, radiation therapy; TAM, tamoxifen.
Endocrine therapy for DCIS
The NSABP B-24 (1,31) and UK/ANZ trials (28) reported on the effectiveness of tamoxifen after partial dissection of the breast with DCIS. The efficacy of tamoxifen was demonstrated in both trials.
In the NSABP B-24 trial, 1 804 patients with DCIS were assigned to a group receiving oral administration of tamoxifen (20 mg/day) for 5 years and a placebo group after partial dissection of the breast with radiotherapy. The median follow-up period was 163 months. The 15-year cumulative incidences of ipsilateral invasive breast tumor recurrence were 8.5% in the tamoxifen group and 10.0% in the placebo group (HR = 0.68, 95% CI: 0.49–0.95, P = 0.025). In addition, the 15-year cumulative incidences of contralateral breast cancers were 7.3% in the tamoxifen group and 10.8% in the placebo group (HR = 0.68, 95% CI: 0.48–0.95, P = 0.023). There were no significant differences in the 15-year cumulative incidence of all-cause mortality and breast cancer mortality between the two groups (1,31). Patients with ER-positive DCIS treated with tamoxifen (vs placebo) showed a significant decrease in the incidence of subsequent breast cancer (HR = 0.64, P = 0.003) (32). In addition, the effect of tamoxifen was more pronounced in patients with positive resection margins. In the UK/ANZ trial, 1 701 patients with DCIS were assigned to the following four groups: partial dissection of the breast alone, partial dissection of the breast + radiotherapy, partial dissection of the breast + tamoxifen, and partial dissection of the breast + radiotherapy + tamoxifen. The median follow-up period was 12.7 years. Ipsilateral breast tumor recurrence (13.2% vs 17.0%, HR = 0.77, 95% CI: 0.59–0.98, P = 0.04) and contralateral breast cancer incidence (0.9% vs 3.1%, HR = 0.27, 95% CI: 0.12–0.59, P = 0.001) were both lower in the partial dissection of the breast + tamoxifen group than in the partial dissection of the breast alone group. There were no significant differences in the death rate across the treatment groups (28,33).
Adverse events of tamoxifen were reported in the NSABP B-24 trial (31). The adverse events were hot flashes (placebo group vs tamoxifen group, 59.0% vs 69.6%), vaginal secretion (20.0% vs 32.4%), deep venous thrombosis (0.2% vs 1.0%), and endometrial carcinoma (0.45/1 000 person-years vs 1.53/1 000 person-years), and these events were more frequently noted in the tamoxifen group than in the placebo group. However, there was no case of fatal pulmonary embolism or death from endometrial cancer. Studies have reported that local control improves with the inclusion of endocrine therapy after partial dissection of the breast, but its effect is larger in breast irradiation (1,28,31,33). For patients who are scheduled for radiotherapy, it is necessary to consider whether its administration is appropriate based on the possibility of risk of recurrence and side effects.
Two double-blind RCTs of anastrozole versus tamoxifen for postmenopausal women with ER-positive DCIS after partial breast excision have been performed (34,35).
In the IBIS-II trial (34), the median follow-up period was 7.2 years. There was no statistically significant difference in overall recurrence between anastrozole and tamoxifen (67 recurrences for anastrozole vs 77 for tamoxifen; HR = 0.89, 95% CI: 0.64–1.23). The non-inferiority of anastrozole was established (upper 95% CI < 1.25), but its superiority to tamoxifen was not established (P = 0.49). In the NSABP B-35 trial (35), breast cancer-free interval events were as follows: 122 in the tamoxifen group and 90 in the anastrozole group (HR = 0.73, 95% CI: 0.56–0.96, P = 0.0234). Additionally, there was a significant interaction between treatment and age group (P = 0.0379), with anastrozole showing superiority only in women younger than 60 years of age. Endocrine therapy with tamoxifen (for premenopausal and postmenopausal women) or an aromatase inhibitor (for postmenopausal women especially those under 60 years of age or those with concerns of embolism) may be considered as a strategy to reduce the risk of ipsilateral breast cancer recurrence in women with ER-positive DCIS treated with breast-conserving therapy (Category 1 for those undergoing breast-conserving surgery followed by radiation therapy and Category 2A for those undergoing excision alone according to the NCCN Guidelines version 2.2016) (36).
Prognostic factors after surgery
We have presented the factors that indicate a high risk of local recurrence after breast-conserving therapy for DCIS in Table 3. Young age, high nuclear grade, presence of comedo necrosis, large tumor size, close to margin and human epidermal growth factor receptor Type 2 (HER 2) positive have been reported as high risk factors for local recurrence (1,27,37–43). A prognostic index after DCIS surgery created by combining these local recurrence risk factors has been developed. In the University of Southern California/Van Nuys prognostic index (USC/VNPI), each factor is scored, and the total score is used for assisting in the determination of postoperative adjuvant therapy use (43), Table 4. The USC/VNPI divides DCIS into three groups with a statistically significant risk of local recurrence after breast conservation therapy. It recommends treatment choice for each group as follows; excision only for patients with scores of 4, 5 or 6, excision plus radiation therapy for patients with scores of 7, 8 or 9, and mastectomy for patients with scores of 10, 11 or 12.
Table 3.
Risk factors of ipsilateral breast tumor recurrence after breast-conserving therapy for ductal carcinoma in situ
Table 4.
The USC/Van Nuys Prognostic Index scoring system (43)
| Score | 1 | 2 | 3 |
|---|---|---|---|
| Size (mm) | ≦15 | 16–40 | ≧41 |
| Margin width (mm) | ≧10 | 1–9 | <1 |
| Pathologic classification | NG 1, 2 | NG 1, 2 | NG 3 |
| Necrosis (–) | Necrosis (+) | Necrosis (–/+) | |
| Age (year) | >60 | 40–60 | <40 |
Overdiagnosis and overtreatment for DCIS
Natural history of DCIS
The natural history of DCIS has not been well elucidated, because follow-up after DCIS diagnosis with no treatment is not always possible.
DCIS was noted in 8.9% (0–14.7%) of cases in a survey of autopsy cases of women who died of other diseases (40–70 years of age) (44). According to a review of follow-up studies of DCIS patients who were not treated owing to misdiagnosis as benign in the initial biopsy, 14–53% of DCIS cases progressed to invasive cancer (5), indicating that about 50% of DCIS cases do not progress to invasive cancer.
Furthermore, Eusebi et al. classified DCIS in nuclear morphology and examined the prognosis of each. It has been reported that DCIS with pleomorphic nuclei (poorly differentiated cyto-nuclear morphology) progressed to invasive carcinoma more often than monomorphic nuclei (well-differentiated cyto-nuclear morphology), and that OS was poor (P = 0.0002) (45). Additionally, among the cases of DCIS with pleomorphic nuclei (n = 14), six cases of IDC were confirmed, and all of these showed cancer death. However, among the cases of DCIS with monomorphic nuclei (n = 66), five cases of IDC were confirmed, and only one showed breast cancer death, while the other four cases were associated with other causes (45).
These findings indicate that the growth rate of low-intermediate grade DCIS is extremely slow even in cases of proliferation; therefore, the patient might die from other diseases and the condition might not affect prognosis.
Overdiagnosis for DCIS
Overdiagnosis produces overtreatment, resulting in patient's quality of life declining. These are now being discussed all over the world. Welch et al. defined overdiagnosis as follows: overdiagnosis is the term used when a condition is diagnosed that would otherwise not go on to cause symptoms or death. Cancer overdiagnosis may have of one of two explanations: (1) The cancer never progresses (or, in fact, regresses) or (2) the cancer progresses slowly enough that the patient dies of other causes before the cancer becomes symptomatic (46).
Owing to widespread MMG screening, the frequency of early breast cancer detection has increased. In early breast cancer, increased incidence of DCIS is remarkable.
However, there is not enough reduction of advanced cancer to match it in the United States (47). Recently, research on the tumor size distribution, tumor incidence and tumor size mortality of breast cancer in women over 40 years old has been conducted using data from the SEER program from 1975 to 2012. On comparing the period from 1975 to 1979 and the period from 2008 to 2012, the authors found that the incidence of large tumors decreased by 30 cases of cancer per 100 000 women and the incidence of small tumors increased by 162 cases of cancer per 100 000 women. Among them, DCIS increased remarkably from 10 to 79 cases of cancer per 100 000 women (48). Also, UK research team reported that if 10 000 women aged 50 years received breast cancer screening for 20 years, 43 cases of breast cancer death would be prevented but 129 cases of overdiagnosis would occur (49). On the other hand, retrospective study was conducted to estimate the amount of overdiagnosis by DCIS grade for 4232 women diagnosed with DCIS in the Netherlands from 2007 to 2009.
Overdiagnosis rate was 61% for low-grade, 57% for intermediate-grade, 45% for high-grade DCIS. It has been reported that the overdiagnosis rate was relatively low at 21–29% in younger age and high-grade DCIS, while it was high at 46–66% in low-intermediate grade DCIS (50).
In overdiagnosis, it is reported that the following third definition can be considered in addition to the above two definitions; the cancer needs to be treated someday in her lifetime, but it does not affect the treatment method or prognosis without treatment immediately (51). Low risk DCIS is likely to be categorized as the above definition of overdiagnosis, and high risk DCIS and small invasive cancer of luminal A type are considered to be possibly classified as the third definition.
Overtreatment for DCIS
A study on omission of breast irradiation of DCIS completely resected using strictly controlled criteria was reported in a single Japanese facility (52). The incidences of ipsilateral and contralateral breast cancer and ipsilateral invasive breast cancer at 10 years were 10.8%, 9.1% and 3.6%, respectively. No patient died of breast cancer. They reported that If complete resection of DCIS can be ensured, the annual incidence of ipsilateral breast cancer, even without irradiation, can be limited to approximately 1%, which equals the incidence of contralateral breast cancer.
The Radiation Therapy Oncology Group (RTOG) 9804 trial (53,54) is the only RCT to verify the efficacy of radiotherapy after partial resection of the breast for low-risk DCIS. The Eastern Cooperative Oncology Group (ECOG) 5194 trial is a single-arm trial (55). The eligibility criteria of the two trials were almost the same (NG 1, NG 2, tumor diameter less than 2.5 cm, and negative for resection margin). In the RTOG 9804 trial, at 7 years, the ipsilateral local failure rates were 0.9% (95% CI: 0.0–2.2%) in the RT arm and 6.7% (95% CI: 3.2–9.6%) in the observation arm (HR = 0.11, 95% CI: 0.03–0.47, P < 0.001). The OS and DFS rates were not different between the two arms. In the ECOG 5094 trial, the 5-year rate of ipsilateral breast events in 565 eligible patients in the low/intermediate grade stratum was 6.1% (95% CI: 4.1–8.2%). From these results, it was confirmed that the life prognosis of low risk DCIS was extremely good regardless of whether or not radiation therapy was enforced. The possibility of omission of radiation therapy may also be suggested to reduce overtreatment of DCIS.
Recently, it was reported that low-grade DCIS showed no difference in the breast cancer-specific survival rate with or without surgery. Previously, a retrospective longitudinal cohort study was performed using the Surveillance Epidemiology and End Results database (56).
Between 1988 and 2011, 57 222 eligible cases of DCIS were identified. Patients were divided into a surgery or non-surgery group, and propensity score weighting was used to balance clinico-pathologic factors between the groups. The weighted 10-year BCSS of low-grade DCIS was similar between patients managed with and those managed without surgery (98.8% vs 98.6%, respectively; P = 0.93). This is a very important report on whether the current standard treatment (surgery) can be omitted. However, this was a retrospective study, and at present, no clinical trials have validated the usefulness of a non-surgical approach for DCIS.
Awareness of DCIS in patients
A previous survey was conducted on 394 healthy women in the United States of America, and they were asked ‘Which approach among surgery, medication, and active surveillance would you choose if you are diagnosed with DCIS?’ (57). In this scenario, the alternative expression of DCIS was presented as ‘non-invasive breast cancer also called pre-invasive breast cancer,’ ‘breast lesion also called pre-invasive breast cancer,’ or ‘abnormal cells also called pre-invasive breast cancer.’ As expected, non-surgery (endocrine therapy or active surveillance) was nearly 70% chosen when not using the word ‘cancer’. However, even with the word ‘non-invasive cancer,’ 53% of women selected treatments other than surgery.
Although DCIS has a very good prognosis, the psychological impact of the word ‘cancer’ is considered large. The word ‘cancer’ has the power to directly remind us of ‘death’. Therefore, neither patients nor doctors are worried that they will not perform surgery even in ‘non-invasive cancer’. We select treatments similar to those for invasive cancer. In addition, patients feel not only fear of recurrence against cancer but also feeling sorrow, anger and alienation, making it difficult to correctly understand diseases and present their own wishes. However, by presenting the patient with information such as extremely good prognosis and benefits/disadvantages due to each treatment methods, they can more reliably make treatment choices according to their wishes.
Recently, a similar study was reported in Australia. Likewise, preference for non-surgery (active surveillance) was 67% in ‘abnormal cells’ group and 60% in ‘pre-invasive breast cancer cells’ group (58).
As the above reports show, it is important to note that nearly half of the women want to avoid surgery if they have a good prognosis after the diagnosis of DCIS.
Future perspectives
In recent clinical practice, the following treatments are common; partial resection of the breast without lymph node surgery + whole breast radiation therapy, mastectomy with sentinel node biopsy. Regarding the administration of endocrine therapy for patients with ER-positive breast cancer, we discuss the merit of reducing the risk of recurrence and the disadvantage of side effects with patients and decide whether or not to administer them.
However, in order to reduce the harm caused by overdiagnosis and overtreatment in the future, we must now consider various approaches. To reduce overdiagnosis, review of the close examination required criteria is necessary in screening MMG. In addition, development of minimally invasive treatment is urgently needed for avoiding overtreatment. From the result of RTOG 9804 trial and ECOG 5194 trial, the possibility of omission of radiation therapy may also be suggested to reduce overtreatment of DCIS.
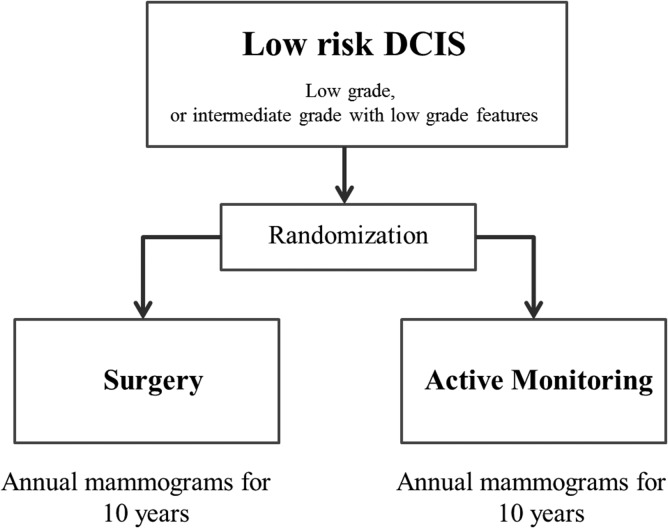
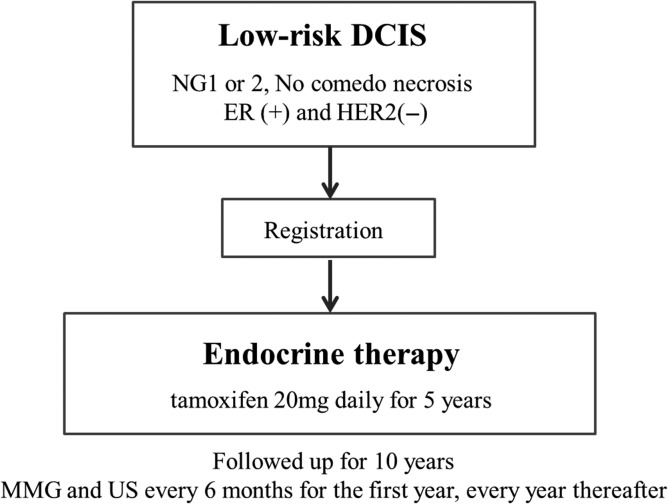
Furthermore, clinical trials of omission of surgery have also begun. In Europe, two non-inferiority studies are attempting to compare active surveillance (no treatment) to standard therapy, including surgery, for low-risk DCIS. In April 2014, the LORIS trial (59,60), Figure 1 started registration in the UK, while the LORD trial (61,62) in the Netherlands is preparing to register as of November 2016. Furthermore, in Japan, a single-arm confirmatory trial of endocrine therapy alone for estrogen receptor-positive, low-risk DCIS of the breast is planned, and registration is scheduled to start in June 2017 (JCOG 1505: LORETTA trial) (Fig. 2).
Figure 1.
LORIS trial.
Figure 2.
JCOG 1505 (LORETTA trial).
Conclusion
DCIS has an extremely good prognosis, and it is reported that some DCIS cases do not progress to invasive cancer even without treatment. However, presently, it is not possible to reliably identify a population that does not progress to invasive cancer even without treatment.
It is necessary to establish new less invasive treatment strategies for DCIS without compromising the good prognosis obtained with the current treatment approach.
Funding
This report was supported in part by the National Cancer Center Research and Development Fund (26-A-4).
Conflict of interest statement
None declared.
References
- 1. Wapnir IL, Dignam JJ, Fisher B, et al. . Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for dshis. J Natl Cancer Inst 2011;103:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010;41:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Projected cancer incidence in Japan 2016: Center for Cancer Control and Information Service, National Cancer Center http://ganjoho.jp/reg_stat/statistics/stat/short_pred.html
- 4. Stuart KE, Houssami N, Taylor R, Hayen A, Boyages J. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer 2015;15:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 2006;97:135–44. [DOI] [PubMed] [Google Scholar]
- 6. Shaitelman SF, Wilkinson JB, Kestin LL, et al. . Long-term outcome in patients with ductal carcinoma in situ treated with breast—conserving therapy: implications for optimal follow-up strategies. Int J Radiat Oncol Biol Phys 2012;83:e305–12. [DOI] [PubMed] [Google Scholar]
- 7. Vidali C, Caffo O, Aristei C, et al. . Conservative treatment of breast ductal carcinoma in situ: results of an Italian multi-institutional retrospective study. Radiat Oncol 2012;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boughey JC, Gonzalez RJ, Bonner E, Kuerer HM. Current treatment and clinical trial developments for ductal carcinoma in situ of the breast. Oncologist 2007;12:1276–87. [DOI] [PubMed] [Google Scholar]
- 9. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010;102:170–8. [DOI] [PubMed] [Google Scholar]
- 10. Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer 2003;98:2105–13. [DOI] [PubMed] [Google Scholar]
- 11. Meretoja TJ, Heiltkilti PS, Salmenkivi K, Leidenius MH. Outcome of patients with ductal carcinoma in sim and sentinel node biopsy. Ann surg Oncol 2012;19:2345–51. [DOI] [PubMed] [Google Scholar]
- 12. Zavagno G, Carcoforo P, Marconato R, et al. . Role of axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. BMC Cancer 2005;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osako T, Iwase T, Kimura K, Masumura K, Horii R, Akiyama F. Incidence and possible pathogenesis of sentinel node micrometastases in ductal carcinoma in situ of the breast detected using molecular whole lymph node assay. Br J Cancer 2012;106:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackman RJ, Burbank F, Parker SH, et al. . Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology 2001;218:497–502. [DOI] [PubMed] [Google Scholar]
- 15. Renshaw AA. Predicting invasion in the excision specimen from breast core needle biopsy specimens with only ductal carcinoma in situ. Arch Pathol Lab Med 2002;126:39–41. [DOI] [PubMed] [Google Scholar]
- 16. Hoorntje LE, Schipper ME, Peeters PH, Bellot F, Storm RK, Rinkes IH. The finding of invasive cancer after a preoperative diagnosis of ductal carcinoma-in-situ: causes of ductal carcinoma-in-situ underestimates with stereotactic14-gauge needle biopsy. Ann Surg Oncol 2003;10:748–53. [DOI] [PubMed] [Google Scholar]
- 17. Yen TW, Hunt KK, Ross MI, et al. . Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg 2005;200:516–26. [DOI] [PubMed] [Google Scholar]
- 18. Mittendorf EA, Arciero CA, Gutchell V, Hooke J, Shriver CD. Core biopsy diagnosis of ductal carcinoma in situ: an indication for sentinel lymph node biopsy. Curr Surg 2005;62:253–7. [DOI] [PubMed] [Google Scholar]
- 19. Wilkie C, White L, Dupont E, Cantor A, Cox CE. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg 2005;190:563–6. [DOI] [PubMed] [Google Scholar]
- 20. Goyal A, Douglas-Jones A, Monypenny I, Sweetland H, Stevens G, Mansel RE. Is there a role of sentinel lymph node biopsy in ductal carcinoma in situ? Analysis of 587 cases. Breast Cancer Res Treat 2006;98:311–4. [DOI] [PubMed] [Google Scholar]
- 21. Huo L, Sneige N, Hunt KK, Albarracin CT, Lopez A, Resetkova E. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer 2006;107:1760–8. [DOI] [PubMed] [Google Scholar]
- 22. Miyake T, Shimazu K, Ohashi H, et al. . Indication for sentinel lymph node biopsy for breast cancer when core biopsy shows ductal carcinoma in situ. Am J Surg 2011;202:59–65. [DOI] [PubMed] [Google Scholar]
- 23. Park AY, Gweon HM, Son EJ, Yoo M, Kim JA, Youk JH. Ductal carcinoma in situ diagnosed at US-guided 14-gauge core-needle biopsy for breast mass: preoperative predictors of invasive breast cancer. Eur J Radiol 2014;83:654–9. [DOI] [PubMed] [Google Scholar]
- 24. Brennan ME, Turner RM, Ciatto S, et al. . Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011;260:119–28. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe T, Yamaguchi T, Tsunoda H, et al. . Ultrasound image classification of Ductal Carcinoma in Situ (DCIS) of the breast: analysis of 705 DCIS lesions. Ultrasound Med Biol 2017;43:918–25. [DOI] [PubMed] [Google Scholar]
- 26. Japan Association of Breast and Thyroid Sonology Guideline for Breast Ultrasound-Management and Diagnosis. 3rd edn Japan: Nankodo, 2014. [Google Scholar]
- 27. Bijker N, Meijnen P, Peterse JL, et al. . Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006;24:3381–7. [DOI] [PubMed] [Google Scholar]
- 28. Cuzick J, Sestak I, Pinder SE, et al. . Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holmberg L, Garmo H, Granstrand B, et al. . Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol 2008;26:1247–52. [DOI] [PubMed] [Google Scholar]
- 30. Correa C, McGale P, Taylor C, et al. . Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010;2010:162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fisher B, Dignam J, Wolmark N, et al. . Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 1999;353:1993–2000. [DOI] [PubMed] [Google Scholar]
- 32. Allred DC, Anderson SJ, Paik S, et al. . Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 2012;30:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. UK Coordinating Committee on Cancer Research; Ductal Carcinoma in situ Working Party; DCIS trialists in the UK, Australia, and New Zealand. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomized controlled trial. Lancet 2003;362:95–102. [DOI] [PubMed] [Google Scholar]
- 34. Forbes JF, Sestak I, Howell A, et al. . Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 2016;387:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Margolese RG, Cecchini RS, Julian TB, et al. . Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 2016;387:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NCCN Guidelines version 2.2016, NCCN Recommendations for Primary Treatment and after Primary Treatment of DCIS.
- 37. Warren JL, Weaver DL, Bocklage T, et al. . The frequency of ipsilateral second tumors after breast-conserving surgery for DCIS: a population based analysis. Cancer 2005;104:1840–48. [DOI] [PubMed] [Google Scholar]
- 38. Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer 2006;106:2104–12. [DOI] [PubMed] [Google Scholar]
- 39. Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the national surgical adjuvant breast and bowel project experience. Semin Oncol 2001;28:400–18. [DOI] [PubMed] [Google Scholar]
- 40. Silverstein MJ, Lagios MD, Groshen S, et al. . The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med 1999;340:1455–61. [DOI] [PubMed] [Google Scholar]
- 41. Kepple J, Henry-Tillman RS, Klimberg VS, et al. . The receptor expression pattern in ductal carcinoma in situ predicts recurrence. Am J Surg 2006;192:68–71. [DOI] [PubMed] [Google Scholar]
- 42. Barnes NL, Khavari S, Boland GP, Cramer A, Knox WF, Bundred NJ. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res 2005;11:2163–68. [DOI] [PubMed] [Google Scholar]
- 43. Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg 2003;186:337–43. [DOI] [PubMed] [Google Scholar]
- 44. Welch HG, Black WC. Using autopsy series to estimate the disease ‘reservoir: for ductal carcinoma in situ of the breast: how much more breast cancer can we find. Ann Intern Med 1997;127:1023–28. [DOI] [PubMed] [Google Scholar]
- 45. Eusebi V, Feudale E, Foschini MP, et al. . Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol 1994;11:223–5. [PubMed] [Google Scholar]
- 46. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605–13. [DOI] [PubMed] [Google Scholar]
- 47. Bleyer A. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998–2005. [DOI] [PubMed] [Google Scholar]
- 48. Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47. [DOI] [PubMed] [Google Scholar]
- 49. Independent UK. Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778–86. [DOI] [PubMed] [Google Scholar]
- 50. van Luijt PA, Heijnsdijk EA, Fracheboud J, et al. . The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast Cancer Res 2016;18:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shimada T. Over-diagnosis in Breast Cancer Screening. J Breast Cancer 2015;30:377–43. [Google Scholar]
- 52. Sakai T, Iwase T, Teruya N, et al. . Surgical excision without whole breast irradiation for complete resection of ductal carcinoma in situ identified using strict, unified criteria. Am J Surg 2016;[Epub ahead of print]. Published online: November 29, 2016. [DOI] [PubMed] [Google Scholar]
- 53. McCormick B, Winter K, Hudis C, et al. . RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 2015;33:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.RTOG9804: http://jco.ascopubs.org/content/suppl/2015/01/20/JCO.2014.57.9029.DC1/Protocol_JCO.2014.57.9029.pdf
- 55. Hughes LL, Wang M, Page DL, Gray R, Solin LJ, Davidson NE, et al. . Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:5319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sagara Y, Mallory MA, Wong S, et al. . Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 2015;150:739–45. [DOI] [PubMed] [Google Scholar]
- 57. Omer ZB, Hwang ES, Esserman LJ, Howe R, Ozanne EM. Impact of ductal carcinoma in situ terminology on patient treatment preferences. JAMA Intern Med 2013;173:1830–31. [DOI] [PubMed] [Google Scholar]
- 58. McCaffery K, Nickel B, Moynihan R, et al. . How different terminology for ductal carcinoma in situ impacts women's concern and treatment preferences: a randomised comparison within a national community survey. BMJ Open 2015;5:e008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Francis A, Bartlett JMS, Billingham LJ, et al. . The LORIS trial: a multicentre, randomized phase III trial of standard surgery versus active monitoring in women with newly diagnosed low risk ductal carcinoma in situ. Cancer Res 2013;73:OT2–3-01. [Google Scholar]
- 60. Francis A, Thomas J, Fallowfield L, et al. . Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 2015;51:2296–303. [DOI] [PubMed] [Google Scholar]
- 61. Elshof LE, Tryfonidis K, Slaets L, et al. . The LORD trial: a randomized, non-inferiority trial, between active surveillance versus standard treatment in patients with low risk ductal carcinoma in situ. SABCS 2014;75:OT3–6-01. [Google Scholar]
- 62. Elshof LE, Tryfonidis K, Slaets L, et al. . Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ – the LORD study. Eur J Cancer 2015;51:1497–510. [DOI] [PubMed] [Google Scholar]