The aim of this study was to determine the frequency of perception of curability among cancer patients living with advanced cancer. The predictive factors associated with an inaccurate perception of curability are also examined.
Keywords: Perception of curability, Prognostic awareness, Cancer, Palliative care, Decision‐making preference
Abstract
Background.
There are limited data on illness understanding and perception of cure among advanced cancer patients around the world. The aim of the study was to determine the frequency and factors associated with inaccurate perception of curability among advanced cancer patients receiving palliative care across the globe.
Materials and Methods.
Secondary analysis of a study to understand the core concepts in end‐of‐life care among advanced cancer patients receiving palliative care from 11 countries across the world. Advanced cancer patients were surveyed using a Patient Illness Understanding survey and Control Preference Scale. Descriptive statistics and multicovariate logistic regression analysis were performed.
Results.
Fifty‐five percent (763/1,390) of patients receiving palliative care inaccurately reported that their cancer is curable. The median age was 58, 55% were female, 59% were married or had a partner, 48% were Catholic, and 35% were college educated. Sixty‐eight percent perceived that the goal of therapy was “to get rid of their cancer,” and 47% perceived themselves as “seriously ill.” Multicovariate logistic regression analysis shows that accurate perception of curability was associated with female gender (odds ratio [OR] 0.73, p = .027), higher education (OR 0.37, p < .0001), unemployment status (OR 0.69, p = .02), and being from France (OR 0.26, p < .0001) and South Africa (OR 0.52, p = .034); inaccurate perception of curability was associated with better Karnofsky performance status (OR 1.02 per point, p = .0005), and being from Philippines (OR 15.49, p < .0001), Jordan (OR 8.43, p < .0001), Brazil (OR 2.17, p = .0037), and India (OR 2.47, p = .039).
Conclusion.
Inaccurate perception of curability in advanced cancer patients is 55% and significantly differs by gender, education, performance status, employment status, and country of origin. Further studies are needed to develop strategies to reduce this misperception of curability in advanced cancer patients.
Implications for Practice.
The findings of this study indicate that inaccurate perception of curability among advanced cancer patients is 55%. Inaccurate perception of curability significantly differs by gender, education, performance status, employment status, and country of origin. There is great need to facilitate improved patient–physician communication so as to improve health care outcomes and patient satisfaction.
Introduction
In recent years, with advances in cancer treatment, there is a higher frequency of cancer patients living with advanced cancer [1]. High symptom distress and poor quality of life are frequent in advanced cancer patients [2], [3], [4], [5], [6]. However, various issues including patient–physician communication with regard to understanding of illness and perception of curability (UIPC) remain unresolved [4], [7]. These issues could potentially prevent early integration of palliative care [8], [9], [10]. Prior studies suggest timely UIPC discussions can potentially improve quality of life and coping, and facilitate patients to make informed decisions and set appropriate priorities with regard to diagnosis, treatment, and end‐of‐life care [11], [12], [13], [14].
Prior studies also suggest that many advanced cancer patients have an inaccurate perception of curability of their cancer [4], [15], [16], [17], [18]. However, there are limited studies to evaluate the role of patient characteristics and clinical factors on perception of curability in advanced cancer patients across the world. This is important in the context of provision of cancer care in the global health care environment because many patients are receiving cancer care in places other than their country of origin.
The primary aim of this study was to determine the frequency of perception of curability among advanced cancer patients receiving palliative care across the globe. We also examined the predictive factors associated with inaccurate perception of curability.
Materials and Methods
Participants
We conducted an international study in advanced cancer patients receiving palliative care from 11 countries across the world to understand how core concepts in end‐of‐life care such as decisional control preferences and terminal illness understanding differ by country. This present report is a secondary analysis of this study, with a focus on patient understanding of illness and perception of curability [19].
The participating centers included the following: UT MD Anderson Cancer Center, Houston, Texas, U.S.; King Hussein Cancer Center, Amman, Jordan; Benavides Cancer Institute, Manila, Philippines; Hôpitaux Sud Centre Hospitalier Lyon‐sud, Lyon, France; HCA Hospice Care, Singapore; Highway Hospice, Durban, Kwazulu‐natal, South Africa; Tata Memorial Hospital, Mumbai, India; Hospital De Câncer De Barretos, Barretos, Brazil; LBJ Hospital, Houston, Texas, U.S.; Pontificia Universidad Catolica De Chile, Santiago, Chile; Fundación FEMEBA, Buenos Aires, Argentina; and Hospital Centro De Cuidados Laguna, Madrid, Spain. The participating centers were a part of a research collaborative network to advance cancer research.
We received approval from the Institutional Review Board of The University of Texas MD Anderson Cancer Center and collaborating institutions to conduct this study.
Patients were enrolled into the study if they met the following eligibility criteria: (a) diagnoses of advanced cancer (defined as locally recurrent or metastatic incurable cancer); (b) 18 years of age or older; (c) having normal cognitive status as assessed by clinician as per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria; (d) willing to participate in the study and sign informed consent; and (e) all participants were seen by the palliative care consultation team for at least one visit, and have resided in the study country for at least 5 years.
Regarding data collection, the study principal investigator (PI) and site PIs and their research team had regular teleconferences one to two times a month. The PI also met the coinvestigators in person to review the protocol and data to ensure data are collected in an accurate manner.
Measures
Data collection occurred between December 15, 2013, and February 25, 2016. The research coordinator from each study site completed the assessments, after the consent process, by patient interview and review of medical records. The assessments included collection of demographic information including age, gender, race, religion, cancer type, cancer treatment, education, Karnofsky performance status, marital status, and employment status. The research coordinator then supervised the patients’ completion of the patient illness understanding (PIU) survey and Control Preference Scale (CPS) questionnaire.
The PIU survey was used to evaluate the patients’ level of understanding of their illness and prognosis. This survey has been used in prior studies [4], [20], [21], [22] to assess patients' perception of curability, goals of therapy, and health status in various cultures [18].
The CPS questionnaire was used to assess patients’ decisional control preferences (DCP) [23], [24]. CPS was commonly used to assess DCP in research settings and has been previously used by our team [25], [26], [27]. We used the triadic form (patient‐family‐physician) of this scale, which assesses the patient's decisional control preferences with respect to family and the physician. The assessment is based on the following question: “In my opinion, decisions about my care should be made by…”. Patients had 15 answer options and were instructed to choose 1 option, which was later categorized as a passive, active, or shared decisional control preference.
For this study, the Patient Illness Understanding survey and Control Preference Scale were linguistically validated by bilingual investigators of our team and independent back‐translation by bilingual natives to determine semantic and linguistic equivalence between the English and native versions (Spanish, French, Hindi, Portuguese, Arabic, Tagalog, and Zulu).
Statistical Analysis
Descriptive statistics were used to summarize patient demographic and clinical characteristics, PIU survey items, and CPS items. Univariate/multicovariate logistic regression analysis was used to assess the effect of categorical and continuous covariates for the question “My cancer is curable” in the PIU survey. The variables included were age, gender (female vs. male), education (college or advanced degree vs. less), Karnofsky performance status, marital status (married or with partner as reference), religion including Catholicism, Christianity, Islam, Hinduism, Buddhism, others (Catholicism as reference), occupation (working as reference), countries including Argentina, Brazil, Chile, France, India, Singapore, South Africa, Spain, U.S., Philippines, Jordan (with U.S. as reference), and passive decisional control preference. All computations were carried out in SAS 9.3 (SAS Institute Inc., Cary, NC) and R‐3.1.1
Results
Sixty‐six percent (1,390/2,094) of advanced cancer patients approached were evaluable. Reasons for exclusion were as follows: (a) Patients declined to consent due to severe symptoms or feeling ill (n = 433); lack of time (n = 49); lack of interest in research (n = 71); and/or lack of interest in the study (n = 51). (b) Patient was not able to complete the PIU survey (n = 100). The mean age was 58, 55% were female, 59% were married, 48% were Catholic, and 35% were college educated (Table 1). Fifty‐five percent (763/1,390) of patients inaccurately reported that their cancer is curable, 68% perceived that the goal of therapy was “to get rid of their cancer,” 90% perceived that the goal of therapy was to “help them live longer,” and 95% perceived that the goal of therapy was to “make them feel better.” Forty‐seven percent of patients perceived themselves as “seriously ill.”
Table 1. Demographics and clinical characteristics.
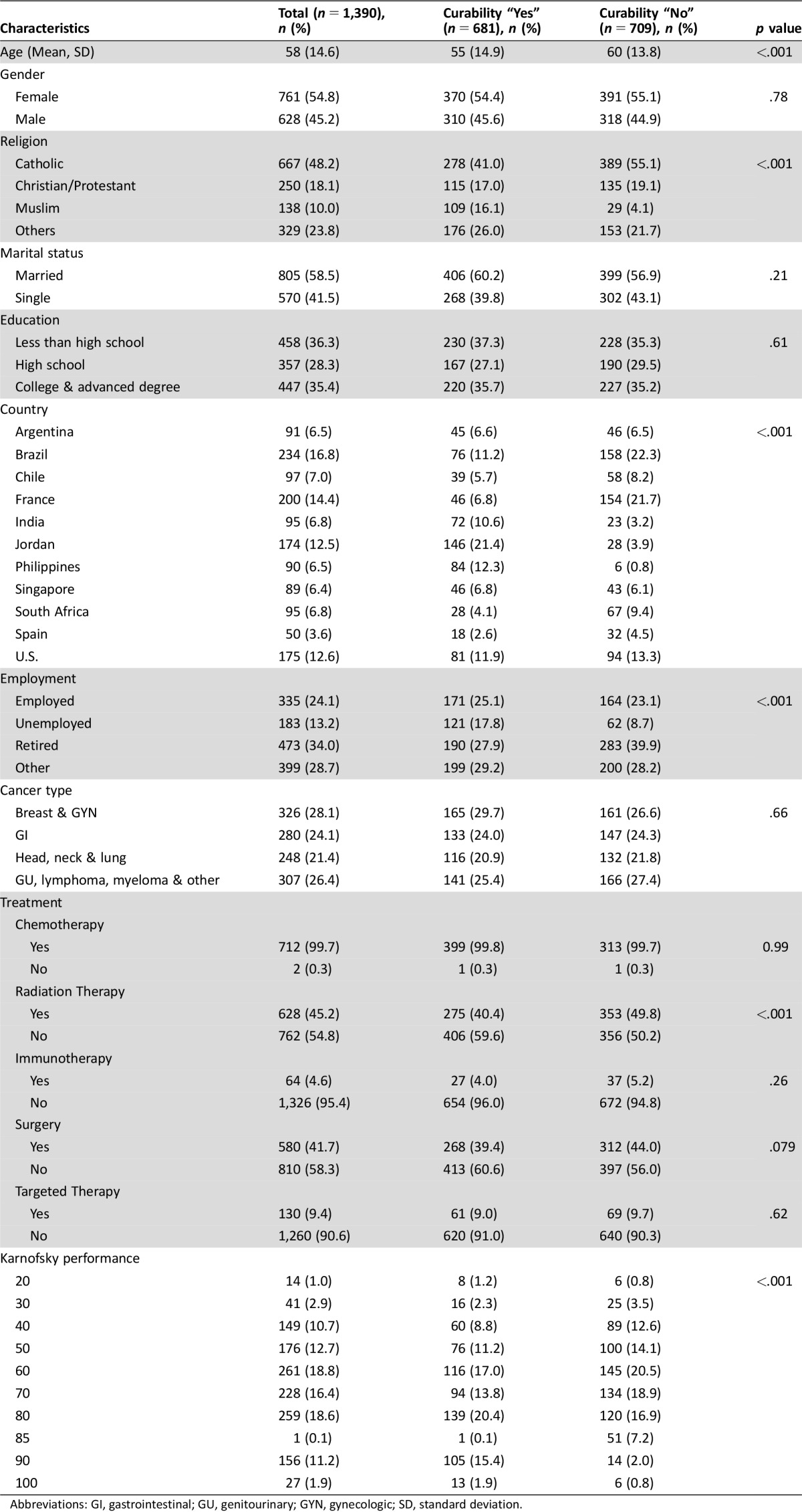
Abbreviations: GI, gastrointestinal; GU, genitourinary; GYN, gynecologic; SD, standard deviation.
The frequency of shared (patient prefers to make shared decisions after consulting physician or family), active (patient prefers to make decision by him or herself), and passive DCP (patient prefers physician or family to make decision for him/her) was 33%, 44%, and 23%, respectively. Table 2 shows the results from multicovariate logistic regression analysis. Accurate perception of curability was associated with female gender (OR 0.73, p = .027), higher education (OR 0.37, p < .0001), unemployment status (OR 0.69, p = .02), and being from countries such as France (OR 0.26, p < .0001) and South Africa (OR 0.52, p = .034). Inaccurate perception of curability was associated with better Karnofsky performance status (OR 1.02 per point, p = .0005) and being from countries such as Philippines (OR 15.49, p < .0001), Jordan (OR 8.43, p < .0001), Brazil (OR 2.17, p = .0037), and India (OR 2.47, p = .039).
Table 2. Predictors of perception of curability in advanced cancer patients.
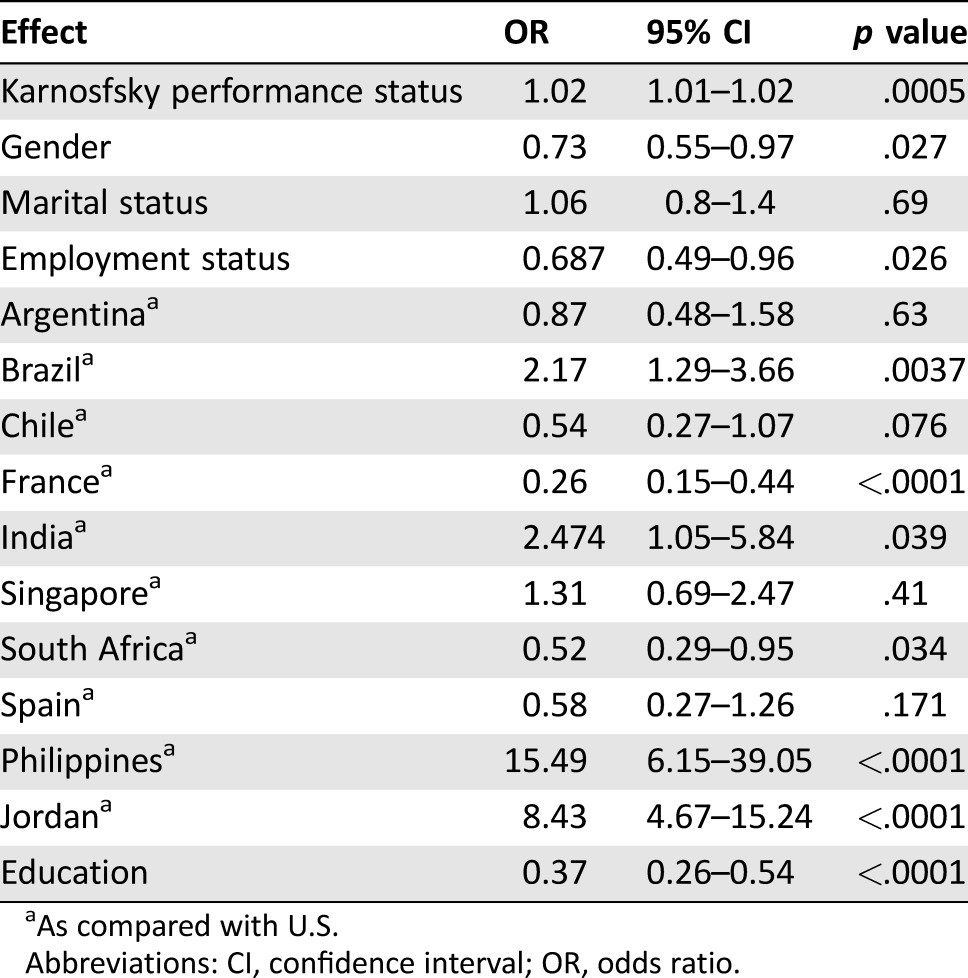
As compared with U.S.
Abbreviations: CI, confidence interval; OR, odds ratio.
Age, marital status, religion, and passive DCP were not significantly associated with perception of curability.
Discussion
The findings of this study suggest that there is a high frequency of inaccurate perception of curability among advanced cancer patients receiving palliative care (55%). Patients’ gender, education, performance status, employment status, and country were significantly associated with inaccurate perception of curability.
Our study is the first to evaluate the perception of curability in a global setting in advanced cancer patients receiving palliative care. The patient population for this study were recruited from 11 countries in 5 different continents [15]. In a previous study by Weeks et al. [4], which was conducted in various cancer centers in the U.S., 69%–81% of advanced lung and colon cancer patients inaccurately reported that chemotherapy was likely to cure their cancer. Craft et al. found 30.4% of 163 advanced cancer patients inaccurately reported that the aim of treatment was to cure their illness [17]. A study by Pronzato et al. found that 47% (41/87) of advanced cancer patients reported that the aim of cancer treatment was curative [28]. The finding that 55% of patients perceived that the goal of therapy was a cure is in line with these previous reports. This information was particularly surprising given that these results were reported in patients receiving palliative care. Compared with previous studies [18], [29], [30], patients in our study were younger, with approximately equal distribution with regard to gender, marital status, education status, employment status, cancer types, and performance status. Similar to prior studies, we found that perception of curability is more inaccurate in men [16], [21].
The findings of our study are also important because it is the first multicenter, international study to evaluate the role of country (cultural background), religion, and decision‐making preferences in patient's perception of curability in advanced cancer patients [18], [29], [30]. In our study, the country of origin was strongly associated with perception of curability, whereas religion and decisional control preference showed no association. These results highlight the need to have a personalized approach to understand the patient's understanding of illness and perception of curability.
Further studies are needed to determine the association between patient information needs, patient report of information provided by their physician, and perceptions of curability. Also, further research is needed to better understand the association between perception of curability among palliative patients and outcomes such as timing of palliative consult, enrollment to hospice, and choice of more aggressive therapies. Because the availability of such services varies according to the countries, our group is unable to conduct analysis of these outcomes for this study. Future studies are needed to investigate the role of checklists, audio, or video decisional aids in improving the misperception of curability. Results from recent studies suggest that perception of curability in palliative care patients can be improved by early access to palliative care [31] and improved patient–physician communication, especially with regard to prognosis and life expectancy [20].
More research is necessary to understand why there was a significant difference among many of the countries with regard to perception of curability. The traditional difference between developed and developing countries does not seem to apply in the case of perception of curability because patients in developing countries such as Philippines, Jordan, Brazil, and India had increased expectation, whereas others such as South Africa, France, Chile, and Argentina had lower expectation (Table 2). More research is needed to understand the role of culture in misperception of curability in palliative care patients, and whether the notion of loss of hope, stigma of cancer, and discussion of nearing death in a given culture seem to factor into this misperception [32], [33], [34], [35]. Results of our study showed that after controlling for education and performance status, country remained independently associated with perception of curability, suggesting that the other cultural factors that are not directly related to socioeconomic status may play a role in the perception of curability.
Prior studies suggest that patients' inaccurate perception of curability plays a significant role in the greater use of chemotherapy [4]. However, in our study, we were unable to find any significant association with greater use of chemotherapy, given that these patients were unlikely to get chemotherapy if they were receiving palliative care in some of these countries. A recent study by Nipp et al. [36] in early advanced lung and colorectal cancer patients found that patients’ cancer treatment goal of “to cure my cancer” was significantly associated with better quality of life and less anxiety, suggesting that their misperception could be a coping strategy, which could play a role in the greater use of chemotherapy. However, future well‐designed studies should investigate how misperception affects the greater use of chemotherapy.
Our data also suggest that misperception with regard to curability is not a barrier to receiving palliative care, given that we sampled only palliative care recipients. Thus, further studies are needed to investigate how inaccurate perception of the curability of their cancer affects the patient or family member's end‐of‐life care planning, quality of death, and even possibly bereaved caregiver outcomes.
Conclusion
There is a high frequency of inaccurate perception of curability among advanced cancer patients receiving palliative care (55%). The perception of curability significantly differs by gender, education, performance status, employment status, and country of origin. Integration of supportive/palliative care services can be more complex in these patients. Further studies are needed to develop strategies to reduce this misperception of curability among advanced cancer patients.
Acknowledgments
This work was supported by Sister Intuition Network Fund.
Author Contributions
Conception/design: Sriram Yennurajalingam, Eduardo Bruera
Administrative support: Sriram Yennurajalingam
Collection and/or assembly of data: Sriram Yennurajalingam, Luis Fernando Rodrigues, Omar M. Shamieh, Colombe Tricou, Marilène Filbet, Kyaw Naing, Akhileshwaran Ramaswamy, Pedro Emilio Perez‐Cruz, Mary Jocylyn S. Bautista, Sofia Bunge, Mary Ann Muckaden, Vikash Sewram, Sarah Fakrooden, Antonio Noguera‐Tejedor, Shobha S. Rao, Diane Liu, Minjeong Park, Janet L. Williams, Zhanni Lu, Hilda Cantu, Eduardo Bruera
Data analysis and interpretation: Sriram Yennurajalingam, Diane Liu, Minjeong Park, Eduardo Bruera
Manuscript writing: Sriram Yennurajalingam, Luis Fernando Rodrigues, Omar M. Shamieh, Colombe Tricou, Marilène Filbet, Kyaw Naing, Akhileshwaran Ramaswamy, Pedro Emilio Perez‐Cruz, Mary Jocylyn S. Bautista, Sofia Bunge, Mary Ann Muckaden, Vikash Sewram, Sarah Fakrooden, Antonio Noguera‐Tejedor, Shobha S. Rao, Diane Liu, Minjeong Park, Janet L. Williams, Zhanni Lu, Hilda Cantu, David Hui, Suresh K. Reddy, Eduardo Bruera
Final approval of manuscript: Sriram Yennurajalingam, Luis Fernando Rodrigues, Omar M. Shamieh, Colombe Tricou, Marilène Filbet, Kyaw Naing, Akhileshwaran Ramaswamy, Pedro Emilio Perez‐Cruz, Mary Jocylyn S. Bautista, Sofia Bunge, Mary Ann Muckaden, Vikash Sewram, Sarah Fakrooden, Antonio Noguera‐Tejedor, Shobha S. Rao, Diane Liu, Minjeong Park, Janet L. Williams, Zhanni Lu, Hilda Cantu, David Hui, Suresh K. Reddy, Eduardo Bruera
Critical revision of the manuscript for important intellectual content: Sriram Yennurajalingam, Eduardo Bruera
Obtained Funding: Sriram Yennurajalingam
Study Supervision: Sriram Yennurajalingam
Disclosures
Eduardo Bruera: Helsinn (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One 2010;5:e9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yennurajalingam S, Urbauer DL, Casper KL et al. Impact of a palliative care consultation team on cancer‐related symptoms in advanced cancer patients referred to an outpatient supportive care clinic. J Pain Symptom Manage 2011;41:49–56. [DOI] [PubMed] [Google Scholar]
- 3. Teunissen SC, Wesker W, Kruitwagen C et al. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage 2007;34:94–104. [DOI] [PubMed] [Google Scholar]
- 4. Weeks JC, Catalano PJ, Cronin A et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prigerson HG, Bao Y, Shah MA et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen RC, Falchook AD, Tian F et al. Aggressive care at the end‐of‐life for younger patients with cancer: Impact of ASCO's Choosing Wisely campaign. J Clin Oncol 2016;34(suppl 18):LBA10033a. [Google Scholar]
- 7. El‐Jawahri A, Traeger L, Park ER et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014;120:278–285. [DOI] [PubMed] [Google Scholar]
- 8. Greer JA, Jackson VA, Meier DE et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349–363. [DOI] [PubMed] [Google Scholar]
- 9. Gaertner J, Weingartner V, Wolf J et al. Early palliative care for patients with advanced cancer: How to make it work? Curr Opin Oncol 2013;25:342–352. [DOI] [PubMed] [Google Scholar]
- 10. Yennurajalingam S, Lu Z, Williams JL et al. Characteristics of patients with advanced lung cancer referred to a rapid‐access supportive care clinic. Palliat Support Care 2017;15:197–204. [DOI] [PubMed] [Google Scholar]
- 11. Wright AA, Zhang B, Ray A et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopman P, Rijken M. Illness perceptions of cancer patients: Relationships with illness characteristics and coping. Psychooncology 2015;24:11–18. [DOI] [PubMed] [Google Scholar]
- 13. Mack JW, Cronin A, Keating NL et al. Associations between end‐of‐life discussion characteristics and care received near death: A prospective cohort study. J Clin Oncol 2012;30:4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruera E, Yennurajalingam S. Palliative care in advanced cancer patients: How and when? The Oncologist 2012;17:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mackillop WJ, Stewart WE, Ginsburg AD et al. Cancer patients' perceptions of their disease and its treatment. Br J Cancer 1988;58:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Temel JS, Greer JA, Admane S et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non‐small‐cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326. [DOI] [PubMed] [Google Scholar]
- 17. Craft PS, Burns CM, Smith WT et al. Knowledge of treatment intent among patients with advanced cancer: A longitudinal study. Eur J Cancer Care 2005;14:417–425. [DOI] [PubMed] [Google Scholar]
- 18. Applebaum AJ, Kolva EA, Kulikowski JR et al. Conceptualizing prognostic awareness in advanced cancer: A systematic review. J Health Psychol 2014;19:1103–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yennu S, Rodrigues LF, Shamieh OM et al. A multicenter study of patients decisional control preferences in patients with advanced cancer. J Clin Oncol 2016;34(suppl 15):6578a. [Google Scholar]
- 20. Epstein AS, Prigerson HG, O'Reilly EM et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol 2016;34:2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fletcher K, Prigerson HG, Paulk E et al. Gender differences in the evolution of illness understanding among patients with advanced cancer. J Support Oncol 2013;11:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prigerson HG. Socialization to dying: Social determinants of death acknowledgement and treatment among terminally ill geriatric patients. J Health Soc Behav 1992;33:378–395. [PubMed] [Google Scholar]
- 23. Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res 1997;29:21–43. [PubMed] [Google Scholar]
- 24. Degner LF, Sloan JA. Decision making during serious illness: What role do patients really want to play? J Clin Epidemiol 1992;45:941–950. [DOI] [PubMed] [Google Scholar]
- 25. Bruera E, Sweeney C, Calder K et al. Patient preferences versus physician perceptions of treatment decisions in cancer care. J Clin Oncol 2001;19:2883–2885. [DOI] [PubMed] [Google Scholar]
- 26. Bruera E, Willey JS, Palmer JL et al. Treatment decisions for breast carcinoma. Cancer 2002;94:2076–2080. [DOI] [PubMed] [Google Scholar]
- 27. Yennurajalingam S, Parsons HA, Duarte ER et al. Decisional control preferences of Hispanic patients with advanced cancer from the United States and Latin America. J Pain Symptom Manage 2013;46:376–385. [DOI] [PubMed] [Google Scholar]
- 28. Pronzato P, Bertelli G, Losardo P et al. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer 1994;2:242–244. [DOI] [PubMed] [Google Scholar]
- 29. Diamond EL, Corner GW, De Rosa A et al. Prognostic awareness and communication of prognostic information in malignant glioma: A systematic review. J Neurooncol 2014;119:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith AK, McCarthy EP, Paulk E et al. Racial and ethnic differences in advance care planning among patients with cancer: Impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol 2008;26:4131–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 32. Daher M. Cultural beliefs and values in cancer patients. Ann Oncol 2012;23(suppl 3):66–69. [DOI] [PubMed] [Google Scholar]
- 33. Searight HR, Gafford J. Cultural diversity at the end of life: Issues and guidelines for family physicians. Am Fam Physician 2005;71:515–522. [PubMed] [Google Scholar]
- 34. Giger JN, Davidhizar RE, Fordham P. Multi‐cultural and multi‐ethnic considerations and advanced directives: Developing cultural competency. J Cult Divers 2006;13:3–9. [PubMed] [Google Scholar]
- 35. Kagawa‐Singer M, Blackhall LJ. Negotiating cross‐cultural issues at the end of life: “You got to go where he lives”. JAMA 2001;286:2993–3001. [DOI] [PubMed] [Google Scholar]
- 36. Nipp RD, Greer JA, El‐Jawahri A et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Oncol 2017;35:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]


