Patients with cancer are at increased risk for venous thromboembolism, and therapeutic anticoagulation decreases its associated mortality and morbidity. This review summarizes three case scenarios that are commonly seen in clinical practice to illustrate considerations for anticoagulation in these patients. Available evidence and practical algorithms for use in these situations are presented.
Keywords: Venous thromboembolism, Therapeutic anticoagulation, Malignant glioma, Brain metastasis
Abstract
Patients with primary or metastatic brain tumors are at increased risk of developing venous thromboses. However, the potential benefit of therapeutic anticoagulation in these patients must be weighed against the deadly complication of intracranial hemorrhage. In this review, we summarize available evidence and recent studies of intracranial bleeding risks in primary and metastatic tumors and the impact of therapeutic anticoagulation. We find that for the majority of primary and treated metastatic brain tumors, the risk of spontaneous bleeding is acceptable and not further increased by careful therapeutic anticoagulation with low molecular weight heparin or direct oral anticoagulants, although thrombocytopenia (platelet count less than 50,000/μL) and other coagulopathies are relative contraindications. Patients with brain metastasis from melanoma, renal cell carcinoma, choriocarcinoma, thyroid carcinoma, and hepatocellular carcinoma have a higher tendency to bleed spontaneously than noted in patients with other malignancies, and thus warrant routine brain imaging and alternative strategies such as inferior vena cava filter placement in the acute setting of venous thromboembolism before consideration of therapeutic anticoagulation.
Implications for Practice.
Malignant gliomas are associated with increased risks of both venous thromboses and intracranial hemorrhage, but the additional bleeding risk associated with therapeutic anticoagulation appears acceptable, especially after treatment of primary tumors. Most patients with treated brain metastasis have a low risk of intracranial hemorrhage associated with therapeutic anticoagulation, and low molecular weight heparin is currently the preferred agent of choice. Patients with untreated brain metastasis from melanoma, renal cell carcinoma, thyroid cancer, choriocarcinoma, and hepatocellular carcinoma have a higher propensity for spontaneous intracranial bleeding, and systemic anticoagulation may be contraindicated in the acute setting of venous thromboembolism.
Introduction
Patients with cancer are at increased risk for venous thromboembolism (VTE), and therapeutic anticoagulation is known to decrease VTE's associated mortality and morbidity [1]. The biggest risk of systemic anticoagulation is bleeding, including intracranial hemorrhage, which must be balanced against the recommended chronic anticoagulation for at least the duration of active cancer [1], [2]. As discussed below, those with primary central nervous system tumors and those with tumors that metastasize to the brain have a particularly high rate of VTE. Thus, clinicians often encounter a patient with a brain tumor who requires anticoagulation for a deep venous thromboembolism (DVT) or pulmonary embolism (PE) or for cardiac reasons, such as atrial fibrillation or peripheral vascular disease. As an alternative, an inferior venal cava filter reduces the risk of nonfatal pulmonary embolism, although it does not correct the underlying coagulation defects and can itself thrombose or induce thrombosis of the lower extremity [3]. In recent years, accumulating evidence suggests that anticoagulation can be used safely and effectively in most of these patients [4], [5]. Patients with persistent thrombocytopenia, recent neurosurgery, and tumor types prone to bleeding may require special consideration. In this narrative review, we use three case scenarios commonly seen in the clinical practices of hematologists and oncologists, as well as general internists, to illustrate considerations for anticoagulation in these patients. We summarize available evidence and offer practical algorithms in these situations.
Patient 1
A 42‐year‐old man with a history of grade III astrocytoma, whose status is after resection and adjuvant radiation and who is now on maintenance temozolomide, presents with macrocytic anemia and peripheral blasts. Bone marrow biopsy reveals acute myeloid leukemia transformed from a background of myelodysplasia. Cytogenetics show an unfavorable inversion of chromosome 3. He is started on decitabine induction chemotherapy. What are the risk factors predisposing this patient to intracranial hemorrhage?
Because it occurs in a closed space, intracranial hemorrhage is one of the most feared complications of cancer and its treatment. Two of the most common causes of bleeding are spontaneous intratumoral hemorrhage and coagulopathy, with less common causes including trauma, hypertension, or hemorrhagic transformation of venous thrombosis [6]. In a large contemporary series of patients with cancer with intracranial bleeding, 68% of patients had solid tumors metastatic to the brain, 16% had primary brain tumors, and another 16% had hematologic malignancies. Intratumoral hemorrhage (61%) and/or coagulopathy (46%) accounted for the majority of cases, whereas bleeding associated with hypertension (5%) was rare [7]. The mechanisms underlying intratumoral hemorrhage include abnormal tumor vasculature, tumor invasion of blood vessels, and tumor cell necrosis [6], [8]. In addition, local imbalances in factors involved in coagulation and fibrinolytic cascades likely contribute directly to intratumoral hemorrhage [9].
The role of platelets in intracranial hemorrhage is less well defined. Thrombocytopenia increases the risk of intracranial tumor bleeding considerably, especially when it is severe (defined as less than 50,000/μL) [10]. Notably, several studies in acute leukemia and other hematologic malignancies have found an elevated prothrombin time, rather than thrombocytopenia, to be more predictive of intracranial bleeding [11], [12]. Nevertheless, an ongoing prospective, nested, case‐control study of intracranial hemorrhage in patients with thrombocytopenic hematology will help delineate the exact role of thrombocytopenia and identify additional risk factors for bleeding [13]. Finally, given the potential bleeding risk, the concurrent use of antiplatelet agents during anticoagulation, including nonsteroidal anti‐inflammatory drugs, should be avoided if possible.
Patient 1 continued: The patient receives four cycles of decitabine chemotherapy and achieves partial response. His transfusion requirement lessens, and his platelet count is stable above 100,000. However, he complains of left leg swelling, and a left lower extremity venous Doppler ultrasound reveals an acute deep vein thrombosis. Should you initiate anticoagulation?
VTE is highly prevalent in high‐grade gliomas, especially around the perioperative period, with a pooled incidence rate of 7%–28% that is among the highest in all cancers [14]. A recent meta‐analysis estimated the risk of VTE to be 1.5%–2.0% per month of survival in high‐grade gliomas [15]. A myriad of additional VTE risk factors related to the patient, such as immobility, or to the treatment, such as antiangiogenic therapy, have been identified [13], [16]. In addition, tumor‐specific factors, such as elevated factor VIII levels, certain ABO blood types, and thrombin generation, were also shown to be risk factors for VTE in patients with glioma [17], [18]. Recently, an elegant biochemical analysis revealed that increased expression of podoplanin, a cell surface glycoprotein, in malignant glioma can trigger platelet aggregation in mouse models, thus contributing to hypercoagulability [19].
A recent meta‐analysis estimated the risk of VTE to be 1.5%–2.0% per month of survival in high‐grade gliomas. A myriad of additional VTE risk factors related to the patient, such as immobility, or to the treatment, such as antiangiogenic therapy, have been identified.
Management of VTE in patients with intracranial malignancies is particularly challenging, due to the fear of intracranial hemorrhage. Although early studies suggested that carefully managed anticoagulation does not appear to increase the risk of intracranial bleeding [20], [21], [22], a recent meta‐analysis of several retrospective cohort studies indicated an elevated risk of intracranial hemorrhage associated with anticoagulation in patients with glioma (odds ratio 3.75) [5]. This risk increased even further in the setting of supratherapeutic anticoagulation or inadequate preoperative correction of coagulation abnormalities [8], [23]. In more contemporary series, the risk of intracranial bleeding associated with therapeutic anticoagulation in glioma is approximately 4%–5% [5], [24], [25]. Therefore, balancing the risks of thrombosis and hemorrhage is essential, and anticoagulation is generally avoided in patients with a history of previous intracranial hemorrhage, bleeding diathesis, thrombocytopenia (<50,000/μL), coagulopathy, or ongoing life‐threatening extracranial bleeding [24]. A recent matched cohort analysis found that the platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, and stroke score for intracranial hemorrhage, initially developed for risk stratification in patients without cancer, accurately predicted intracranial bleeding in patients with malignant glioma [26]. However, this model must be validated by independent datasets before it can be adopted widely.
Anticoagulation with low molecular weight heparin (LMWH) or warfarin is the recommended treatment for established VTE in patients with primary brain tumors [4]. Due to warfarin's significant drug‐drug interactions and the need for frequent laboratory monitoring, LMWH has been increasingly used for treatment and prevention of VTE and has been shown to be more effective than warfarin in reducing recurrent VTE [27], [28]. The duration of anticoagulation treatment depends on the continued presence of predisposing factors. An indefinite duration of treatment is generally recommended for patients with primary brain tumor or active systemic malignancy. In patients who are no longer considered at risk for increased hypercoagulability, such as those with grossly resected meningioma, primary central nervous system lymphoma with complete response, systemic malignancies with durable response to chemotherapy, or metastatic germ cell tumors fully treated with chemotherapy, anticoagulation duration is usually 6 months [4], [27].
Finally, patients with thrombocytopenia present an additional challenge for therapeutic anticoagulation. An individualized approach, taking into account the etiology, the severity, the expected duration, and the potential reversibility of thrombocytopenia, is recommended [29]. In the acute setting of VTE, full therapeutic anticoagulation may be initiated at platelet count of ≥50,000 and may be supported by platelet transfusion, whereas in the subacute or chronic treatment periods, dose reduction of LMWH for platelet count <50,000 and discontinuation for platelet count <25,000 have been endorsed by guidelines [29].
Case follow‐up: The patient received therapeutic enoxaparin for treatment of his DVT. However, his acute myelogenous leukemia soon progressed, and he had a brief trial of lenalidomide before enrolling in hospice care.
Patient 2
A 69‐year‐old woman with metastatic non‐small cell lung cancer on second‐line chemotherapy presents with acute onset dyspnea and chest pressure for 2 days. A chest computed tomography (CT) angiogram shows a segmental PE in the left lower lobe, and a Doppler study reveals a lower extremity DVT. She had brain metastases that were treated with gamma knife therapy, although a brain magnetic resonance imaging (MRI) 1 week ago showed a new, 10‐mm frontal metastasis, and she is awaiting additional gamma knife therapy. Should you initiate anticoagulation?
As discussed above, the overall intracranial bleeding risk is usually determined by the presence of prior intracranial bleeding, pre‐existing bleeding diathesis (i.e., thrombocytopenia and inherited or acquired bleeding diathesis), and coagulopathy. However, brain metastases from melanoma, choriocarcinoma, thyroid carcinoma, and renal cell carcinoma have particularly high propensities for spontaneous hemorrhage in a small case series of 15 patients [30], although brain metastases from other primary tumors (e.g., lung, breast) generally do not bleed spontaneously [4], [31]. In addition, hepatocellular carcinoma also appears to have increased risk of spontaneous intracranial bleeding [32]. In the case of non‐small cell lung cancer, a large single‐institution series of 776 patients with non‐small cell lung cancer with brain metastasis at M.D. Anderson reveals a spontaneous intracranial bleeding risk of only 1.2% over 580 person‐years of follow‐up [33]. The authors attribute the low rate to the almost universal application of radiation therapy, including gamma knife, which is known to blunt angiogenesis, normalizing tumor vasculature and decreasing the risk of tumor hemorrhage [34].
Several studies have suggested that the risk of tumor‐associated intracranial hemorrhage may not be significantly increased in patients with metastatic brain tumors if the degree of anticoagulation is carefully monitored [5]. For example, in a series of 49 patients with intracranial malignancies and VTE who received either inferior vena cava filter (42 patients) or warfarin anticoagulation (15 patients), none of the latter patients had proven hemorrhagic complications, but the complication rate of inferior vena cava filter was higher than commonly perceived and may outweigh the risk of anticoagulation [35]. In another series of 51 patients with known brain metastases and VTE, 3 developed intracranial hemorrhage, 2 of whom had supratherapeutic anticoagulation (above a target international normalized ratio of 2–3). Therefore, therapeutic anticoagulation was more effective than inferior vena cava filter in reducing recurrent VTE [36]. In another large Spanish prospective cohort study of 203 patients with cancer with symptomatic VTE that included 45 with brain metastases, only 1 patient developed an intracranial hemorrhage following anticoagulation with LMWH [37]. Similarly, in a recent large retrospective matched cohort study of 293 patients with brain metastases in which about one third of the patients received therapeutic LMWH, there was no significant difference in the cumulative incidence of intracranial hemorrhage at 1 year among patients treated with LMWH compared with controls (19% vs. 21% for measurable hemorrhage and 44% vs. 37% for total hemorrhage) [38]. These studies establish LMWH as a safe, and perhaps preferred, anticoagulant for VTE in patients with cancer with treated brain metastasis and without significant coagulopathy or thrombocytopenia. Thrombolytics are considered contraindicated in patients with brain metastasis who present with hemodynamically significant acute PE, although prospective supporting evidence is lacking [39].
The risk of inducing intracranial hemorrhage in a patient with cancer raises questions about whether such patients should undergo brain imaging prior to the use of antiplatelet agents or anticoagulants for VTE or other indications. Unfortunately, there are insufficient data to answer this question, and our practical approach is based upon the risk of brain metastasis being present and the likelihood of such metastases to bleed with anticoagulation. We suggest brain imaging, preferably with MRI, for patients whose primary malignancy is melanoma, renal cell carcinoma, choriocarcinoma, hepatocellular carcinoma, or thyroid cancer, and we suggest that anticoagulation be delayed if brain metastasis is found. Patients with other cancers should also be imaged if anticoagulation can be safely delayed while awaiting imaging or if there are any symptoms suggesting brain metastasis (e.g., headache, mental status changes, seizures, other neurologic symptoms). For patients with occult metastases from other primary tumors, the indication for anticoagulation (e.g., stroke prophylaxis in atrial fibrillation, acute PE, DVT) must be weighed against the risk of hemorrhage. Serial brain imaging during anticoagulation for VTE is currently not supported by prospective evidence, but imaging should be considered in patients with new or changing neurologic symptoms.
The risk of inducing intracranial hemorrhage in a patient with cancer raises questions about whether such patients should undergo brain imaging prior to the use of antiplatelet agents or anticoagulants for VTE or other indications. Unfortunately, there are insufficient data to answer this question, and our practical approach is based upon the risk of brain metastasis being present and the likelihood of such metastases to bleed with anticoagulation.
Case follow‐up: The patient received therapeutic enoxaparin for treatment of her PE and DVT after a successful gamma knife surgery for her isolated brain metastasis. A repeat MRI 1 month later revealed no signs of intracranial bleeding.
Patient 3
A 71‐year‐old man with metastatic melanoma presents with falls and right lower extremity swelling. A Doppler study shows a DVT in the right femoral vein. Before starting anticoagulation, a noncontrast enhanced head CT reveals several large brain metastases with microhemorrhages and associated vasogenic edema. Targeted therapy and immunotherapy with anti‐programmed death 1 antibody have previously failed for this patient. How should we manage the anticoagulation?
Patients with malignant melanoma have a high prevalence of brain metastasis and increased risk of spontaneous intracranial hemorrhage. Donato et al. found a significantly higher risk of intracranial bleeding (40%–50% at 1 year) for brain metastases from melanoma and renal cell carcinoma, with or without LMWH [38]. However, a single‐institution, retrospective cohort study of 74 patients with melanoma with brain metastases and VTE, in which 57 received systemic anticoagulation, mainly with LMWH, showed that only 2 patients (4%) developed intracranial bleeding [40]. This discrepancy is likely due to the increased sensitivity of modern imaging and longer follow‐up in the former study, as well as differences in the radiographic and clinic definition of intracranial hemorrhage. A recent meta‐analysis of nine retrospective cohort studies revealed that there was no additional statistically significant increase in intracranial hemorrhage associated with therapeutic anticoagulation in patients with brain metastasis from solid tumors, including melanoma and renal cell carcinoma, suggesting that therapeutic anticoagulation should be considered even for these patients [5].
An acceptable alternative to anticoagulation is the placement of an inferior vena cava (IVC) filter in patients at high risk for intracranial bleeding. However, the use of IVC filters in patients with primary and metastatic brain tumors has been associated with substantial complications, as well as lower efficacy in reducing recurrent VTE [35], [36], [41]. For example, in a series of 42 such patients, 12% had recurrent pulmonary emboli and 57% developed IVC or filter thrombosis, recurrent deep vein thrombosis, or post‐thrombotic syndrome [35]. In another series of 51 patients with known brain metastases and VTE, 10 were initially treated with a Greenfield filter (Boston Scientific, Marlborough, MA); 4 (40%) had recurrent nonfatal thromboembolic events (2 PE and 2 DVT), and none of the 39 patients who were initially treated with anticoagulation developed recurrent VTE [36].
Novel direct oral anticoagulants (DOACs) that directly inhibit thrombin (such as dabigatran) or factor Xa, including rivaroxaban, apixaban, and edoxaban, have the advantages of being administered orally, not requiring laboratory monitoring, and infrequent dose adjustment. Overall, these agents appear to have a slightly lower risk of bleeding than warfarin or LMWH in the unselected (i.e., noncancer) population [42]. These agents can effectively treat and prevent VTE but are only now under study extensively in patients with cancer or in comparison with LMWH. In addition, the safety of DOAC use in brain tumors is not well established. Apixaban given for 12 weeks is a safe and efficacious option for the prevention of VTE in ambulatory patients with advanced metastatic cancer undergoing first‐ or second‐line chemotherapy, although the rate of intracranial hemorrhage is unknown [43]. Recently, the first prospective study of rivaroxaban for treating patients with cancer with VTE was reported with acceptable efficacy and safety; however, the rate of intracranial bleeding was not reported [44]. Two recent papers reported cancer subgroup analysis of rivaroxaban and apixaban for treatment of VTE, which showed similar efficacy and reduced rate of bleeding compared with warfarin and LMWH [45], [46]. Given the additional advantage of avoiding self‐injection and the increased availability of reversal agents, DOACs will likely see increased use in patients with cancer, although their safety and efficacy in patients with primary brain tumor and secondary brain metastasis warrant prospective investigation.
Case follow‐up: Given his limited life expectancy and the high risk of intracranial hemorrhage, Patient 3 received inferior vena cava filter placement for treatment of DVT and salvage whole brain radiation therapy. He maintained acceptable quality of life until passing away 4 months later.
Conclusion
Given the available retrospective and prospective evidence, carefully monitored systemic anticoagulation appears safe in all patients with primary and metastatic brain tumors and VTE, except for patients with untreated tumors with a high rate of intracranial hemorrhage (i.e., metastases from melanoma, choriocarcinoma, thyroid carcinoma, hepatocellular carcinoma, and renal cell carcinoma). LMWH is the preferred agent of anticoagulation over warfarin, given its known safety and efficacy in patients with cancer. The optimal duration of anticoagulation is 6 months, although continuous treatment is recommended for active systemic and/or intracranial malignancies. This is also supported by a recent systematic review and meta‐analysis of five prospective trials comparing LMWH with warfarin in patients with cancer with VTE, which found that the overall risk of intracranial hemorrhage was <1% and that anticoagulation with LMWH for ≤6 months did not increase this risk [47].
For patients with metastatic brain tumors that have an increased risk of hemorrhage, it may be appropriate to place an inferior vena cava filter if there is significant untreated disease in the brain, with an understanding that a high rate of complications from these filters is possible. For those whose metastatic disease has already been surgically removed or treated effectively with radiotherapy, systemic anticoagulation may be warranted even for tumors with high bleeding risks. These recommendations are consistent with the published American Society of Clinical Oncology guidelines for the prevention and treatment of VTE in patients with cancer [48], as well as opinions from other experts in the field [49], and are summarized in Table 1.
Table 1. Anticoagulation considerations in patients with malignant brain tumors.
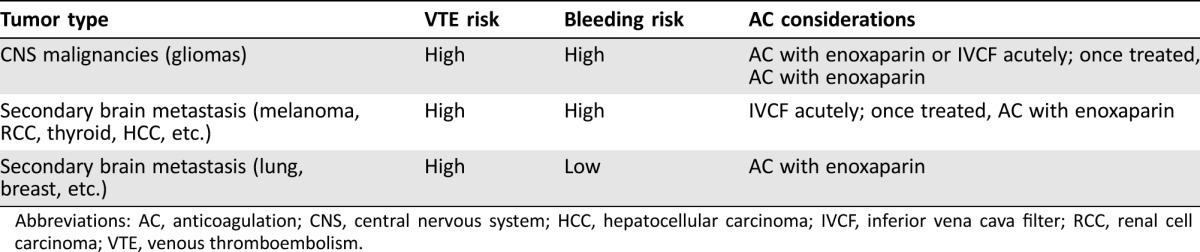
Abbreviations: AC, anticoagulation; CNS, central nervous system; HCC, hepatocellular carcinoma; IVCF, inferior vena cava filter; RCC, renal cell carcinoma; VTE, venous thromboembolism.
Acknowledgments
We thank Drs. Lawrence Gardner, Michael Grossbard, and Franco Muggia for comments on the manuscript. Dr. Lin is currently affiliated with Memorial Sloan Kettering Cancer Center.
This article was published online on 20 November 2017. After online publication, the author affiliations have been updated. This notice is included in the online and print versions to indicate that both have been corrected 11 April 2018.
Footnotes
For Further Reading: Eoin Donnellan, Alok A. Khorana. Cancer and Venous Thromboembolic Disease: A Review. The Oncologist 2017;22:199–207.
Implications for Practice: This article discusses venous thromboembolism (VTE) in patients with malignancy. Practical guidance is offered on how to prevent, diagnose, and treat VTE in cancer patients. The management of “challenging” cases of VTE is also discussed.
Author Contributions
Conception/design: Richard J. Lin, David L. Green, Gunjan L. Shah
Provision of study material or patients: Richard J. Lin, David L. Green, Gunjan L. Shah
Collection and/or assembly of data: Richard J. Lin, David L. Green, Gunjan L. Shah
Data analysis and interpretation: Richard J. Lin, David L. Green, Gunjan L. Shah
Manuscript writing: Richard J. Lin, David L. Green, Gunjan L. Shah
Final approval of manuscript: Richard J. Lin, David L. Green, Gunjan L. Shah
Disclosures
The authors indicated no financial relationships.
References
- 1. Streiff MB. Thrombosis in the setting of cancer. Hematology Am Soc Hematol Educ Program 2016;2016:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farge D, Debourdeau P, Beckers M et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost 2013;11:56–70. [DOI] [PubMed] [Google Scholar]
- 3. Schwarz RE, Marrero AM, Conlon KC et al. Inferior vena cava filters in cancer patients: Indications and outcome. J Clin Oncol 1996;14:652–657. [DOI] [PubMed] [Google Scholar]
- 4. Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol 2006;24:1310–1318. [DOI] [PubMed] [Google Scholar]
- 5. Zwicker JI, Leaf RK, Carrier M. A meta‐analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation. J Thromb Haemost 2016;14:1736–1740. [DOI] [PubMed] [Google Scholar]
- 6. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep 2012;14:373–381. [DOI] [PubMed] [Google Scholar]
- 7. Navi BB, Reichman JS, Berlin D et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology 2010;74:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magnus N, D'Asti E, Garnier D et al. Brain neoplasms and coagulation. Semin Thromb Hemost 2013;39:881–895. [DOI] [PubMed] [Google Scholar]
- 9. Jung S, Moon KS, Jung TY et al. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor‐associated intracerebral hemorrhage. J Neurooncol 2006;76:257–263. [DOI] [PubMed] [Google Scholar]
- 10. Neunert C, Noroozi N, Norman G et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systemic review. J Thromb Haemost 2015;13:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CY, Tai CH, Tsay W et al. Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Ann Oncol 2009;20:1100–1104. [DOI] [PubMed] [Google Scholar]
- 12. Chen CY, Tai CH, Cheng A et al. Intracranial hemorrhage in adult patients with hematological malignancies. BMC Med 2012;10:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estcourt LJ, Stanworth SJ, Collett D, Murthy MF. Intracranial haemorrhage in thrombocytopenic haematology patients—A nested case‐control study: The InCiTe study protocol. BMJ Open 2014;4:e004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry JR. Thromboembolic disease in patients with high‐grade glioma. Neuro Oncol 2012;14:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: An evidence based review. Cancer 2000;89:640–646. [DOI] [PubMed] [Google Scholar]
- 16. Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost 2014;40:325–331. [DOI] [PubMed] [Google Scholar]
- 17. Streiff MB, Segal J, Grossman SA et al. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer 2004;100:1717–1723. [DOI] [PubMed] [Google Scholar]
- 18. Streiff MB, Ye X, Kickler TS et al. A prospective multicenter study of venous thromboembolism in patients with newly‐diagnosed high‐grade glioma: Hazard rate and risk factors. J Neurooncol 2015;124:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riedl J, Preusser M, Nazari PM et al. Podoplanin expression in primary brain tumors induced platelet aggregation and increases risk of venous thromboembolism. Blood 2017;129:1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983;13:334–336. [DOI] [PubMed] [Google Scholar]
- 21. Choucair AK, Silver P, Levin VA. Risk of intracranial hemorrhage in glioma patients receiving anticoagulant therapy for venous thromboembolism. J Neurosurg 1987;66:357–358. [DOI] [PubMed] [Google Scholar]
- 22. Altschuler E, Moosa H, Selker RG et al. The risk and efficacy of anticoagulant therapy in the treatment of thromboembolic complications in patients with primary malignant brain tumors. Neurosurgery 1990;27:74–76; discussion 77. [DOI] [PubMed] [Google Scholar]
- 23. Lazio BE, Simard JM. Anticoagulation in neurosurgical patients. Neurosurgery 1999;45:838–847; discussion 847–848. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins EO, Schiff D, Mackman N et al. Venous thromboembolism in malignant gliomas. J Thromb Haemost 2010;8:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yust‐Katz S, Mandel JJ, Wu J et al. Venous thromboembolism (VTE) and glioblastoma. J Neurooncol 2015;124:87–94. [DOI] [PubMed] [Google Scholar]
- 26. Mantia C, Uhlmann EJ, Puligandla M et al. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood 2017;129:3379–3385. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt F, Faul C, Dichgans J et al. Low molecular weight heparin for deep vein thrombosis in glioma patients. J Neurol 2002;249:1409–1412. [DOI] [PubMed] [Google Scholar]
- 28. Lee AY, Levine MN, Baker RI et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 29. Carrier M, Khorana AA, Zwicker JI et al. Management of challenging cases of patients with cancer‐associated thrombosis including recurrent thrombosis and bleeding: Guidance from the SSC of the ISTH. J Thromb Haemost 2013;11:1760–1765. [DOI] [PubMed] [Google Scholar]
- 30. Mandybur TI. Intracranial hemorrhage caused by metastatic tumors. Neurology 1977;27:650–655. [DOI] [PubMed] [Google Scholar]
- 31. Wakai S, Yamakawa K, Manaka S et al. Spontaneous intracranial hemorrhage caused by brain tumor: Its incidence and clinical significance. Neurosurgery 1982;10:437–444. [DOI] [PubMed] [Google Scholar]
- 32. Hsieh MJ, Lu CH, Tsai NW et al. Prediction, clinical characteristics and prognosis of intracerebral hemorrhage in hepatocellular carcinoma patients with intracerebral metastasis. J Clin Neurosci 2009;16:394–398. [DOI] [PubMed] [Google Scholar]
- 33. Srivastava G, Rana V, Wallace S et al. Risk of intracranial hemorrhage and cerebrovascular accidents in non‐small cell lung cancer brain metastasis patients. J Thorac Oncol 2009;4:333–337. [DOI] [PubMed] [Google Scholar]
- 34. Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: Exploring mechanisms of interaction. Clin Cancer Res 2003;9:1957–1971. [PubMed] [Google Scholar]
- 35. Levin JM, Schiff D, Loeffler JS et al. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993;43:1111–1114. [DOI] [PubMed] [Google Scholar]
- 36. Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer 1994;73:493–498. [DOI] [PubMed] [Google Scholar]
- 37. Monreal M, Zacharski L, Jiménez JA et al. Fixed‐dose low‐molecular‐weight heparin for secondary prevention of venous thromboembolism in patients with disseminated cancer: A prospective cohort study. J Thromb Haemost 2004;2:1311–1315. [DOI] [PubMed] [Google Scholar]
- 38. Donato J, Campigotto F, Uhlmann EJ et al: Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: A matched cohort study. Blood 2015;126:494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Norris LK, Grossman SA. Treatment of thromboembolic complications in patients with brain tumors. J Neurooncol 1994;22:127–137. [DOI] [PubMed] [Google Scholar]
- 40. Alvarado G, Noor R, Bassett R et al. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res 2012;22:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olin JW, Young JR, Graor RA et al. Treatment of deep vein thrombosis and pulmonary emboli in patients with primary and metastatic brain tumors. Anticoagulants or inferior vena cava filter? Arch Intern Med 1987;147:2177–2179. [PubMed] [Google Scholar]
- 42. Di Minno MN, Ambrosino P, Lupoli R et al. Direct oral anticoagulants for the treatment of unprovoked venous thromboembolism: A meta‐analysis of randomised controlled trials. Blood Transfus 2015;13:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levine MN, Gu C, Liebman HA et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012;10:807–814. [DOI] [PubMed] [Google Scholar]
- 44. Mantha S, Laube E, Miao Y et al. Safe and effective use of rivaroxaban for treatment of cancer‐associated venous thromboembolic disease: A prospective cohort study. J Throm Thrombolysis 2017;43:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agnelli G, Buller HR, Cohen A et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: Results from the AMPLIFY trial. J Thrombo Haemost 2015;13:2187–2191. [DOI] [PubMed] [Google Scholar]
- 46. Prins MH, Lensing AW, Bauersachs R et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rojas‐Hernandez CM, Oo TH, García‐Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: A systematic review and meta‐analysis. J Thromb Thrombolysis 2017;43:233–240. [DOI] [PubMed] [Google Scholar]
- 48. Lyman GH, Khorana AA, Falanga A et al. American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490–5505. [DOI] [PubMed] [Google Scholar]
- 49. Weinstock MJ, Uhlmann EJ, Zwicker JI. Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb Res 2016;140(suppl 1):60–65. [DOI] [PubMed] [Google Scholar]


