This brief communication reports on the activity of lapatinib plus trastuzumab after acquired disease resistance to trastuzumab plus pertuzumab in patients with HER2 colorectal cancer. HER2 amplification represents a distinct molecular subgroup of colorectal cancers that is associated with anti‐EGFR resistance and sensitivity to dual HER2 targeting. Three such cases are described.
Abstract
Human epidermal growth factor 2 (HER2) amplification represents a distinct molecular subgroup of colorectal cancers that is associated with anti‐epidermal growth factor receptor resistance and sensitivity to dual HER2 targeting. Although clinical trials have reported activity for trastuzumab/pertuzumab and trastuzumab/lapatinib combinations, there are no reports on lapatinib plus trastuzumab activity after resistance to trastuzumab plus pertuzumab. Presented are three cases of HER2 amplified colorectal cancer that developed acquired refractoriness to trastuzumab pertuzumab with subsequent clinical benefit to lapatinib plus trastuzumab, highlighting the potential for HER2 tyrosine kinase inhibition plus trastuzumab in overcoming trastuzumab/pertuzumab resistance.
Introduction
Human epidermal growth factor 2 (HER2) amplification occurs in 2%–3% of colorectal cancers (CRCs) and is enriched in patients with RAS wild‐type (WT) and BRAF WT left colon or rectum primary tumors [1], [2]. HER2‐amplified CRC tumors have been associated with anti‐epidermal growth factor receptor (EGFR) resistance in preclinical and clinical studies [1], [3]. Preclinical models support a role for dual HER2 targeting, whether with trastuzumab plus pertuzumab or with trastuzumab plus lapatinib [1], [4]. Two phase II clinical studies have now reported on these combinations in HER2‐amplified metastatic CRC with significant responses and disease stabilizations [3], [5]. The HERACLES study investigated the combination of lapatinib plus trastuzumab in patients with metastatic CRC cancer with HER2 overexpression (HER2:chromosome enumeration probe 17 [CEP17] ratio >2 by fluorescence in situ hybridization [FISH] or human epidermal growth factor expression of 3+ by immunohistochemistry [IHC]) [3]. The response rate to treatment was 30%, with an additional 44% of patients attaining stable disease. The median progression‐free survival (PFS) was 5 months in the overall population and exceeded 7 months in patients with a HER2 copy ≥9.45 [3]. The My Pathway clinical trial investigated the combination of trastuzumab plus pertuzumab in patients with HER2‐amplified tumors, defined as having 3+ staining by IHC, FISH or chromogenic in situ hybridization ratio >2, or >6 HER2 copies by next‐generation sequencing [5]. Thirty‐four patients with HER2‐overexpressed metastatic CRC were treated on study. The response rate, PFS, and overall survival (OS) on preliminary analysis were 38%, 4.6 months, and 10.3 months, respectively [5]. Patients with left‐sided primaries or with RAS wild‐type tumors appeared to derive an improved outcome in comparison with patients with right‐sided and RAS‐mutated tumors [5]. Both HERACLES and My Pathway establish the potential utility of dual HER2 targeting in HER2‐amplified CRC. The combination of trastuzumab plus pertuzumab will be compared with cetuximab plus irinotecan in an Southwest Oncology Group (SWOG) randomized phase II clinical trial in the second‐ and third‐line treatment of RAS wild‐type, HER2‐amplified metastatic CRC (SWOG 1613). Yet the optimal regimen for HER2 targeting in this population remains undefined. In addition, the utility of HER2 tyrosine kinase inhibition plus trastuzumab after trastuzumab plus pertuzumab failure, or vice versa, remains unknown. In this communication, we report on three cases of HER2‐amplified metastatic CRC with acquired refractoriness to trastuzumab plus pertuzumab with documented clinical benefits on salvage lapatinib plus trastuzumab, highlighting the potential for HER2 tyrosine kinase inhibitor (TKI) and trastuzumab combination in overcoming acquired resistance to dual HER2 monoclonal antibody targeting (i.e., trastuzumab plus pertuzumab).
Case Series
Three patients with HER2‐amplified colorectal cancer whose disease progressed on fluoropyrimidine, irinotecan, and oxaliplatin were treated with trastuzumab and pertuzumab, as described by the My Pathway trial [5]. All patients developed an initial documented clinical benefit followed by on‐treatment disease progression. The patients’ characteristics are listed in Table 1. All three patients were treated with trastuzumab intravenously at 6 mg/kg every 3 weeks in combination with lapatinib 1000 mg by mouth daily, all within 4 weeks of documented disease progression on trastuzumab plus pertuzumab. All three patients experienced major reductions in carcinoembryonic antigen (CEA) from their baseline (Table 1). The CEA nadirs were 10 ng/mL at 17 weeks, 2 ng/mL at 9 weeks, and 9 ng/mL at 6 weeks, in cases 1, 2, and 3, respectively. One patient (case 1) achieved an ongoing partial response, and two patients achieved an ongoing stable disease (Figs. 1, 2, 3). Case 1 represents a patient with metastatic rectal cancer to the lungs and the left chest wall and pleura. The patient had progressed on trastuzumab plus pertuzumab with a significant increase in his left chest mass and left pleural tumor deposits. After three cycles of lapatinib plus pertuzumab, all previously progressing lesions experienced major regression, with resolution of tumor‐related chest pain (Fig. 1). Case 2 represents a patient with sigmoid colon cancer with extensive hepatic metastases and low‐volume metastatic disease to the lungs. The patient developed disease progression in the liver after a protracted benefit from trastuzumab plus pertuzumab. The patient experienced regression in all existing metastases in the liver without satisfying an objective response by RECIST guidelines (Fig. 2). Case 3 represents a patient with rectal cancer with low volume metastatic disease to the lungs and pelvic lymph nodes and whose disease progressed in the lungs while on trastuzumab pertuzumab. The patient had disease stabilization after four cycles of lapatinib plus pertuzumab (Fig. 3). The patients in cases 1, 2, and 3 remain on lapatinib plus pertuzumab at 5, 4.5, and 9 months of treatment, respectively. Their treatment continues to be well tolerated without dose modifications.
Table 1. Patients’ baseline characteristics.
HER2 copy number was assessed on the primary tumors and was performed by a commercially validated assay (Foundation One; Foundation Medicine, Cambridge, MA).
Abbreviations: CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor 2; NGS, next‐generation sequencing; WT, wild type.
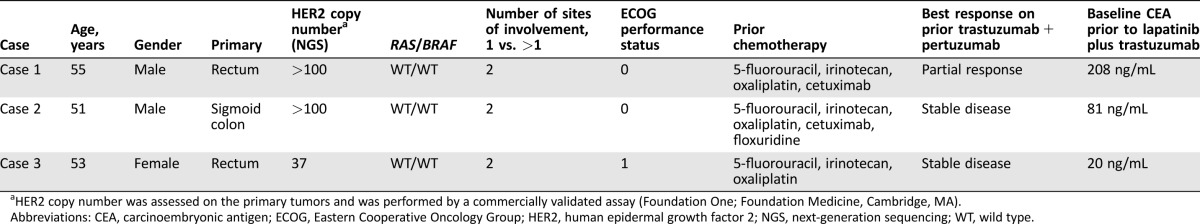
Figure 1.
Case 1. (A): At baseline, pleural carcinomatosis and left rib involvement. (B): After three cycles of trastuzumab plus lapatinib, with resolution of pleural thickening, sclerosis of left rib, and regression of associated soft tissue mass.
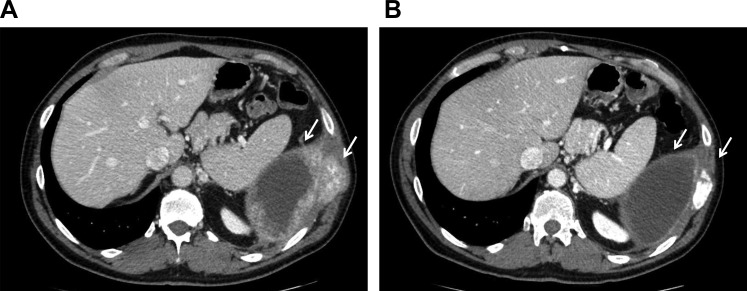
Figure 2.
Case 2. (A): At baseline, hepatic metastases, including a large lesion in the caudate lobe. (B): After nine cycles of trastuzumab plus lapatinib, with a decrease in caudate lobe metastatic disease and an increase in treatment‐related calcification.
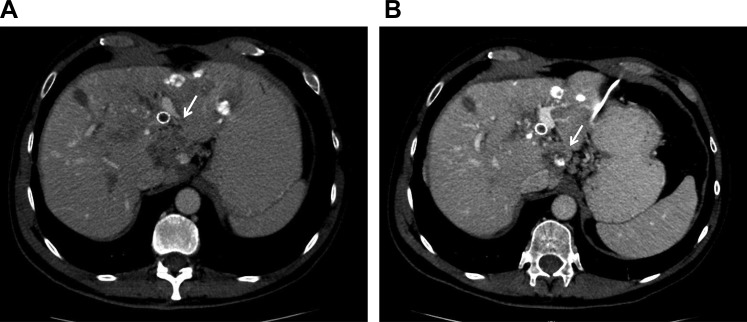
Figure 3.
Case 3. (A): At baseline, pulmonary metastases. (B): After four cycles of trastuzumab plus lapatinib, with stable pulmonary metastatic disease.
Discussion
Several preclinical studies have evaluated the combinations of lapatinib and trastuzumab or lapatinib plus pertuzumab in HER2‐amplified colorectal patient‐derived xenograft models [1], [4]. These experiments have confirmed the need for dual HER2 targeting to achieve an optimal tumor response. The combination of lapatinib plus trastuzumab leads to more effective human epidermal growth receptor 3 and EGFR signaling inhibition than seen with either agent alone, therefore leading to the superior antitumor activity of the combination [4]. Similar synergistic activity has been proposed based on clinical trials conducted in patients with breast cancer [6], [7]. The EGF104900 study evaluated the combination of lapatinib plus trastuzumab in patients with HER2 amplified breast cancer who progressed after first‐line trastuzumab‐based therapy [6]. Lapatinib plus trastuzumab was significantly better than lapatinib alone for both PFS and OS endpoints. The findings from EGF104900 represent a proof of concept of the potential synergy of HER2 TKI plus trastuzumab after trastuzumab failure.
Here, we report the first case series on the off‐label use and activity of lapatinib plus trastuzumab after trastuzumab plus pertuzumab progression in HER2‐amplified metastatic CRC. These findings, although preliminary, indicate that the mechanisms of resistance to trastuzumab and pertuzumab are not cross‐resistant with lapatinib plus trastuzumab. In addition, these findings indicate that HER2 remains a main driver of tumor growth after trastuzumab plus pertuzumab failure. Unfortunately, our patients did not undergo tumor biopsies before and after treatment to interrogate the mechanisms of resistance to trastuzumab plus pertuzumab and/or the associated mechanisms of the activity of lapatinib plus trastuzumab. Our clinical findings have potential clinical implications for the treatment of HER2‐amplified colorectal cancer and form the rationale for developing salvage therapies in this population after trastuzumab plus pertuzumab progression.
Footnotes
For Further Reading: Andrew Rankin, Samuel J. Klempner, Rachel Erlich et al. Broad Detection of Alterations Predicted to Confer Lack of Benefit From EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer. The Oncologist 2016;21:1306–1314; first published on September 28, 2016.
Implications for Practice: Comprehensive genomic profiling (CGP) detects diverse genomic alterations associated with lack of benefit to anti‐epidermal growth factor receptor therapy in advanced colorectal cancer (CRC), as well as targetable alterations in many other genes. This includes detection of a broad spectrum of activating KRAS alterations frequently missed by focused molecular hotspot testing, as well as other RAS/RAF pathway alterations, mutations shown to disrupt antibody binding, RTK activating point mutations, amplifications, and rearrangements, and activating alterations in downstream effectors including PI3K and MEK1. The use of CGP in clinical practice is critical to guide appropriate selection of targeted therapies for patients with advanced CRC.
Disclosures
The author indicated no financial relationships.
References
- 1. Bertotti A, Migliardi G, Galimi F et al. A molecularly annotated platform of patient‐derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab‐resistant colorectal cancer. Cancer Discov 2011;1:508–523. [DOI] [PubMed] [Google Scholar]
- 2. Richman SD, Southward K, Chambers P et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: Analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 2016;238:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sartore‐Bianchi A, Trusolino L, Martino C et al. Dual‐targeted therapy with trastuzumab and lapatinib in treatment‐refractory, KRAS codon 12/13 wild‐type, HER2‐positive metastatic colorectal cancer (HERACLES): A proof‐of‐concept, multicentre, open‐label, phase 2 trial. Lancet Oncol 2016;17:738–746. [DOI] [PubMed] [Google Scholar]
- 4. Leto SM, Sassi F, Catalano I et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2‐amplified gastrointestinal carcinomas. Clin Cancer Res 2015;21:5519–5531. [DOI] [PubMed] [Google Scholar]
- 5. Hurwitz H, Raghav KPS, Burris HA et al. Pertuzumab + trastuzumab for HER2‐amplied/overexpressed metastatic colorectal cancer (mCRC): Interim data from MyPathway. J Clin Oncol 2017;35(suppl 4):676a. [Google Scholar]
- 6. Blackwell KL, Burstein HJ, Storniolo AM et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer: Final results from the EGF104900 Study. J Clin Oncol 2012;30:2585–2592. [DOI] [PubMed] [Google Scholar]
- 7. Robidoux A, Tang G, Rastogi P et al. Lapatinib as a component of neoadjuvant therapy for HER2‐positive operable breast cancer (NSABP protocol B‐41): An open‐label, randomised phase 3 trial. Lancet Oncol 2013;14:1183–1192. [DOI] [PubMed] [Google Scholar]