Pharmacovigilance is required for all approved biologic agents, but because of the limited clinical experience with biosimilars at the time of approval, long‐term safety is a particular concern. Evidence of the safety of biosimilars requires the monitoring of adverse events. This report is an analysis of two phase III confirmatory studies, performed to strengthen the evidence on the safety profile of the biosimilar filgrastim in the breast cancer population.
Keywords: Biosimilar, Filgrastim, EP2006, Granulocyte colony‐stimulating factor
Abstract
Background.
Evaluation of adverse events (AEs) in pivotal registration trials and ongoing postmarketing surveillance is important for all biologics, including biosimilars. A combined analysis of two pivotal registration studies was performed to strengthen evidence on safety for biosimilar filgrastim EP2006 in patients with breast cancer receiving myelosuppressive chemotherapy, a sensitive clinical setting to confirm biosimilarity of filgrastim.
Materials and Methods.
Data were combined from two phase III studies of biosimilar filgrastim EP2006. The U.S. registration study was a randomized, double‐blind comparison of biosimilar and reference filgrastim in women aged ≥18 years with breast cancer, receiving (neo)adjuvant treatment with TAC (docetaxel + doxorubicin + cyclophosphamide). The European Union registration study was a single‐arm, open‐label study of biosimilar filgrastim in women aged ≥18 years with breast cancer receiving doxorubicin + docetaxel. Patients received filgrastim as a subcutaneous injection on day 2 of each cycle for <14 days or until the absolute neutrophil count reached 10 × 109/L after the expected nadir. Results were combined for cycles 1–4.
Results.
A total of 277 patients received biosimilar filgrastim EP2006. Patients had a mean (± standard deviation) age of 51.1 (± 10.8) years, and 78.7% of patients had stage II or III breast cancer. A total of 46 (20.6%) patients receiving biosimilar filgrastim had AEs considered filgrastim‐related. The most frequently reported filgrastim‐related AEs were musculoskeletal or connective tissue disorders (15.2%), including bone pain (7.2%). One death (due to pulmonary embolism) occurred of a patient receiving biosimilar filgrastim (not considered filgrastim‐related). No patient developed antidrug antibodies during the study.
Conclusion.
Biosimilar filgrastim has a safety profile consistent with previous filgrastim studies and is effective in preventing febrile neutropenia in patients with breast cancer.
Implications for Practice.
The biosimilar filgrastim EP2006 (Zarzio, Zarxio, biosimilar filgrastim‐sndz) has been approved in Europe since 2009 and in the U.S. since 2015. This combined analysis of two phase III studies provides additional clinical evidence that the biosimilar filgrastim EP2006 has a safety profile consistent with previous studies of reference filgrastim and supports large postmarketing studies of EP2006 in Europe. Strengthening the evidence for biosimilar filgrastim can help improve acceptance of biosimilars and increase patient access to biologics.
摘要
背景.对关键注册试验和正在进行的上市后监测中的不良事件(AE)进行评估对所有生物制剂(包括生物仿制药)而言都很重要。对两项关键注册研究进行了综合分析, 以加强接受骨髓抑制化疗的乳腺癌患者使用生物仿制药非格司亭EP2006(这是一个敏感的临床情况, 目的是确定非格司亭的生物相似性)的安全性证据。
材料与方法.数据来自生物仿制药非格司亭EP2006的两个III期研究。美国注册研究在接受TAC(多西他赛+多柔比星+环磷酰胺)(新)辅助治疗的年满18岁女性乳腺癌患者中进行了生物仿制药非格司亭和参比非格司亭的随机双盲比较。欧盟注册研究是在接受多柔比星+多西他赛治疗的年满18岁女性乳腺癌患者中进行生物仿制药非格司亭的单组、开放标签研究。患者在每个周期的第2天接受非格司亭皮下注射<14天或者直到绝对中性粒细胞计数达到预期最低点后的10 x 109/L。将1‐4周期的结果合并。
结果.共有277名患者接受了生物仿制药非格司亭EP2006给药。患者平均(±标准差)年龄为51.1(±10.8)岁, 78.7%的患者患有II期或III期乳腺癌。共46例(20.6%)接受生物仿制药非格司亭治疗的患者发生了与非格司亭相关的AE。最常报告的与非格司亭相关的AE是肌肉骨骼或结缔组织疾病(15.2%), 包括骨痛(7.2%)。有一例接受生物仿制药非格司亭治疗的患者死亡(死亡原因为肺栓塞, 认为与非格司亭无关)。在研究期间没有患者产生抗药物抗体。
结论.生物仿制药非格司亭的安全性特征与以前的非格司亭研究一致, 可有效预防乳腺癌患者的发热性中性粒细胞减少症。
对临床实践的提示: 生物仿制药非格司亭EP2006(Zarzio, Zarxio, 生物仿制药非格司亭‐sndz)已于2009年在欧洲获得批准, 于2015年在美国获得批准。两项III期研究的综合分析提供了额外的临床证据, 证明生物仿制药非格司亭EP2006的安全性特征与以前的参比非格司亭研究一致, 并且支持欧洲EP2006的大型上市后研究。加强生物仿制药非格司亭的安全性证据可帮助提高生物仿制药的接受程度, 增加患者获得生物制剂的机会
Introduction
Biosimilars are approved biologics with comparable quality, safety, and efficacy to a reference product after loss of patent protection [1]. One rationale for the development of biosimilars is the enhanced sustainability of cancer treatment, including increased patient access to expensive biological treatments [2]. Several biosimilar products have now been approved in Europe and elsewhere, including different versions of recombinant human filgrastim, based on the reference product. These have been available in Europe since 2008, and the U.S. Food and Drug Administration approved the first biosimilar, Zarxio/EP2006 (marketed in the U.S. as Filgrastim Hexal and elsewhere as Zarzio), in March 2015. Zarzio/Zarxio is a granulocyte‐colony stimulating factor (G‐CSF), indicated to reduce the duration of neutropenia and the incidence of febrile neutropenia in patients treated with established cytotoxic chemotherapy for malignancy.
Approval of biosimilar products requires a totality of evidence approach [3], [4]. This involves integrating various complementary tests in a sequential fashion to confirm the biosimilarity of the proposed biosimilar product compared with the reference product. Such a sequential approach starts with extensive structural and functional characterization of both the proposed biosimilar and reference products [5]. This is then followed by further testing, as appropriate, including pharmacokinetic and pharmacodynamic assessments and, typically, a final confirmation in a study population receiving a treatment regimen that is adequately sensitive for predicting a difference between the proposed biosimilar and the reference product [5], [6]. Guidelines for biosimilar medicinal products also recommend that the patient population should be homogenous and that clinical endpoints used must be the most sensitive for detecting differences in efficacy and safety between the proposed biosimilar and the reference. As the safety and efficacy of the reference product has already been established in the most sensitive patient population, based on the totality of evidence, indications of the reference product may be extrapolated to the biosimilar product [4].
Breast cancer treated by myelosuppressive chemotherapy is considered a sensitive indication in which similarity can be confirmed and any clinically meaningful differences between G‐CSF products and their biosimilars can be resolved. Treatment guidelines support the use of docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy as a standard curative treatment in patients with early breast cancer [7], [8], [9]. However, TAC chemotherapy has a dose‐limiting hematological toxicity with grade 3–4 neutropenia reported in approximately 65.5% of patients [10] and a median duration of grade 4 neutropenia of 7 days without G‐CSF support [11]. Treatment guidelines therefore recommend primary prophylaxis with G‐CSF as supportive care for TAC chemotherapy [12], [13], which has been proven to be effective in reducing neutropenia in women with early stage breast cancer [14]. Similarly, chemotherapy with doxorubicin and docetaxel has a risk of febrile neutropenia (FN) of 33%–48%, and thus primary prophylaxis with G‐CSF is recommended as supportive care for patients undergoing this chemotherapy regimen [15], [16]. Duration of severe neutropenia is directly proportional to the risk of infection and has become a well‐established and objective measure of efficacy to study and compare products in the G‐CSF class. Regarding the safety profile of G‐CSF, studies in reference filgrastim show that the most frequently reported adverse events (AEs) considered related to filgrastim are bone and skeletal pain, which is generally mild or moderate [17], [18].
Pharmacovigilance is required for all approved biologic agents, but because biosimilars generally have limited clinical experience at the time of approval, long‐term safety is a particular concern for biosimilar use [19]. This highlights the need for evidence regarding the safety of biosimilars, including ongoing monitoring of AEs to detect safety signals and provide reassurance for prescribers [19]. This combined analysis of two phase III confirmatory studies was performed to strengthen the evidence on the safety profile of biosimilar filgrastim (Zarzio/Zarxio) in the breast cancer population.
Materials and Methods
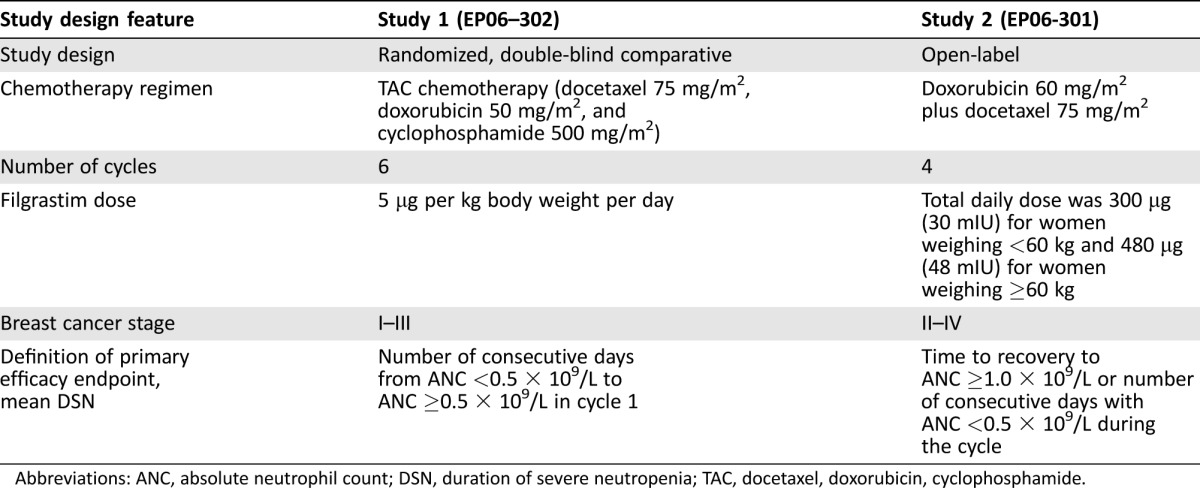
Data were combined from two phase III registration studies of biosimilar filgrastim. Study 1 (EP06‐302; NCT01519700) [20], the U.S. registration trial, was a randomized, double‐blind comparative study of biosimilar filgrastim and U.S.‐licensed reference filgrastim in women aged ≥18 years with breast cancer, receiving neoadjuvant or adjuvant treatment with TAC chemotherapy (docetaxel 75 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2). Study 2 (EP06‐301) [21], the European Union registration trial, was a single‐arm, open‐label study of biosimilar filgrastim in women aged ≥18 years with breast cancer, receiving doxorubicin 60 mg/m2 plus docetaxel 75 mg/m2. In both studies, patients received filgrastim as a subcutaneous (s.c.) bolus injection from day 2 of each cycle for up to 14 days or until absolute neutrophil count (ANC) reached 10 × 109/L after the expected nadir. In study 1, patients received filgrastim at a dose of 5 μg per kg of body weight per day s.c. In study 2, the total daily dose of filgrastim was 300 µg (30 mIU) for women weighing <60 kg and 480 µg (48 mIU) for women weighing ≥60 kg.
Both studies were conducted in accordance with the International Conference on Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice, applicable local regulations, and the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Key common inclusion criteria in the two studies included the following: chemotherapy‐naïve adult women with documented breast cancer, an estimated life expectancy of >6 months, Eastern Cooperative Oncology Group performance status ≤2, ANC ≥1.5 × 109/L, and platelet count ≥100 × 109/L. Staging of the patients differed between trials; study 1 included patients between stages I and III, whereas study 2 included patients from stage II to IV.
Common exclusion criteria in the two studies were the following: previous treatment with a G‐CSF, a previous or concurrent malignancy; concurrent or prior radiotherapy within 4 weeks of study start, total bilirubin higher than the upper limit of normal, creatinine >1.5 × the upper limit of normal, prior bone marrow or stem cell transplant, and systemic anti‐infective treatment within 72 hours of chemotherapy. In study 2, exclusion criteria also included severe neutropenia and white blood cell count >50 × 109/L.
Data of the patients treated with biosimilar filgrastim were only combined for cycles 1–4. Differences between the two studies are summarized in Table 1.
Table 1. Differences in study design between studies 1 and 2.
Abbreviations: ANC, absolute neutrophil count; DSN, duration of severe neutropenia; TAC, docetaxel, doxorubicin, cyclophosphamide.
Endpoints
This combined analysis was performed to further evaluate safety outcomes in study groups that received biosimilar filgrastim. Safety was assessed through the incidence and severity of treatment‐emergent adverse events (TEAEs). TEAEs were defined as adverse events that were reported after initiation of the study drug treatment. Local tolerability at the injection site was also assessed. Immunogenicity of filgrastim was assessed by a radioimmunoprecipitation assay for screening and confirmation and a cell‐based neutralization antibody assay.
The primary efficacy endpoint was the mean duration of severe (grade 4) neutropenia (DSN) during cycle 1 of chemotherapy, defined as the number of consecutive days with an ANC <0.5 × 109/L.
Secondary efficacy assessments included the number of patients who reported ≥1 fever episode (oral temperature ≥38.3°C), total number of days of fever, incidence of FN (oral temperature ≥38.3°C and ANC <0.5 × 109/L on the same day), and hospitalization due to FN; these were analyzed descriptively for each cycle and across all cycles. The depth of ANC nadir was analyzed descriptively for each cycle (median and day on which the nadir occurred). The time to ANC recovery (time after ANC nadir to ANC ≥2 × 109/L) was analyzed descriptively for cycle 1. The frequency of infections (recorded as AE, coded to system organ class of infections and infestations) and incidence of hospitalizations due to FN were analyzed descriptively for each cycle and across all cycles. Results for the secondary endpoints are not presented in this publication.
Statistical Analysis
All data were summarized using descriptive statistics. Results were reported as number of patients, mean, standard deviation, median, minimum and maximum for continuous variables and frequency and percentage for categorical data. Two‐sided 95% confidence intervals were calculated for mean or median values as appropriate. Incidences of AEs were calculated by chemotherapy cycle and for all cycles. All statistical analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC).
Results
A total of 277 patients received biosimilar filgrastim across the two studies. Patient disposition is shown in Figure 1. Patients had a mean (± standard deviation [SD]) age of 51.1 (± 10.8) years, and 78.7% of patients had stage II or III breast cancer. Baseline characteristics of patients are summarized in Table 2.
Figure 1.
Patient disposition.
Abbreviations: AE, adverse event; N, number of patients in a treatment group or analysis set; n, number of patients with at least one episode.
Table 2. Patient demographics and baseline characteristics (full analysis set/safety analysis set).
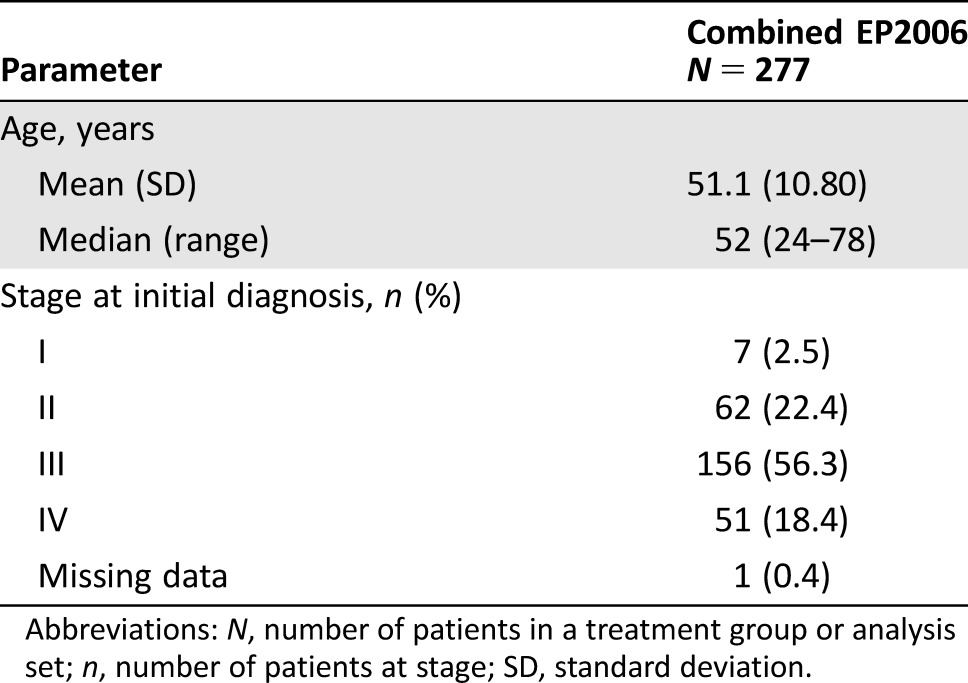
Abbreviations: N, number of patients in a treatment group or analysis set; n, number of patients at stage; SD, standard deviation.
In cycles 1–4, TEAEs were reported in 98.7% of patients receiving biosimilar filgrastim, the majority of which were related to chemotherapy (96.9%; Table 3), including alopecia, nausea, asthenia, fatigue, neutropenia, and leukopenia. Serious TEAEs were reported in 10.3% of patients. The most frequently reported serious TEAE was febrile neutropenia (cycle 1: n = 16, 7.2%). No serious TEAEs were reported as having a suspected relationship to filgrastim. In cycles 1–4, one death was reported of a patient receiving biosimilar filgrastim, which was due to a pulmonary embolism (day 6, cycle 1). This was not suspected to be related to study treatment.
Table 3. TEAEs reported in cycles 1–4 (safety analysis set).

All TEAEs except injection site reactions.
Abbreviations: N, number of patients in a treatment group or analysis set; n, number of patients with at least one episode; TEAE, treatment‐emergent adverse event.
A total of 46 (20.6%) patients receiving biosimilar filgrastim had TEAEs with a suspected relationship to filgrastim in cycles 1–4 (Table 4). The most frequently reported TEAEs related to study treatment were musculoskeletal and connective tissue disorders (n = 34, 15.2%), including bone pain (n = 16, 7.2%), myalgia (n = 16, 7.2%), musculoskeletal pain (n = 4, 1.8%), and arthralgia (n = 4, 1.8%). Other TEAEs related to study treatment included general disorders and administration site conditions, gastrointestinal disorders, blood and lymphatic system disorders, metabolism and nutrition disorders, skin and subcutaneous tissue disorders, nervous system disorders, and vascular system disorders.
Table 4. Treatment‐emergent adverse events with a suspected relationship to study drug by system organ class and preferred term reported in cycles 1–4 (safety analysis set).
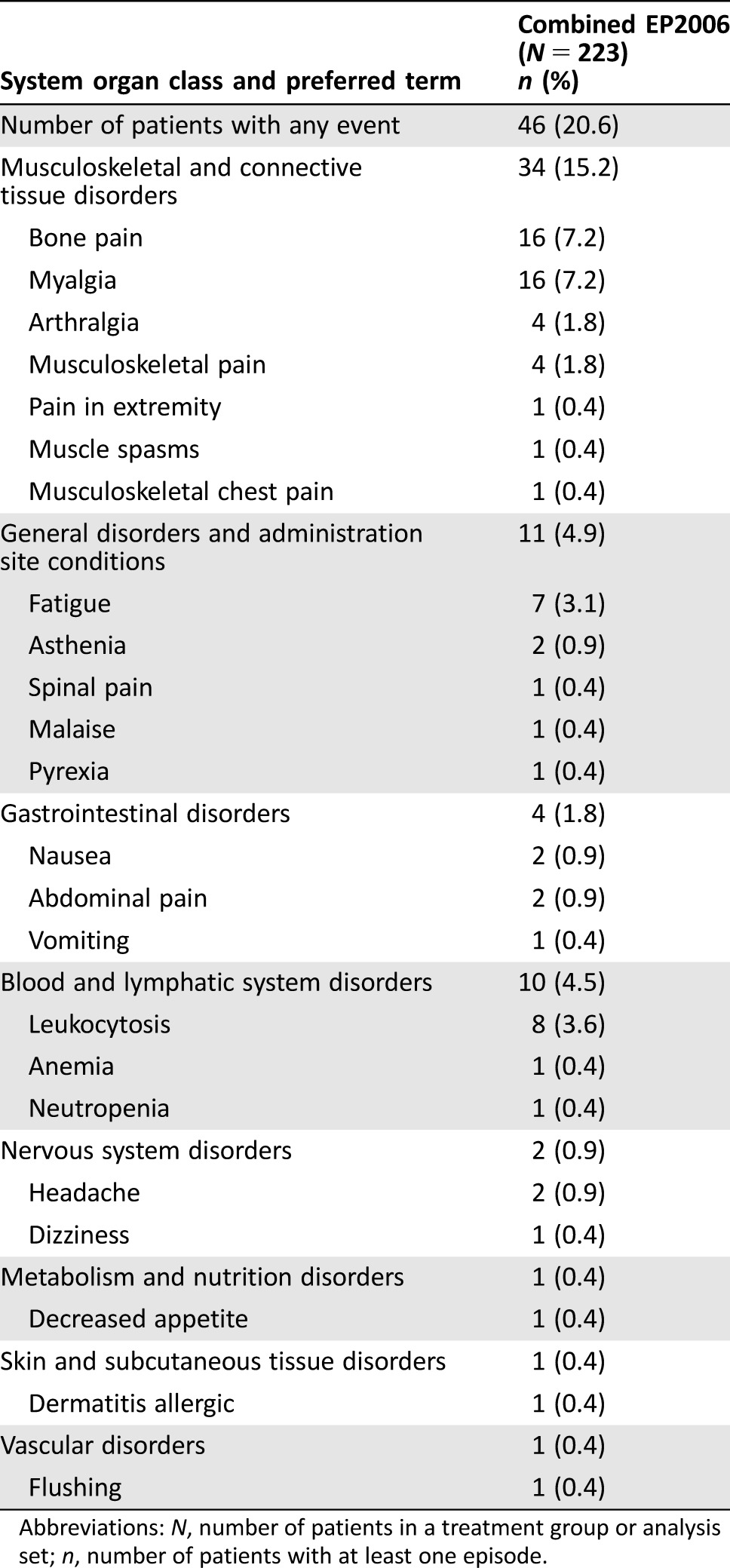
Abbreviations: N, number of patients in a treatment group or analysis set; n, number of patients with at least one episode.
No patient developed binding or neutralizing antibodies against G‐CSF at any time during the study.
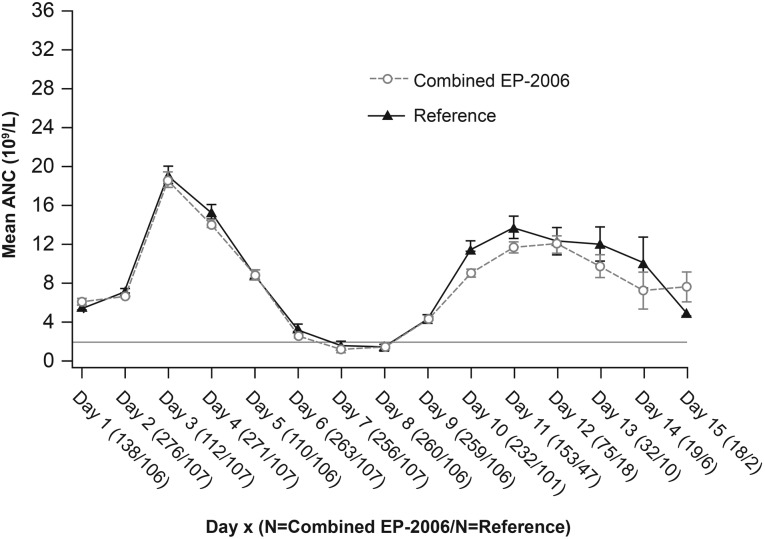
Mean (± SD) duration of severe neutropenia (primary endpoint) was 1.04 (± 1.51) in cycle 1. The mean ANC time course showed the expected increase at day 3 and subsequent decrease with nadir at days 7–8, with recovery from day 10 (Fig. 2).
Figure 2.
Time course of mean ANC in cycle 1 (full analysis set).
Abbreviations: ANC, absolute neutrophil count; N, number of evaluable patients.
Discussion
After approval of a biologic agent, pharmacovigilance is required to detect AEs that only emerge during long‐term use of the biologic in a large population [22]. Although this step is necessary for all approved biologics, including reference products, concerns regarding the safety of biosimilars means that ongoing pharmacovigilance is particularly important to continue to monitor the long‐term safety of biosimilars and build confidence in their use. Although EP2006 is well studied in the pharmacovigilance setting [23], [24], [25], [26], this combined analysis of phase III confirmatory studies aimed to broaden the evidence base in breast cancer for the safety profile of biosimilar filgrastim and confirm that reports of TEAEs in the included studies are consistent with the known safety profile of filgrastim. As expected, TEAEs were reported in a similar incidence in the combined biosimilar group (98.7%), compared with the reference group (96.2%) (Table 5). The majority of these TEAEs were due to chemotherapy.
Table 5. TEAEs reported in cycles 1–4 in the EP2006 and reference groups (safety analysis set).
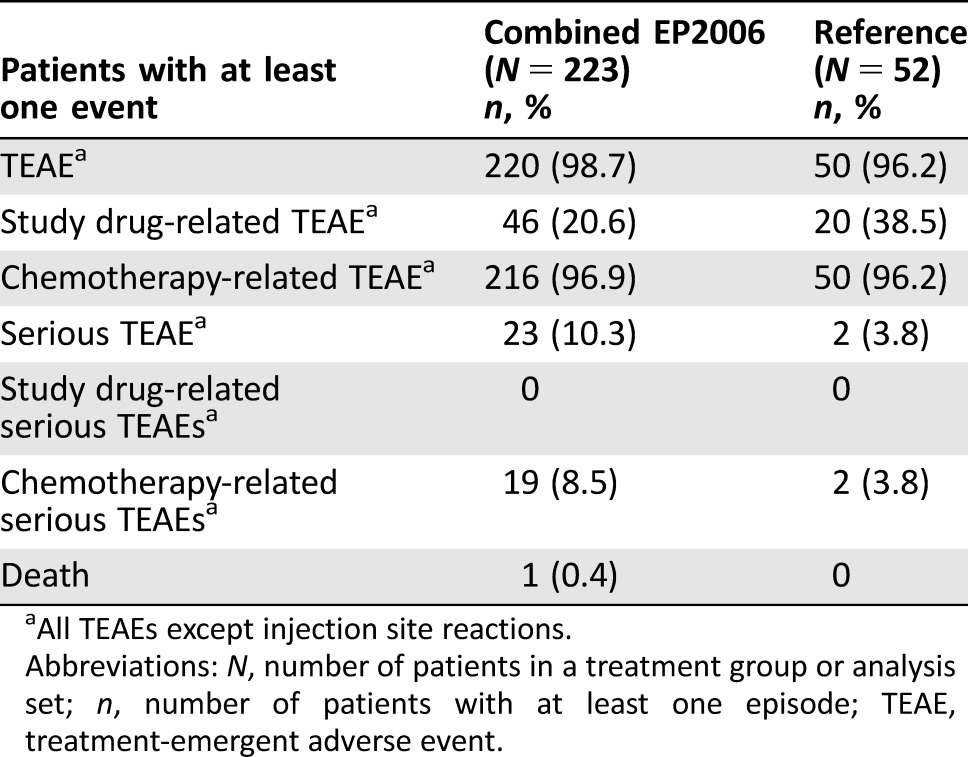
All TEAEs except injection site reactions.
Abbreviations: N, number of patients in a treatment group or analysis set; n, number of patients with at least one episode; TEAE, treatment‐emergent adverse event.
Bone pain is widely and consistently reported as the most frequently observed side effect associated with G‐CSF treatment [27]. Indeed, bone pain was the most frequent AE in our combined analysis and was reported at a higher incidence in the reference group (15%), compared with the combined biosimilar group (5.8%). However, these levels are lower than reports from several other randomized controlled studies of patients with breast cancer receiving reference filgrastim (26%–42%) [14], [17], [18], [28], [29]. Studies of patients with breast cancer receiving other G‐CSF products (lipegfilgrastim and pegfilgrastim) have reported incidences of bone pain of 10%–38% [27], [30], [31], [32], [33]. This highlights the considerable variation in the incidence of bone pain between studies of G‐CSF, likely representative of differences in patient populations, study design, chemotherapy regimens, or how bone pain was defined and reported.
There are also differences in incidences of bone pain from large, prospective, observational studies of biosimilar filgrastim; musculoskeletal or bone pain was reported in 5.6% of patients in the HEXAFIL study [34], compared with 24.7% of patients reporting bone pain in the MONITOR‐GCSF study [26]. An incidence of 24.7%, as reported in MONITOR‐GCSF, is in line with clinical trial results. It has been suggested that, compared with randomized clinical trials, bone pain may be reported at lower levels during routine use in long‐term safety studies, because bone pain is a well‐known TEAE associated with G‐CSF use and therefore less likely to be recorded than rarer TEAEs [22]. This may explain the incidence of 5.6% in the HEXAFIL study. However, there is also evidence to suggest that musculoskeletal pain is reported at a higher incidence in everyday practice than in clinical trials [27], [35]. This may reflect the variation in how G‐CSF is used in real‐life clinical practice compared with clinical trials, including administering G‐CSF for shorter periods of time and starting administration after the first cycle of chemotherapy. Because there is limited evidence to define the best practice for prevention and management of bone pain, these differences emphasize the need for long‐term safety studies and monitoring of TEAEs in G‐CSF products.
Duration of severe neutropenia is a well‐established and objective measure of efficacy to study and compare products in the G‐CSF class. Because it has a continuous nature and its measurement uses repeat sampling, it is a sensitive variable to detect differences compared with categorical clinical endpoints. Comparing efficacy results for biosimilar filgrastim with efficacy results for reference filgrastim showed similar findings for the primary endpoint for the two groups included in our combined analysis (mean ± SD DSN for cycle 1: combined biosimilar, 1.04 ± 1.51; reference: 1.20 ± 1.02; cycles 1–4: combined biosimilar, 2.2 ± 2.91; reference: 3.4 ± 3.11). These values were generally in line with other randomized controlled studies with reference filgrastim (1.3–2.5 days) [14], [17], [18], [28]. There were no clinically meaningful differences between the combined biosimilar and reference groups for secondary endpoints, including infections and hospitalizations. The majority of patients did not experience FN over cycles 1–4 (biosimilar: 94.2%; reference: 98.1%). However, there was a higher incidence of FN in the combined biosimilar group, with 10 patients (4.5%) experiencing 1 day of FN, compared with 1 patient (1.9%) in the reference group (cycles 1–4). Two patients in the biosimilar group experienced longer periods of FN: one patient experienced FN of 2 days’ duration and one patient experienced FN of 9 days’ duration. However, there are limitations to making comparisons between the combined biosimilar and reference groups because no patient in study 2 received the reference; therefore, the reference arm was not “combined” between the two studies. Furthermore, there are differences between the populations of the two studies, including the chemotherapy regimen, number of cycles, breast cancer stage, and definition of primary efficacy endpoint (mean DSN). Considering real world evidence, results from MONITOR‐GSCF showed that incidence of FN was similar to that reported in our study (5.9% vs. 5.4%, respectively) [26]. Hospitalization because of FN were reported in 6.1% of patients in MONITOR‐GCSF, compared with 3.1% of patients in our study. This difference may have been due to differences in the definition of hospitalization because of FN, as in MONITOR‐GCSF this also included hospitalization because of chemotherapy‐induced neutropenia. Thus, differences in endpoints mean that there are limitations to comparing data between our analysis and MONITOR‐GCSF. Indeed, MONITOR‐GCSF did not report DSN, which was the primary endpoint in our study.
One concern with use of biosimilars is that they may induce a different immunological response than the reference product [22]. No anti‐recombinant human G‐CSF (anti‐rhG‐CSF) antibodies were detected in either study included in this combined analysis, confirming that biosimilar filgrastim had no increased immunogenic potential compared with reference filgrastim. This was expected, given the low immunogenic potential of filgrastim, and is in line with previous studies and postmarketing surveillance of biosimilar filgrastim in Europe, where no anti‐rhG‐CSF antibodies have been reported across the clinical program, with more than 3,300 samples tested [22]. Likewise, no safety concerns have been observed in an ongoing safety surveillance study [36].
Conclusion
This combined analysis provides additional clinical evidence that the biosimilar filgrastim EP2006 has a safety profile consistent with previous studies of G‐CSF and is effective in the prevention of febrile neutropenia in patients with breast cancer undergoing myelosuppressive chemotherapy. These findings are in line with real‐world evidence from the MONITOR‐GCSF study, showing that the safety profile of biosimilar filgrastim is similar to historical safety data for reference filgrastim. The results of our study also support the use of biosimilar G‐CSF, which in turn may lead to cost savings compared with the use of reference G‐CSF. Data from 17 European Union countries suggest that switching from reference to biosimilar G‐CSF led to overall savings of €85 million, a finding supported by a cost‐effectiveness study that reported that biosimilar G‐CSF was more cost‐effective than reference G‐CSF across treatment regimens [22], [37], [38].
Acknowledgments
Editorial support was provided by Caroline McGown of Spirit Medical Communications Ltd. and supported by Sandoz GmbH, Kundl, Austria. Final approval of the manuscript rested solely with the scientific authors. This study was supported by Sandoz GmbH, Austria.
Author Contributions
Conception/design: Nadia Harbeck, Pere Gascón, Andriy Krendyukov, Nadja Hoebel, Sreekanth Gattu, Kimberly Blackwell
Provision of study material or patients: Nadia Harbeck, Pere Gascón, Kimberly Blackwell
Collection and/or assembly of data: Nadia Harbeck, Pere Gascón, Nadja Hoebel, Kimberly Blackwell
Data analysis and interpretation: Nadia Harbeck, Pere Gascón, Andriy Krendyukov, Nadja Hoebel, Sreekanth Gattu, Kimberly Blackwell
Manuscript writing: Nadia Harbeck, Pere Gascón, Andriy Krendyukov, Nadja Hoebel, Sreekanth Gattu, Kimberly Blackwell
Final approval of manuscript: Nadia Harbeck, Pere Gascón, Andriy Krendyukov, Nadja Hoebel, Sreekanth Gattu, Kimberly Blackwell
Disclosures
Nadia Harbeck: Amgen, Hexal AG (C/A); Andriy Krendyukov: Hexal AG, a Sandoz company (E); Nadja Hoebel: Hexal AG, a Sandoz company (E); Sreekanth Gattu: Hexal AG, a Sandoz company (E); Kimberly Blackwell: Sandoz GmbH (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Gascon P. Presently available biosimilars in hematology‐oncology: G‐CSF. Target Oncol 2012;7(suppl 1):S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabernero J, Vyas M, Giuliani R et al. Biosimilars: A position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open 2016;1:e000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kozlowski S, Woodcock J, Midthun K et al. Developing the nation's biosimilars program. N Engl J Med 2011;365:385–388. [DOI] [PubMed] [Google Scholar]
- 4. Weise M, Kurki P, Wolff‐Holz E et al. Biosimilars: The science of extrapolation. Blood 2014;124:3191–3196. [DOI] [PubMed] [Google Scholar]
- 5.Committee for Medicinal Products for Human Use, European Medicines Agency . Guideline on Similar Biological Medicinal Products. October 23, 2014. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed July 14, 2017.
- 6.Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services . Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. April 2015. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed July 14, 2017.
- 7.Arbeitsgemeinschaft Gynäkologische Onkologie E.V. Guidelines of the AGO Breast Committee. Updated March 1, 2016. Diagnosis and treatment of patients with primary and metastatic breast cancer. Available at http://www.ago-online.de/en/guidelines-mamma/march-2016/. Accessed July 14, 2017.
- 8. Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO clinical practice guidelines. Ann Oncol 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. Version 3.2017. Updated November 10, 2017. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed December 20, 2017.
- 10. Martin M, Pienkowski T, Mackey J et al. Adjuvant docetaxel for node‐positive breast cancer. N Engl J Med 2005;352:2302–2313. [DOI] [PubMed] [Google Scholar]
- 11. Nabholtz JM, Mackey JR, Smylie M et al. Phase II study of docetaxel, doxorubicin, and cyclophosphamide as first‐line chemotherapy for metastatic breast cancer. J Clin Oncol 2001;19:314–321. [DOI] [PubMed] [Google Scholar]
- 12. Aapro MS, Bohlius J, Cameron DA et al. 2010 update of EORTC guidelines for the use of granulocyte‐colony stimulating factor to reduce the incidence of chemotherapy‐induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011;47:8–32. [DOI] [PubMed] [Google Scholar]
- 13. Smith TJ, Khatcheressian J, Lyman GH et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence‐based clinical practice guideline. J Clin Oncol 2006;24:3187–3205. [DOI] [PubMed] [Google Scholar]
- 14. Park KH, Sohn JH, Lee S et al. A randomized, multi‐center, open‐label, phase II study of once‐per‐cycle DA‐3031, a biosimilar pegylated G‐CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early‐stage breast cancer. Invest New Drugs 2013;31:1300–1306. [DOI] [PubMed] [Google Scholar]
- 15. Alba E, Martín M, Ramos M et al. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first‐line treatment of metastatic breast cancer: A Spanish Breast Cancer Research Group (GEICAM‐9903) phase III study. J Clin Oncol 2004;22:2587–2593. [DOI] [PubMed] [Google Scholar]
- 16. Nabholtz JM, Falkson C, Campos D et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first‐line chemotherapy for metastatic breast cancer: Results of a randomized, multicenter, phase III trial. J Clin Oncol 2003;21:968–975. [DOI] [PubMed] [Google Scholar]
- 17. Green MD, Koelbl H, Baselga J et al. A randomized double‐blind multicenter phase III study of fixed‐dose single‐administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003;14:29–35. [DOI] [PubMed] [Google Scholar]
- 18. Holmes FA, Jones SE, O'Shaughnessy J et al. Comparable efficacy and safety profiles of once‐per‐cycle pegfilgrastim and daily injection filgrastim in chemotherapy‐induced neutropenia: A multicenter dose‐finding study in women with breast cancer. Ann Oncol 2002;13:903–909. [DOI] [PubMed] [Google Scholar]
- 19. Gascon P. The evolving role of biosimilars in haematology‐oncology: A practical perspective. Ther Adv Hematol 2015;6:267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackwell K, Semiglazov V, Krasnozhon D et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: A phase III, randomized, double‐blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol 2015;26:1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gascon P, Fuhr U, Sörgel F et al. Development of a new G‐CSF product based on biosimilarity assessment. Ann Oncol 2010;21:1419–1429. [DOI] [PubMed] [Google Scholar]
- 22. Gascón P, Tesch H, Verpoort K et al. Clinical experience with Zarzio in Europe: What have we learned? Support Care Cancer 2013;21:2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aapro M, Bokemeyer C, Ludwig H et al. Chemotherapy‐induced (febrile) neutropenia prophylaxis with biosimilar filgrastim in elderly versus non‐elderly cancer patients: Patterns, outcomes, and determinants (MONITOR‐GCSF study). J Geriatr Oncol 2017;8:86–95. [DOI] [PubMed] [Google Scholar]
- 24. Aapro M, Ludwig H, Bokemeyer C et al. Predictive modeling of the outcomes of chemotherapy‐induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR‐GCSF study). Ann Oncol 2016;27:2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bokemeyer C, Gascón P, Aapro M et al. Over‐ and under‐prophylaxis for chemotherapy‐induced (febrile) neutropenia relative to evidence‐based guidelines is associated with differences in outcomes: Findings from the MONITOR‐GCSF study. Support Care Cancer 2017;25:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gascón P, Aapro M, Ludwig H et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy‐induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR‐GCSF study). Support Care Cancer 2016;24:911–925. [DOI] [PubMed] [Google Scholar]
- 27. Lambertini M, Del Mastro L, Bellodi A et al. The five “Ws” for bone pain due to the administration of granulocyte‐colony stimulating factors (G‐CSFs). Crit Rev Oncol Hematol 2014;89:112–128. [DOI] [PubMed] [Google Scholar]
- 28. Holmes FA, O'Shaughnessy JA, Vukelja S et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high‐risk stage II or stage III/IV breast cancer. J Clin Oncol 2002;20:727–731. [DOI] [PubMed] [Google Scholar]
- 29. Waller CF, Semiglazov VF, Tjulandin S et al. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie 2010;33:504–511. [DOI] [PubMed] [Google Scholar]
- 30. Blackwell K, Donskih R, Jones CM et al. A comparison of proposed biosimilar LA‐EP2006 and reference pegfilgrastim for the prevention of neutropenia in patients with early‐stage breast cancer receiving myelosuppressive adjuvant or neoadjuvant chemotherapy: Pegfilgrastim randomized oncology (supportive care) trial to evaluate comparative treatment (PROTECT‐2), a phase III, randomized, double‐blind trial. The Oncologist 2016;21:789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bondarenko I, Gladkov OA, Elsaesser R et al. Efficacy and safety of lipegfilgrastim versus pegfilgrastim: A randomized, multicenter, active‐control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 2013;13:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harbeck N, Lipatov O, Frolova M et al. Randomized, double‐blind study comparing proposed biosimilar LA‐EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 2016;12:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sveikata A, Liutkauskienė S, Juozaitytė E et al. An open‐label multicenter safety, tolerability, and efficacy study of recombinant granulocyte colony‐stimulating factor in the prevention of neutropenic complications in breast cancer patients. Medicina (Kaunas) 2011;47:428–433. [PubMed] [Google Scholar]
- 34. Tesch H, Ulshöfer T, Vehling‐Kaiser U et al. Prevention and treatment of chemotherapy‐induced neutropenia with the biosimilar filgrastim: A non‐interventional observational study of clinical practice patterns. Oncol Res Treat 2015;38:146–152. [DOI] [PubMed] [Google Scholar]
- 35. Kirshner JJ, Hickock J, Hofman M. Pegfilgrastim‐induced bone pain: Incidence, risk factors and management in a community practice. Comm Oncol 2007;4:455–458; discussion 459. [Google Scholar]
- 36. Becker P, Schwebig A, Brauninger S et al. Healthy donor hematopoietic stem cell mobilization with biosimilar granulocyte‐colony‐stimulating factor: Safety, efficacy, and graft performance. Transfusion 2016;56:3055–3064. [DOI] [PubMed] [Google Scholar]
- 37.IQvia . MIDAS Global Biologics database, 2012. Available at https://www.iqvia.com/. Accessed August 8, 2017.
- 38. Aapro M, Cornes P, Abraham I. Comparative cost‐efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy‐induced febrile neutropenia. J Oncol Pharm Pract 2012;18:171–179. [DOI] [PubMed] [Google Scholar]