A case series is presented of conservative, fertility‐sparing management of well‐differentiated G1 endometrioid adenocarcinoma of the endometrium with minimal infiltration of the myometrium, preventively treated with hysteroscopic resection and hormone therapy. This pilot study may offer a treatment option that preserves fertility in patients with superficial myometrial invasion.
Abstract
Three women with a well‐differentiated grade 1 endometrioid adenocarcinoma of the endometrium with minimal myometrial infiltration were treated with hysteroscopic resection and hormone therapy. The presence of myometrial infiltration has often been mentioned as an exclusion criterion for conservative management in young patients because of worsening cancer prognosis. The subsequent 5‐year follow‐up and the pregnancies achieved may confirm the choice of this temporary treatment and indicate a new option for fertility‐sparing treatment in highly motivated patients.
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy, and the 5‐year survival rate of stage IA EC is >93% [1]. Among women with an EC diagnosis, approximately 20% are in premenopausal age and 5% are under 40 years of age [2], highlighting the importance of fertility preservation in these patients, whose medical options are currently limited to treatment with megestrol acetate or medroxyprogesterone acetate, with conflicting outcomes [3].
Recently, hysteroscopic resections accompanied by hormonal therapy and followed by several pregnancies in young women with early‐stage EC have been reported [4], [5]. Consequently, conservative management was proposed to these patients based on pre‐established criteria: grade 1 (G1) well‐differentiated tumor, absence of infiltration of lymphovascular spaces, no evidence of myometrial invasion, and no evidence of metastatic disease or suspicious adnexal mass [4].
In patients with EC with minimal myometrial invasion, who refuse hysterectomy and wish to maintain their fertility, it is difficult to select a safe therapeutic alternative to traditional surgery. The literature reports only one case of a hysteroscopic endomyometrial resection with a 5‐year survival, which was, however, in the different context of a postmenopausal patient with EC [6].
We report here for the first time a case series about conservative management for sparing fertility in women with a well‐differentiated G1 endometrioid adenocarcinoma of the endometrium with minimal myometrial infiltration preventively treated with hysteroscopic resection and hormone therapy.
Case Series
Three women with primary infertility were referred to our center with a polypoid lesion in the uterine cavity, discovered by ultrasound. S.C., D.M., and M.E. were 37‐, 38‐, and 32‐year‐old women, respectively, with normal body mass indices, regular menses, and negative gynecological examination findings.
Diagnostic hysteroscopy was performed with a 5‐mm hysteroscope with a 30° forward oblique lens (office hysteroscope; Karl Storz, Tuttlingen, Germany) and saline solution. In all patients, an exophytic lesion 12–15 mm in diameter with atypical vascularization was observed, whereas the remaining endometrium was regular. A lesion biopsy was performed with operating instruments of 5 French gauge (Fr). Histologic examination evidenced a well‐differentiated G1 endometroid adenocarcinoma of the endometrium. Enhanced pelvic magnetic resonance imaging (MRI) was then performed and showed no metastatic disease, pelvic or para‐aortic lymph node enlargement, or suspicious adnexal mass but expressed a strong suspicion of minimal myometrial infiltration at the site of primary disease, taking into account the recognized limits of this method [7] in evaluating the degree of myometrial infiltration. All patients strongly refused hysterectomy. After accurate counseling on fertility‐sparing treatment and the risks associated with nonstandard treatment, patients provided written informed consent to the conservative procedure, accepting a close follow‐up and a possible subsequent hysterectomy. Operative hysteroscopy was performed under general anesthesia; a cervical dilatation of 10 mm with Hegar dilators was accomplished, and a 26‐Fr resectoscope (Karl Storz, Tuttlingen, Germany) with a 0° lens was introduced. The uterus was distended with 3,000 mL of 0.54% mannitol and 2.7% sorbitol urologic irrigation (Baxter Healthcare Corporation, Rome, Italy). Intrauterine pressure was automatically controlled (80–100 mm Hg) by an electronic irrigation and suction device (Hamou Endomat Irrigation Suction Pump, Karl Storz). The endometrial lesion (step 1) and a small portion (about 3–4 mm) of the underlying myometrium (step 2; Fig. 1) and the endometrium and myometrium surrounding the lesion (step 3) were cautiously resected with a 5‐mm cutting loop electrode and 100 watts of pure cutting output power, according to the technique of Mazzon et al. [8]. Furthermore, multiple random endometrial biopsies were taken on each uterine wall (step 4). The material from each step was sent in separate containers for histologic examination. The diagnosis of well‐differentiated G1 endometrioid adenocarcinoma of the endometrium was confirmed histologically. The polypoid lesion also confirmed minimal invasion within the myometrium (1–2 mm), despite the absence of lympho‐vascular invasion and with free resection margins (Fig. 2). All other endometrial samples were negative for atypia and malignancy. After diagnosis, an intramuscular LH‐RH agonist (leuprorelin 3.75 mg, Enantone, Takeda, Tokyo, Japan) and megestrol acetate (160 mg daily) were administered for 9 months. Patients underwent office hysteroscopic follow‐up with endometrial biopsies to monitor potential recurrence of the disease every 3 months. A regular endometrial cavity with proliferative endometrium or atrophic glands and pseudodecidualized stroma during hysteroscopic controls was found according to progestin therapy. Three months after progestin cessation, patients underwent an additional office hysteroscopy with multiple endometrial biopsies, all of which were negative. Therefore, a complete remission of the lesion was suggested. Patients were authorized to seek pregnancy spontaneously or via assisted reproductive technology (ART) and were closely followed by further office hysteroscopies with tailored biopsies every 3 months for the first year and every 6 months for the subsequent 4 years.
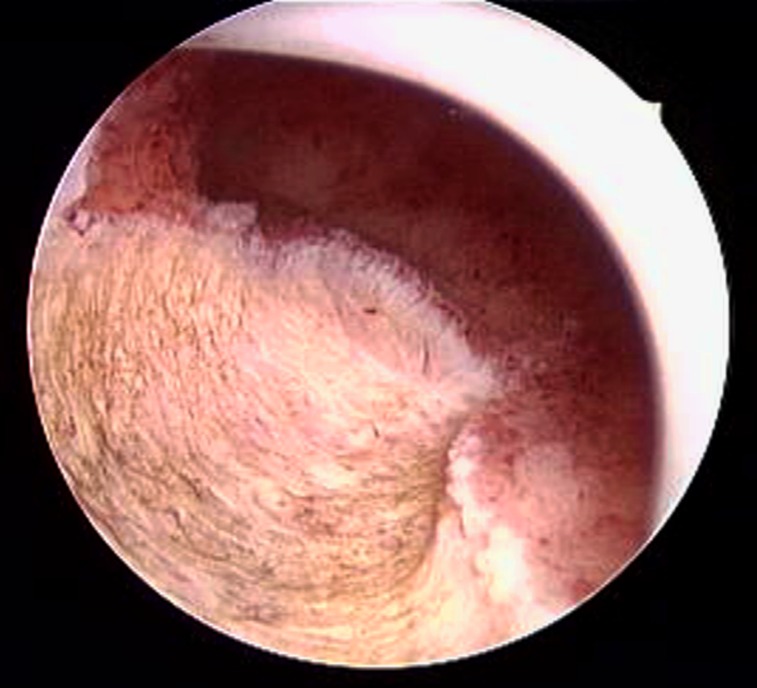
Figure 1.
Hysteroscopic image of endometrial lesion resection. The resection margin reaches the healthy myometrium below the lesion.
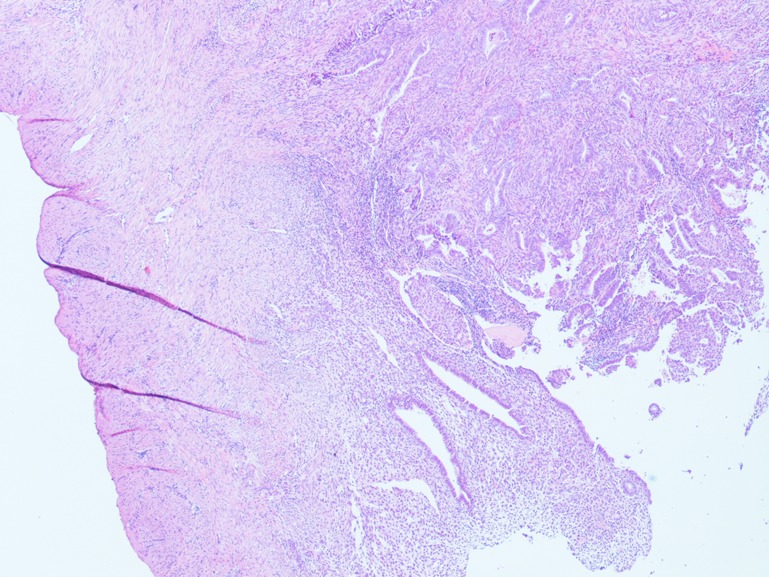
Figure 2.
Resection margin. Representative pathology slides showing endometrioid adenocarcinoma of the endometrium with polypoid pattern of growth and infiltration up to myometrial stroma. The resection margin is clearly assessable and appears free from disease. (Stained with hematoxylin/eosin, ×20 magnification.)
S.C. became pregnant after 18 months with ART and delivered by cesarean section. Subsequently she achieved two further pregnancies, ending in abortions. After 5 years of follow‐up, an atypical hyperplasia was found at hysteroscopic control, and the patient agreed to undergo definitive surgery (hysterectomy and bilateral salpingo‐oophorectomy). The definitive histological examination was negative for myometrial infiltration. D.M. had a spontaneous pregnancy after 12 months that ended in abortion. In the following 4 years of negative follow‐up, she underwent some ART cycles unsuccessfully and continued to refuse definitive surgery. M.E. tried to conceive spontaneously and refused ART cycles. After 5 years of negative follow‐up, an atypical hyperplasia was reported at hysteroscopic control and she decided to undergo hysterectomy and bilateral salpingo‐oophorectomy. The final histological examination was negative for myometrial infiltration.
Discussion
The European Society for Medical Oncology, European Society of Gynecological Oncology, and European Society for Radiotherapy and Oncology Consensus Conference [9] on EC recently affirmed that in absence of randomized trials on large populations, there are no conservative minimally invasive surgical procedures approved for the EC treatment with low histological grade (G1, well differentiated), in young women requiring fertility, although the need for a particularly accurate and precise diagnosis by MRI has been highlighted to exclude a diffusion in the myometrium and ovaries. Two options were proposed to preserve fertility: (a) progestin therapy alone [3] and (b) the same in combination with operative hysteroscopy considered as preliminary surgery, depending on the center [4], [5], [9].
Mazzon et al. [4] first proposed hysteroscopic resection followed by hormone therapy and close postsurgical follow‐up as a new therapeutic option for young women with stage IA EC who wish to preserve fertility. Falcone et al. [5] also used the same procedure in a greater number of women with initial stage IA EC, obtaining very promising outcomes in terms of both 5‐year survival and pregnancy rate.
Otherwise, there are no reports of low‐grade EC with minimal myometrial invasion that for reproductive needs were treated with hormonal therapy and hysteroscopic surgery. The presence of myometrial infiltration is an exclusion criterion for conservative treatment, as the prognosis is worsening and the lymphonodular involvement rises to 34% for pelvic and 25% for para‐aortic lymph nodes [10]. In only one case, Vilos et al. [6] reported an hysteroscopic endomyometrial resection, which was performed in a postmenopausal patient.
Our data show for the first time a case series of three young women seeking pregnancy with diagnosed well‐differentiated G1 endometrioid adenocarcinoma with minimal myometrial invasion treated with conservative hysteroscopic surgery. Patients were made aware by proper counseling of the risks of intervention; the progression and recurrence of disease, estimated at 34% after conservative management [11]; and the need for a close follow‐up. In agreement with the patients’ strong desire for pregnancy, even in the presence of myometrial invasion, we performed the above‐mentioned conservative treatment. The choice of our conservative management was confirmed by the 5‐year follow‐up that was negative for neoplasia and by the number of pregnancies achieved, although the decision to proceed with this management may be associated with multiple complexities, such as the risk of inadequate cancer staging, risk of synchronous or metasynchronous cancer, increased risk of inherited genetic predisposition to malignancy, and lack of uniformity in the medical management and surveillance. We firmly believe in both the diagnostic and the therapeutic roles of this technique, which appears to be a reasonable short‐term alternative before the definitive surgical management according to Mazzon et al. [4], [8]. Moreover, resectoscopic lesion removal before medical treatment was proposed as a preliminary surgery to achieve neoplastic cytoreduction and detailed histopathologic diagnosis [9].
Despite all the limitations of our series because of the provisional nature of the intervention, this pilot study may represent a new option for fertility‐sparing treatment in strictly selected patients with EC with superficial myometrial invasion, thus offering a chance to women who have not yet fulfilled their desire to have children.
Disclosures
The authors indicated no financial relationships.
References
- 1. Creasman WT, Odicino F, Maisonneuve P et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95(suppl. 1):S105–S143. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Xu J et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 3. Liou WS, Yap OW, Chan JK et al. Innovations in fertility preservation for patients with gynecologic cancers. Fertil Steril 2005;84:1561–1573. [DOI] [PubMed] [Google Scholar]
- 4. Mazzon I, Corrado G, Masciullo V et al. Conservative surgical management of stage 1A endometrial carcinoma for fertility preservation. Fertil Steril 2010;93:1286–1289. [DOI] [PubMed] [Google Scholar]
- 5. Falcone F, Laurelli G, Losito S et al. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol 2017;28:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vilos GA, Ettler HC, Edris F et al. Endometrioid adenocarcinoma treated by hysteroscopic endomyometrial resection. J Minim Invasive Gynecol 2007;14:119–122. [DOI] [PubMed] [Google Scholar]
- 7. Rieck GC, Bulman J, Whitaker R et al. A retrospective review of magnetic resonance imaging in assessing the extent of myometrial infiltration for patients with endometrial carcinoma. J Obstet Gynaecol 2005;25:765–768. [DOI] [PubMed] [Google Scholar]
- 8. Mazzon I, Corrado G, Morricone D et al. Reproductive preservation for treatment of stage IA endometrial cancer in a young woman: Hysteroscopic resection. Int J Gynecol Cancer 2005;15:974–978. [DOI] [PubMed] [Google Scholar]
- 9. Colombo N, Creutzberg C, Amant F et al. ESMO‐ESGO‐ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow‐up. Ann Oncol 2016;27:16–41. [DOI] [PubMed] [Google Scholar]
- 10. Euscher E, Fox P, Bassett R et al. The pattern of myometrial invasion as a predictor of lymph node metastasis or extrauterine disease in low‐grade endometrial carcinoma. Am J Surg Pathol 2013;37:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiva L, Lapuente F, González‐Cortijo L et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol 2008;111(suppl 2):S101–S104. [DOI] [PubMed] [Google Scholar]