Abstract
Lessons Learned.
Motivating patients to enroll in chemopreventive studies is challenging.
Chemoprevention with toxic drugs is not feasible.
Background.
LKB1 mutations are the underlying genetic abnormality causing Peutz‐Jeghers syndrome (PJS) and are a potential target for everolimus. In this phase II study, the efficacy of everolimus on polyp and tumor growth in PJS patients was investigated.
Methods.
Adult patients with a proven LKB1 mutation and who were suitable for everolimus treatment were included in two different PJS cohorts: (a) patients with unresectable malignancies and (b) patients with high‐risk polyps. Treatment in both groups was oral everolimus, 10 mg daily. Response rates were primary endpoints for both cohorts.
Results.
Between October 2011 and April 2016, only two patients were enrolled, one in each cohort. A 49‐year‐old patient with advanced pancreatic cancer in cohort 1 was progressive after 2 months. A 52‐year‐old male patient in cohort 2 experienced severe toxicity and refused treatment after 4 months, even though endoscopy suggested stabilization of polyps. Adverse events included dental inflammations, mucositis, and rash. In 2016, the trial was aborted for lack of accrual, despite extensive accrual efforts in an area where PJS is highly prevalent and care is highly centralized.
Conclusion.
Due to accrual problems, no conclusions can be drawn about the value of everolimus in PJS treatment, questioning the feasibility of this agent for chemoprevention.
Abstract
经验总结
• 激励患者参加化学预防研究具有挑战性。
• 使用毒性药物进行化学预防不可行。
摘要
背景.LKB1突变是导致Peutz‐Jeghers综合征(PJS)的潜在基因异常, 且是依维莫司的潜在靶标。在本项II期研究中, 考察了依维莫司对PJS患者息肉和肿瘤生长的疗效。
方法.将携带经证实的LKB1突变且适于依维莫司治疗的成年患者纳入两个不同的PJS队列:(a)不可切除恶性肿瘤患者和(b)高风险息肉患者。两组的治疗均为口服依维莫司, 每日 10 mg。缓解率为这两个队列的主要终点。
结果.2011年10月至2016年4月, 仅两例患者入组, 每个队列各一例。队列1中的一例49岁晚期胰腺癌患者在2个月后出现进展。队列2中的一例52岁男性患者在4个月后出现严重毒性反应并拒绝治疗, 但内镜显示息肉稳定。不良事件包括牙齿炎症、黏膜炎和皮疹。2016年, 虽然在PJS高发且治疗高度集中的地区进行了广泛的招募工作, 但因招募人数不足中止了试验。
结论.由于招募问题, 未能得出有关依维莫司在PJS治疗中的价值的结论, 此药物用于化学预防的可行性存疑。
Discussion
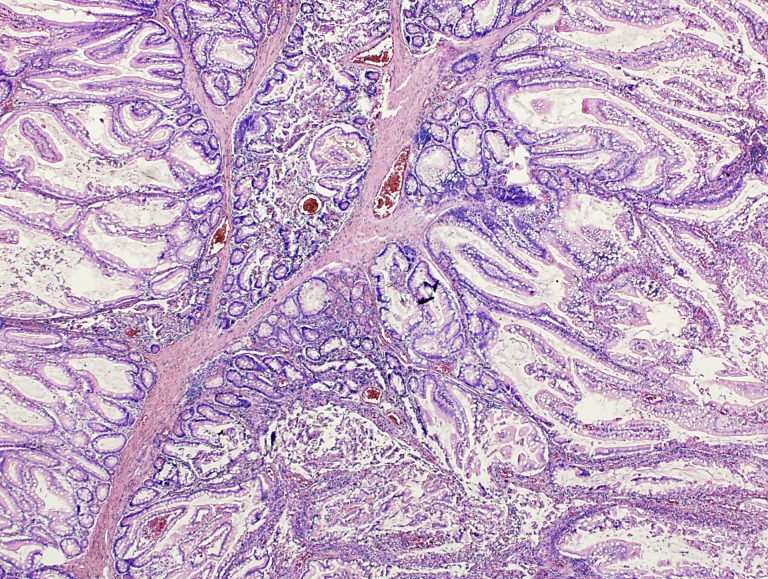
Peutz‐Jeghers syndrome is caused by a mutation in the LKB1 gene, a tumor suppressor gene located on chromosome 19. This mutation results in a decreased inhibition of mammalian target of rapamycin (mTOR), with uncontrolled cell growth as a result, manifesting as intestinal polyps (Fig. 1) and malignancies. Oral selective mTOR inhibitors such as rapamycin and everolimus have been successfully used in several exploratory studies [1], [2]. The report of a successful treatment of a PJS patient with pancreatic cancer with everolimus was the starting point for a more comprehensive study: the EVAMP trial [3]. Our hypothesis was that treating PJS patients with everolimus would result in reduced growth of intestinal polyps and tumors. In 2011, researchers from the University of Utah initiated a similar study on the role of chemoprevention agents in PJS, which was stopped prematurely because of poor accrual [4].
Figure 1.
A Peutz‐Jeghers polyp of the small intestine with arborizing smooth muscle fibers and non‐neoplastic epithelium.
Due to the rare nature of the disease, the intent was to start with a pilot study including 15 patients, executed in the two largest PJS centers in The Netherlands (Academic Medical Center, Amsterdam, and Erasmus Medical Center, Rotterdam). To obtain relevant information about activity of everolimus, we selected a high‐risk population consisting of patients with either fast‐growing gastric or small bowel polyps requiring therapeutic enteroscopy at least once every 2 years with resection of >4 polyps larger than 15 mm, or patients suffering from PJS‐related malignancies.
Despite the clear study design, the selection of high‐risk patients, and well‐targeted medication, our study met some major obstacles. First, it turned out to be very difficult to find enough relevant patients, and secondly, the chosen treatment turned out to have both poor tolerability and (although in only one patient) a disappointing lack of efficacy. Even in two areas where PJS is highly prevalent (The Netherlands and Utah), researchers were not even close to reaching enough patients to perform a trial. Also, extensive accrual efforts, including the provision of additional trial information in national medical journals and during two patient information days in the accrual period, did not lead to increased patient participation. We presume that the currently existing intense surveillance programs do already diminish the rate of symptomatic polyps and malignancies and, therefore, are considered by patients to be efficacious, which probably hampers the willingness of these patients to enroll in chemoprevention treatment studies. In addition, everolimus often induces cumbersome side effects, which further decreases long‐term use in prevention. Furthermore, the need for dose reduction in both The Netherlands and Utah confirmed the poor tolerability of everolimus therapy. Therefore, it is not surprising that both experiences raise the question of whether use of this drug for chemoprevention in PJS patients is feasible. Potential future options are a lower dose of everolimus, or another targeted agent.
Trial Information
- Disease
Advanced cancer
- Disease
Peutz‐Jeghers syndrome
- Stage of Disease/Treatment
Prevention
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Toxicity
- Investigator's Analysis
Poorly tolerated/not feasible
Drug Information for Phase II Advanced Malignancies
- Drug 1
- Generic/Working Name
Everolimus
- Trade Name
Afinitor
- Company Name
Novartis
- Dose
10 mg per flat dose
- Route
p.o.
Patient Characteristics for Phase II Advanced Malignancies
- Number of Patients, Male
1
- Number of Patients, Female
0
- Age
Median (range): 48
- Number of Prior Systemic Therapies
Median (range): 0
- Performance Status: ECOG
-
0 — 1
1 — 0
2 — 0
3 — 0
Unknown —
Patient Characteristics for Phase II High‐Risk Polyps
- Number of Patients, Male
1
- Number of Patients, Female
0
- Age
Median (range): 53
- Number of Prior Systemic Therapies
Median (range): 0
- Performance Status: ECOG
-
0 — 0
1 — 1
2 — 0
3 — 0
Unknown —
Primary Assessment Method for Phase II Advanced Malignancies
- Title
Total patient population
- Number of Patients Screened
1
- Number of Patients Enrolled
1
- Number of Patients Evaluable for Toxicity
1
- Number of Patients Evaluated for Efficacy
0
- Evaluation Method
RECIST 1.1
- Response Assessment PD
n = 1 (100%)
- (Median) Duration Assessments PFS
7 weeks
- (Median) Duration Assessments TTP
7 weeks
- (Median) Duration Assessments OS
6 months
- (Median) Duration Assessments Duration of Treatment
7 weeks
Primary Assessment Method for Phase II High‐Risk Polyps
- Title
Total patient population
- Number of Patients Screened
1
- Number of Patients Enrolled
1
- Number of Patients Evaluable for Toxicity
1
- Number of Patients Evaluated for Efficacy
1
- Evaluation Method
RECIST 1.1
- Response Assessment SD
n = 1 (100%)
- (Median) Duration Assessments PFS
8 months
- (Median) Duration Assessments Response Duration
8 months
- (Median) Duration Assessments Duration of Treatment
4 months
- Adverse Events: Phase II Advanced Malignancies
None
- Adverse Events: Phase II High‐Risk Polyps
Root cavity inflammation, three times
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Did not fully accrue
- Investigator's Assessment
Poorly tolerated/not feasible
Mutation spectrum: Peutz‐Jeghers syndrome (PJS) is an autosomal dominant condition characterized by multiple hamartomatous polyps of the gastrointestinal tract, the presence of mucocutaneous hyperpigmentation, and an elevated lifetime risk to develop cancer, varying between 37% and 93% [1]. The genetic abnormality responsible for the syndrome is a mutation in the LKB1/STK11 gene, which maps to 19p13 [2]. This LKB1/STK11 gene activates adenine monophosphate‐activated protein kinase, a necessary element in cell metabolism that is required for maintaining energy homeostasis, which in turn activates tumor suppressors tuberous sclerosis complex 1 and 2, leading to mammalian target of rapamycin (mTOR) inhibition. Two well‐characterized downstream targets of mTOR are S6 kinase and eukaryotic translation initiation factor 4E‐binding protein 1. In genetic analysis of PJS families, around 150 different mutations in the LKB1 gene have been found, ranging from truncation to missense mutations [3], [4]. The kind of mutation does not clearly correlate with the disease course, although loss of heterozygosity is observed more frequently in PJS carcinomas than in PJS hamartomas. No data on specific LKB1 mutations and the sensitivity to mTOR inhibition is available.
Suitability of everolimus: Elevated levels of phospho‐S6 (pS6) kinase and pS6 are detected in the polyps from LKB1 +/− mice, suggesting that hyperactivation of mTOR signaling accounts for the development of the PJS‐characteristic hamartomatous lesions. These observations suggested that mTOR inhibitors such as rapamycin and its analogues could be useful for the treatment of polyps arising from patients with PJS. Wei et al. investigated in 2008 the benefit of mTOR inhibition in the development of polyps in PJS mice [5]. During this trial, rapamycin was given in LKB1 +/− mice at 9 months of age (after the onset of polyposis) at the dose of 2 mg/kg per day for a 2‐month period. The efficacy of rapamycin was assessed by measuring polyp sizes and tumor burden. It was found that rapamycin effectively suppresses PJS polyposis in a mouse model, suggesting that mTOR inhibitors may represent a new targeted therapy for the treatment of PJS.
Shackelford et al. analyzed 11‐month‐old LKB1 +/+ and LKB1 +/− mice by fludeoxyglucose (FDG)‐positron emission tomography (PET) to scan for the presence of gastrointestinal (GI) polyps [6]. Images showed increased FDG uptake in focal masses located in the LKB1 +/− mice where the stomach and pylorus are located, whereas the LKB1 +/+ were negative (p = .06) for FDG signal in this area. Several of the LKB1 +/− mice were killed after imaging, and it was confirmed that these animals had large polyps in the pylorus and stomach corresponding exactly to the regions of greatest FDG uptake. Treatment of animals with rapamycin for 4 weeks abolished the FDG‐PET signal. Immediate autopsy of the animals imaged by FDG‐PET revealed that the rapamycin‐treated mice had minimal detectable GI polyps, whereas the vehicle‐treated mice all exhibited the presence of large GI polyps. These results suggest that rapamycin reverses polyp growth in LKB1 +/− mice.
Our study is based on the observation by Franz et al. that rapamycin, U.S. Food and Drug Administration approved for use in orthotopic transplant recipients, was successfully used in an off‐label study of five individuals with tuberous sclerosis [7]. This disease, which is related to PJS, is caused by germline mutations in the tuberous sclerosis complex 1 or 2, downstream of LKB1, also leading to aberrant mTOR activation. All of the patients in this study had subependymal giant cell astrocytomas, which exhibited regression and necrosis on well‐tolerated treatment with oral rapamycin. Faivre et al. suggested in 2006 the benefit of mTOR inhibitors in the treatment of patients with PJS [8]. In a recent case of a patient with PJS suffering from advanced pancreatic cancer, we observed an impressive response to oral treatment with everolimus monotherapy [9]. Therefore, mTOR inhibition might be a potential anticancer treatment in Peutz‐Jeghers‐related malignancies and needs confirmation in a larger patient cohort.
Toxicity: Several trials with everolimus have already been performed. Yee et al. performed a phase I/II study with everolimus 10 mg daily in hematological malignancies and observed the following adverse events in more than 15% of the patients, in descending order of frequency: anorexia, oral aphthous ulcers, diarrhea, fatigue, dermatologic, dysgeusia, constipation, and cramps [10]. Blood tests also showed hyperglycemia, hyperlipidemia, elevated hepatic parameters, hypophosphatemia, hypomagnesemia, and hypocalcemia in more than 15% of the patients. Motzer et al. executed a phase III trial in patients with renal‐cell cancer [11]. Stomatitis, rash, fatigue, asthenia, diarrhea, and anorexia occurred in more than 15% of the participants, using 10 mg everolimus per day. Most of the aforementioned adverse events are grade 1 or 2. Toxicities of grade 3 and 4 are rare, with stomatitis and fatigue in less than 10%. In the latter study, everolimus toxicity led to treatment discontinuation for 10% of the patients. Furthermore, 34% of the patients required a dose interruption and 5% had a dose reduction.
Chemoprevention: The use of chemoprophylaxis is not a new phenomenon. The ability to prevent cancer has been demonstrated before, and some agents are already administrated for cancer prevention in clinical practice, with aspirin as the best‐known example. Low‐dose aspirin is proven to be successful in reducing cancer incidence by about 10% in men and 7% in women [12]. Because the side‐effect profile is favorable, the benefits of aspirin treatment outweigh the adverse events in patients without risk factors for gastrointestinal bleeding. Another chemoprophylactic drug used is tamoxifen, which is effective against breast cancer [12]. Serious adverse events such as endometrial cancer and venous thromboembolism are rare, making this drug suitable for therapeutic prevention in patients with an elevated breast cancer risk.
In the setting of a preventive intervention with potential severe adverse events, the selection of high‐risk patients is essential. The presence of a mutation resulting in an enhanced cancer risk might be a suitable target for chemoprevention. Squarize et al. investigated the use of a chemopreventive agent targeting a directly associated mutation, a situation comparable to the one in our trial [13]. The administration of rapamycin in mice with the Cowden's disease mutation resulted in a decrease of tumor growth and prolonged survival. A chemoprevention trial on patients with PJS, executed in 2011, did not lead to statistical analysis due to insufficient polyp burden and poor accrual [14].
Footnotes
ClinicalTrials.gov Identifier: NCT01178151
Sponsor(s): Novartis
Principal Investigator: H.J. Klümpen
IRB Approved: Yes
Disclosures
Evelien Dekker: FujiFilm, Tillots (C/A); FujiFilm (RF); Olympus: Other [endoscopic equipment loan]. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. van Lier MG, Wagner A, Mathus‐Vliegen EM et al., High cancer risk in Peutz‐Jeghers syndrome: A systematic review and surveillance recommendations. Am J Gastroenterol 2010;105:1258–1264; author reply 1265. [DOI] [PubMed] [Google Scholar]
- 2. Jenne DE, Reimann H, Nezu J et al. Peutz‐Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 1998;18:38–43. [DOI] [PubMed] [Google Scholar]
- 3. Korsse SE, Peppelenbosch MP, van Veelen W. Targeting LKB1 signaling in cancer. Biochim Biophys Acta 2013;1835:194–210. [DOI] [PubMed] [Google Scholar]
- 4. van Veelen W, Korsse SE, van de Laar L et al. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene 2011;30:2289–2303. [DOI] [PubMed] [Google Scholar]
- 5. Wei C, Amos CI, Zhang N et al. Suppression of Peutz‐Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res 2008;14:1167–1171. [DOI] [PubMed] [Google Scholar]
- 6. Shackelford DB, Vasquez DS, Corbeil J et al. mTOR and HIF‐1alpha‐mediated tumor metabolism in an LKB1 mouse model of Peutz‐Jeghers syndrome. Proc Natl Acad Sci U S A 2009;106:11137–11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franz DN, Leonard J, Tudor C et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 2006;59:490–498. [DOI] [PubMed] [Google Scholar]
- 8. Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 2006;5:671–688. [DOI] [PubMed] [Google Scholar]
- 9. Klumpen HJ, Queiroz KC, Spek CA et al. mTOR inhibitor treatment of pancreatic cancer in a patient with Peutz‐Jeghers syndrome. J Clin Oncol 2011;29:e150–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yee KW, Schittenhelm M, O'Farrell AM et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD‐positive leukemic cells. Blood 2004;104:4202–4209. [DOI] [PubMed] [Google Scholar]
- 11. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 12. Cuzick J. Preventive therapy for cancer. Lancet Oncol 2017;18:e472–e482. [DOI] [PubMed] [Google Scholar]
- 13. Squarize CH, Castilho RM, Gutkind JS. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res 2008;68:7066–7072. [DOI] [PubMed] [Google Scholar]
- 14. Kuwada SK, Burt R. A rationale for mTOR inhibitors as chemoprevention agents in Peutz‐Jeghers syndrome. Fam Cancer 2011;10:469–472. [DOI] [PubMed] [Google Scholar]