Abstract
Although the hepatomitogenic activity of T3 is well established, the wide range of harmful effects exerted by this hormone precludes its use in regenerative therapy. The aim of this study was to investigate whether an agonist of TRβ, KB2115 (Eprotirome), could exert a mitogenic effect in the liver, without most of the adverse T3/TRα-dependent side effects. F-344 rats treated with KB2115 for 1 week displayed a massive increase in bromodeoxyuridine incorporation (from 20% to 40% vs. 5% of controls), which was associated with increased mitotic activity in the absence of significant signs of liver toxicity. Noteworthy, while cardiac hypertrophy typical of T3 was not observed, beneficial effects, such as lowering blood cholesterol levels, were associated to KB2115 administration. Following a single dose of KB2115, hepatocyte proliferation was evident as early as 18 h, demonstrating its direct mitogenic effect. No increase in serum transaminase levels or apoptosis was observed prior to or concomitantly with the S phase. While KB2115-induced mitogenesis was not associated to enhance expression of c-fos, c-jun, and c-myc, cyclin D1 levels rapidly increased. In conclusion, KB2115 induces hepatocyte proliferation without overt toxicity. Hence, this agent may be useful for regenerative therapies in liver transplantation or other surgical settings.
Key words: Thyromimetics, Cyclin D1, Regenerative medicine, Hepatocyte proliferation
INTRODUCTION
An increasing number of agents capable of inducing hepatocyte proliferation without causing liver injury (primary mitogens) has been identified in the past 20 years1. Unlike liver regeneration after cell loss/injury, the proliferative process induced by primary mitogens represents the initial event resulting in an increased cell number. Among these primary mitogens, ligands of nuclear receptors of the steroid/thyroid hormone superfamily are the most represented since they include agonists of peroxisome proliferator-activated receptor-α (PPARα)2,3, constitutive androstane receptor (CAR)4,5, all-trans retinoic acid receptors (RARs)6, and thyroid hormone receptors (TRs)7.
Among the nuclear receptor ligands, 3,5,3′-triiodothyronine (T3) has been long recognized as a potent hepatomitogen7–10. Although the molecular mechanisms through which T3 induces hepatocyte proliferation are still unclear, its mitogenic effect seems to be mediated by TRβ11. Opposite to what is observed in liver regeneration after two-thirds surgical partial hepatectomy (PH), T3-induced liver cell proliferation occurs in the absence of activation of transcription factors, such as AP-1, nuclear factor-κB (NF-κB), or signal transducer and activator of transcription 3 (STAT3), and is not associated with an increased expression of immediate early genes, such as c-fos, c-jun, or c-myc9.
More recently, an additional mechanism responsible for T3-induced mitogenesis has implicated β-catenin, an important nuclear effector of the Wnt signaling pathway12,13. Indeed, while in wild-type mice administration of T3 induces a robust wave of hepatocyte proliferation, no mitogenic response is seen in the hepatocyte-specific β-catenin knockout mice14.
T3 not only is mitogenic for intact liver but also improves the regenerative response of rodent livers after 70% or 90% hepatectomy and stimulates liver regeneration in aged rats when given prior to or after 70% PH15–18.
Although the hepatomitogenic effect of T3 could represent a useful tool in pathological conditions characterized by an impaired regenerative ability (i.e., aged livers) or when a rapid growth stimulation of the liver is required (i.e., small for size transplantation), its use is hampered by its toxic effects (tachyarrhythmias, heart failure, bone and muscle catabolism, and mood disturbances)19–21.
Since the α isoform of TRs is the main responsible for harmful T3 effects on heart, muscle, and bone, several efforts have been made to develop agonists of the β-isoform that could induce some beneficial effects [triglyceride (TG), cholesterol (CH), obesity, and body mass lowering] without most of the adverse T3-dependent side effects22,23,24. Among the several analogs so far generated, GC-1, KB2115, and the Hep-Direct prodrug MBO07811 have reproduced most of the effects of T3 in the absence of deleterious effects25–27.
KB2115, commercially known as Eprotirome, is characterized by high liver selectivity and no extrahepatic side effects and entered human clinical trials for dyslipidemia, displaying encouraging results in the absence of detrimental effects typically associated with high levels of thyroid hormones26,28–31. Despite the promising activity on LDL, CH, and TG reduction, a phase III trial was terminated because of alterations of dog cartilage following chronic treatment (see Karo bio web site, www.karobio.com). In addition, reduced T4 levels, as well as liver toxicity, occurred in homozygous patients affected by familiar hypercholesterolemia, treated with 50 or 100 μg of Eprotirome for only 6 weeks32.
Nevertheless, a subchronic treatment with KB2115 might still be clinically useful in conditions where a rapid liver regeneration is required or when the regenerative capacity of this organ is impaired. Since no information is available on the effect of KB2115 on hepatocyte proliferation, we analyzed the effect of acute and subchronic treatment of KB2115 on liver cell proliferation.
MATERIALS AND METHODS
Animals and Treatments
Five-week-old male F-344 rats purchased from Charles River (Milano, Italy) were maintained on a standard laboratory diet (Ditta Mucedola, Milano, Italy). The animals were given food and water ad libitum with a 12-h light/dark daily cycle and were acclimated for 1 week before the start of the experiment. All procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Italian Ministry of Health. Animals were treated with daily intraperitoneal (IP) or intragastric (IG) injections for a week of T3 [20 μg/100 g body weight (b.w.)] or KB2115 (25 and 12.5 μg/100 g b.w) or vehicle (DMSO 5% in corn oil). In other experiments, rats were fed a KB2115-supplemented diet at a final concentration of 0.8 or 1.6 mg/kg of diet. For the measurement of hepatocyte proliferation, animals received bromodeoxyuridine (BrdU; 1 g/1 L) in drinking water during the 1-week treatment period.
For the kinetics experiments, animals treated with a single IG dose of KB2115 (12.5 μg/100 g b.w.) or vehicle were sacrificed at 18, 24, 36, and 48 h after treatment. BrdU (100 mg/kg; Sigma-Aldrich, Milan, Italy) was administered by IP injection 2 h before sacrifice.
For gene expression analysis, rats treated with 12.5 μg of KB2115 or vehicle were sacrificed 1, 3, and 6 h after treatment. An additional group included animals subjected to two-thirds PH performed according to Higgins and Anderson33 and sacrificed at the same time points.
Immediately after sacrifice, sections of the liver were fixed in 10% buffered formalin and processed for staining with hematoxylin–eosin (H&E) or immunohistochemistry (IHC). The remaining liver was snap frozen in liquid nitrogen and kept at −80°C until use.
Histology and Immunohistochemistry
Liver sections were fixed in 10% of buffered formalin and processed for staining with H&E or IHC. For BrdU detection, paraffin-embedded 4-μm sections were deparaffinized, treated with HCl 2 N for 1 h, and then with 0.1% trypsin at 37°C. Sections were sequentially incubated with goat serum (Sigma-Aldrich), mouse monoclonal anti-BrdU antibody (Becton Dickinson, San Jose, CA, USA), and Dako EnVision+® System Labelled Polymer-horseradish peroxidase (HRP) anti-mouse (Dako Corporation, Carpinteria, CA, USA). Peroxidase-binding sites were detected by Vector Novared Peroxidase (HRP) Substrate Kit (Vector Labs, Burlingame, CA, USA). Harris hematoxylin solution (Sigma-Aldrich) was used to counterstain liver sections. Labeling index (LI) was expressed as the number of BrdU+ hepatocyte nuclei/100 nuclei. Mitotic index was calculated as the number of mitotic figures/1,000 nuclei. Results were expressed as the means ± SE of three to five animals per group. At least 3,000 hepatocyte nuclei per liver were scored.
Determination of Apoptosis
The incidence of apoptotic bodies was quantified on H&E-stained sections according to the following morphological criteria: cytoplasmic eosinophilia and fragmentation of nuclei. Only membrane-surrounded apoptotic bodies containing nuclear fragments were recorded. The apoptotic index (AI) was calculated as the number of apoptotic bodies/field at 40× magnification. At least 25 fields per rat liver were scored. Apoptosis was also determined by IHC analysis of the cleaved form of caspase 3 (CAS-3), as described by Eckle et al.34, using rabbit monoclonal anti-CAS-3 antibody (Cell Signaling, Danvers, MA, USA).
RNA Extraction and Quantitative PCR Analysis
Total RNA was extracted from 50–70 mg of frozen liver with TRIzol reagent (Gibco Thermo Scientific, Gaithersburg, MD, USA) following recommended procedures and quantified with NanoDrop ND1000 (Thermo Scientific). RNA (2 μg) was reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA). The expression of Cnnd1, Jun, Myc, Fos, Dio1, G6pc, Ctnnb1, Lgr5, Axin-2, and Glul was assessed by real-time PCR analysis of 10 ng of cDNA mixed with 2× TaqMan Gene expression Master Mix and 20× specific TaqMan gene expression assays (Rn0432360_m1, Rn99999045_s1, Rn00561507_m1, Rn02396759_m1, Rn00572183_m1, Rn00689876_m1, Rn00584431_g1, Rn01509662_m1, Rn00577441_m1, and Rn01483107_m1, respectively) with an ABI PRISM 7300 Thermocycler (Life Technologies). Each sample was run in triplicate, and gene expression analysis of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as endogenous control. Relative quantification analysis for each gene was calculated by the 2−ΔΔCt method.
Western Blot Analysis
Total cell extracts were prepared from frozen livers. Equal amounts of liver tissue (about 80 mg) per sample were resuspended in 1.2 ml of Triton lysis buffer. Several protease inhibitors were added to the extraction buffer to minimize protein degradation during the isolation protocol. Western blot was performed as previously described9. The following antibodies were used: mouse monoclonal antibodies directed against cyclin D1 (72-13 G) and PCNA (PC-10) and rabbit polyclonal antibody directed against cyclin A (C-19) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). As loading control, mouse monoclonal antibody directed against actin (clone AC-40; Sigma-Aldrich) was used. Anti-mouse and anti-rabbit HRP-conjugated IgGs were from Santa Cruz Biotechnology. Immunoreactive bands were identified with chemiluminescence detection system, as described by the manufacturer (SuperSignal Chemiluminescent Substrate; Pierce, Rockford, IL, USA). Densitometric analysis was performed using ImageJ software.
Analysis of Serum Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Triglycerides (TGs), and Cholesterol (CH)
Immediately after sacrifice, blood samples were collected from the abdominal aorta, and serum was separated by centrifugation (2,000 × g for 20 min) and tested for TGs, CH, AST, and ALT using a commercially available kit from Boehringer (Mannheim, Germany).
Statistical Analysis
All data were expressed as the mean ± SE unless otherwise indicated. Differences between groups were compared using either unpaired two-tail Student’s t-test or ANOVA for multiple-group comparison.
RESULTS
Effect of KB2115 on Liver and Heart Weight
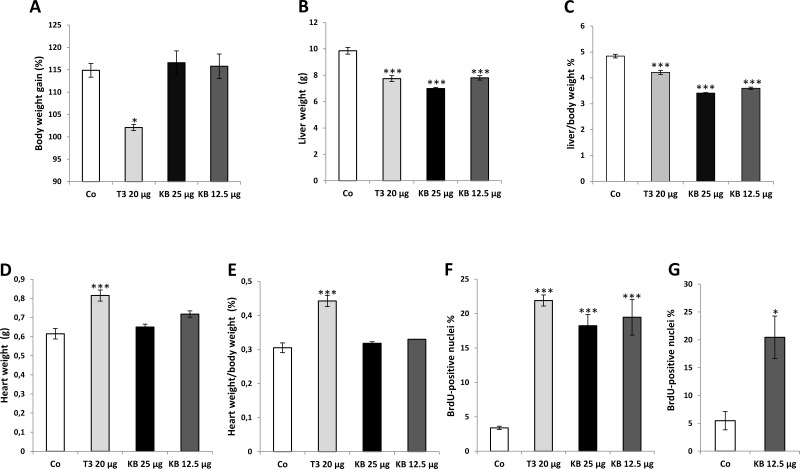
Initial studies were performed to explore the effect of different doses of KB2115 on the heart, an organ typically affected by T3. To this aim, rats were treated with IP injections of two different concentrations of KB2115 (25 and 12.5 μg/100 g b.w., once a day for 1 week). Two other groups were treated with either T3 (20 μg/100 g b.w.) or vehicle. At sacrifice, administration of T3 resulted in a much lower gain of body weight compared to controls, while no such effect was observed with any of the doses of KB2115 (Fig. 1A). A decreased liver weight was observed following T3 treatment, as well as KB2115, independently of the administered dose (Fig. 1B). As a result, liver/body weight ratio was decreased in T3- and KB2115-treated animals compared to controls. Since T3 administration was associated with a decreased body weight compared to KB2115-treated rats, the decrease in liver/body weight ratio resulted more pronounced in the latter group (Fig. 1C).
Figure 1.
Effect of KB2115 on body weight gain, liver weight, heart weight, and hepatocyte proliferation. F-344 male rats were treated intraperitoneally (IP) daily for a week with KB2115 (25 and 12.5 μg/100 g b.w.) or T3 (20 μg/100 g b.w.) or vehicle (DMSO 5% in corn oil): (A) body weight, (B) liver weight, (C) liver/body weight ratio, (D) heart weight, and (E) heart/body weight ratio. Labeling index (LI) of hepatocytes from F-344 rats subjected for a week to a daily treatment with (F) IP injections of KB2115 (25 or 12.5 μg/100 g b.w.) or T3 (20 μg/100 g b wt) or (G) intragastric (IG) injections of 12.5 μg/100 g b.w. KB2115. All animals received bromodeoxyuridine (BrdU) (1 g/L) in drinking water all throughout the treatment period. LI was expressed as the number of BrdU+ hepatocyte nuclei/100 nuclei. At least 3,000 hepatocyte nuclei per liver were scored. Results were expressed as means ± SE of three to five rats per group. Statistically significant for *p < 0.05; ***p < 0.001.
As expected, T3 administration caused a strong increase in heart weight and, as a consequence, an enhanced heart/body weight ratio (Fig. 1D and E). Indeed, following T3, a marked hypertrophy of the left ventricle and the septum with increase in muscle stiffness and a marked reduction in left ventricular cavity size was observed during necroscopy.
No such effect was found in the heart of animals treated with KB2115 at both doses (Fig. 1D and E), showing that the preferential binding to, and activation of, TRβ does not result in cardiac hyperthrophy.
Effect of KB2115 on Hepatocyte Proliferation
Since T3 exerts a powerful mitogenic effect in the liver, we investigated whether KB2115 could also elicit a proliferative response in this organ. As shown in Figure 1F, 1-week treatment with IP injections of KB2115 (25 and 12.5 μg/100 g b.w.) induced a strong hepatocyte proliferation, as assessed by BrdU incorporation. Indeed, LI displayed a five- to sixfold increase compared to controls. Notably, the extent of hepatocyte proliferation elicited by KB2115 was similar to that observed by repeated injections of T3.
Next, we assessed whether a similar mitogenic effect could be elicited also by IG administration of the drug. Since the results obtained by IP injections of 25 and 12.5 μg of KB2115 displayed a similar LI (see Fig. 1F), only the lower dose was selected for this experiment. The results showed that KB2115 retains a similar mitogenic activity also when given intragastrically (Fig. 1G).
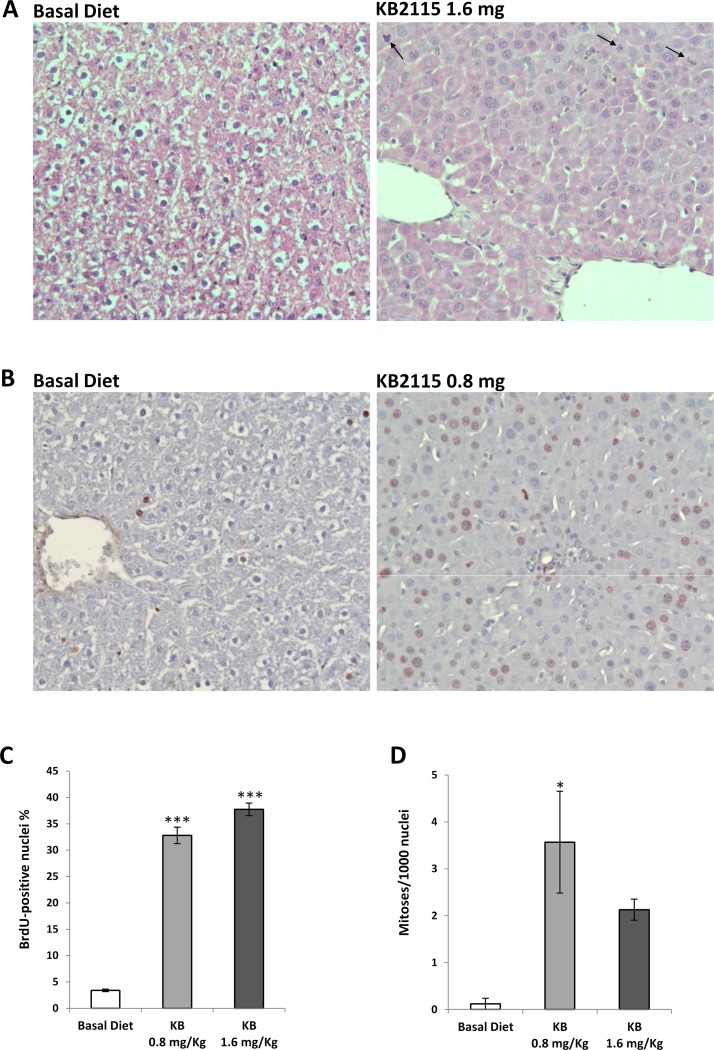
The proliferative activity of KB2115 was also assessed by incorporating the drug in a basal diet, at a final concentration of 1.6 or 0.8 mg/kg of diet, corresponding to a daily dose of 12.5 and 6.25 μg/100 b.w., respectively. As shown in Table 1, feeding KB2115 did not affect the growth of the animals as assessed by a similar body weight in drug and control rats. Histologic examination showed an almost complete depletion of cytoplasmic components in the hepatocytes of KB2115-treated rats, due to the catabolic effect of the thyroid hormone analog, thus leading to an increased nuclear/cytoplasmic ratio. Apart from these changes, the examination did not show any clear evidence of liver damage in treated animals compared to basal diet-fed animals (Fig. 2A). Lack of severe liver toxicity was confirmed by measuring the levels of serum transaminases (Table 1) that, although slightly elevated, were within the range of normal values for F-344 rats of the same age (Charles River datasheet). While no change in the content of serum TGs was observed, serum CH levels were significantly reduced by the lower dose of KB2115 (41.3 vs. 70 mg/dl of controls) (Table 1).
Table 1.
Serum Levels of Hepatic Transaminases, Triglycerides, and Cholesterol in Rats Fed Basal Diet or KB2115 Diet for a Week
| Basal | KB2115 0.8 mg/kg | KB2115 1.6 mg/kg | |
|---|---|---|---|
| Body weight (g) | 203.8 ± 2.4 | 192.5 ± 4.8 | 200.0 ± 10 |
| AST (UI/L) | 104.5 ± 2.5 | 171.8 ± 3.4* | 203.0 ± 12.1* |
| ALT (UI/L) | 66.0 ± 4.0 | 87.0 ± 3.4* | 113.7 ± 10.3* |
| γGT (UI/L) | 6.7 ± 0.9 | 5.1 ± 0.3 | 9.0 ± 4.1 |
| Triglycerides (mg/dl) | 80.5 ± 1.5 | 73.8 ± 2.7 | 76.7 ± 3.8 |
| Cholesterol (mg/dl) | 70.0 ± 15 | 41.3 ± 2.7* | 57.3 ± 4.6* |
Values are expressed as mean ± SE of three to four animals per group.
Significantly different from controls (p < 0.01).
Figure 2.
Effect of a KB2115-supplemented diet on the S phase and mitotic activity of hepatocytes. Rats were fed a KB2115 diet (1.6 or 0.8 mg/kg of diet) for 1 week. (A) Representative microphotograph showing the liver of controls and KB2115-fed rats [arrows indicate the presence of mitoses in the liver of drug-fed animals; hematoxylin–eosin (H&E): 20×]. (B) Microphotograph illustrating the presence of several BrdU+ hepatocyte nuclei in the liver of KB2115-fed rats (0.8 mg/kg of diet) [sections stained with anti-BrdU antibody and counterstained with hematoxylin (20×)]. (C) LI and (D) mitotic index (MI). To label hepatocytes, animals received BrdU (1 g/L) in drinking water all throughout the treatment period. LI was expressed as the number of BrdU+ hepatocyte nuclei/100 nuclei. MI was calculated as the number of mitotic figures/1,000 nuclei. Results were expressed as means ± SE of three to five rats per group. At least 3,000 hepatocytes per liver were scored. *p < 0.05; ***p < 0.001.
Feeding KB2115 at both concentrations for a week induced a very striking enhancement of hepatocyte proliferation, as assessed by LI that was increased by sevenfold compared to rats fed a basal diet (Fig. 2B and C). Notably, while scoring the LI, a reduction of hepatocyte size due to loss of cytoplasmic material was observed, leading to an increased number of cells/field in KB2115-treated rats (120 cells/field vs. 93 of control liver). The increased LI was accompanied by a statistically significant higher number of cells undergoing mitosis with 0.8 mg/kg of KB2115 diet compared to controls (Fig. 2A and D). Importantly, the proliferative effect exerted by KB2115 affected only hepatocytes, as, in contrast to what is seen in liver regeneration after PH, almost no labeling was found in other types of liver cells.
Time Course Analysis of KB2115-Induced Liver Cell Proliferation
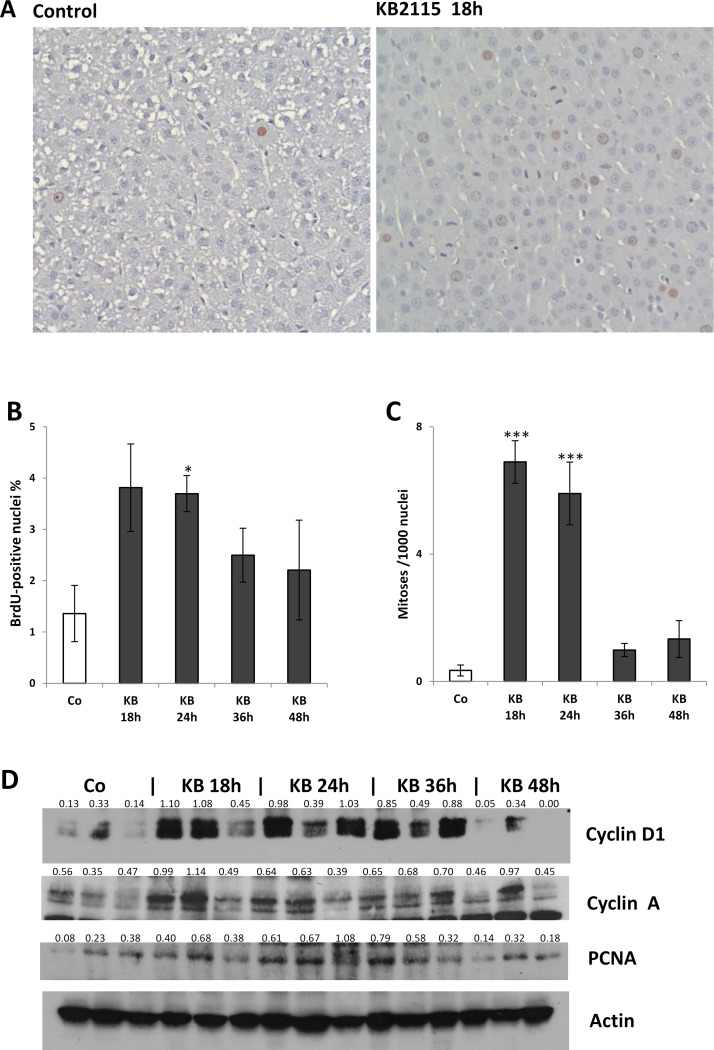
Next, we evaluated the kinetics of KB2115-induced hepatocyte proliferation. To this aim, F-344 male rats were treated with a single IG dose of KB2115 (12.5 μg/100 g b.w.) or vehicle and sacrificed at 18, 24, 36, and 48 h afterward. In this experiment, BrdU was given IP 2 h before sacrifice. As shown in Figure 3A and B, 18 h after treatment with KB2115 there was a strong induction of DNA synthesis that, as determined by LI analysis, displayed a trend to a return to control values at later times. Interestingly, mitotic index peaked at 18 h, suggesting that the entry into the S phase induced by KB2115 most likely occurred at an even earlier time (Fig. 3A and C).
Figure 3.
Time course analysis of KB2115-induced liver cell proliferation. Animals treated with a single IG dose of KB2115 (12.5 μg/100 g b.w.) or DMSO (5% in corn oil, vehicle) were sacrificed at 18, 24, 36, and 48 h after treatment. BrdU was given by IP injection (100 mg/kg) 2 h before sacrifice. (A) Immunohistochemical staining of BrdU in the liver of rats sacrificed 18 h after vehicle or KB2115 showing the presence of several mitoses [counterstained with hematoxylin (20×)]. (B) LI, (C) MI, and (D) Western blot analysis of cell cycle proteins. LI was expressed as number of BrdU+ hepatocyte nuclei/100 nuclei. Mitotic index was calculated as the number of mitotic figures/1,000 nuclei. Results were expressed as means ± SE of three to five rats per group. At least 3,000 hepatocytes per liver were scored. For Western blot, total cell extracts were prepared from frozen livers, and Western analysis was performed as described in Materials and Methods. Actin was used as loading control. Numbers below the blots represent quantification of the bands normalized to actin. Each lane represents an individual sample. Co, controls (DMSO-treated animals). *p < 0.05; ***p < 0.001.
The mitogenic effect of KB2115 was further confirmed by Western blot analysis of the cell cycle-related proteins, cyclin D1, cyclin A, and PCNA. Indeed, an increased content of these proteins was observed in KB-2115-treated rats compared to animals receiving vehicle alone (Fig. 3D).
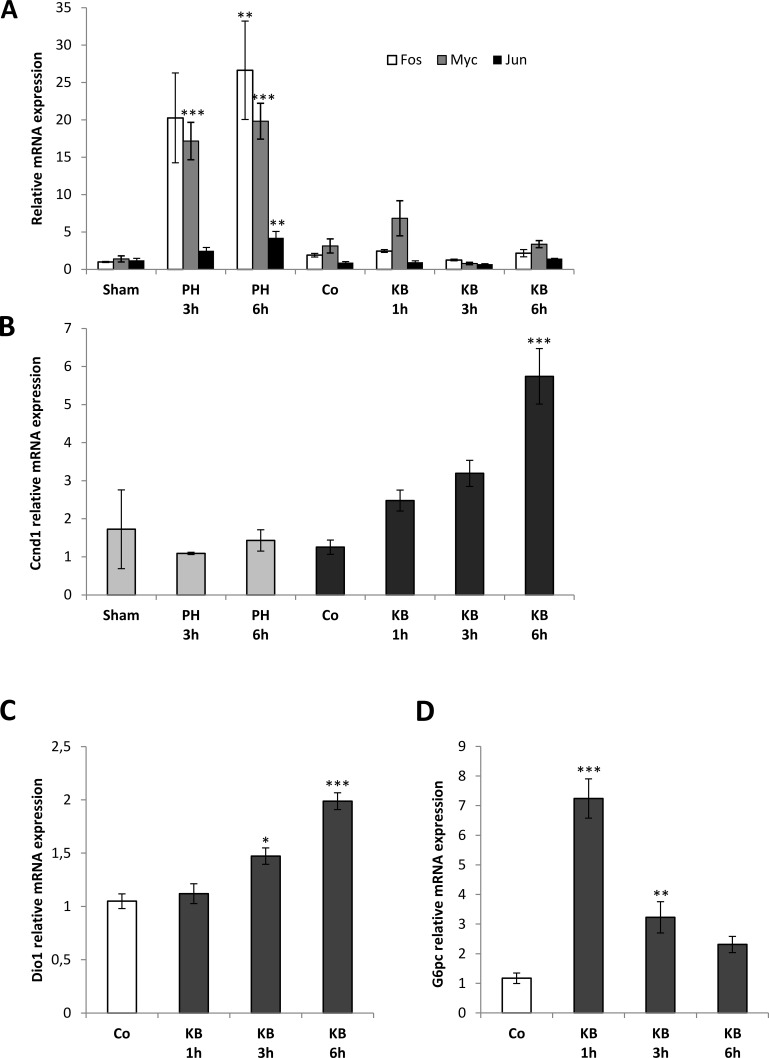
Next, to explore the molecular changes preceding the hepatocyte entry into the cell cycle, we evaluated the expression of some immediate early genes/cell cycle-related genes at 1, 3, and 6 h after a single IG administration of KB2115 (12.5 μg/100 g b.w.). In this experiment, rats subjected to two-thirds PH were used as well-established proliferating controls. As shown in Figure 4A, while in agreement with the literature, the expression of c-jun, c-fos, and c-myc was strongly increased in liver regeneration following PH, no such increase was observed at any time within the first 6 h after KB2115 treatment. On the other hand, while the expression of Cnnd1 rapidly increased after KB2115 and continued to raise in the following hours, no induction of the expression of this gene was observed in the first 6 h after surgery (Fig. 4B).
Figure 4.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of the expression of immediate early genes, Ccnd1, and TRβ target genes. Animals treated with a single IG dose of KB2115 (12.5 μg/100 g b.w.) or DMSO (5% in corn oil, Co) were sacrificed 1, 3, or 6 h after treatment. qRT-PCR analysis was performed also in the liver of rats subjected to two-thirds PH or laparotomy (sham operation). Expression of (A) c-fos, c-jun, and c-myc; (B) Ccnd1; (C) deiodinase 1 (Dio1) and glucose-6-phosphatase (G6pc) in rats subjected to laparotomy, PH, vehicle (Co), or KB2115 and sacrificed at the indicated times. Each sample was run in triplicate, and gene expression analysis of GAPDH was used as endogenous control. Relative quantification analysis for each gene was calculated by the 2−ΔΔCt method. *p < 0.05; **p < 0.01; ***p < 0.001.
KB2115 Treatment Induces the Expression of TRs Target Genes
Although it has been postulated that the existence of rapid nongenomic mechanisms initiated at the cell membrane could be involved in mediating the actions of thyroid hormones35, most of the effects of these hormones require the presence of TRs. To assess whether hepatocyte proliferation induced by KB2115 is preceded by activation of TRs, we evaluated the expression of two well-established TR target genes, deiodinase 1 (Dio1) and glucose-6-phosphatase (G6pc). Quantitative reverse transcriptase PCR (qRT-PCR) analysis revealed a statistically significant induction of the two genes analyzed following treatment with KB2115 (Fig. 4C and D).
KB2115-Induced Hepatocyte Proliferation Is Not a Consequence of Liver Damage
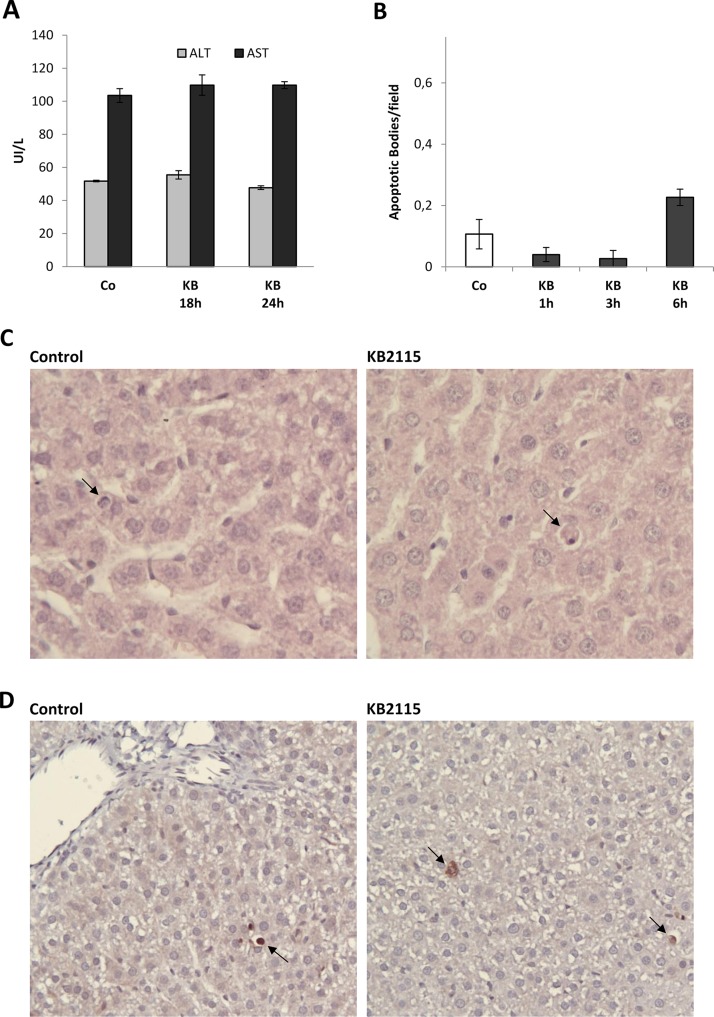
To further confirm that hepatocyte proliferation induced by KB2115 is not a compensatory response of the liver consequent to cell damage, we assessed the levels of serum transaminases and the expression of cleaved CAS-3. The results showed that no changes in AST/ALT occurred at 18 and 24 h after treatment, times corresponding to maximal DNA synthesis and mitotic activity (Fig. 5A). Moreover, no significant difference in the number of apoptotic bodies, as determined by histological analysis (Fig. 5B and C), was observed within the first 6 h after drug administration. Accordingly, only a few hepatocytes were positive when immunostained for the cleaved form of CAS-3 in both vehicle- and KB2115-treated rats (Fig. 5D).
Figure 5.
Effect of a single treatment with KB2115 on serum transaminases and liver apoptosis. (A) Graph representing the levels of serum transaminases 18 and 24 h after treatment with a single IG dose of DMSO (5% in corn oil, Co) or KB2115 (12.5 μg/100 g b.w.). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined in blood samples collected from the abdominal aorta. (B) Apoptotic index (AI) and (C) representative microphotographs showing the presence of apoptotic bodies (ABs) indicated by the arrows (H&E: 40×). AI was calculated as the number of ABs per field at 40× magnification. At least 25 fields per rat liver were scored. Results were expressed as means ± SE of three rats per group. (D) Representative microphotographs showing immunostaining for the cleaved form of caspase 3 (CAS-3) in the liver of rats treated with the vehicle or a single dose of KB2115 (12.5 μg/100 g b.w.) and sacrificed 3 h later. Arrows indicate single CAS-3+ cells (20×).
KB2115-Induced Hepatocyte Proliferation Is Not Associated With Increased Expression of β-Catenin or its Target Genes Axin-2, Glul, and Lgr5
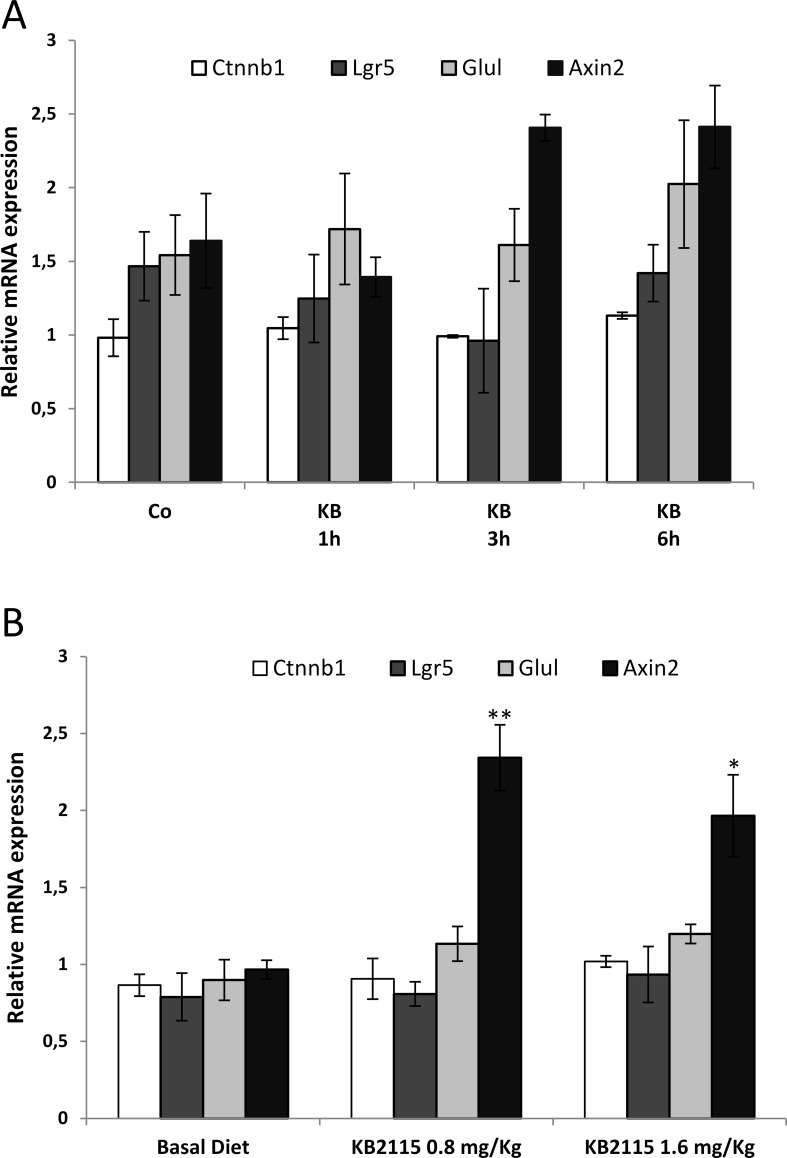
Previous works have shown that β-catenin is crucial for T3-induced hepatocyte proliferation14. Indeed, T3 was unable to stimulate hepatocyte proliferation in mice knockout for β-catenin. To investigate whether an increased β-catenin-dependent pathway could be involved in KB2115 mitogenesis, we performed qRT-PCR analyses at early time points after a single administration of KB2115 (12.5 μg/100 g b.w.) or 1 week after a continuous feeding with two different concentrations of the drug (0.8 or 1.6 mg/kg). As shown in Figure 6, no enhanced levels of β-catenin and of its target genes were observed in both the regimens with the exception of an approximately twofold increase in Axin-2 mRNA level in rats fed KB2115 for 7 days.
Figure 6.
qRT-PCR analysis of the expression of β-catenin (Ctnnb1) and of its target genes (Axin2, Lgr5, and Glul). Expression of β-catenin, Axin2, Lgr5, and Glul in rats treated with (A) a single IG dose of KB2115 (12.5 μg/100 g b.w.) or DMSO (5% in corn oil, Co) and sacrificed at 1, 3, or 6 h or (B) fed a KB2115-supplemented diet (0.8 or 1.6 mg/kg) and sacrificed 1 week later. Each sample was run in triplicate, and gene expression analysis of GAPDH was used as endogenous control. Relative quantification analysis for each gene was calculated by the 2−ΔΔCt method. *p < 0.05; **p < 0.01.
DISCUSSION
The present study shows that the TRβ agonist, KB2115 (Eprotirome), exerts a mitogenic effect in rat liver in the absence of one of the most T3 harmful side effects, namely, cardiac hyperthropy. The liver proliferative response induced by KB2115, by IP, IG, or dietary administration, was not associated to liver damage, but it was the result of a direct effect of this drug.
Similar to T3 and other ligands of nuclear receptors acting as primary mitogens, such as agonists of PPARs, CAR, and RARs1, KB2115 did not induce the expression of immediate early genes, considered to be a key event during liver regeneration following hepatocyte loss/death. On the other hand, KB2115 caused a very rapid increase in the mRNA levels of the cell cycle regulator cyclin D1 that was associated to an earlier entry into the S phase, compared to what was observed during liver regeneration after two-thirds PH9. The hepatocyte proliferation response driven by KB2115 was preceded by activation of TR target genes, suggesting that the mitogenic effect of this drug is to be attributed to the binding and activation of TRs, rather than to extragenomic mechanisms.
KB2115 has been evaluated in humans as a selective thyromimetic able to reduce hypercholesterolemia with no evidence of adverse thyrotoxic effects on the heart, skeletal muscle, bone, or pituitary gland26,28–31. Despite those encouraging results, development of KB2115 was interrupted, due to a toxicological study demonstrating dog cartilage damage after a 12-month treatment (Karo bio web site, www.karobio.com); however, no indication of such potential effects in humans with thyroid dysfunction has been found. In addition, reduction of T4, as well as some degree of liver toxicity, occurred in homozygous patients affected by familiar hypercholesterolemia, treated with Eprotirome for 6 weeks32. Nevertheless, our data, showing a liver mitogenic effect of KB2115, suggest that an acute and/or subchronic treatment with the drug might be clinically useful in regenerative medicine to manage conditions whereby a rapid liver regeneration is required, such as in the living-related transplantation.
Another condition where inducers of hepatocyte proliferation devoid of toxicity can be envisaged is the elderly status that is characterized by an impaired liver regenerative capacity36,37. Indeed, our previous studies have shown that although liver regeneration occurring after PH is greatly reduced in aged mice, hyperplasia induced by xenobiotic mitogens was found to be age independent38; furthermore, T3 is not only mitogenic for intact liver but also improves the regenerative response of rodent livers after 70% or 90% hepatectomy and stimulates liver cell proliferation in aged rats when given prior to or after 70% PH15–18.
On this basis, the possible introduction in the clinic of the thyromimetic KB2115 provided with a powerful hepatocyte mitogenic capacity in the absence of T3-associated harmful side effects could represent an important improvement in the field of regenerative therapies.
Recently, it was demonstrated that the mitogenic activity exerted by T3 and GC-1 is, at least in part, mediated by activation of the Wnt/β-catenin signaling. Indeed, T3 was unable to stimulate hepatocyte proliferation in mice knockout for β-catenin14, and a follow-up study using conditional knockout mice for Wnt co-receptors, LRP5–6, also showed dampened hepatocyte proliferative response to GC-1 and T339. In the present work, we were unable to find evidence for an increased activation of the Wnt/β-catenin in KB2115-induced hepatocyte proliferation. Studies to determine whether KB2115 could still elicit a proliferative response in mice knockout for β-catenin are needed to clarify whether an intact Wnt/β-catenin pathway is a sine qua non condition for the mitogenic activity of this drug.
Another relevant point raised by these results is the potential usefulness of T3 analogs as therapeutic anticancer drugs, as animal studies support the possible use of TRβ agonists to interfere with hepatocellular carcinoma (HCC) development and progression. Indeed, (i) a short-term treatment with GC-1 of rats carrying preneoplastic nodules induced a rapid disappearance of the vast majority of these lesions in two distinct experimental protocols40, and (ii) treatment with KAT-681, another liver-selective thyromimetic with hypocholesterolemic properties, showed inhibitory effects in the early and late phases of hepatocarcinogenesis41. In addition, our initial experiments suggest that also KB2115, in spite of its mitogenic effect, reduces the number of GSTP+ preneoplastic lesions induced in rats. These results, although preliminary, are in line with those obtained with T3 and GC-1 and suggest the antitumorigenic effect of KB2115, probably by inducing a differentiation program in preneoplastic hepatocytes.
In conclusion, although caution about the use of these analogs should be maintained, as they could have some yet unknown deleterious effects, still the possibility of their therapeutic use in regenerative medicine and in a disease that currently does not offer any satisfactory alternative, such as HCC, might be considered.
ACKNOWLEDGMENTS
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC; grant IG-15279 to A.C.) and Fondazione Banco di Sardegna (to A.C. and A.P.).
REFERENCES
- 1. Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118–28. [DOI] [PubMed] [Google Scholar]
- 2. Rao MS, Reddy JK. Peroxisome proliferators and hepatocarcinogenesis. Carcinogenesis 1987;8:631–6. [DOI] [PubMed] [Google Scholar]
- 3. Marsman DS, Cattley RC, Conway G, Popp JA. Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and [4-chloro-6-(2,3-xilidino)-2-pirimidynilthio]acetic acid (WY14643) in rats. Cancer Res. 1988;48:6739–44. [PubMed] [Google Scholar]
- 4. Dragani TA, Manenti G, Galliani G, Della Porta G. Promoting effect of 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene in mouse hepatocarcinogenesis. Carcinogenesis 1985;6:225–8. [DOI] [PubMed] [Google Scholar]
- 5. Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin-D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of mitogen 1,4-bis[2-3,5-dichloropyridyloxy)]benzene (TCPOBOP). Am J Pathol. 2000;156:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ledda-Columbano GM, Pibiri M, Molotzu F, Cossu C, Sanna L, Simbula G, Perra A, Columbano A. Induction of hepatocyte proliferation by retinoic acid. Carcinogenesis 2004;25:2061–6. [DOI] [PubMed] [Google Scholar]
- 7. Short J, Brow RF, Husakova A, Gilbertson JR, Zemel R, Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of intact animal. J Biol Chem. 1972;247:1757–66. [PubMed] [Google Scholar]
- 8. Francavilla A, Carr BI, Azzarone A, Polimeno L, Wang Z, Van Diehl DH, Subbotin V, Prelich JG, Starzl TE. Hepatocyte proliferation and gene expression induced by triiodothyronine in vivo and in vitro. Hepatology 1994;20:1237–41. [PubMed] [Google Scholar]
- 9. Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A. Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEB J. 2001;15:1006–13. [DOI] [PubMed] [Google Scholar]
- 10. Malik R, Habib M, Tootle R, Hodgson H. Exogenous thyroid hormone induces liver enlargement, whilst maintaining regenerative potential—A study relevant to donor preconditioning. Am J Transplant. 2005;5:1801–7. [DOI] [PubMed] [Google Scholar]
- 11. Kowalik MA, Perra A, Pibiri M, Cocco MT, Samarut J, Plateroti M, Ledda-Columbano GM, Columbano A. TRbeta is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J Hepatol. 2010;53:686–92. [DOI] [PubMed] [Google Scholar]
- 12. Sylvie J, Ellen C, Kris V. The role of Wnt in cell signaling and cell adhesion during early vertebrate development. Front Biosci. 2011;16:2352–66. [DOI] [PubMed] [Google Scholar]
- 13. Nejak-Bowen KN and Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Triiodothyronine induces hepatocyte proliferation by protein kinase A-dependent-β-catenin activation in rodents. Hepatology 2014;59:2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Columbano A, Simbula M, Pibiri M, Perra A, Deidda M, Locker J, Pisanu A, Uccheddu A, Ledda-Columbano GM. Triiodothyronine stimulates hepatocyte proliferation in two models of impaired liver regeneration. Cell Prolif. 2008;41:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Zhou WH, Zhang W, Xing CY, Yang Z, Zang K, Zheng SS. Tri-iodothyronine enhances liver regeneration after living donor liver transplantation in rats. J Hepatobiliary Pancreat Sci. 2011;18:806–14. [DOI] [PubMed] [Google Scholar]
- 17. Bockhorn M, Frilling A, Benko T, Best J, Sheu SY, Trippler M, Schlaak JF, Broelsch CE. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39:58–63. [DOI] [PubMed] [Google Scholar]
- 18. Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, Nejak-Bowen K, Monga SP. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. [DOI] [PubMed] [Google Scholar]
- 20. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–53. [DOI] [PubMed] [Google Scholar]
- 22. Baxter JD, Dillmann WH, West BL, Huber R, Furlow JD, Fletterick RJ, Webb P, Apriletti JW, Scanlan TS. Selective modulation of thyroid hormone receptor action. J Steroid Biochem Mol Biol. 2001;76:31–42. [DOI] [PubMed] [Google Scholar]
- 23. Webb P. Selective activators of thyroid hormone receptors. Expert Opin Investig Drugs 2004;13:489–500. [DOI] [PubMed] [Google Scholar]
- 24. Baxter JD, Webb P. Thyroid hormone mimetics: Potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–20. [DOI] [PubMed] [Google Scholar]
- 25. Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5:299–306. [DOI] [PubMed] [Google Scholar]
- 26. Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 2008;105:663–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 2007;104:15490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meruvu S, Ayers SD, Winnier G, Webb P. Thyroid hormone analogues: Where do we stand in 2013? Thyroid 2013;23:1333–44. [DOI] [PubMed] [Google Scholar]
- 29. Tancevski I, Rudling M, Eller P. Thyromimetics: A journey from bench to bed-side. Pharmacol Ther. 2011;131:33–9. [DOI] [PubMed] [Google Scholar]
- 30. Webb P. Selective activators of thyroid hormone receptors. Expert Opin Investig Drugs 2004;13:489–500. [DOI] [PubMed] [Google Scholar]
- 31. Angelin B, Kristensen JD, Eriksson M, Carlsson B, Klein I, Olsson AG, Chester Ridgway E, Landerson PW. Reductions in serum levels of LDL cholesterol apolipoprotein B, triglycerides and lipoprotein(a) in hypercholesterolaemic patients treated with the liver-selective thyroid hormone receptor agonist eprotirome. J Intern Med. 2015;277:331–42. [DOI] [PubMed] [Google Scholar]
- 32. Sjouke B, Langslet G, Ceska R, Nicholls SJ, Nissen SE, Öhlander M, Ladenson PW, Olsson AG, Hovingh GK, Kastelein JJ. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): A randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2014;2:455–63. [DOI] [PubMed] [Google Scholar]
- 33. Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following partial removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 34. Eckle VS, Buchmann A, Bursch W, Schulte-Hermann R, Schwarz M. Immunohistochemical detection of activated caspases in apoptotic hepatocytes in rat liver. Toxicol Pathol. 2004;32:9–15. [DOI] [PubMed] [Google Scholar]
- 35. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–21. [DOI] [PubMed] [Google Scholar]
- 36. Bucher NL, Swaffield MN, Ditroia JF. The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res. 1964;24:509–12. [PubMed] [Google Scholar]
- 37. Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci USA 2001;98:11468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ledda-Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A. Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology 2004;40:981–8. [DOI] [PubMed] [Google Scholar]
- 39. Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, Nejak-Bowen K, Monga SP. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perra A, Kowalik MA, Pibiri M, Ledda-Columbano GM, Columbano A. Thyroid hormone receptor ligands induce regression of rat preneoplastic liver lesions causing their reversion to a differentiated phenotype. Hepatology 2009;49:1287–96. [DOI] [PubMed] [Google Scholar]
- 41. Hayashi M, Tamura T, Kuroda J, Ohnota H, Shibata N, Akahane M, Kashida Y, Mitsumori K. Different inhibitory effects in the early and late phase of treatment with KAT-681, a liver selective thyromimetic, on rat hepatocarcinogenesis induced by 2-acetylaminofluorene and partial hepatectomy after diethylnitrosamine initiation. Toxicol Sci. 2005;84:22–8. [DOI] [PubMed] [Google Scholar]