Abstract
By their interaction with IgG immune complexes FcγR and complement link innate and adaptive immunity showing functional redundancy. In complement-deficient mice not only IgG downstream effector functions are often impaired but also adaptive immunity. Based on a variety of model systems utilising FcγR KO mice, it has been concluded that FcγR are also key regulators of both innate and adaptive immunity. However, several of the model-systems under-pinning these conclusions suffer from flawed experimental design. To address this issue in the absence of these caveats we generated a novel mouse model deficient for all FcγR (FcγRI/II/III/IV-/- mice).
These mice displayed normal development and lymphoid and myeloid ontogeny. Although IgG-effector pathways were impaired, adaptive immune responses to a variety of challenges, including bacterial infection and IgG-immune complexes, were not. Like FcγRIIb-deficient mice, FcγRI/II/III/IV-/- mice developed higher Ab titres, but no autoantibodies. These observations indicate a redundant role for activating FcγRs in the modulation of the adaptive immune response in vivo. We conclude that FcγR are downstream IgG-effector molecules with a restricted role in the ontogeny and maintenance of the immune system as well as regulation of adaptive immunity.
Introduction
Adequately defining the in vivo role of the receptors for IgG, FcγR, is severely hampered not only by the complexity of the FcγR gene family itself but also because of their functional redundancy with the complement system. FcγR and complement link innate and adaptive immunity on two levels. First of all, they mediate the activation of downstream effector pathways of innate immune cells by antigen (Ag) specific IgG. Secondly, they are involved in the IgG immune complex (IC) mediated regulation of adaptive immunity.
Four different FcγR, have been identified in the mouse. The IgG binding α-chains of the activating FcγRI, FcγRIII and FcγRIV, are associated with the FcR γ chain, a signal transduction subunit which is also required for cell surface expression (1). The activating FcγR are counterbalanced by the inhibiting receptor FcγRIIb. The four FcγR, are expressed in different combinations on a variety of immune cells, mainly myeloid effector cells.
The in vivo role of FcγR has been extensively studied by analyzing the phenotype of mice deficient either for one or combinations of two or three FcγR or the FcR γ chain. By establishing a variety of disease models such as arthritis, hemolytic anemia, anaphylaxis and lupus like disease in these KO mice, we and others have shown that FcγR play an important role in the downstream antibody (Ab) effector pathways which drive the pathogenesis in these diseases (2). However, by using antibodies with a mutation in their Fc domain, destroying FcγR binding without affecting interactions with complement, it has recently been shown that several IgG downstream effector functions can be mediated also by complement (3).
Mice deficient in the early pathway components C1q, C3 and C4 and the complement receptors Cr1/Cr2 have impaired humoral responses to T cell dependent and T cell independent Ag (4–6) indicating that the complement system plays an important role in priming and regulation of the adaptive immune response (7). Moreover, C1q deficient mice develop spontaneously lupus like disease. A series of observations suggest that FcγR also play a role in priming and regulation of adaptive immunity and the maintenance of immune tolerance. Ag-specific IgG1, IgG2a, and IgG2b enhance Ab and CD4+ T cell responses to soluble protein Ag via activating FcγRs, probably by increasing Ag presentation by dendritic cells to Th cells (8). With Ag-specific IgG3, an IgG subclass not interacting with FcγR, this process is complement dependent (9). In FcR γ chain KO mice, immunized with the model Ag KLH, the delayed-type hypersensitivity (DTH) response after challenge is significantly decreased compared to the DTH in WT mice. Moreover, the secondary responses of CD4+ T cells to Ag and Ab formation were also reduced in these mice (10). These data suggest that activating FcγRs on antigen presenting cells (APCs) facilitate Ag presentation resulting in efficient priming of Th cell responses in vivo in an IC-dependent manner which is required for a full-blown Ab response. We and others have shown that soluble IgG-IC enhance cross presentation by DCs resulting in a strong induction of the proliferation of antigen specific CTLs (11–14). It is generally believed that FcγR play an important role in this process (15).
Combined, these observations suggest an important role of activating FcRs in modulating the adaptive immune response. In addition, cross-linking the B cell receptor with FcγRIIb by IgG-IC results in down regulations of Ab production. FcγRIIb deficient mice develop higher Ab titers compared to WT mice (16). Moreover it has been shown that FcγRIIb deficient mice when backcrossed into C57BL/6 background develop spontaneously lupus like disease (17).
In conclusion, many in vivo observations in WT and FcγR KO mice suggest a pleiotropic role of FcγR in the immune system. However, many of these studies have some flaws. Several studies were performed in FcR γ chain deficient mice. The FcR γ chain is a promiscuous signal transduction subunit, associated with at least nine other receptor complexes (18). Most FcγR KO mice have been generated by gene targeting in 129-derived ES cells, and subsequently backcrossed into C57BL/6 background. We have shown that after backcrossing the remaining 129-derived sequences flanking the FcγRIIb KO allele, including the hypomorphic autoimmune susceptibility SLAM locus (19) cause the autoimmune phenotype of the FcγRIIb KO mouse on mixed 129/C57BL/6 background whereas the FcγRIIb deficiency only enhances the lupus like disease (20). In many in vivo cross-presentation studies bone marrow derived DCs ex vivo loaded with IgG-IC of the model antigen chicken Ovalbumine (OVA) induced strong proliferation of adoptively transferred OVA antigen specific T cells (11,12,14). However, the endogenous anti-OVA cytotoxic T cell response was very low (21). Moreover, in vivo cross presentation of IgG-IC derived Ag was impaired in C1q deficient mice (22).
To address these issues in the absence of these caveats we generated a novel C57BL/6 mouse model deficient for all four FcγR but expressing the FcR γ chain and analyzed its phenotype. Although, as expected, we could confirm that a variety of IgG downstream effector pathways were impaired, the overall characteristics of the immune system of these mice and WT control mice were very similar. Their B and T cell responses were not impaired. Like FcγRIIb-deficient mice, FcγRI/II/III/IV-/- mice developed higher Ab titres, but no autoantibodies with age. We conclude that, in contrast to complement, FcγR have little or no role in the ontogeny and the maintenance of the immune system. Their role in priming and regulation of the adaptive immune response appears redundant.
Material and Methods
Mice
Mice were housed and all experiments were performed at the SPF animal facilities of the laboratory animal facility (PDC) of the Leiden University Medical Center (LUMC) or University of Cambridge (Salmonella infection). The health status of the animals was monitored over time. Animals tested negative for all agents listed in the FELASA (Federation of European Laboratory Animal Science Associations) guidelines for SPF mouse colonies (23).
All mouse studies were approved by the animal ethics committee of the LUMC. Experiments were performed in accordance with the Dutch Act on Animal Experimentation and EU Directive 2010/63/EU ('On the protection of animals used for scientific purposes'). C57BL/6J mice were purchased from Charles River the Netherlands. All FcγR KO mice were generated in the transgenic mouse facility of the LUMC (Figures 1 and S1). The EIIaCre deleter strain (n=20 on C57BL/6J background), was a kind gift of Dr. Heiner Westphal. The Flp deleter strain C57BL/6-Tg(CAG-flpe)36Ito/ItoRbrc, was purchased from Jackson Laboratories Bar Harbor, Me. Mice were routinely checked for their genotype by PCR.
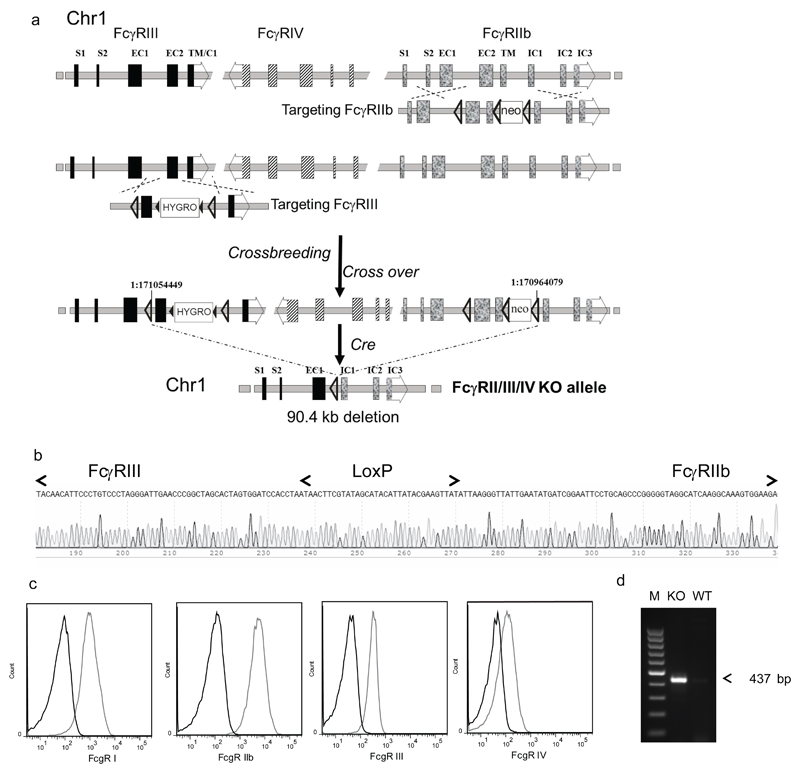
Fig.1. Generation of the FcγRI/FcγRII/FcγRIII/FcγRIV quadruple KO (FcγRI/II/III/IV-/-) mouse strain.
FcγRIIbfl/fl mice (13) were crossed with FcγRIIIfl/fl mice (supplemental figure S1). Offspring was selected for crossover between both floxed alleles. By crossing FcγRIIbfl/fl / FcγRIIfl/fl mice with the EIIaCre deleter strain a 90.4 kb fragment between the two most distant loxP sites, containing the main part of the FcγRIIb and FcγRIII gene and the complete FcγRIV gene, was removed resulting in a FcγRII/III/IV KO allele. The presence of the deletion was confirmed by PCR and DNA sequencing. The FcγRII/III/IV-/- mice were crossed with our previously generated FcγRI-/- mice (16) and FcγRI/II/III/IV-/- offspring was selected. The absence of all four FcγR was confirmed by FACS analysis.
a. From top to bottom are depicted the genomic structure of the WT FcγRIIb/FcγRIV/FcγRIII gene cluster on chromosome 1, the gene targeting strategy for the generation of the floxed FcγRIIb and FcγRIII genes, the genomic structure after the crossover between the two floxed genes and subsequently after Cre mediated recombination. The locus is shown in reverse orientation in relation to the chromosomal nucleotide numbering. The exact location of the borders of the deletion (NC_000067.6:g 171054449_170964079del according to HGVS nomenclature) on chromosome 1 are depicted based on the mouse reference genome build GRCm38.p3 (C57BL/6J) provided by the Genome Reference Consortium.
b. Core sequence flanking the remaining LoxP site within the 437 bp PCR fragment.
c. Flow cytometry of thioglycolate elicited peritoneal cells from FcγRI/II/III/IV-/- (black lines) and WT C57BL/6 mice (grey lines) stained with fluorescent labeled antibodies specific for F4.80 and CD11b and antibodies specific for the different FcγR as indicated. d. Agarose gel electrophoresis of the unique PCR fragment bridging the 90.4 kb deletion. By using a FcγRIII specific ‘Geno Fw’:
GAGGGCATCCGATTTCATTA and a FcγRIIb specific ‘Null B Rev’ GCTTCCATTGACCTGCCTAC primer, and genomic DNA from a FcγRII/III/IV-/-mouse as a template, a unique 437 bp fragment with the remaining LoxP site was synthesized. M: 100bp ladder, KO: FcγRII/III/IV-/- mouse; WT: WT control mouse.
Cells and cell lines
B3Z is an ovalbumin (OVA) 257-264 (SIINFEKL) –specific H-2Kb restricted co-stimulatory independent T cell hybridoma cell line. Intraperitoneal thioglycolate elicited macrophages of WT C57BL/6 and FcγRI/II/III/IV-/- mice were isolated by abdominal lavage with 5 ml PBS 48h after intraperitoneal injection of 1.5 ml 4% thioglycollate medium (Becton Dickinson, Mountain View CA).
Collagen Ab Induced Arthritis (CAIA)
Mice were injected intravenously (i.v.) with 4 mg of a cocktail of four different mouse anti-mouse collagen type II IgG monoclonal Abs (Equimolar mix of M2139 (IgG2b) + CIIC1 (IgG2a) + CIIC2 (IgG2b) + UL1 (IgG2b) on day 0 and in addition on day 3 with 100μg of LPS from E.coli 055:B5 (Sigma-Aldrich L2880) in 100μl of PBS intraperitoneally. At day 10 an additional amount of 4 mg of a cocktail of four different mouse anti-mouse collagen type II IgG monoclonal Abs was injected IP to boost the response. From day 7 onwards development of arthritis was monitored daily in a blind manner using a caliper to measure footpad swelling (24).
Anaphylaxis
Mice were sensitized by injecting iv 400 μg of pyrogen-free mouse anti-TNP IgG2a in saline and challenged 4 hrs. later by i.v. injection of 1 mg pyrogene-free DNP-HSA (2,4-dinitrophenylated human serum albumin) (A6661 Sigma-Aldrich) in saline per mouse (25). For the monitoring of blood pressure, the mice were anesthetized by i.p. injection of ketamine (75mg/kg), dexdomitor (0.2mg/kg) and atropine (0.5mg/kg) in saline. After induction of anesthesia, the femoral artery and femoral vein were catheterized. The artery catheter was connected to the blood pressure monitor and blood pressure was allowed to stabilize for ≥5 min. Subsequently, the mice were injected i.v. via the femoral vein catheter with DNP-HSA. Blood pressure was monitored for ≥30 min after OVA injection, using a physiological pressure transducer (AD Instruments, Colorado Springs, CO). The signal was acquired and digitized in PowerLab (AD Instruments), sampled at 200 Hz, and analyzed offline using LabChart (AD Instruments).
Ab dependent Cellular phagocytosis (ADCP)
WT C57Bl/6 and FcγRI/II/III/IV-/- mice were injected with 25 µg of the rat IgG2b 2.43 Ab (in-house production) intraperitoneally to deplete CD8+ T cells. One day before, and three days after the depleting Ab injection CD8+ T cell numbers were analyzed in blood using flowcytometry and quantified as a percentage of total CD3+ cells. As determined with Surface Plasmon Resonance, the rat IgG2b antibody has a binding preference for activating mouse FcγR (activating-to-inhibitory FcγR binding [A/I] = 40) (26).
In vitro uptake and cross presentation of immune complex derived Ag
In vitro uptake and cross-presentation of OVA-IC derived Ag by DCs were studied by pre-forming complexes of OVA and rabbit polyclonal anti-OVA ab (Cappel) in a ratio of 1 to 30, and incubating 5 µg of these complexes with 25.000 BM-DC cells in normal DC conditioned medium, as described (27). Rabbit IgG binds to all mouse FcγR (27,28). For uptake, Alexa488-labeled OVA was used, and measured using flow cytometry, with or without quenching of extracellularly bound fluorescent OVA by addition of trypan blue (Sigma-Aldrich). For Cross presentation, 25.000 BMDC were incubated with 50.000 B3Z cells. B3Z cells recognize the minimal SIINFEKL OVA-CTL epitope in MHC class I. Recognition leads to up-regulation of the transcription factor NFAT which activates a LacZ-reporter gene by binding to its IL-2 promoter (29). After overnight incubation with BM-DC the B3Z cells were incubated with a lysis buffer containing the CPRG substrate for β-gal (PBS +1% 9 mg/mL CPRG + 0.9% 1m MgCl2 + 0.125% NP40 + 0.71% 14.3m β-mercaptoethanol) at 37 °C until the color reaction had progressed sufficiently for readout in a plate reader measuring the optical density at 590 nm. A peptide with the minimal OVA epitope SIINFEKL (100 ng/mL in PBS) that directly binds to MHC class I was used as a positive control and unstimulated D1 cells (a dendritic cell line, as described (27) as negative controls.
In vivo cross-presentation of immune complex derived Ag
CD8+ T cells were isolated from spleen and lymph nodes from OT-I/CD45.1 mice with the BD CD8+ lymphocyte enrichment kit and labeled with CFSE. Three million OT-1 T cells were i.v. injected in recipient mice. One day later either 200 µg rabbit polyclonal anti-OVA Ab (Cappel) or non-specific rabbit sera (negative control) was injected i.v. followed 30 minutes later by 5 µg OVA (Worthington Biochemicals) or in house synthesized peptide with the minimal SIINFEKL OVA epitope (positive control). Three days later mice were sacrificed, spleens were isolated and proliferation of CFSE-labelled OT-1 T lymphocytes was analysed in single cell suspensions by flow cytometry gated on CD8+ and CD45.1+ cells.
Quantitation of Immune complex clearance
Age- and weight-matched naive mice received an i.v. injection of 100 μg rabbit
IgG anti-ovalbumin (Cappel) followed by an i.v. injection of 5 μg Alexa Fluor 488/647 labeled ovalbumin (Life Technologies) 15 minutes later. At indicated time points blood was drawn and serum was collected. 5 μl serum was mixed with sample buffer, heated at 95° C for 5 minutes and loaded on SDS/PAGE. Fluorescent ovalbumin was quantified directly from the SDS/PAGE gels by a Typhoon 9410 Variable mode imager (GE Healthcare Bio-Sciences) and ImageQuant TL v8.1 software (GE Healthcare Life Sciences).
At different time-points after injection, mice were sacrificed and a single lobe of liver was isolated and imaged by IVIS Spectrum (PerkinElmer) using excitation at 605 nm and measuring emission at 680 nm with an exposure time of 2 seconds.
Flow cytometry
Single cell suspensions were made from spleen, thymus, lymph nodes, bone marrow and from lavage of peritoneal cavity 24hr after injection of 1.5ml thioglycolate. For analysis of myeloid cells from spleen, organs were incubated for 30 minutes with Liberase (Sigma-Aldrich) according to manufacturer’s protocol. Cells were blocked with 10% normal mouse serum. 7-AAD (Life Technologies) was used to exclude dead cells.
Abs for the following surface markers were used in this study: CD11c (clone HL3), CD8b (clone 53-5.8), CD19 (clone 1D3), CD90.1 (clone H1S51), and FcγRI clone X54-5/7.1 (all from Becton Dickinson). CD3ε (145-2c11), B220 (RA3-6B2), and CD45.2 (clone 104) (all from eBioscience). CD4 (clone RM4-4), F4-80 (clone BM8), Ly6C (HK1.4), and Ly6G (clone 1A8) (all from Biolegend), FcgRIIb (clone Ly17.2 produced in house); FcγRIII (clone 275003, from R&D) and FcγRIV (clone 012, from Sino Biological).
Serum levels of IgG subclasses
Serum was collected from 10 months old naive FcγRI/II/III/IV-/- and WT C57Bl/6 mice.
Elisa was performed with goat anti mouse IgG (Becton Dickinson), and goat anti mouse IgG, IgG1, IgG2a or IgG2b HRP (Southern Biotech) and TMB substrate (Dako). Reaction was stopped with 1M H2SO4 and absorption was measured at 450 nm.
For antigen-specific antibody titres in serum; Mice were immunized with 50 μg TNP-BSA in 100 μl CFA (1:1 emulsion with PBS) injected s.c. at day 0 and boosted at day 14 and 28 with 25 μg TNP-BSA in 100 μl IFA (1:1 emulsion with PBS). Ab titres in sera collected at day 36 were assessed with ELISA. Streptavidin-coated 96-wells plates were incubated with 1nmol/ml biotin-BSA and blocked with 5% non-fat milk. Secondary Ab was goat-anti-mouse horseradish peroxidase (HRP). Substrate ABTS (Code no. S1599, Dako) was added, and absorption was measured at 415nmSerum levels of IgG class autoantibodies were determined using ELISA plates coated with 5μg/ml of dsDNA (Sigma-Aldrich) or 5μg/ml of histone (Sigma-Aldrich) or 4μg/ml of chromatin, respectively, as previously described (30). Serum levels of binding activities against dsDNA, histone, and chromatin were expressed in units by reference to a standard curve obtained by serial dilution of a standard serum pool from (NZB × NZW)F1 mice ages >8 months, containing 1,000 unit activities/ml. Serum levels of IgG Abs were measured using HRP conjugated anti-mouse IgG secondary Abs (Southern Biotech, Birmingham AL, USA) and detected at OD450 nm by using TMB Substrate Reagent(BD).
Infection with Salmonella live vaccine
Mice were infected with the Salmonella Typhimurium (STm) SL3261, and attenuated aroA strain (31). Live bacteria for parenteral immunization were prepared from a 16 hr. static culture of STm SL3261 in LB Broth, diluted 1/100 in PBS and administered by i.v. injection into the tail vein (~106 CFU/mouse). Actual inoculum dose was determined by plating dilutions on LB Agar.
Salmonella induces Th1 T-cell responses. IFNγ and IL2 production correlate well with T-h1 responses to Salmonella. T-cell stimulation assays and cytokine measurement after infection with Salmonella live vaccine were performed as follows: CD4+ T cells were positively enriched from spleens using magnetic bead-conjugated Abs (Miltenyi Biotec), according to manufacturer’s instructions. Purity was assessed by flow cytometry on a FACSCalibur machine (Becton Dickinson). CD4+ T cells were stimulated with Salmonella Ag or anti-CD3e (clone 145-2C11, eBioscience) and anti-CD28 (clone 37.51, eBioscience) as positive control in the presence of mitomycin C-treated (25 mg/ml; 37°C for 30 min) splenic Ag presenting cells. Salmonella Ag was alkali-treated Salmonella Typhimurium SL1344. Levels of IFN-γ and IL-2 produced at 72 hr and 24 hr were determined using Duoset ELISA kits (R & D Systems) according to manufacturer’s instructions.
Anti-LPS Abs after infection with Salmonella live vaccine were detected by ELISA as follows: Salmonella Typhimurium LPS (Sigma-Aldrich) was dissolved in water containing sodium deoxycholate (0.5% w/v). Microtitre plates (Greiner Bio-One) were coated overnight at 37°C with LPS at 5 μg/ml in carbonate buffer. Serum sample serial dilutions in PBS-Tween+1% BSA were applied in duplicate and incubated. Plates were washed and total Ab detected with HRP-conjugated goat anti-mouse Ab (Southern Biotech), detection was with SigmaFast OPD substrate (Sigma-Aldrich) with absorbance read FLUOstar Omega (BMG Labtech).
Complement analysis
Plasma samples were collected from CO2 euthanized mice via heart puncture and put directly on ice. EDTA-plasma was collected with syringes pre-treated with EDTA and tubes with final EDTA concentration of 10mM. Blood was kept on ice for 30-120 min, centrifuged twice at 3000-5000g for 10 minutes at 4°C. Samples were pooled and aliquoted to single use batches and stored at -80°C.
Measurement of functional pathway activities was performed in plasma of mice as described (32) In brief, complement activation was induced by incubation of serial dilutions in ELISA plates (Nunc Maxisorp plates, Thermo Fisher Scientific) coated with human IgM, mannan and LPS to induce Classical Pathway, Lectin Pathway and Alternative Pathway respectively. Activation of complement was either quantified at the level of C3 deposition, using an Ab directed against mouse C3b/C3c/iC3b, or at the level of C9 deposition using a rabbit anti-mouse C9 polyclonal Ab. Complement activity in the experimental samples was calculated using CD1 serum as a standard which was put at 100 AU/ml.
Complement factors in plasma were quantified using specific sandwich ELISAs. C3 was quantified in the form of C3b/C3c/iC3b as previously described. C1q was quantified using rabbit anti-mouse C1q pAb (33). Mouse properdin was measured using coating with an anti-mouse properdin mAb, and detection with Rabbit anti-mouse properdin pAb-DIG, whereas C6 and C9 were quantified using rabbit polyclonal anti-mouse C6 and rabbit polyclonal anti-mouse C9 (34,34).
Histology
Complete necropsy was performed following standard procedures. Tissues were fixed in 4% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin and evaluated by light microscopy. Histopathological analysis was performed by a European board certified veterinary pathologist. All main organs were analysed. Light microscopy pictures were taken with a DP26 Olympus camera.
Metabolic parameters
In 12 weeks-old mice, body weight was measured and lean and fat mass was assessed by MRI-based body composition analysis (Echo MRI, Echo Medical Systems, Houston, TX, USA). Blood was drawn from overnight fasted mice via tail vein into paraoxon (Sigma-Aldrich, St. Louis, MO) coated capillary tubes. After centrifugation, plasma was collected and triglyceride (TG), total cholesterol (TC), free fatty acid (FA), glucose and insulin levels were determined using commercially available kits (11488872 and 236691, Roche Molecular Biochemicals, Indianapolis; NEFA-C Wako Chemicals GmbH, Neuss, Germany; ab83390, Abcam, Cambrigde, UK; Instruchemie, Delfzijl, The Netherlands and Crystal Chem Inc., IL, USA, respectively). Indirect calorimetry measurements were performed using metabolic cages (LabMaster System, TSE Systems, Bad Homburg, Germany) as previously described (35).
FcγR expression during embryonic development
Total RNA was extracted using QIAzol (5346994; Qiagen). 1 µg of total RNA was used for reverse transcription with the RevertAid H Minus First Strand cDNA Synthesis Kit (K1632; Thermo). qRT-PCR was performed in triplicate on a C1000TM Thermal cycler (Bio-Rad) with SYBR Green (170-8887; Bio-Rad). Data was normalized to β-actin. The following primers were used:
β-Actin_F: GGCTGTATTCCCCTCCATCG; β-Actin_R: CCAGTTGGTAACAATGCCATGT;
FcγRI_F: AAGTGCTTGGTCCCCAGTC; FcγRI_R: CTGCAGCCTGTGTATTTTCA;
FcγRIIb_F: AATTGTGGCTGCTGTCACTG; FcγRIIb_R: GTTTCCTGGGAGAGCTGGA;
FcγRIII_F: TGGGGACTACTACTGCAAAGG; FcγRIII_R:AGAAATAAAGGCCCGTGTCC
FcγRIV_F: TGGAATGTACAGGTGCCAGA; FcγRIV_R: TTCCGTACAGGTCTGTTTTGC
Results
Generation of the FcγRI/II/III/IV quadruple KO mouse model
To overcome the drawbacks of the existing FcγR KO mouse models we generated a novel mouse model in C57BL/6 background deficient for all four FcγR ligand binding chains while maintaining the promiscuous FcR γ signal transduction subunit. To this end we crossed a newly generated mouse model with a 90.4 Kb deletion on chromosome 1 deficient for the FcγRII/III/IV gene cluster (Fig.1) with our previously generated mouse model with a deletion of the FcγRI gene (25) located on chromosome 3 (Fig.1 and Fig.S1). The FcγRI/II/III/IV quadruple KO (FcγRI/II/III/IV-/-) offspring developed normally and showed normal breeding characteristics. The phenotype of the FcγRI/II/III/IV-/- mouse was analyzed in a series of in vitro and in vivo assays
IgG downstream effector functions are impaired in FcγRI/II/III/IV-/- mice
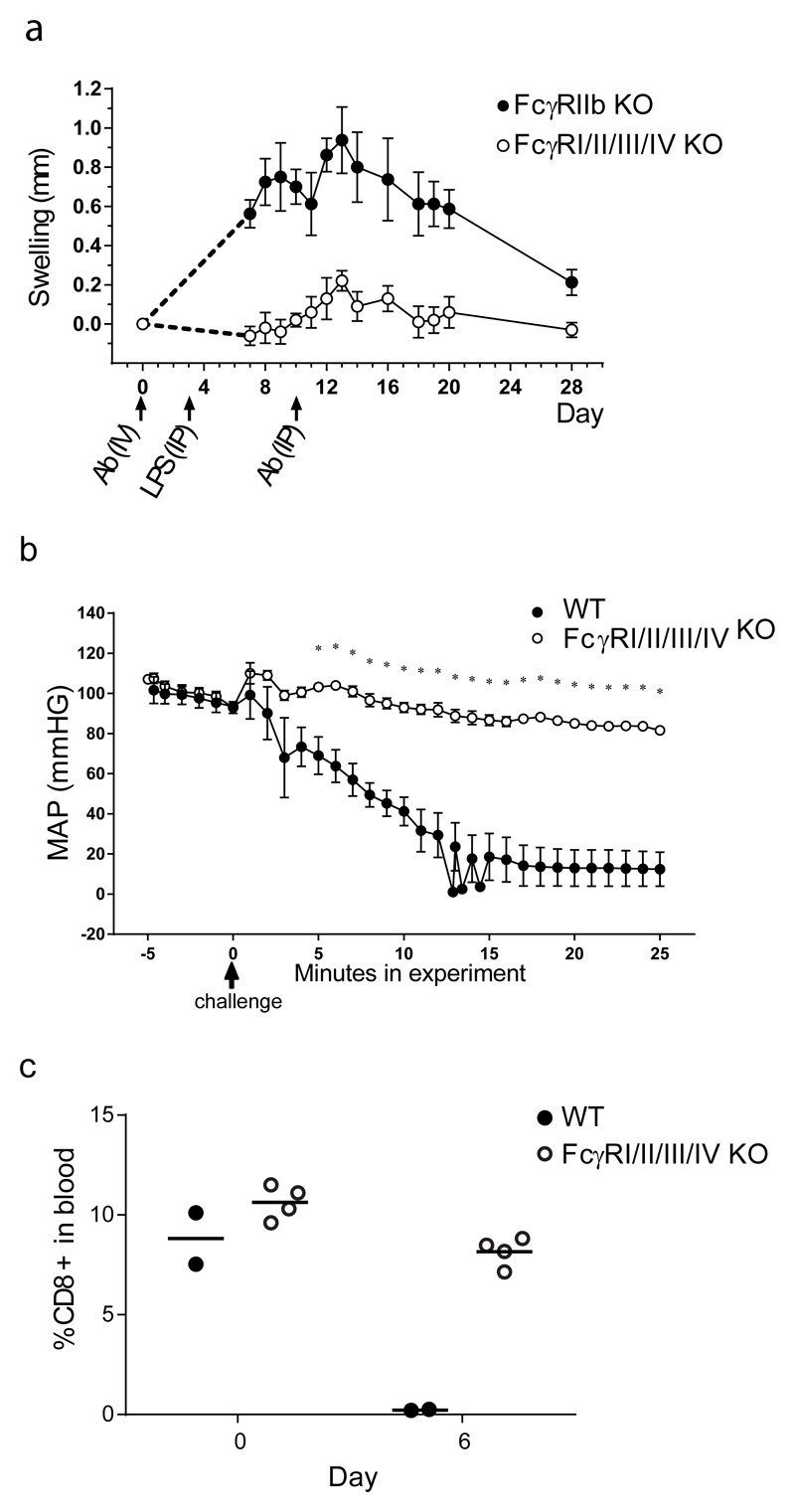
In order to confirm that the novel FcγRI/II/III/IV-/- mouse model had impaired known FcγR dependent IgG downstream effector functions, we studied IgG collagen Ab induced arthritis (CAIA), IgG induced passive systemic anaphylaxis and IgG induced antibody dependent cell mediated phagocytosis (ADCP) in these mice. As expected, FcγRI/II/III/IV-/- mice were almost completely resistant to CAIA initiated by intravenous (i.v.) injection of a cocktail of four different anti-collagen IgG Abs (Fig.2.a). CAIA cannot be induced easily in WT C57BL/6 mice, whereas FcγRIIb-/- mice are more sensitive (36). We therefore compared FcγRI/II/III/IV-/- mice, which lack FcγRIIb, with FcγRIIb-/- mice. In contrast to FcγRIIb-/- mice, FcγRI/II/III/IV-/- mice showed little footpad swelling. This confirms our previous results with K/BXN serum induced arthritis (37).
Fig.2. IgG downstream effector functions are impaired in FcγRI/II/III/IV-/- mice.
a. CAIA. From each phenotype footpad swelling was measured using a caliper. The average of combined left and right footpad swelling of the forepaws was plotted and expressed as Mean and SEM of increase in footpad thickness in mm. The area under the curve was calculated per mouse from day 7 until day 28 and a Mann-Witney test was performed for statistics. The response of the FcγRI/II/III/IV-/- mice was significantly lower compared to the response of FcγRIIb-/- mice (P=0.0159). Five mice per group. One representative experiment out of two performed is shown.
b. Passive systemic anaphylaxis. Time course of blood pressure, expressed in mm HG, as mean arterial pressure (MAP) plus SEM, in FcγRI/II/III/IV-/- and WT C57BL/6 mice passively sensitized by i.v. injection of mouse anti-TNP IgG2a, and challenged 4 hours later with DNP-HSA. Six mice per group. Each time point is analyzed by a separate T test, and the curves are significantly different (p<0.01) from 5 minutes onwards, indicated with *.
c. ADCP. Mice were injected with CD8+ depleting Ab (2.43). Before and after Ab injection, the number of CD8+ T cell in blood was determined by flow cytometry and depicted as percentage of CD8+/CD3+ cells of total lymphocyte population. Data shown are from one out of two experiments with similar results. Four FcγRI/II/III/IV-/- and two WT mice per group.
Data was statistically analyzed with a T test at each time point, p=0.15 at day 0, and 0.0001 at day 6 as compared to WT C57BL/6 mice.
FcγRI/II/III/IV-/- mice were also resistant to passive systemic anaphylaxis, induced by challenging mice, sensitized by i.v. injection of IgG2a anti-TNP, with DNP-HSA, whereas WT C57BL/6 mice were not (Fig 2.b). The in vivo phagocytosis of CD8+ T cells by ADCP after intraperitoneal (i.p.) injection of rat IgG2b anti-CD8 Ab was completely abrogated in FcγRI/II/III/IV-/- mice (Fig.2.c) Together these results confirm that in our FcγRI/II/III/IV-/- mouse model IgG downstream effector functions are strongly impaired in a variety of in vivo experimental IgG induced inflammation models.
In vivo Cross-presentation of soluble IgG-IC derived Ag is normal in FcγRI/II/III/IV-/- mice
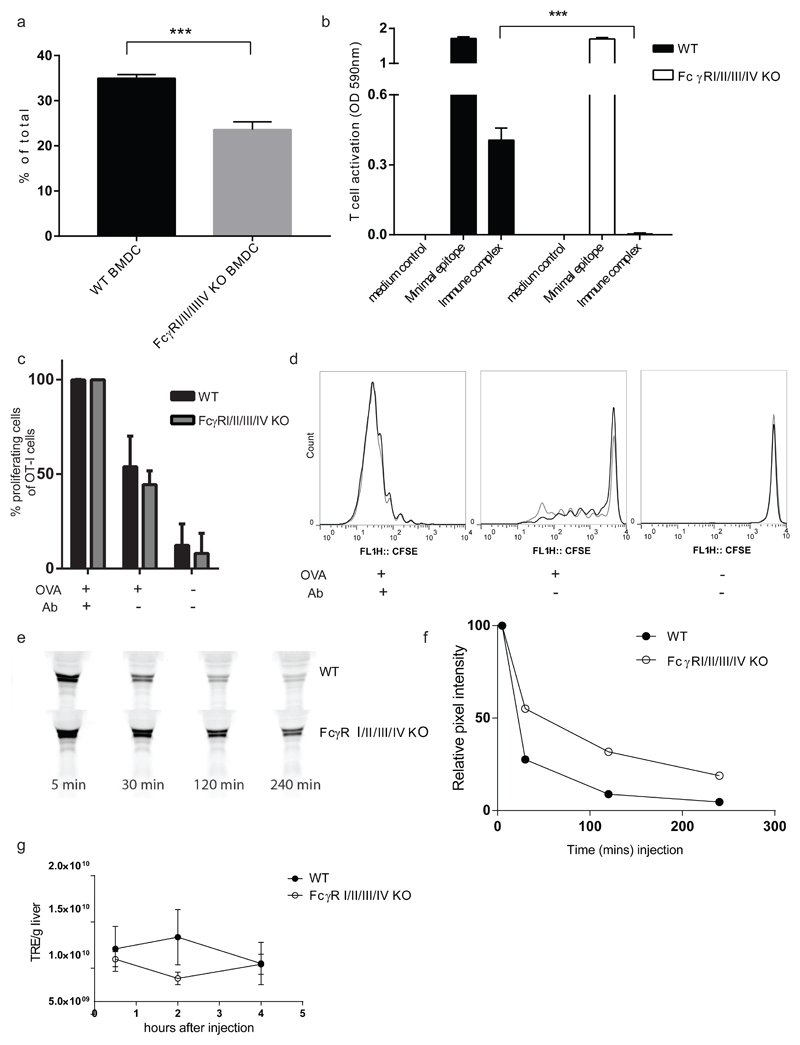
It has been demonstrated with a variety of in vitro and in vivo experiments that FcγR on Ag presenting cells facilitate the presentation of soluble IC-derived Ag to cytotoxic T cells, the process of cross presentation (12,14,15). In accordance with these observations the uptake of fluorescent labeled OVA-IgG IC by dendritic cells from FcγRI/II/III/IV-/- mice was strongly impaired in vitro compared to the uptake by dendritic cells from WT C57BL/6 mice (Fig.3.a). Moreover, as shown in Fig.3.b, the in vitro presentation of SIINFEKL peptide processed from OVA-ICs to B3Z hybridoma cells by FcγRI/II/III/IV-/- BMDCs was strongly inhibited. SIINFEKL synthetic peptide was used as control, and was presented by BMDCs from FcγRI/II/III/IV-/- and WT C57BL/6 control mice with similar efficiency indicating that MHC Class I expression was comparable between genotypes. These data show that FcγR are required for the in vitro IC uptake and subsequent MHC class I-restricted presentation of IC-derived peptides.
Fig.3. FcγR involvement in the cross-presentation of IgG-IC derived Ag.
a. Uptake of IgG-IC derived Alexa488 labeled Ag by bone-marrow derived dendritic cells (BM-DC) from WT and FcγRI/II/III/IV-/- mice measured by flow cytometry, presented as mean plus SD of three samples. Extracellular binding was quenched by the addition of trypan blue. Depicted is percentage of Alexa488 positive cells out of total cell count. One representative experiment of two experiments performed is shown, statistical analysis was performed with T test. Asterisks indicate significant differences (***p<0.001) as compared to WT C57BL/6.
b. BM-DC from FcγRI/II/III/IV-/- and WT C57BL/6 mice were incubated with OVA-IgG IC and subsequently co-cultured with T cell hybridoma B3Z which recognized an OVA-CTL epitope in MHC class I. Recognition leads to activation of the LacZ reporter gene which was measured with a β-galactosidase assay, and analyzed as absorption of light at OD590 nm. Minimal SIINFEKL OVA epitope was included as an MHC class I loaded positive control in both DC types. Presented as mean plus SEM, 4 samples per group. Statistical analysis was performed with a T test, (***p<0.001 for the mice that received immune complexes as compared to WT C57BL/6 mice
c and d. WT C57BL/6 and FcγRI/II/III/IV-/- mice were injected with CFSE labeled OT-I T cells and subsequently injected with OVA with or without anti-OVA IgG. In vivo cross presentation was determined by analyzing the CFSE dilution of OT-I cells using flow-cytometry. Depicted are percentage of proliferating OT-I cells (CFSE fluorescence is diluted at least once) of total OT-I gated cells as mean of group plus SD (c) and representative CFSE plots (d). Data shown are from one out of two experiments with similar results. Five mice per group. Statistical analysis with T test per condition revealed no differences, p=0.63, 0.15, 0.73 respectively for OVA+Ab, OVA alone and naïve mice compared to WT C57BL/6 mice.
e and f. Western blot analysis of the presence of Alexa488 labeled OVA in serum of mice at different time-points after i.v. injection of the OVA anti-OVA IgG IC (e) and quantification of fluorescent OVA in Western Blot samples (f). Data shown are representative samples from three mice per experiment. Three experiments with similar results were performed.
g. At different time-points after injection, mice were sacrificed and a single lobe of liver was isolated and imaged. Signal quantification of Alexa488 labeled IgG IC was performed. The fluorescent signal is shown as the total radiant efficiency (TRE), expressed in (photons/second)/(µW/cm2). The TRE/g liver in the time is shown. Data shown are from one out of two experiments with similar results. Three mice per group.
Surprisingly, proliferation of adoptively transferred OT-1 CD8+ T cells was indistinguishable between FcγRI/II/III/IV-/- and WT C57BL/6 control mice. No difference in CFSE division was observed after activation by IC, which were formed in situ by i.v. administration of OVA and subsequently anti-OVA Abs (Fig.3.c and d). Compared to WT C57BL/6 mice FcγRI/II/III/IV-/- mice showed a delay in clearance of IgG-IC from circulation (Fig.3.e and f) while the uptake of IgG-IC by the liver appeared to be decreased (Fig.3.g). These observations imply that in vivo, FcγR are dispensable for cross presentation of IgG-IC-derived Ag, but involved in clearance of IgG-IC from circulation.
Adaptive immunity is not impaired in FcγRI/II/III/IV-/- mice
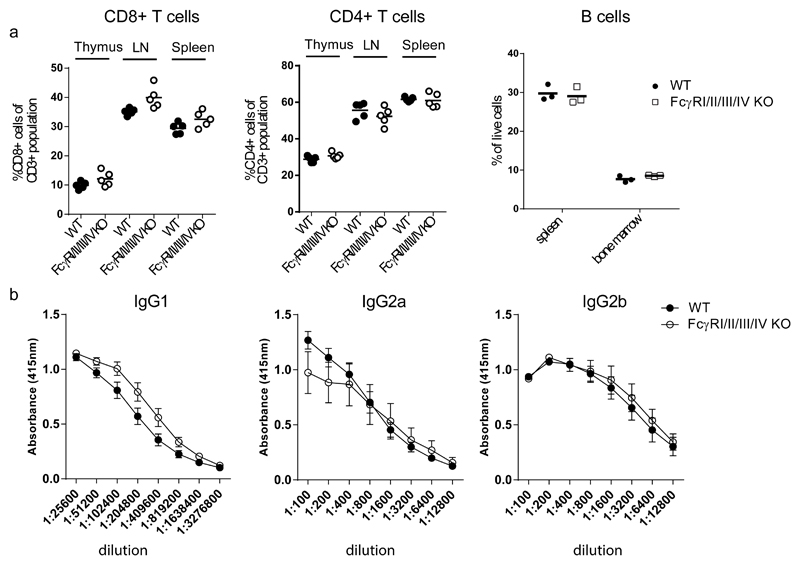
In order to further characterize the adaptive immune system in the FcγRI/II/III/IV-/- mice the B and T lymphocyte compartments were analyzed by flowcytometry using a panel of fluorescent labeled Abs specific for B and T lymphocyte surface markers. No differences in percentages of CD8+ and CD4+ T cells in the thymus, lymph nodes or spleen were found between WT C57BL/6 and FcγRI/II/III/IV-/- mice (fig.4.a). Also, CD19+ B220+ B cell numbers in the spleen and bone marrow were comparable between WT C57BL/6 and FcγRI/II/III/IV-/- mice.
Fig.4. Adaptive immune system is normal in FcγRI/II/III/IV-/- mice.
a. Lymphoid organs were harvested from two months old FcγRI/II/III/IV-/- and WT mice. Single cell suspensions were labeled with fluorescent Abs and analyzed using flow-cytometry. Each symbol represents an individual mouse. Data shown are from one out of two experiments with similar results. b. Mice were immunized three times with TNP-BSA. Serum samples were taken 8 days after first boost and 7 and 12 days after second boost. Titres of anti-BSA antibodies were determined with ELISA. Data of day 12 are shown. Other time points showed similar results. Eight mice per group, shown is mean plus SEM.
After vaccination with BSA-TNP in CFA no gross differences were observed in BSA Ag specific Ab titers between WT C57BL/6 and FcγRI/II/III/IV-/- mice except a small increase in IgG1 in the FcγRI/II/III/IV-/- mice compared to WT C57BL/6 mice (Fig. 4.b), which can be explained by the absence of FcγRIIb on B cells in FcγRI/II/III/IV-/- mice. These results are in agreement with the results of a previous experiment using a milder immunisation protocol (long synthetic peptide in saline with CpG) in our FcγRI/II/III/IV -/- mice (38) indicating that FcγRs are dispensible for T cell dependent B cell responses against a protein Ag.
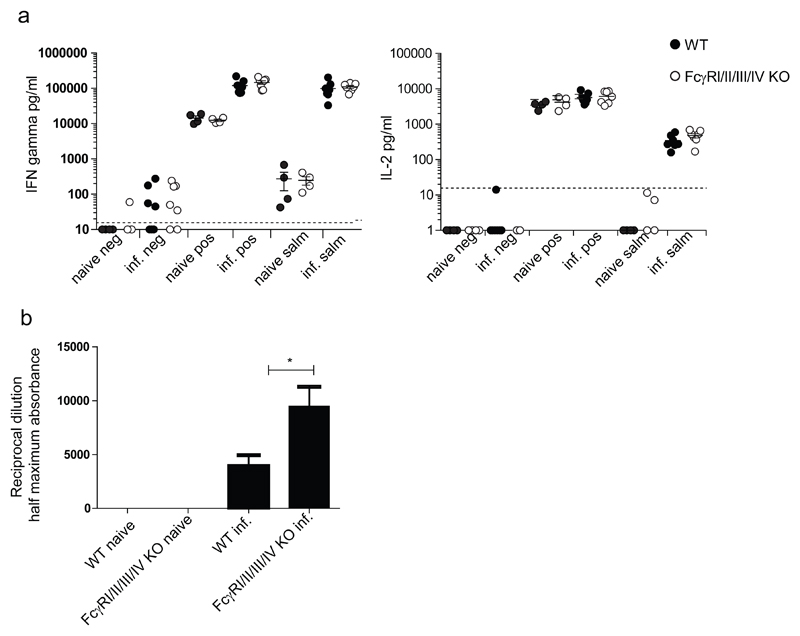
In order to test further the functionality of the adaptive immune response in FcγRI/II/III/IV-/- mice, the ability of these mice to respond to a bacterial infection was analyzed. We inoculated FcγRI/II/III/IV-/- and WT C57BL/6 mice with a live vaccine consisting of the non-virulent SL3261 attenuated aroA Salmonella Typhimurium strain, and analyzed the T and B cell responses (Fig. 5. a and b). We did not observe significant differences in the induction of T cell responses between the groups. B cell responses were not hampered in FcγRI/II/III/IV-/- mice. The higher Salmonella-specific Ab responses detected in these mice are in keeping with the absence of FcγRIIb on their B cells (Fig.5.b).
Fig.5. T and B cell responses to Salmonella infection were similar in FcγRI/II/III/IV-/- and WT control mice.
a. CD4+ T cells were positively enriched from splenocytes of groups of seven WT C57BL/6 and seven FcγRI/II/III/IV-/- mice infected 10 weeks earlier with STm SL3261. Groups of four WT C57BL/6 and four FcγRI/II/III/IV-/- naïve mice were also included in the experiment. The cells from individual mice were exposed to Salmonella Ag (salm) or anti-CD3 and anti-CD28 (pos) as a positive control, or medium (neg), as negative control. IFNγ (left panel) and IL2 (right panel) were measured in the supernatants by ELISA after 72 and 24 hours respectively. Data of one representative experiment out of two performed are shown. Statistical analysis using ANOVA did not show significant differences between WT C67BL/6 and FcγRI/II/III/IV-/-mice.
b. Anti-STm LPS Abs were measured by ELISA in the sera of groups of five WT C57BL/6 and five FcγRI/II/III/IV-/- mice infected as in fig 5a. Groups of four naïve mice were included as controls. Ab titers are expressed as the reciprocal of the dilutions giving a reading equal to half of the maximal absorbance. Data of one representative experiment out of two performed are shown. Statistical analysis was performed with T-test. Asterisks indicate significant difference. (* p< 0.05) as compared to WT C57BL/6.
Normal Ab levels and no auto-Ab formation in aging FcγRI/II/III/IV-/- mice
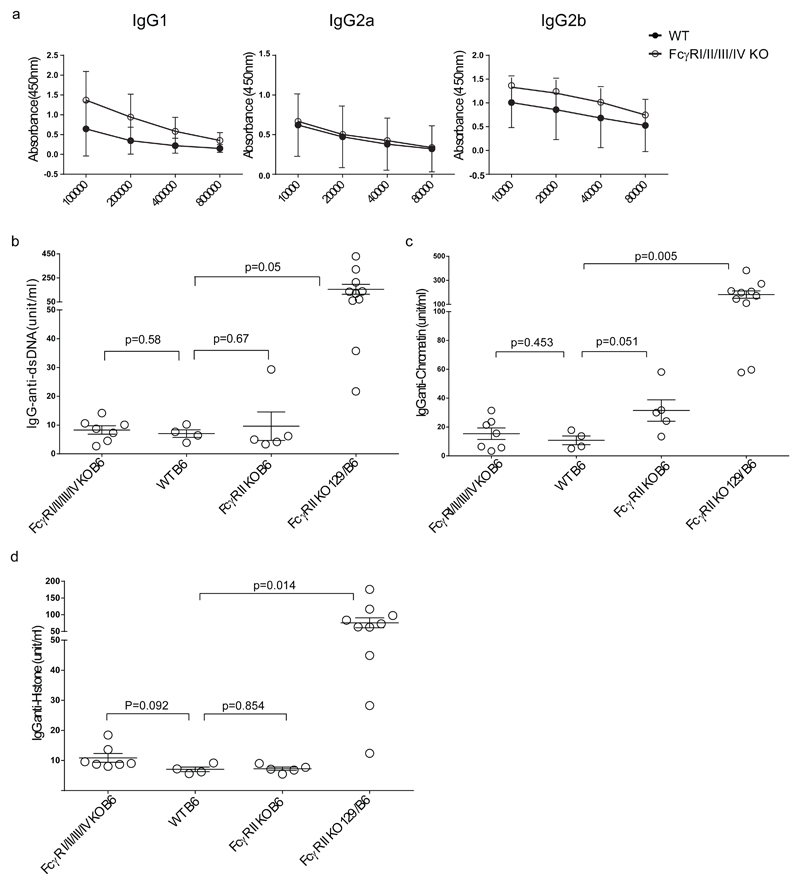
Serum titers of IgG in 10 months old naïve mice were comparable between FcγRI/II/III/IV-/- and WT C57BL/6 mice (Fig.6.a). FcγRIIb-/- mice on mixed 129/C57BL/6 background develop high anti-nuclear Ab (ANA) titers with age (17) whereas FcγRIIb-/- mice on pure C57BL/6 background hardly develop ANA (20). The FcγRII/III/IV deletion (on Chr1) has been generated in C57BL/6 derived ES cells whereas the FcγRI-/- mice were generated by gene targeting of the FcγRI gene (on Chr3) in 129 derived ES cells (25) and subsequent backcrossing into C57BL/6 background (n>12). We compared ANA titres in the serum of old FcγRI/II/III/IV-/- mice with ANA titres in old FcγRIIb-/- mice on mixed 129/C57Bl/6 background (n≥ 8) and FcγRIIb-/- mice on pure C57BL/6 background. Only FcγRIIb-/- mice generated by gene targeting in 129 derived ES cells and backcrossed into C57BL/6 background developed high ANA titres (Fig. 6.b, c and d) confirming that 129 derived FcγRIIb-flanking sequences (SLE16 on Chr1) (39) determine the development of ANA. The absence of FcγRIIb does not lower the threshold for the development of autoimmunity in FcγRI/II/III/IV-/- mice.
Fig.6. IgG titres in older FcγRI/II/III/IV-/- and WT control mice.
a. IgG1, IgG2a and IgG2b titres were determined in sera of FcγRI/II/III/IV-/- and WT C57BL/6 mice with ELISA, three mice per group. One representative experiment out of two performed is shown.
b, c and d. IgG anti-dsDNA Ab titers (b) IgG anti-Chromatin titers (c) and IgG anti Histone titers (d), were determined by ELISA using anti-mouse γ chain-specific secondary Abs. Each symbol represents a mouse. Mean and SEM are shown with respective p-values.
FcγR deficiency does not affect the development and homeostasis of the myeloid cell compartment
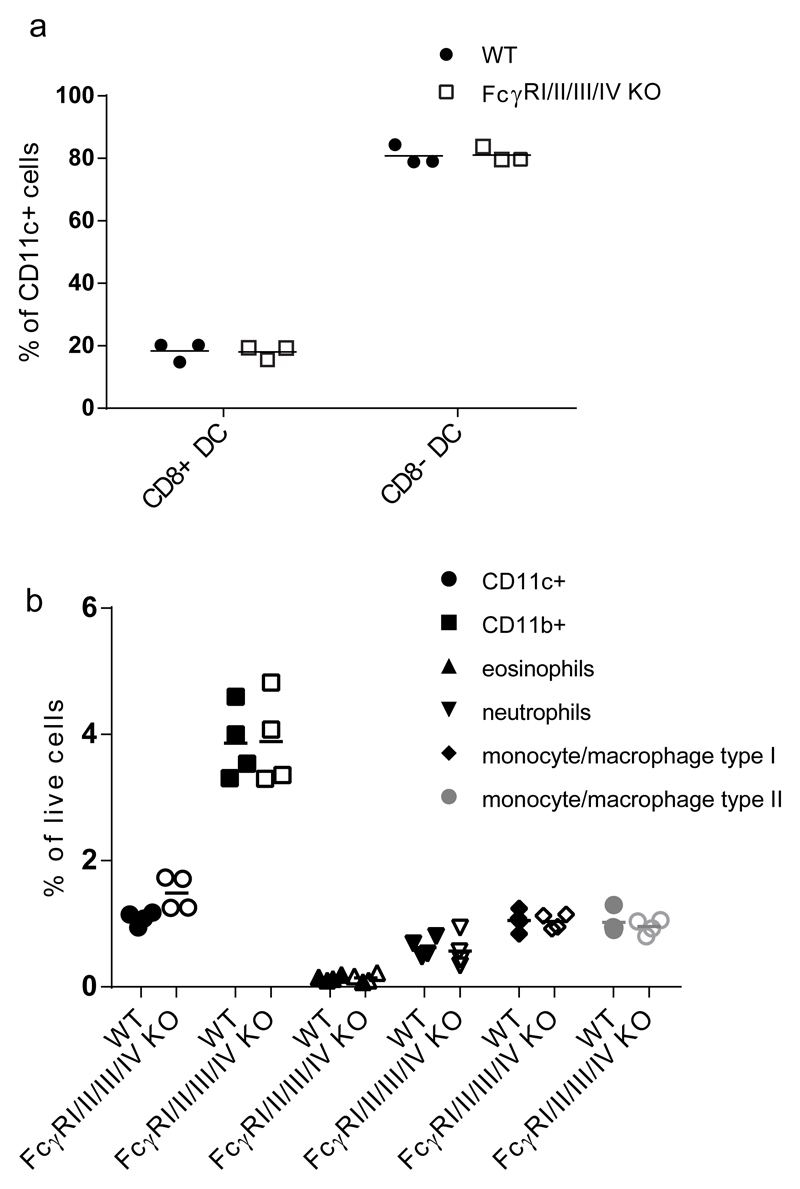
Since the adaptive immune system was not impaired in FcγRI/II/III/IV-/- mice, we focused on the innate immune system. Considering the extensive expression of FcγR on myeloid cells, we envisaged that FcγR mediated interactions might influence the development or differentiation of myeloid cells, and that the absence of these receptors in FcγRI/II/III/IV-/- mice could cause alterations in the innate immune compartment. We therefore evaluated the relative numbers of CD11c+/CD8a+ and CD11c+/CD8a- dendritic cells in bone-marrow and spleen (Fig.7.a) and the relative numbers of the different myeloid subsets in spleen, using a gating strategy described by Shawn Rose et al. (40) (Fig 7.b). We found no variances in percentages of either subset of cells between FcγRI/II/III/IV-/- and WT C57BL/6 mice. These results indicate that FcγR deficiency does not influence the development, differentiation or homeostasis of the cells on which they are normally most prominently expressed.
Fig.7. No differences in myeloid cell compartments between FcγRI/II/III/IV-/- and WT C57BL/6 control mice.
a. Spleens of three months old FcγRI/II/III/IV-/- and WT C57BL/6 mice were incubated with Liberase, and single cell suspensions were labeled and analyzed by flow-cytometry. Graphs show the percentage CD8 positive or negative cells of CD11c+/CD19-/ B220-/7-AAD- cells. Three mice per group. Data shown are from one out of two experiments with similar results.
b. Spleens of three months old FcγRI/II/III/IV-/- and WT C57BL/6 mice were incubated with Liberase, and single cell suspensions were labeled and analyzed by flow-cytometry. Gating strategy was according to Shawn Rose et al (40), in short, gated on 7-aad-/CD19-/CD3- and followed by CD11c+ for CD11c+ group, CD11b+ for CD11b+ group, F4-80 +/-/ Ly6G+ for neutrophils, F4-80+/ Ly6G-/Ly6C+/-, SSC high for eosinophils, F4-80+/ Ly6G-/Ly6C+, SSC low monocyte/macrophage type I, F4-80+ /Ly6G-/Ly6C-, SSC low for monocyte/macrophage type II. Four mice per group. Data shown are from one out of two experiments with similar results. Statistical analysis performed with Sidak’s multiple comparisons test, showed that all groups were not significantly different from each other.
No difference in complement, overall organ architecture and metabolic homeostasis between FcγRI/II/III/IV-/- and WT C57BL/6 control mice
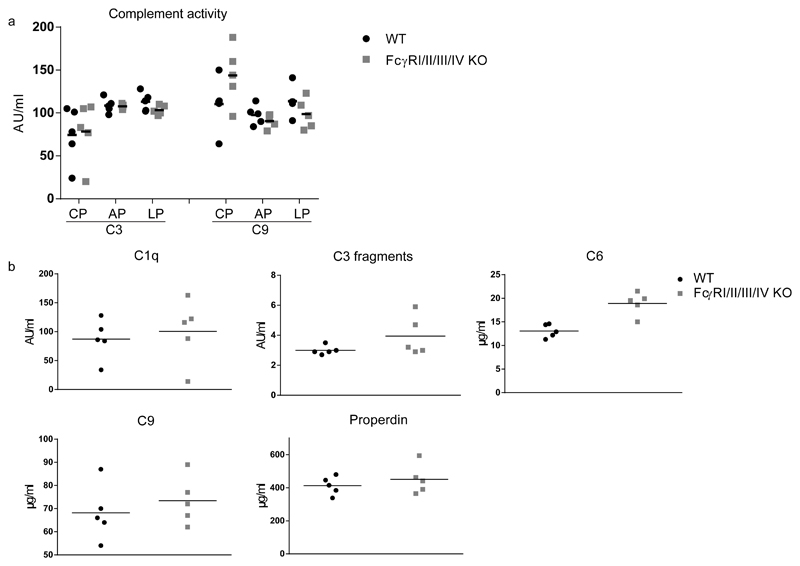
Since a direct connection between complement and FcγR effector pathway activation has been reported (41) we analyzed the complement system in FcγRI/II/III/IV-/- mice. We quantified the complement activity in an ELISA based system (32), upon initiation of the three pathways of complement activation. Both at the level of C3 and C9 deposition, there were no differences in complement activity between FcγRI/II/III/IV-/- and WT C57BL/6 mice (Fig. 8a). In line with this, also circulating levels of individual components, including C1q, properdin, activated C3 or C9 were not different between FcγRI/II/III/IV-/- mice and WT C57BL/6 control mice (Fig.8b). Circulating plasma levels of C6 were somewhat higher in FcγRI/II/III/IV-/- mice (Fig.8b), however this did not result in an increased terminal pathway complement activity (Fig.8a). Similarly, also complement activity measured at the level of C3 deposition was comparable between both groups.
Fig. 8. No differences in plasma concentrations and activity of complement factors between FcγRI/II/III/IV-/- mice and WT C57BL/6 control mice.
a. Functional complement activity in plasma of WT C57BL/6 and FcγRI/II/III/IV-/- mice was determined for all three pathways (classical (CP), alternative (AP) and lectin pathway (LP), both at the level of C3 deposition and at the level of C9 deposition. Samples were tested in serial dilutions, quantified in comparison with a standard CD1 serum and depicted as AU/ml. Five mice per group. Statistical analysis with Sidak’s multiple comparison’s test revealed no significant difference between FcγRI/II/III/IV-/-.and WT C57/BL/6 mice.
b. Individual complement factors were quantified in plasma of five mice per genotype using specific ELISAs. This includes C1q as inducer of the classical pathway, properdin as stabilizer of the alternative pathway, the central component C3 and C6 and C9 as part of the terminal pathway. Data are shown as the amounts per ml serum of the indicated factors either in μg (properdin, C6 and C9) or in (AU) arbitrary units (C1q and C3 fragment).
Furthermore, we examined histological sections of several organs, including kidney, liver, lung, and spleen of 47 weeks old female mice. In keeping with the flow cytometry data, there were no abnormalities in overall architecture of these organs detectable in the FcγRI/II/III/IV-/- mice compared to WT C57BL/6 control mice (Fig.S2). There were no differences also regarding bronchus-associated lymphoid tissue (BALT) composition in representative lung sections between the 2 groups. Glomerular and kidney pathology was also absent. In representative liver sections from both groups lymphoid aggregates were absent.
It has been postulated that the adaptive immune system, the intestine and microbiota govern a homeostatic metabolic function (42). B cells and pathogenic IgG promote insulin resistance in mice fed a high-fat diet (43). Moreover, we have recently shown that mice deficient for the FcR γ chain are protected against diet-induced obesity and insulin resistance (35), suggesting a role of activating FcR in intestinal and systemic metabolic homeostasis. Therefore, we measured a series of metabolic parameters in the FcγRI/II/III/IV-/- mice. Statistical analysis using unpaired t-tests did not reveal significant differences between measured parameters of FcγRI/II/III/IV-/- and WT C57BL/6 control mice. (Table S1).
Discussion
The novel mouse model presented here is the first C57BL/6 model exclusively and completely deficient for all four FcγR. This enabled us for the first time to study the consequences of complete FcγR deficiency without confounding factors. The phenotype of the FcγRI/II/III/IV-/- mouse demonstrates the dominant role of FcγR in IgG downstream effector pathways whereas complement is dispensable. These results confirm older studies with single, double or triple FcγR KO mouse strains or FcR γ-/- mice, lacking all three activating receptors. However, the overall immune system of the FcγRI/II/III/IV-/- mice was surprisingly normal. Lymphoid organs, subsets of lymphoid and myeloid cells, complement and metabolic homeostasis were comparable to WT mice.
Mammals are exposed to the Abs of their mother before birth. Also, before birth they have developed immune cells expressing a variety of FcγR (Fig.S3) which can directly interact with the Fc part of these Abs. The high affinity FcγRI binds monomeric IgG whereas the other FcγR are low affinity receptors which bind immune complexes. After birth the animals develop their own Abs in response to threats from the outside world. The lack of aberration in the phenotype of the FcγRI/II/III/IV-/- mouse suggests that the absence of all FcγR has little impact on the ontogeny and functionality of the immune system of these mice except their downstream Ab-mediated inflammatory effector functions.
Biological systems have a strong tendency to bypass a blockade in development and functionality by adaptation (44). The complement system and FcγR are redundant in the downstream effector pathways of IgG. However, the loss of FcγR was not compensated by increased activity of the complement system. We did not find indications for other compensation mechanisms in FcγRI/II/III/IV-/- mice.
Surprisingly, in vivo cross presentation of IgG-IC derived protein Ag, the adaptive immune response against Salmonella and the antibody response against a model antigen were almost indistinguishable between FcγRI/II/III/IV-/- and WT mice. This suggests strong redundancy in the involved adaptive immune pathways.
The role of FcγR in cross presentation is still puzzling. We and others (11–14) have shown that the cross presentation by DCs, in vitro loaded with IgG-IC, is FcR γ chain dependent. In contrast, more recently we demonstrated that the enhanced in vivo cross presentation of protein Ag derived from injected pre-formed IgG-IC is partially but not crucially dependent on the FcR γ chain (22) which is in line with previous data. Den Haan and Bevan showed that in the absence of FcR γ chain the uptake of i.v. injected IgG-IC and enhanced cross presentation by DC was not impaired (45). An obvious explanation for this discrepancy is that an in vivo dominant FcR γ chain independent - most likely complement dependent - pathway is bypassed by loading DCs with IC in vitro. Our cross-presentation experiments with the FcγRI/II/III/IV-/- mice directly demonstrated that the IgG mediated enhanced cross presentation by DC in vitro loaded with IgG-IC is exclusively dependent on FcγR and not on other FcR γ chain associated receptor molecules. In contrast in vivo, FcγRs are not required, neither the inhibiting FcγRIIB nor the activating FcγRs. For the uptake of exogenous Ag DCs display besides FcγR a large variety of receptors such as c-type lectin receptors, TLR and complement receptors. We have found a pivotal role for C1q in the presentation of Ag derived from i.v. administered IgG-IC to CD8+ T cells in vivo (22) indicating that the complement system provides alternative pathways in IgG-dependent cross presentation.
Remarkably, in comparison to WT control mice, FcγRI/II/III/IV-/- mice were not hampered in B cell responses but rather developed somewhat higher Ag-specific Ab titres upon immunization. This is not in agreement with the previous observation that FcR γ chain KO mice, lacking functional expression of all three activating receptors, develop lower Ag specific Ab titers compared to WT mice (10). The discrepancy between these results might be explained by the use of different FcγR deficient mouse strains. The FcR γ chain is associated with at least nine other receptor complexes (18). Therefore, FcR γ chain deficiency might cause a more pleiotropic effect in immunity than FcγRI/II/III/IV deficiency. On the other hand, the FcγRI/II/III/IV-/- mouse lacks not only the activating FcγR but also the regulatory inhibiting FcγRIIb. The higher IgG titres in FcγRI/II/III/IV-/- mice suggest that the Ab response in these mice is affected by the deficiency of FcγRIIb on B cells, but not the deficiency of the three activating FcγR expressed on APCs. These data indicate that activating FcγRs on antigen presenting cells (APCs) are not required for the development of a full- blown antibody response by facilitating presentation of IC derived Ag resulting in efficient priming of Th cell responses as was suggested by previous results with FcR γ chain KO mice (10).
Combined, our data suggest that in vivo the role of activating FcγR in the regulation of adaptive immunity by facilitating APC mediated presentation of IgG-IC derived Ag is dispensable. The role of FcR has been implicated in enhancing an anti-tumor response by facilitating antigen-presentation of IC derived tumor antigen after anti-tumor antibody therapy (46,47). In light of our findings, indicating that other FcγR-independent mechanisms play a dominant role, most likely complement associated, we propose to study IgG-IC mediated immune modulation in more detail in our FcγRI/II/III/IV-/- mice, as it is the first C57BL/6 model in which these questions can be answered without confounding factors. The use of antibodies with a mutation in their Fc domain, destroying FcγR binding without affecting interactions with complement, is limited to passive models (3), whereas our FcγRI/II/III/IV-/- mouse enables to study active models such as vaccination and infection.
Supplementary Material
Acknowledgements
The authors would like to thank Amanda Pronk for technical assistance, the Drs Alies Snijders and Mark Cragg for critical reading the manuscript, Dr. Ron Wolterbeek for support with the statistical calculation and the personnel of the animal facility (PDC) at the LUMC for excellent animal care.
Grant support: The work was supported by: Dutch Cancer Society UL 2014-6828 (M.F.F. and M.C. and J.W.K.); STW project 10412 (W. van M.); EU grant FP7 MCA-ITN 317445 (H. S. S. and H. B.); Grant-in-Aid (26460493) from the Ministry of Education, Science, Technology, Sports and Culture of Japan (Q.L.); Grant-in-Aid (15K08432) from the Ministry of Education, Science, Technology, Sports and Culture of Japan and a grant for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (S.H.); The Swedish Foundation for Strategic Research (RH), the Knut and Alice Wallenberg foundation (RH) and the Swedish Research Council (RH); Grant number BB/I002189/1 from the United Kingdom Biotechnology and Biological Sciences Research Council (C.C.); Grant from the Center of Medical Systems Biology (CMSB) (L. van B.) LUMC fellowship (L.D. and V. van H.)
Footnotes
The authors have no financial conflict of interest.
Reference List
- 1.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 2.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Romain G, Yan W, Watanabe M, Charab W, Todorova B, Lee J, Triplett K, Donkor M, Lungu OI, Lux A, et al. IgG Fc domains that bind C1q but not effector Fcgamma receptors delineate the importance of complement-mediated effector functions. Nat Immunol. 2017;18:889–898. doi: 10.1038/ni.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 5.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 6.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 8.Heyman B. Antibodies as natural adjuvants. Curr Top Microbiol Immunol. 2014;382:201–219. doi: 10.1007/978-3-319-07911-0_9. [DOI] [PubMed] [Google Scholar]
- 9.Hjelm F, Carlsson F, Verbeek S, Heyman B. IgG3-mediated enhancement of the antibody response is normal in Fc gammaRI-deficient mice. Scand J Immunol. 2005;62:453–461. doi: 10.1111/j.1365-3083.2005.01684.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamano Y, Arase H, Saisho H, Saito T. Immune complex and Fc receptor-mediated augmentation of antigen presentation for in vivo Th cell responses. J Immunol. 2000;164:6113–6119. doi: 10.4049/jimmunol.164.12.6113. [DOI] [PubMed] [Google Scholar]
- 11.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol. 2002;168:2240–2246. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 13.Yada A, Ebihara S, Matsumura K, Endo S, Maeda T, Nakamura A, Akiyama K, Aiba S, Takai T. Accelerated antigen presentation and elicitation of humoral response in vivo by Fcgamma. Cell Immunol. 2003;225:21–32. doi: 10.1016/j.cellimm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 15.Platzer B, Stout M, Fiebiger E. Antigen cross-presentation of immune complexes. Front Immunol. 2014;5:140. doi: 10.3389/fimmu.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 17.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 18.Fodor S, Jakus Z, Mocsai A. ITAM-based signaling beyond the adaptive immune response. Immunol Lett. 2006;104:29–37. doi: 10.1016/j.imlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Boross P, Arandhara VL, Martin-Ramirez J, Santiago-Raber ML, Carlucci F, Flierman R, van der Kaa J, Breukel C, Claassens JW, Camps M, Lubberts E, et al. The inhibiting Fc receptor for IgG, FcgammaRIIB, is a modifier of autoimmune susceptibility. J Immunol. 2011;187:1304–1313. doi: 10.4049/jimmunol.1101194. [DOI] [PubMed] [Google Scholar]
- 21.van Montfoort N, Mangsbo SM, Camps MG, van Maren WW, Verhaart IE, Waisman A, Drijfhout JW, Melief CJ, Verbeek JS, Ossendorp F. Circulating specific antibodies enhance systemic cross-priming by delivery of complexed antigen to dendritic cells in vivo. Eur J Immunol. 2012;42:598–606. doi: 10.1002/eji.201141613. [DOI] [PubMed] [Google Scholar]
- 22.van Montfoort N, de Jong JM, Schuurhuis DH, van der Voort EI, Camps MG, Huizinga TW, van Kooten C, Daha MR, Verbeek JS, Ossendorp F, Toes RE. A novel role of complement factor C1q in augmenting the presentation of antigen captured in immune complexes to CD8+ T lymphocytes. J Immunol. 2007;178:7581–7586. doi: 10.4049/jimmunol.178.12.7581. [DOI] [PubMed] [Google Scholar]
- 23.Mahler CM, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, Raspa M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014;48:178–192. doi: 10.1177/0023677213516312. [DOI] [PubMed] [Google Scholar]
- 24.Boross P, van Lent PL, Martin-Ramirez J, van der Kaa J, Mulder MH, Claassens JW, van den Berg WB, Arandhara VL, Verbeek JS. Destructive arthritis in the absence of both FcgammaRI and FcgammaRIII. J Immunol. 2008;180:5083–5091. doi: 10.4049/jimmunol.180.7.5083. [DOI] [PubMed] [Google Scholar]
- 25.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, Roozendaal R, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 26.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28:543. doi: 10.1016/j.ccell.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shashidharamurthy R, Bozeman E, Patel J, Kaur R, Meganathan J, Selvaraj P. Analysis of cross-species IgG binding to human and mouse Fcgamma receptors (FcγRs) (138.29. J Immunol. 2010 Apr 1;184(1 Supplement):138.29. [Google Scholar]
- 29.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Fujio K, Jiang Y, Zhao J, Tada N, Sudo K, Tsurui H, Nakamura K, Yamamoto K, Nishimura H, Shira T, et al. Dissection of the role of MHC class II A and E genes in autoimmune susceptibility in murine lupus models with intragenic recombination. Proc Natl Acad Sci U S A. 2004;101:13838–13843. doi: 10.1073/pnas.0405807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 32.Kotimaa JP, van Werkhoven MB, O'Flynn J, Klar-Mohamad N, van GJ, Schilders G, Rutjes H, Daha MR, Seelen MA, van Kooten C. Functional assessment of mouse complement pathway activities and quantification of C3b/C3c/iC3b in an experimental model of mouse renal ischaemia/reperfusion injury. J Immunol Methods. 2015;419:25–34. doi: 10.1016/j.jim.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Trouw LA, Seelen MA, Duijs JM, Benediktsson H, van Kooten C, Daha MR. Glomerular deposition of C1q and anti-C1q antibodies in mice following injection of antimouse C1q antibodies. Clin Exp Immunol. 2003;132:32–39. doi: 10.1046/j.1365-2249.2003.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotimaa J, Klar-Mohammad N, Gueler F, Schilders G, Jansen A, Rutjes H, Daha MR, van Kooten C. Sex matters: Systemic complement activity of female C57BL/6J and BALB/cJ mice is limited by serum terminal pathway components. Mol Immunol. 2016;76:13–21. doi: 10.1016/j.molimm.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 35.van Beek L, Vroegrijk IO, Katiraei S, Heemskerk MM, van Dam AD, Kooijman S, Rensen PC, Koning F, Verbeek JS, Willems van Dijk K, van Harmelen V. FcRgamma-chain deficiency reduces the development of diet-induced obesity. Obesity (Silver Spring) 2015;23:2435–2444. doi: 10.1002/oby.21309. [DOI] [PubMed] [Google Scholar]
- 36.Nandakumar KS, Andren M, Martinsson P, Bajtner E, Hellstrom S, Holmdahl R, Kleinau S. Induction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIB. Eur J Immunol. 2003;33:2269–2277. doi: 10.1002/eji.200323810. [DOI] [PubMed] [Google Scholar]
- 37.Hobday PM, Auger JL, Schuneman GR, Haasken S, Verbeek JS, Binstadt BA. Fcgamma receptor III and Fcgamma receptor IV on macrophages drive autoimmune valvular carditis in mice. Arthritis Rheumatol. 2014;66:852–862. doi: 10.1002/art.38311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quakkelaar ED, Fransen MF, van Maren WW, Vaneman J, Loof NM, van Heiningen SH, Verbeek JS, Ossendorp F, Melief CJ. IgG-mediated anaphylaxis to a synthetic long peptide vaccine containing a B cell epitope can be avoided by slow-release formulation. J Immunol. 2014;192:5813–5820. doi: 10.4049/jimmunol.1302337. [DOI] [PubMed] [Google Scholar]
- 39.Bygrave AE, Rose KL, Cortes-Hernandez J, Warren J, Rigby RJ, Cook HT, Walport MJ, Vyse TJ, Botto M. Spontaneous autoimmunity in 129 and C57BL/6 mice-implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, Fraser-Liggett C, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudmann DG. On-target and off-target-based toxicologic effects. Toxicol Pathol. 2013;41:310–314. doi: 10.1177/0192623312464311. [DOI] [PubMed] [Google Scholar]
- 45.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.