Abstract
In this paper we describe the use of Cu-mediated [18F]fluorodeboronation for the automated production of PET radiotracers suitable for clinical use. Two recurrent issues with the method, low radiochemical conversion (RCC) on automation and protoarene byproduct purification issues, have been successfully addressed. The new method was utilized to produce sterile injectable doses of [18F]-(±)-IPMICF17, a PET radiotracer for tropomyosin receptor kinase B/C, using an automated synthesis module. Product was isolated in 1.9 ± 0.1 % isolated radiochemical yield, excellent radiochemical purity (>99%) and high specific activity (5294±1227 Ci/mmol). Quality control testing confirmed doses were suitable for clinical use.
Keywords: late-stage fluorination, fluorine-18, F18, automation, radiochemistry, automated radiosynthesis, PET chemistry
Graphical abstract
This paper describes the use of Cu-mediated [18F]fluorodeboronation for the automated production of PET radiotracers for clinical use. Two recurrent issues with the method, low radiochemical conversion (RCC) on automation and protoarene byproduct purification issues, have been successfully addressed. The new method was utilized to produce sterile injectable doses of [18F]-(±)-IPMICF17, a PET radiotracer for tropomyosin receptor kinase B/C, using an automated synthesis module that are suitable for in vivo clinical PET imaging.
1. Introduction
The use of arylboron compounds as precursors for radiofluorination has increased the scope of late-stage radiofluorination to include electron neutral and rich arenes (and related substrates) [1, 2]. This, in turn, has increased the number of accessible scaffolds that can be radiofluorinated to generate radiotracers for use in functional positron emission tomography (PET) imaging. Based on pioneering work conducted by the Sanford lab [3], our recent report demonstrated the copper-mediated nucleophilic radiofluorination of arylboron species, including boronic acids, pinacol boronate esters and BF3 salts, can be achieved in good radiochemical conversion (RCC; % of integrated area corresponding to product versus total radioactivity in a radio-TLC trace) [2]. The reaction utilizes a small quantity of aryl boron precursor (4 μmol), which facilitates adaption of the chemistry for automation and purification by semi-preparative HPLC and solid phase extraction (SPE). Subsequently, we and others have also optimized this chemistry further, including exploring substrate scope, functional group tolerance and developing alternate labeling conditions [4, 5, 6, 7, 8]. However, most of these reports have conducted the majority of the chemistry in a “manual” format, wherein a stock solution of nucleophilic [18F]fluoride is divided into aliquots and used in multiple reactions carried out in sealed vials or in a synthesis module.
These proof-of-concept studies encouraged us to automate the reaction to facilitate its use with starting radioactivity required for production of clinical radiotracer doses. Such syntheses typically utilize multiple Curies of cyclotron-produced [18F]fluoride (~1 – 30 Ci or 37 – 1110 GBq), and safety requirements at these scales usually preclude manual manipulation. Instead, for such production runs [18F]fluoride is purified, azeotropically dried, reacted with the precursor, purified by semi-preparative HPLC and/or SPE, and formulated as a sterile isotonic injectable dose, all using an automated synthesis module housed in a commercial “hot-cell” that provides adequate shielding and containment. Safety and current Good Manufacturing Practice (cGMP) standards require automated methods with data tracking, so it is of utmost importance that any new chemistry, such as the [18F]fluorination of organoborons, be validated according to the pertinent regulations (e.g. 21CFR212 in the United States) prior to synthesizing clinical doses [9]. Adapting a manual method for automation can present several challenges including: differences in the presence/absence of atmospheric air and/or water (e.g. automated synthesis module reactors are usually under an inert atmosphere, while manual reactions on the bench are often performed under ambient atmosphere); [18F]fluoride source including the fluoride processing reagents like K2CO3 or, in our case, KOTf (as stated above, automated reactions frequently require larger amounts of such reagents); the total activity of [18F]fluoride used (typically automated reactions employ Ci amounts, while manual reactions employ mCi or μCi amounts); and the presence of leftover reactants and/or reaction byproducts that need to be removed during purification of the PET radiotracer. All of these issues can potentially lead to significant differences in observed manual RCC and the isolated radiochemical yield (RCY) achieved upon automation. For example, in our initial efforts to automate our radiofluorination of boronic acids and pinacol boronate esters, two complicating factors were quickly identified: A) we noted a reduction in RCC when azeotropically dried fluoride was used instead of the [18F]fluoride stock solution used in manual reactions, and B) we observed the formation of a protoarene impurity (formed via protodeboronation) that often co-eluted with the [18F]fluoroarene and thus complicated HPLC purification of radiotracers. This paper reports our efforts to address these two issues and thereby enable the routine automated production of clinical radiotracer doses using our previously published Cu-mediated radiofluorination of arylborons. Proof-of-concept is demonstrated through the synthesis of [18F]-(±)-IPMICF17 (2-F), a radiolabeled inhibitor of tropomyosin receptor kinase B/C (TrkB/C) that is currently being developed for PET imaging of TrkB/C [10, 11].
2. Experimental
Full details of (radio)synthesis procedures and quality control methods, as well as all associated analytical data, can be found in the Supporting Information.
2.1 General Radiofluorination Details
Fluorine-18 was produced via the 18O(p,n)18F nuclear reaction using a GE PETTrace cyclotron. The [18F]fluoride was then processed and either employed in manual reactions, or automated syntheses using a TRACERLab FXFN radiochemistry synthesis module, according to the methods described in the Supporting Information. Total recovered activity at end-of-synthesis was measured with a Capintec dose calibrator.
2.2 Quality Control Analysis
Radiochemical reactions were analyzed by radio-TLC and/or HPLC using a Bioscan AR-2000 TLC scanner or Shimadzu LC-2010A HT system equipped with a Bioscan B-FC-1000 radiation detector, respectively, according to the methods described in the Supporting Information.
3. Results and Discussion
The general decrease (and unpredictability) in RCC, when copper-mediated fluorodeborylation is automated in a synthesis module, along with the formation of the protoarene side product, were first noted in our earlier report [2]. While we note that several groups have since used this chemistry as-is to produce a number of different radiotracers [12, 13, 14, 15], we nevertheless felt that these two issues left opportunities for improvement. As such, we have focused upon optimizing the chemistry for routine use in the automated production of doses in high yield, purity, and specific activity.
The primary differences between reactions conducted manually in vials using batch-produced [18F]fluoride versus those within an automated synthesis module, are: A) the general absence of atmospheric air in a synthesis module due to the use of argon or helium push gas, and B) the reaction in a synthesis module starts off with cyclotron-produced [18F]fluoride that is dried alongside a significant amount of eluent salt (typically K2CO3, and in our case KOTf), which is not dissolved prior to addition of solutions of reactants and reagents. The need for atmospheric air (dioxygen) in our method was ruled out in our prior report [2], and thus could not account for the difference in reactivity between manual and automated reactions. Thus, we hypothesized that problems were originating from differences in [18F]fluoride preparation between manual and automated processes.
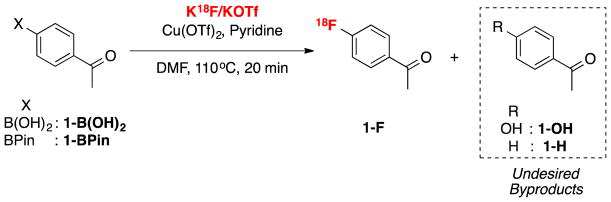
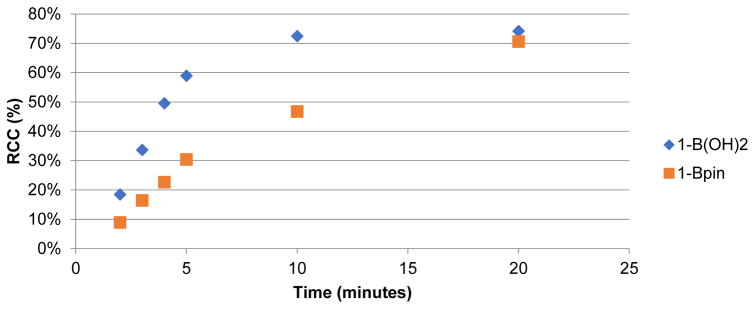
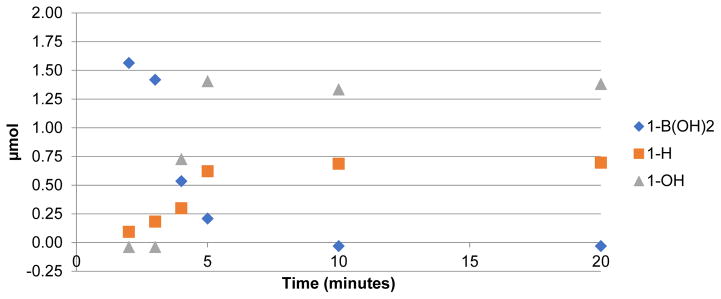
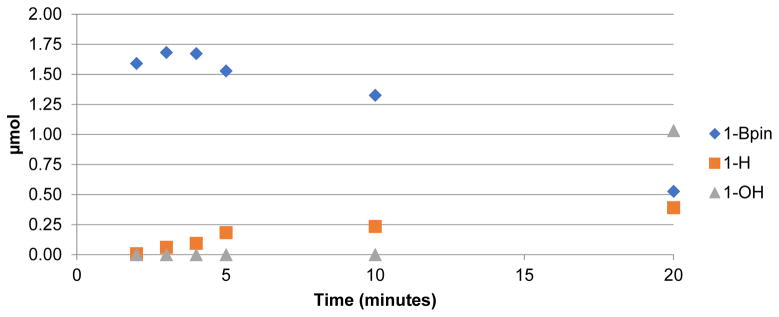
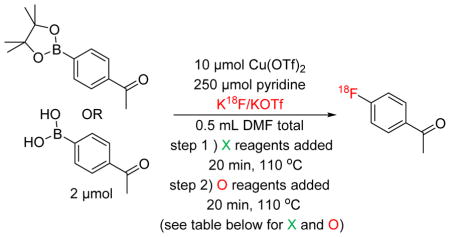
The high concentration of QMA eluent salt represents one possible origin of the lower yields. During an automated run, 5 mg of KOTf is present, and this represents only ~1/50th of that present during a typical manual reaction. To test for this possibility, we conducted manual reactions run with concurrent addition of 5 mg KOTf. However, as shown in Supporting Information S3.3.3, pg S10, the RCCs were nearly identical (74% vs 75% for 1B(OH)2; 75% vs. 78% for 1Bpin). Therefore, the form of the [18F]fluoride reagent in the reaction ([18F]KF) was implicated. In considering the reaction further, we hypothesized that the only way product formation could be significantly impacted by the use of dry [18F]fluoride instead of dissolved [18F]fluoride is if dissolution of dry [18F]KF occurred more slowly than the conversion of the arylboron precursor into non-fluorinated byproducts. To investigate this, a time study was conducted, wherein manual reactions in vials were heated and removed at various timepoints during the 20 min reaction time. The synthesis of [18F]4-acetophenone ([18F]FAP, 1-F) from 4B(OH)2-acetophenone (1-B(OH)2) or 4Bpin-acetophenone (1-Bpin) was used as the test reaction in this study for consistency with our earlier reports [2,4]. In addition to RCC to 1-F, concurrent HPLC analysis was also conducted to identify and quantify the byproducts formed (protoarene (1-H) and phenol (1-OH) impurities, Scheme 1). This experiment showed that when using 1-B(OH)2, the majority of radiofluorination occurred within the first 5 min, with only a modest increase in RCC between 5 and 10 mins, and even less between 10 and 20 mins (Figure 1 and Supporting Information, S.3.3.1, pg S6). Furthermore, the amount of precursor quickly decreased, while the proportion of both impurities increased (Figure 2). Both the rapid increase in RCC and consumption of starting material was less pronounced with 1Bpin (Figures 1 and 3).
Scheme 1.
Radiofluroination of 1-B(OH)2 and 1-Bpin
Figure 1.
RCC over time for radiofluorination of 1-B(OH)2 and 1-Bpin
Figure 2.
Substrate consumption and byproduct production in the radiofluorination of 1-B(OH)2
Figure 3.
Substrate consumption and byproduct production in the radiofluorination of 1-Bpin
To determine whether the consumption of precursor 1-B(OH)2 prior to the dissolution of [18F]KF could adversely affect RCC to the degree observed upon automation, all of the reactants except for [18F]fluoride were heated at 110 °C for 20 min. The [18F]fluoride stock was then added to the solution, and the reaction was re-heated for 20 min at 110 °C. This showed a very pronounced decrease in RCC vs. the control fluorination (1% vs. 73%). As with the time course experiment, the effect was less pronounced for 1-Bpin, with a much smaller decrease in RCC vs. control (34% vs. 52%). This experiment shows that side reactions could indeed be limiting the RCC of the [18F]fluorination if they occur prior to addition of [18F]fluoride to the reaction (or in the case of automated synthesis, prior to dissolution of azeotropically dried [18F]fluoride). Based on these findings, a possible solution to the problem of diminished RCC was to improve the solubility of the azeotropically dried [18F]KF. Interestingly, prior efforts to improve solubility of [18F]KF in these types of reactions using phase transfer catalysts such as kryptofix 2.2.2 or 18-crown-6 have been largely ineffective, providing products in the same (or even diminished) RCCs when they are included. We therefore considered pre-dissolving the azeotropically dried [18F]KF prior to addition of the remaining reactants or, alternately, identifying a more soluble form of [18F]fluoride.
A series of reactions were conducted where [18F]KF was pre-mixed with various reactants, heated for 20 min at 110 °C, and then mixed with any remaining reactants and reheated for an additional 20 min (Table 1, Entries 1 – 8). In a parallel study, various combinations of reagents were pre-mixed and heated for 20 min, followed by the addition of [18F]KF and any remaining reagents/reactants for a second round of heating (Table 1, Entries 9 – 16). The two studies simulated the results of an automated pre-dissolution step in the presence/absence of different reagents/reactants.
Table 1.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| pyridine | X | O | X | X | O | O | X | O |
| Cu(OTf)2 | X | X | O | X | X | O | O | O |
| precursor | X | X | X | O | O | X | O | O |
| [18F]KF stock | X | X | X | X | X | X | X | X |
|
| ||||||||
|
%RCC 1-Bpin |
63±10% | 14±20% | 51±16% | 47±6% | 12±5% | 48±18% | 32±15% | 23±2% |
|
| ||||||||
|
%RCC 1-B(OH)2 |
63±11% | 2±0% | 55±16% | 57±4% | 13±7% | 52±10% | 51±10% | 47±17% |
|
| ||||||||
| Entry | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|
| ||||||||
| pyridine | X | O | X | X | O | O | X | O |
| Cu(OTf)2 | X | X | O | X | X | O | O | O |
| precursor | X | X | X | O | O | X | O | O |
| [18F]KF stock | O | O | O | O | O | O | O | O |
|
| ||||||||
|
%RCC 1-Bpin |
28±6% | 1±0% | 55±26% | 50±3% | 16±8% | 46±13% | 43±23% | 27±3% |
|
| ||||||||
|
%RCC 1-B(OH)2 |
5±4% | 1±0% | 54±17% | 58±10% | 28±19% | 54±14% | 46±19% | 40±16% |
Procedure: step 1) Added
 ; stirred at 110 °C for 20 min; cooled; step 2) added
; stirred at 110 °C for 20 min; cooled; step 2) added
 ; stirred at 110 °C for 20 min; cooled; radio TLC;
; stirred at 110 °C for 20 min; cooled; radio TLC;
RCCs are given as mean ± standard deviation, n = 3.
The results of these order of addition studies were similar to those for a variation of this reaction that we recently reported [4], and are summarized in Table 1. The data demonstrate the importance of addition order due to the dissolution of dried [18F]KF in the automated reaction method. The highest RCCs for both substrates were obtained by mixing everything together well (Entry 1). Pre-mixing [18F]KF with precursor + pyridine (Entry 3), precursor and Cu(OTf)2 (Entry 4), and either precursor (Entry 6) or pyridine (Entry 7) alone, followed by the addition of the remaining reagents, also provided reasonable RCCs. The other permutations had variable degrees of success and appeared to be substrate-dependent. For example, premixing [18F]KF in neat DMF without other reactants worked well for 1-B(OH)2, but this protocol suppressed the yield with 1-Bpin (Entry 8), and this was also the case with Cu(OTf)2 + pyridine premixing (Entry 12). Interestingly, the control reactions, where reactants were premixed at 110 °C followed by the addition of [18F]KF and remaining reagents/reactants also gave some valuable insight into this transformation. Preheating Cu(OTf)2 with either of the substrates and with (Entry 2) or without (Entry 10) [18F]KF led to significantly suppressed yields, suggesting that the role of pyridine may be to counteract deleterious side reactions in addition to acting as a ligand for Cu(OTf)2.
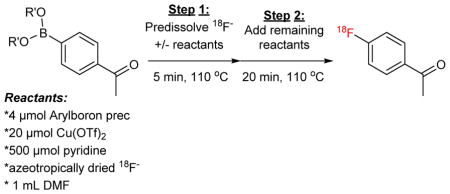
These data served as a roadmap for automation. Several successful premixing procedures were carried out for the synthesis of 1-F from1-B(OH)2 and 1-Bpin (Table 2). The crude (unpurified) reaction mixture was analyzed by radioTLC and radioHPLC to determine RCC (See S4.4, pg S12–13 for RCC as well as total activity values). All reactions were conducted with ~150 mCi starting activity (cyclotron-produced target water from a 2 min beam), and our previously reported data (without a pre-dissolution step [2]) was used as a basis of comparison after appropriate scaling (Table 2, Entry 1). As expected, all variants fared better than the previously reported automated results with no premixing. Furthermore, the automated RCC values started to approach the manual RCC values for fluorination of both 1-B(OH)2 (Table 2, Entries 2–4) and 1-Bpin (Table 2, Entries 5–7). Interestingly, there were differences in the automated fluorination of 1-B(OH)2 vs. the fluorination of 1-Bpin, and these closely followed the results from the order of addition study. For example, with 1-B(OH)2 the best automation result occurred when [18F]KF was pre-dissolved in DMF (Table 2, Entry 2). In contrast, with 1-Bpin, pre-dissolution in DMF (Table 2, Entry 5) was worse than pre-dissolution in either precursor/DMF (Table 2, Entry 6) or precursor/pyridine/DMF (Table 2, Entry 7). This indicates that optimal conditions are different when boronic acid and Bpin precursors are utilized, and the experimental protocol should be modified accordingly.
Table 2.
| |||||
|---|---|---|---|---|---|
| Entry | Substrate | QMA eluent | Fluoride pre-dissolution medium | RCC | n |
| 1 | 1-B(OH)2 | KOTf/K2CO3 | no dissolution step | 10±2%a | 2 |
| 2 | 1-B(OH)2 | KOTf/K2CO3 | DMF | 43±0% | 3 |
| 3 | 1-B(OH)2 | KOTf/K2CO3 | substrate | 30±8% | 2 |
| 4 | 1-B(OH)2 | KOTf/K2CO3 | substrate + pyridine | 39±7% | 2 |
| 5 | 1-Bpin | KOTf/K2CO3 | DMF | 32±24% | 2 |
| 6 | 1-Bpin | KOTf/K2CO3 | substrate | 56±4% | 2 |
| 7 | 1-Bpin | KOTf/K2CO3 | substrate + pyridine | 53±12% | 2 |
| 8 | 1-B(OH)2 | triethylamine | no dissolution step | 34±7% | 2 |
| 9 | 1-B(OH)2 | triethylamine | DMF | 19±6% | 2 |
| 10 | 1-B(OH)2 | triethylamine | substrate | 23±9% | 2 |
| 11 | 1-B(OH)2 | triethylamine | substrate + pyridine | 21±2% | 2 |
Original conditions reported in reference 2.
The use of a more soluble form of [18F]fluoride is an alternate strategy to address the dissolution of [18F]fluoride in DMF. Towards this end, we recently showed that weak nitrogenous bases can be used to elute [18F]fluoride from QMA Sep-Paks to prepare more soluble forms of [18F]fluoride [4]. Specifically, we utilized 0.1 M aq. triethylamine as an eluent in this chemistry, as the subsequent form of [18F]fluoride was expected to be organic-soluble [18F]triethylamine hydrofluoride ([18F]TEAHF). Although triethylamine was previously reported to hinder radiofluorination in this reaction [2], we posited that any residual triethylamine would evaporate during azeotropic drying, leaving only the [18F]TEAHF salt in the reactor. Since such a salt is expected to be much more readily soluble than [18F]KF in organic media, we anticipated that the order of addition would have little impact on reaction yields. This was observed to be the case. For example, 34±7% RCC to 1-F was observed when the automated reaction was conducted with triethylamine eluent without a separate [18F]fluoride dissolution step (Table 2, Entry 8). In fact, lower RCCs were observed when various pre-dissolution steps were implemented with this eluent (Table 2, Entries 9 – 11), and the 1-F activity is over 50% greater when this step is eliminated from the reaction protocol. A generally slightly lower RCC with [18F]TEAHF/TEA vs. [18F]KF/KOTf automated reactions was somewhat surprising, but may be due to the acidity of [18F]TEAHF or residual triethylamine if it was not completely removed during azeotropic drying. As such, we conducted further optimization studies using the KOTf/K2CO3 eluent.
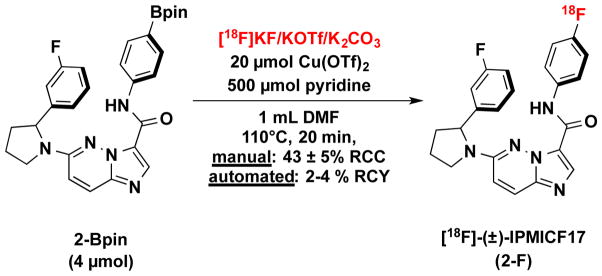
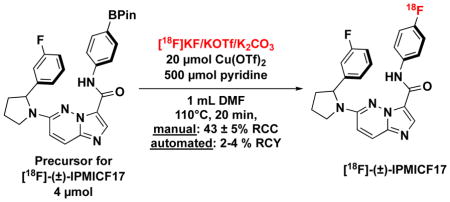
To challenge the chemistry further, the optimized [18F]KF dissolution/order of addition with KOTf/K2CO3 eluent was applied to the synthesis of [18F]-(±)-IPMICF17 (2-F), a PET radiotracer for TrkB/C that is currently being developed by the Schirrmacher and Scott groups (Scheme 2) [10,11]. We expected this molecule to be amenable to such labeling since it has been previously demonstrated that the 6-(pyrrolidin-1-yl)imidazo[1,2-b]pyridazine core does not inhibit Cu-mediated fluorination reactions [6]. Synthesis from the (±)-IPMICF17 Bpin precursor (2-Bpin) was carried out both manually and in an automated format, with pre-dissolution of [18F]KF performed with a precursor/DMF solution. Manual reactions afforded an RCC of 43 ± 5% (see S4.5 pg S22). Analysis of the crude mixture from an automated test reaction in a synthesis module showed 25% RCC to product 2-F, when starting from ~150 mCi of [18F]fluoride. Fully-automated synthesis, semi-preparative HPLC purification, and reformulation in sterile saline led to an isolated RCY of 4.1 ± 0.2 % (61 ± 4 mCi from a starting activity of ~1500 mCi/30 min beam, non-decay corrected, n = 2), which is suitable for ongoing preclinical and clinical studies.
Scheme 2.
Radiosynthesis of [18F]-(±)-IPMICF17 (2-F)
Although an acceptable RCY was obtained in the synthesis of [18F]-(±)-IPMICF17 (2-F), the large size and complexity of this molecule did not allow for the separation of 2-F from the protoarene impurity (2-H) via standard C18 semi-preparative HPLC (see S.4.5.2 pg S25–S27 for HPLC spectra). This impurity is present at a significantly higher mol% than the radiofluorinated product, and it is expected to be approximately equipotent against the same target (TrkB/C) as the 18F-labeled tracer. As such, the lower effective specific activity obtained under these initial conditions is expected to be detrimental to the imaging results. We anticipate this problem could represent a general limitation of the method, particularly when dealing with drug-like molecules. Thus, additional optimization was carried out to achieve separation of product 2-F from protoarene impurity 2-H.
Initial efforts focused on limiting the formation of the protoarene impurity 2-H via chemical means. Alternate solvents, the addition of strong and weak bases, and inclusion of oxidants (e.g. tBuO2) were all explored, based on a recent report showing that these variables can reduce the presence of the protoarene (2-H) and phenol (2-OH) byproducts in related Chan-Lam coupling reactions [16]. These studies showed that the use of stronger bases did result in a decrease in the concentration of protoarene byproduct 2-H. However, this was also accompanied by a significant decrease in RCC to the desired fluorinated product 2-F. We therefore next turned our attention to developing HPLC methods that would allow for the facile separation of protoarene (2-H) from [18F]-(±)-IPMICF17 (2-F).
In prior reports, we have utilized HPLC columns with pentafluorophenyl (PFP) stationary phases to separate polyfluorinated materials such as [18F]N-methyl lansoprazole and its precursor [17] and, more recently, [18F]fluoroarene products from protoarene starting materials in CH-activation chemistry [18]. Building on this work, we reasoned that an appropriately optimized eluent system with a PFP semi-preparative column would allow for the separation of [18F]-(±)-IPMICF17 (2-F) from its protoarene byproduct (2-H). For initial optimization efforts, a crude [18F]-(±)-IPMICF17 radiofluorination reaction mixture spiked with [19F]-(±)-IPMICF17 reference standard was eluted with a range of NH4OAc-buffered H2O/MeCN eluents on a Luna-PFP analytical column. This allowed us to take into account all of the impurities present in the reaction when optimizing HPLC separation, including protoarene 2-H. While peak overlap was still observed at higher %MeCN eluents, lower %MeCN eluents showed separation of the fluoroarene and protoarene peaks (see S.4.5.1 pg S23). The eluent was further optimized with a Kinetex PFP semi-preparative column, and an eluent consisting of 45% MeCN buffered with 10 mM NH4OAc at pH 7.5 was found to separate product 2-F from impurity 2-H in a manual radiolabeling sample of [18F]-(±)-IPMICF17, with a retention time of ~45 min for the byproduct (2-H) and ~52 min for [18F]-(±)-IPMICF17 (2-F). Full production runs were then performed to determine if [18F]-(±)-IPMICF17 could be isolated absent of any protoarene byproduct at an acceptable RCY. Product was isolated with RCY of 1.9 ± 0.1 % (28 ± 2 mCi from a starting activity of ~1500 mCi, non-decay corrected, n = 3), excellent radiochemical purity (>99%) and high specific activity (5294±1227 Ci/mmol), and analytical HPLC gratifyingly showed that no protoarene impurity was present in the final reformulated doses. Quality control testing of [18F]-(±)-IPMICF17 has confirmed suitability of doses for in vivo use in animal and human imaging applications (see S4.5.3, pg S27–S30 for representative semi-preparative and analytical HPLC traces). Biodistribution and microPET imaging studies are underway to evaluate this radiotracer in rodents, primates and human subjects.
The optimization studies in this report have led to more efficient automated Cu-mediated radiofluorination reactions. However, it is noteworthy that the isolated RCYs (1.9–4.1%) of [18F]-(±)-IPMICF17 are still lower than would be expected given the automated RCC (25%). We believe that this discrepancy does not result from the fluorination reaction, but represents challenges associated with the first efforts by our group and others in fitting organometallic chemistry into the confines of automated cGMP PET radiotracer production [9,19]. Our efforts to develop novel transition metal-mediated [18F]fluorination reactions, including new methods for processing [18F]fluoride and automation of reactions, investigation of reaction mechanisms and exploration of substrate scope are ongoing [2, 4,18,20, 21], and will ensure that these new reactions continue to be further optimized for routine PET radiotracer manufacture.
Conclusions
This report updates our recently published copper-mediated [18F]fluorodeboronation method for the automated production of PET radiotracers suitable for clinical use. Two recurrent problems, low RCC upon automation and protoarene byproduct purification issues, have been addressed. Building on our earlier report, a [18F]KF pre-dissolution step has been developed to increase automated RCC with KOTf QMA eluent. An alternative procedure involving triethylamine as the QMA eluent is also effective. The automated method was utilized to produce sterile injectable doses of [18F]-(±)-IPMICF17 in an automated synthesis module in reasonable isolated RCY. Purification of [18F]-(±)-IPMICF17 was achieved with a PFP semi-preparative HPLC column thereby preventing contamination of the dose with the protoarene impurity prevalent in this and similar transition metal-mediated reactions. Doses of [18F]-(±)-IPMICF17 prepared in this fashion are being used in ongoing preclinical and clinical imaging studies.
Supplementary Material
Acknowledgments
This work was supported by NIH (R01EB021155 to M.S.S. and P.J.H.S.), U.S. DOE/NIBIB (DE-SC0012484 to P.J.H.S.), Cancer Research Society and C7 Council (to R.S.), Natural Science and Engineering Research Council of Canada (NSERC, to R.S.) and Weston Brain Institute (to R.S. and P.J.H.S).
Footnotes
Dedication: dedicated to Prof. Dr. Heinz H. Coenen on the occasion of his retirement.
Conflict of Interest
The authors do not report any conflict of interest.
References
- 1.Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, Mercier J, Génicot C, Gouverneur V. A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew Chem Int Ed. 2014;53(30):7751–7755. doi: 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]
- 2.Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, Scott PJH. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org Lett. 2015;17(23):5780–5783. doi: 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Y, Schimler SD, Hanley PS, Sanford MS. Cu(OTf)2-Mediated Fluorination of Aryltrifluoroborates with Potassium Fluoride. J Am Chem Soc. 2013;135(44):16292–16295. doi: 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]
- 4.Mossine AV, Brooks AF, Ichiishi N, Makaravage KJ, Sanford MS, Scott PJH. Development of Customized [18F]Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci Rep. 2017;7:233. doi: 10.1038/s41598-017-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preshlock S, Calderwood S, Verhoog S, Tredwell M, Huiban M, Hienzsch A, Gruber S, Wilson TC, Taylor NJ, Cailly T, Schedler M, Collier TL, Passchier J, Smits R, Mollitor J, Hoepping A, Mueller M, Genicot C, Mercier J, Gouverneur V. Enhanced Copper-Mediated 18F-Fluorination of Aryl Boronic Esters Provides Eight Radiotracers for PET Applications. Chem Commun. 2016;52:8361–8364. doi: 10.1039/c6cc03295h. [DOI] [PubMed] [Google Scholar]
- 6.Taylor NJ, Emer E, Preshlock S, Schedler M, Tredwell M, Verhoog S, Mercier J, Genicot C, Gouverneur V. De-Risking the Cu-Mediated 18F-Fluorination of Heterocyclic PET Radioligands. J Am Chem Soc. 2017;139(24):8267–8276. doi: 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]
- 7.Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E, Endepols H, Neumaier B. Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem Eur J. 2015;21(15):5972–9. doi: 10.1002/chem.201405586. [DOI] [PubMed] [Google Scholar]
- 8.Zischler J, Kolks N, Modemann D, Neumaier B, Zlatopolskiy BD. Alcohol-Enhanced Cu-Mediated Radiofluorination. Chem Eur J. 2017;23(14):3251–3256. doi: 10.1002/chem.201604633. [DOI] [PubMed] [Google Scholar]
- 9.Sanford MS, Scott PJH. Moving Metal-Mediated 18F-Fluorination from Concept to Clinic. ACS Cent Sci. 2016;2(3):128–130. doi: 10.1021/acscentsci.6b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard-Gauthier V, Bailey JJ, Aliaga A, Kostikov A, Rosa-Neto P, Wuest M, Brodeur GM, Bedell BJ, Wuesta F, Schirrmacher R. Development of Subnanomolar Radiofluorinated (2-pyrrolidin-1-yl)imidazo[1,2-b]pyridazine pan-Trk Inhibitors as Candidate PET Imaging Probes. Med Chem Commun. 2015;6(12):2184–2193. [Google Scholar]
- 11.Bernard-Gauthier V, Bailey J, Mossine AV, Lindner S, Vomacka L, Aliaga A, Shao X, Quesada C, Sherman P, Mahringer A, Kostikov A, Grand’Maison M, Rosa-Neto P, Soucy J-P, Thiel A, Kaplan D, Fricker G, Wängler B, Bartenstein P, Schirrmacher R, Scott PJH. A Kinome-Wide Selective Radiolabeled TrkB/C Inhibitor for in Vitro and in Vivo Neuroimaging: Synthesis, Preclinical Evaluation and First-in-Human. J Med Chem. 2017;60(16):6897–6910. doi: 10.1021/acs.jmedchem.7b00396. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Lau J, Kuo HT, Zhang C, Colpo N, Bénard F, Lin KS. Synthesis and Evaluation of 18F-labeled CJ-042794 for Imaging Prostanoid EP4 Receptor Expression in Cancer with Positron Emission Tomography. Bioorg Med Chem Lett. 2017;27(10):2094–2098. doi: 10.1016/j.bmcl.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Lau J, Zhang C, Colpo N, Nocentini A, Supuran CT, Bénard F, Lin KS. Design, Synthesis and Evaluation of 18F-labeled Cationic Carbonic Anhydrase IX Inhibitors for PET Imaging. J Enzyme Inhib Med Chem. 2017;32(1):722–730. doi: 10.1080/14756366.2017.1308928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhang C, Lau J, Colpo N, Bérnard F, Lin K-S. One-step synthesis of 4-[18 F]Fluorobenzyltriphenylphosphonium Cation for Imaging with Positron Emission Tomography. J Label Compd Radiopharm. 2016;59:467–471. doi: 10.1002/jlcr.3436. [DOI] [PubMed] [Google Scholar]
- 15.Tang T, Gill HS, Ogasawara A, Tinianow JN, Vanderbilt AN, Williams S-P, Hatzivassiliou G, White S, Sandoval W, DeMent K, Wong M, Marik J. Preparation and Evaluation of L- and D-5-[18F]fluorotryptophan as PET Imaging Probes for Indoleamine and Tryptophan 2,3-Dioxygenases. Nucl Med Biol. 2017;51:10–17. doi: 10.1016/j.nucmedbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Vantourout JC, Miras HN, Isidro-Llobet A, Sproules S, Watson AJ. Spectroscopic Studies of the Chan-Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity. J Am Chem Soc. 2017;139(13):4769–4779. doi: 10.1021/jacs.6b12800. [DOI] [PubMed] [Google Scholar]
- 17.Fawaz MV, Brooks AF, Rodnick ME, Carpenter GM, Shao X, Desmond TJ, Sherman P, Quesada CA, Hockley BG, Kilbourn MR, Albin RL, Frey KA, Scott PJH. High Affinity Radiopharmaceuticals Based upon Lansoprazole for PET Imaging of Aggregated Tau in Alzheimer’s Disease and Progressive Supranuclear Palsy: Synthesis, Preclinical Evaluation, and Lead Selection. ACS Chem Neurosci. 2014;5(8):718–730. doi: 10.1021/cn500103u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCammant MS, Thompson S, Brooks AF, Krska SW, Scott PJH, Sanford MS. Cu-Mediated C-H 18F-Fluorination of Electron-Rich (Hetero)arenes. Org Lett. 2017;19(14):3939–3942. doi: 10.1021/acs.orglett.7b01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoover AJ, Lazari M, Ren H, Narayanam MK, Murphy JM, va Dam RM, Hooker JM, Ritter T. A Transmetalation Reaction Enables the Synthesis of [18F]5- Fluorouracil from [18F]Fluoride for Human PET Imaging. Organometallics. 2016;35(7):1008–1014. doi: 10.1021/acs.organomet.6b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH. Copper-Catalyzed [18F]Fluorination of (Mesityl)(aryl)iodonium Salts. Org Lett. 2014;16(12):3224–3227. doi: 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH. Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org Lett. 2016;18(20):5440–5443. doi: 10.1021/acs.orglett.6b02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.