Abstract
Background and Aims
The density of epithelial cell extrusion zones in the intestinal lining, also known as gap density (number of gaps/1000 epithelial cells counted) can be quantitated using probe-based confocal laser endomicroscopy (pCLE). Gap density has been reported to be higher than normal in both inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) patients. Epithelial cells destined for extrusion from the intestinal surface would stain positive for either activated caspase-1 or caspase-3 on mucosal biopsy samples. The aim of this study is to determine whether epithelial gap density on pCLE correlates with quantitative analysis of activated caspase staining of mucosal biopsy samples from patients.
Methods
We obtained pCLE images and biopsy samples of the terminal ileum during colonoscopies of healthy controls and patients with either IBD or IBS. The pCLE images and biopsy samples were blindly analyzed for gap density and for cells staining positive for activated caspases, respectively. The degree of correlation was determined using nonparametric statistical tests.
Results
The median results were 10 gaps/1000 cells counted for controls versus 33 gaps/1000 cells counted for chronic intestinal disorder patients (p = 0.02). Activated caspase staining showed 13 positive cells/1000 epithelial cells counted versus 26 positive cells/1000 epithelial cells counted, respectively (p = 0.02), thus showing a strong correlation with a Spearman's coefficient rho of 0.61 (strong correlation for rho = 0.4–0.75, p = 0.01).
Conclusions
Intestinal epithelial gap density via pCLE correlated strongly with quantitative analysis of immunohistochemical staining of mucosal biopsy samples.
Keywords: confocal laser endomicroscopy, small intestine, epithelial extrusion zones, epithelial gaps, inflammatory bowel disease, irritable bowel syndrome
Introduction and Background
Intestinal epithelial cell (IEC) extrusion occurs in both healthy and disease states. Visualization of this extrusion process and the extrusion zones (epithelial gaps) that remain in the human intestine became possible recently with the advent of confocal laser endomicroscopy.1 Our analyses of epithelial gap density via probe-based confocal laser endomicroscopy (pCLE) imaging of the small intestine have found significantly higher rates of epithelial cell extrusion in patients with inflammatory bowel disease (IBD)2 and irritable bowel syndrome (IBS).3 Furthermore, evidence of barrier dysfunction on confocal laser endomicrocopy (increased gap density or ileal fluorescein leakage and microerosions) were predictive of disease relapse in both adult4, 5 and pediatric IBD6 patients. For IBS patients, the increased gap density is the only endoscopic abnormalities identified to date3. In IBS patients with suspected food intolerances, CLE findings of immediate breaks, increased intervillous spaces, and increased intra-epithelial lymphocytes in the mucosa have also been reported.7
When determined using the gold standard of conventional multiphoton confocal microscopy and light microscopy, epithelial gap density is a validated measure of extrusion zones in the intestinal lining in rodent models.8 However, no correlation studies with mucosal biopsy samples have been performed to validate pCLE measurements of epithelial gap density in human patients.
Epithelial cells lining the mammalian intestine extrude because of two major types of cell deaths:9 (a) apoptosis, which is mediated by activation of the caspase-3 enzyme;10, 11 and (b) a second form of cell death (recently identified by our group) that is mediated by caspase-1 activation.12 Therefore, the combined staining for activated caspase-3 and caspase-1 on mucosal biopsy samples is a quantitative measure of the epithelial cells destined to be extruded, which will most likely leave behind corresponding epithelial gaps in the intestinal lining for a period of time. The measurements of activated caspase staining of mucosal biopsy normalized to the total number of cells in the epithelial surface should therefore correlate with the measurements of epithelial gap density determined from pCLE imaging.
The primary aim of this study was to determine the correlation between epithelial gap density on pCLE and staining of mucosal biopsy samples taken from the terminal ileum in human patients. The secondary aims were to examine (1) the etiology of the increase in activated caspase staining observed in patients with chronic intestinal disorders; and (2) the relationships of the activated caspase staining results with the disease type (IBS or IBD), gender and age of the patients.
Methods
Patients
This prospective cohort study was registered at ClinicalTrials.gov (NCT00988273). The Human Ethics Research Review Board at the University of Alberta reviewed and approved our study protocol. The disease group consisted of patients having either biopsy-proven IBD or symptoms consistent with diarrhea-predominant IBS based on the Rome III criteria. For IBD patients, those undergoing surveillance colonoscopy or evaluation of clinical symptoms were included. We performed detailed histories to exclude other causes of diarrhea-predominant IBS symptoms such as lactose/fructose intolerance. Tests for characteristic serum antibodies (anti-tTG or anti-endomysial antibody) and esophagogastroduodenoscopies with biopsy were performed to rule out celiac disease. Finally, serum thyroid stimulating hormone (TSH) levels were checked to rule out thyroid dysfunction. The control group consisted of patients undergoing colonoscopy for colon cancer screening or rectal bleeding but with no other gastrointestinal symptoms. Inclusion criteria were that the patients were over 18 and able to give informed written consent. Exclusion criteria were a known allergy to fluorescein or shellfish, impaired renal function (serum creatinine level over 1.5 mg/dL) or being pregnant or nursing females. Patient demographics, histories, physical examination data, and endoscopic results were maintained in a prospective database.
Confocal Laser Endomicroscopy
Standard colonoscopy with intubation of the terminal ileum was performed in each patient. Patients were intravenously sedated with midazolam and fentanyl and had standard cardiopulmonary monitoring. An antispasmodic agent (glucagon) was used as needed to reduce peristalsis of the gut and movement artifacts. To produce contrast in the images, each patient received an intravenous injection of 5 mL of 10% fluorescein following the intubation. An ultra-high definition pCLE instrument (Coloflex UHD, Mauna Kea Technologies, Paris, France) was used to obtain frame-by-frame confocal images of the terminal ileum. At least five different sites within a 10 cm radius in the terminal ileum were imaged in all patients, beginning at about 10 cm proximal to the ileocecal valve. The duration of the recording of the pCLE images was approximately 10 minutes, yielding over 4,000 images per patient.
Review and analysis of the pCLE images were conducted in a post-hoc manner as previously described.2 Any bias in this analysis was minimized by blinding the two reviewers of the pCLE images to the status of the patient and to the indication for colonoscopy. In brief, villi that had at least 75% of their structure visible within the field of view of the pCLE images and that could be seen on at least three consecutive frames were selected as being adequately imaged. Epithelial cells and epithelial gaps of these villi were then manually counted by the reviewers. For each patient, a minimum of three of these villi with the highest numbers of epithelial gaps were used to calculate the gap density, defined as the number of epithelial gaps per 1000 epithelial cells counted.
Mucosal Biopsy Collection and Preparation
All mucosal biopsies were collected immediately after removal of the pCLE probe, at close to the pCLE imaging site as possible. A total of 624 villus sections were obtained from the 26 patients in the study. The tissue biopsies were taken from normal-appearing mucosa in the terminal ileum at least 5 cm away from any visible pathological lesions. Tissues were immediately transferred to sterile phosphate buffered saline (1× PBS; Fisher BioReagents, Fair Lawn, NJ) kept on ice and were brought to the laboratory for processing in less than 10 min. After careful washing, the specimens were embedded in optimal cutting temperature media (Tissue-Tek, Torrence, CA), quickly frozen using liquid nitrogen and stored at −80 °C. Sections of 5 μm thickness were prepared using a cryostat (CM3050 S; Leica Microsystems, Wetzlar, Germany) and mounted onto Superfrost Plus microscope slides (Fisher Canada, Ottawa, ON, Canada). Two non-adjacent sections from each patient were fixed with cold acetone for 1 min, stained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/mL diluted in PBS wash buffer), and assessed for morphology and orientation.
Immunohistochemistry
Before staining for activated caspase, sections were air dried and fixed with cold acetone for 1 min. The sections were then rehydrated with PBS-Tween wash buffer (0.05% Polysorbate 20; MP Biomedicals, Solon, OH), using two washes of 5 min each. After blocking with Aquablock (East Coast Bio, North Berwick, ME), the sections were stained for 1 h using FAM-FLICA (fluorescent labeled inhibitor of caspases) in vitro kits for activated caspase-1 (catalog #98; Immunochemistry Technologies, Bloomington, MN) and activated caspase-3 (catalog #94; Immunochemistry Technologies, Bloomington, MN). Staining was followed by three washes of 10 min each in the wash buffer previously described. Sections were post-fixed for 20 min at room temperature with 4% paraformaldehyde (PFA; Sigma Aldrich, St. Louis, MO) that had been freshly prepared and adjusted to pH 7.5. After three washes of 7 min each, the samples were permeabilized for 30 min using 0.2% Triton X-100 (Sigma Aldrich, St. Louis, MO), 3% normal goat serum (NGS; diluted from 10%; Invitrogen, Eugene, OR), and 1% bovine serum albumin (BSA; Fisher Scientific, Ottawa, ON) in 1× PBS. The sections were blocked with 2% NGS and 1% BSA in 1× PBS for 1 h and then incubated for 1 h with Alexa Fluor 568 phalloidin (300 units, 1:40 dilution in 1× PBS; Invitrogen, Eugene, OR) to label for F-actin. Excess fluorochrome was removed with three washes of 15 min each, and then tissues were counterstained with DAPI (1 μg/mL diluted in wash buffer) for 1 min to visualize nuclei. After DAPI staining, sections were washed three times (twice with wash buffer followed by double-distilled water) and then mounted with FluorSave reagent (catalog #345789; Calbiochem, EMD Millipore, Billerica, MA) for microscopy examination. Negative controls were processed using 1× PBS in place of FLICA reagents.
Fluorescent Microscopy
On average, eight images of cross-sectional views of villi were obtained for each tissue sample using the Zeiss Axio Observer.Z1 inverted microscope (Carl Zeiss Canada Ltd., Toronto, ON, Canada). Cross-sections of the villi must be visible to differentiate the epithelial surface from the lamina propria. A minimum of three different villi with proper cross-sectional orientation were selected for manual counting from among the eight images obtained for each patient. Wavelengths used for emission/excitation of FLICA were 488/530 nm (green), for phalloidin were 587/600 nm (red), and for DAPI were 358/461 nm (blue). The total number of epithelial cells and the number of cells positively stained for activated caspases were recorded by two reviewers blinded to the status of the specimens. DAPI (blue) staining was used to distinguish individual cells, while phalloidin (red) staining for actin was used to aid in the separation of the lumen from the epithelial surface. All cells in the epithelial surface layer were manually counted for each villus by reviewers blinded to the status of the patient, and cells that stained positively for activated caspase-3 (FLICA-3) or caspase-1 (FLICA-1) were counted separately. The counts of the cells positive for FLICA-1 and FLICA-3 stains were combined and expressed as percentage of the total number of epithelial cells.
Western Blot Analysis
The human biopsy tissues were lysed by sonication in M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, Pittsburgh, PA) containing protease inhibitors. After centrifugation, total cellular proteins from the supernatant (normalized to 20 μg protein per sample) were fractionated on 12% SDS-PAGE gel and transferred to a nitrocellulose membrane. Membranes were blocked with Odyssey blocking buffer (Mandel Scientific Company, Inc., Guelph, ON, Canada) for 1 h at room temperature and then probed overnight at 4 °C with rabbit caspase-1 antibody (Cell Signaling Technology, Danvers, MA) and secondary donkey anti-rabbit IRDye 800CW (Mandel Scientific Company, Inc.). For normalization against housekeeping proteins, the membrane was incubated with primary mouse anti-β-actin (Life Technologies) and secondary goat anti-mouse IRDye 680CW. The prepared membrane was scanned using a Li-Cor Odyssey scanner (Mandel Scientific Company, Inc.)
Statistical Analysis
Sample size calculation
Since we do not have data on caspase staining of mucosal biopsy samples, the sample size calculation was based on the epithelial gap density data obtained from our previous studies of IBD and IBS patients. Assuming a mean gap density difference of 35 gaps/1000 cells between groups and a standard deviation of 28 gaps/1000 cells, a total of 22 patients (11 per group) would be required to achieve 80% power with a type I error (α) of 0.05. Since we anticipated that nonparametric methods of analysis would have to be employed, we increased the patient enrollment by approximately 20% to bring the total to 26 patients.
Analysis of results
The primary end-point of this study was the correlation between epithelial gap density on pCLE and staining of mucosal biopsy samples taken from the terminal ileum in patients. The secondary end-points were to (1) determine whether the increase in activated caspase staining was due to increase in caspase-1 or caspase-3 or combination of both in disease patients; and (2) to assess the relationships of the activated caspase staining with the disease type (IBS or IBD), gender and age of the patients. All variables are summarized using medians (interquartile range). The comparisons are based on nonparametric tests (i.e., Wilcoxon rank sum test, Fisher's exact test and Spearman rank correlation), with p-values less than 0.05 considered to be significant. All analyses were conducted using STATA 12 data analysis and statistical software (StataCorp, College Station, TX).
Results
A total of 26 patients were recruited, 13 disease patients (seven with IBD and six with IBS) and 13 controls. One control patient was found to have colon cancer at the time of colonoscopy and was therefore excluded from further analysis. Of the seven IBD patients, two had Crohn's disease (one with ileo-colonic disease, one had colonic disease), five had ulcerative colitis (two with pan-colitis, two with procto-colitis and one with left-sided colitis). Two patients were undergoing surveillance colonoscopy and five underwent colonoscopy for evaluation of clinical symptoms. Medical therapies for patients at the time of colonoscopies were: 5ASA for 3 patients, immonomodulator for 1 patient, and 3 patients were not on any treatment. Baseline patient characteristics and endoscopic findings for the 25 study patients are shown in Table 1. Technical issues with the confocal probes prevented one disease patient (IBD) and one control patient from undergoing pCLE imaging of the terminal ileum after consenting to the study. Mucosal biopsy samples were obtained for all 25 eligible patients. The main indication for colonoscopy in the disease group was evaluation of symptoms (N = 11: five IBD and six IBS patients), with the remaining two IBD patients undergoing colonoscopy for surveillance of dysplasia. In the control group, the principal indication for the procedure was colon cancer screening (N = 11). The control group was significantly older than the disease group, with the median ages being 59 (57, 69) and 43 (34, 47) years, respectively (p < 0.001). The most common endoscopic findings in the control group were polyps (N = 6) and normal (N = 5).
Table 1. Baseline Patient Characteristics.
| Disease (n=13) | Control (n=12) | p value | |
|---|---|---|---|
| Age (y), median (range) | 43 (34, 47) | 59 (57, 69) | <0.001 |
| % Male | 69 | 33 | 0.12 |
| Endoscopic indications | |||
| Evaluation of symptoms (%) | 11 (85) | ||
| Surveillance | 2 (15) | ||
| Colon cancer screening | 11 (92) | ||
| Rectal bleeding | 1 (8) | ||
| Endoscopic findings | |||
| IBD | |||
| Ileo-colitis | 1 | ||
| Pan-colitis | 2 | ||
| Procto-colitis | 1 | ||
| Proctitis | 1 | ||
| Normal | 3 | ||
| IBS | |||
| Normal | 3 | 5 | |
| Polyps | 1 | 6 | |
| Hemorrhoids | 3 | 1 | |
| Diverticulosis | 3 |
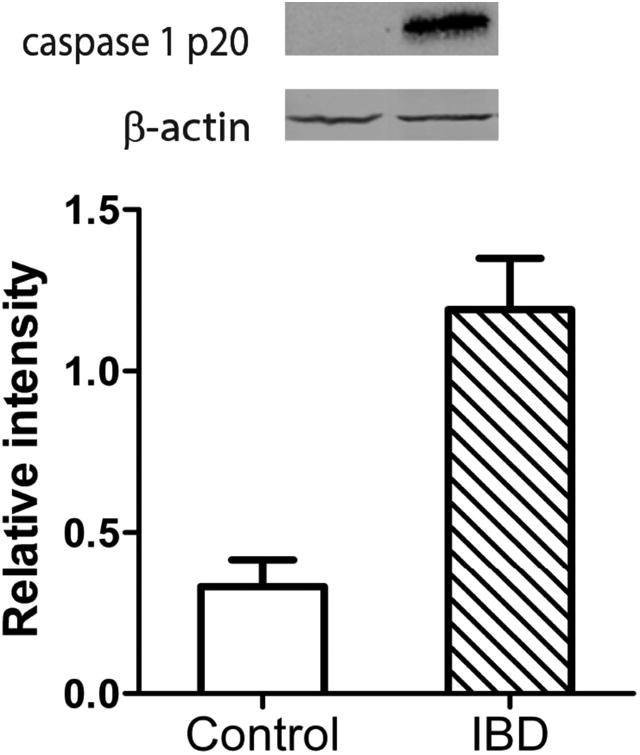
Representative pCLE images from control and disease patients are shown in Figure 1. The median gap density was significantly higher in the disease group compared to control group: 33 (13, 96) gaps/1000 cells versus 10 (6, 16) gaps/1000 cells, respectively (p = 0.02). The distribution of gap densities in the two groups is shown in Figure 2. The median gap density for IBD patients was 90 (0, 250) gaps/1000 cells, while the median gap density for IBS patients was 32 (0, 120) gaps/1000 cells. Representative images of the activated caspase staining of mucosal biopsy samples are in Figure 3. Disease patients had significantly more caspase-positive cells than did the controls (Figure 4a): the median total number of positive cells (combined results of the FLICA-1 and FLICA-3 stains) for disease patients was 26 (18, 41) positive cells/1000 cells counted [2.6% (1.8%, 4.1%) of the total] but for controls was 13 (6, 25) positive cells/1000 cells counted [1.3% (0.6%, 2.5%) of the total] (p = 0.02). The difference in the caspase staining results arose from an increase in activated caspase-1 staining in the disease group compared to the controls (Figure 4b): 16 (10, 25) positive cells/1000 cells counted [1.6% (1%, 2.5%)] versus 4 (3, 9) positive cells/1000 cells counted [0.4% (0.3%, 0.9%)], respectively (p = 0.01). This difference in activated caspase-1 levels was confirmed by Western blot analysis, with disease patients expressing activated caspase-1 (p20 subunit) at significantly higher levels than the controls, shown in Figure 5. On the other hand, the activated caspase-3 staining levels were comparable in the disease and control groups: 13 (6, 16) positive cells/1000 cells counted [1.3% (0.6%, 1.6%)] versus 9 (4, 14) positive cells/1000 cells counted [0.9% (0.4%, 1.4%)], respectively (p = NS).
Figure. 1.
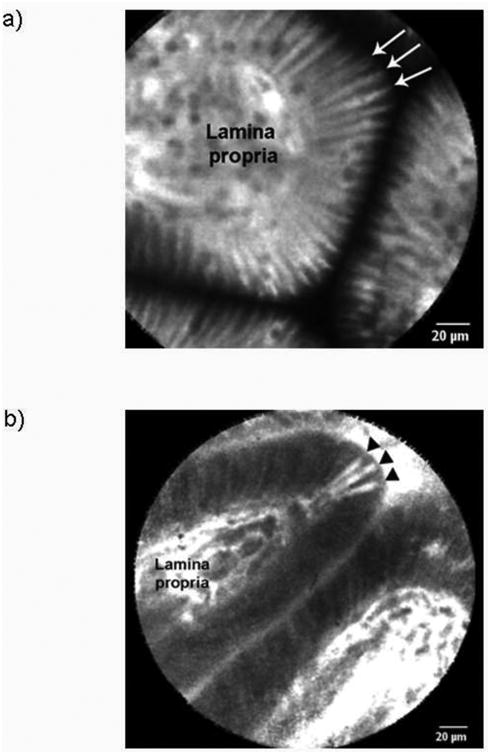
Figure. 2.
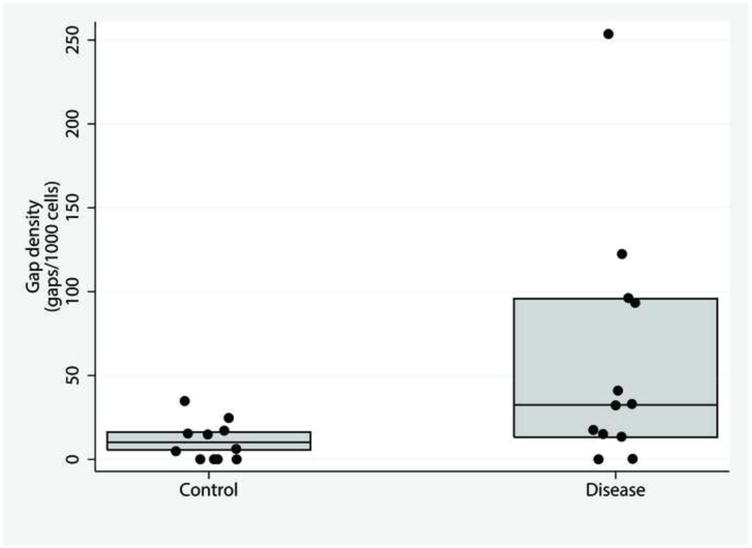
Figure. 3.
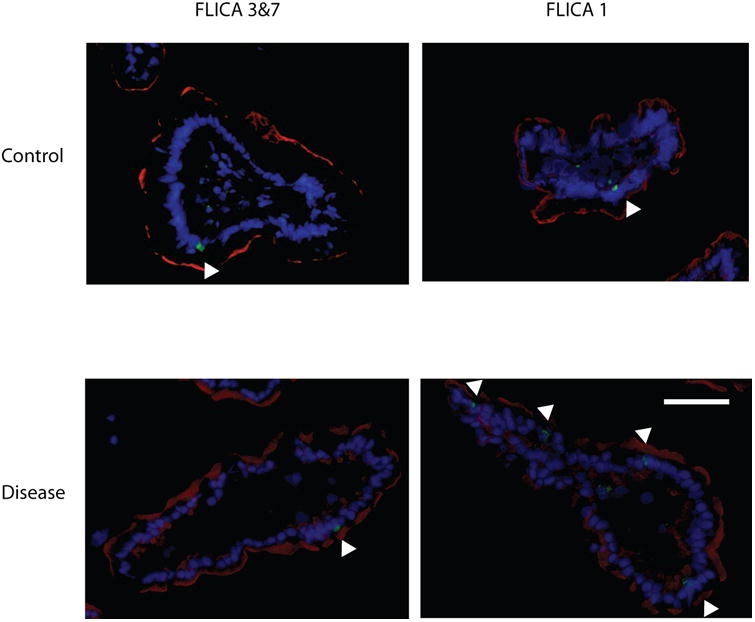
Figure. 4.

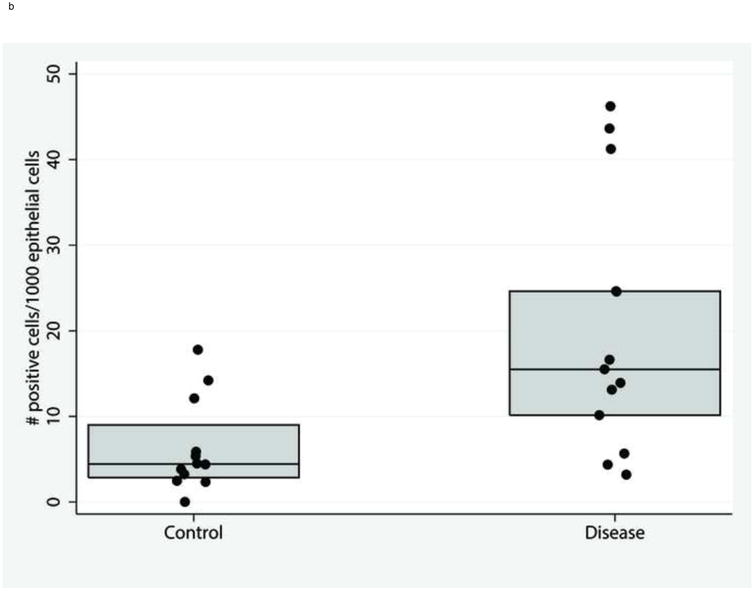
Figure. 5.
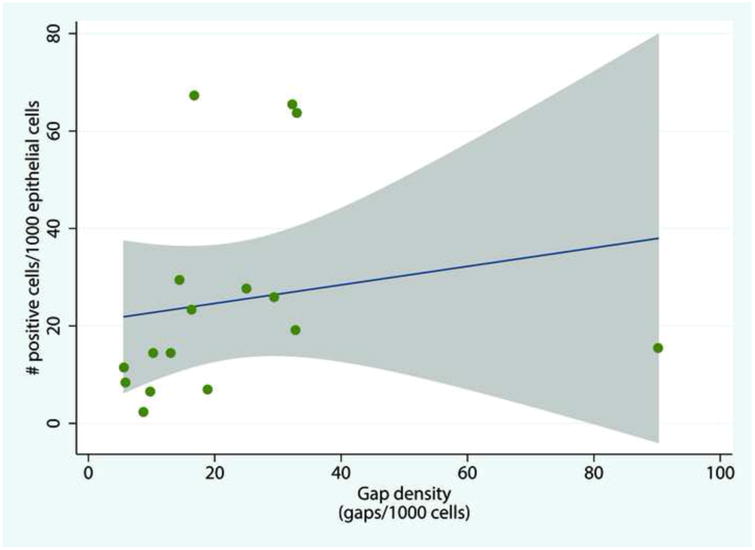
Strong correlation between epithelial gap density and activated caspase staining was observed between the 10th and 90th percentiles of gap density, as shown in Figure 6. The Spearman's correlation coefficient rho was 0.61 (where rho < 0.25 indicates weak correlation, 0.25–0.4 moderate, 0.4–0.75 strong, and >0.75 very strong; p = 0.01). Correlation for the extreme values of gap density (under the 10th and over the 90th percentiles) was weaker because pCLE results were compiled from significantly higher numbers of samples than were the mucosal biopsy results.
Figure. 6.
Discussion
Our results indicate that the higher epithelial gap densities observed in the terminal ileum by pCLE imaging strongly correlate with the results of the quantitative analyses of immunohistochemical staining for activated caspase in mucosal biopsy samples taken from the same patient. The observed increases in the gap density on pCLE imaging may be, in part, due to higher levels of activated caspase-1 staining observed on mucosal biopsies. This study is among the first to validate the results of epithelial gap density determined by pCLE imaging against the conventional gold standard—mucosal biopsy.
Establishing a strong correlation between endoscopic pCLE findings and immunohistochemical staining of mucosal biopsy samples is crucial. Epithelial gap density has been proposed as a surrogate marker for intestinal barrier function, and impaired barrier function has been suggested as being predictive of disease relapse in IBD patients.4, 13 Furthermore, increased epithelial gap density has been the only structural abnormality found to date in endoscopic imaging of IBS patients.3 As noted earlier, the numbers of epithelial gaps left by extruded cells are expected to correlate with the numbers of cells destined to be shed on the intestinal lining. Our results indicate that gap density did correlate with immune-histochemical staining results of mucosal biopsy sample. Furthermore, the increase in gap density appears to be due in part, to an increase in activated caspase-1 staining on biopsy samples. Results of our current study support the notion that gap density on pCLE is a reliable quantitative marker of mucosal barrier function that can be validated against mucosal biopsy staining. Thus, the use of CLE examination of the intestine have significant clinical values for prediction of disease relapse in IBD patients and diagnosis of barrier dysfunction in IBS patients. 3-5, 7
Correlations of increased numbers of epithelial gaps with increased barrier dysfunction in the intestine have been reported in several studies.12-14 In addition to confirming the epithelial gap density findings of our previous study, the current results indicate a potential mechanism for the creation of epithelial barrier defects associated with chronic intestinal disorders such as IBD and IBS: increased activated caspase-1 activity. It is important to note that there is significant overlap in the distribution of gap density and positively stained cells between patients with IBD and IBS. The current study was not designed to evaluate the differences in distribution of gap density nor positively stained cells. The main objective of the study was to confirm the findings of gap density on pCLE with quantitative staining of mucosal biopsy samples, the ultimate gold-standard that endoscopic results are measured against.
Our study has a number of limitations, the most significant of which is that it involves only 26 patients from a single tertiary referral center. This small sample size could partially explain the lack of difference observed in the caspase-3 staining results for the IBD and IBS group. In addition, the study population is heterogeneous because it includes patients with either diarrhea-predominant IBS or IBD. However, we performed standard evaluation tests such as thyroid function tests and celiac serology to exclude other causes of diarrhea in the IBS patients. Other caspases such as caspase-8 have been implicated in epithelial cell death, in particular in the death of paneth cells.15 We did not investigate the activity of caspase-8 in our study because paneth cells represent a small percentage of the overall epithelial cell population and are not likely to be a major contributor to differences in gap density. There were no differences in epithelial gap density between active versus quiescent IBD patients. In our previous studies, we have found that increased gap density in patients with quiescent disease was predictive of disease relapse. The current study was not powered to detect any differences in active or quiescent disease patients.2
In our study, we elected to exclude the extreme values of gap density ranges (<10th and > 90th percentiles). As noted earlier, the poor correlations in these extremes could be attributed to the large differences in sampling error for the two methods used. A minimum of five different sites of the terminal ileum were imaged in the 10-minute pCLE video examination of each patient, with 50 to 200 villi inspected. In contrast, each patient only had two mucosal biopsy samples taken, with 5 to 10 villi examined per patient. Thus, the number of villi sampled using pCLE was many times the number sampled using mucosal biopsy, and in such cases, correlations for paired observations are expected to be good near the median but poor for extreme values. The main advantage pCLE has over immune-histochemical staining is that optical biopsy with pCLE can sample many more sites in the 10 minute span, whereas only a limited number of biopsies can be obtained from each site for staining.
Prospective multicentered clinical trials are needed to validate and ensure the generalizability of the results of this study. The increased numbers of gaps in the intestinal epithelium of IBS and IBD patients, now confirmed here as correlating with increased activated caspase-1 activity, may suggest a potential biological basis for these diseases. Increased caspase-1 activation of intestinal epithelial cells in the small bowel provides not only a potential pathogenic mechanism for chronic intestinal disorders (such as IBD and IBS) but also indicates possible histologic criteria for the diagnosis of barrier dysfunction. As our understanding of IBS and IBD pathogenesis evolves, immunohistochemical staining of mucosal biopsy samples may provide another diagnostic test to further define this complex group of diseases.
In conclusion, we have shown that higher epithelial gap density in the terminal ileum of humans as observed by pCLE imaging correlated with the quantitative analysis of activated caspase staining of mucosal biopsy samples. Furthermore, the increase in gap density in patients with IBD and IBS appears to be due to higher caspase-1 activation of epithelial cells.
Acknowledgments
We would like to thank Ms. Stephanie Mah and Mr. Harsh Thaker for their assistance with the experiments. Dr. Julia Liu is a recipient of the Canadian Institutes of Health Research New Investigator Salary award. Funding support for the study was provided by the Canadian Institutes of Health Research / Canadian Association of Gastroenterology (CIHR/CAG GNO 96692), the University of Alberta Hospital Foundation (UHF 2011) and NIH P20 GM103625 (Center for Microbial Pathogenesis and Host Inflammatory Responses at UAMS). Ms. Theresa Kay was a recipient of a University of Alberta Faculty of Medicine and Dentistry summer studentship.
Grant support: Grant support was provided by the Canadian Institute of Health Research New Investigator Salary Award (JJL) Canadian Association of Gastroenterology (CIHR/CAG GNO 96692 JJL), Crohn's and Colitis Foundation of Canada (JJL), the University of Alberta Hospital Foundation (UHF 2011, JJL), National Institute of Health P20 (GM103625, JJL), Faculty of Medicine and Dentistry summer studentship (TMK), the Canadian Foundation for Innovations (RNF), and Natural Sciences and Engineering Research Council of Canada (RTI).
References
- 1.Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–1778. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Liu JJ, Wong K, Thiesen AL, et al. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174–80. doi: 10.1016/j.gie.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos) Gastrointest Endosc. 2013;77:624–30. doi: 10.1016/j.gie.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Turcotte JF, Wong K, Mah SJ, et al. Increased epithelial gaps in the small intestine are predictive of hospitalization and surgery in patients with inflammatory bowel disease. Clin Transl Gastroenterol. 2012;3:e19. doi: 10.1038/ctg.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karstensen JG, Saftoiu A, Brynskov J, et al. Confocal laser endomicroscopy: a novel method for prediction of relapse in Crohn's disease. Endoscopy. 2015 doi: 10.1055/s-0034-1393314. [DOI] [PubMed] [Google Scholar]
- 6.Shavrov A, Kharitonova AY, Claggett B, et al. A pilot study of the Predictive value of probe-based confocal laser endomicroscopy for relapse in pediatric inflammatory bowel disease patients. Journal of Pediatric Gastroenterology and Nutrition. 2015 doi: 10.1097/MPG.0000000000001022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147:1012–20 e4. doi: 10.1053/j.gastro.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Liu JJ, Rudzinski JK, Mah SJ, et al. Epithelial gaps in a rodent model of inflammatory bowel disease: a quantitative validation study. Clin Transl Gastroenterol. 2011;2:e3. doi: 10.1038/ctg.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayhew TM, Myklebust R, Whybrow A, et al. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol Histopathol. 1999;14:257–67. doi: 10.14670/HH-14.257. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–57. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 11.Watson AJ, Chu S, Sieck L, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–12. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Davis EM, Wine E, et al. Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm Bowel Dis. 2013;19:912–21. doi: 10.1097/MIB.0b013e3182807600. [DOI] [PubMed] [Google Scholar]
- 13.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim LG, Neumann J, Hansen T, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2014;20:892–900. doi: 10.1097/MIB.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 15.Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]


