Abstract
Background
New health policies may have intended and unintended consequences. Active surveillance of population-level data may provide initial signals of policy effects for further rigorous evaluation soon after policy implementation.
Objective
This study evaluated the utility of sequential analysis for prospectively assessing signals of health policy impacts. As a policy example, we studied the consequences of the Food and Drug Administration's warnings cautioning that antidepressant use could increase suicidal risk in youth.
Method
This was a retrospective, longitudinal study, modeling prospective surveillance, using the maximized sequential probability ratio test (maxSPRT). We used historical data (2000-2010) from 11 health systems in the US Mental Health Research Network. The study cohort included adolescents (ages 10-17) and young adults (ages 18-29), who were targeted by the warnings, and adults (ages 30-64) as a comparison group. Outcome measures were observed and expected events of two possible unintended policy outcomes: psychotropic drug poisonings (as a proxy for suicide attempts) and completed suicides.
Results
We detected statistically significant (p<0.05) signals of excess risk for suicidal behavior in adolescents and young adults within 5-7 quarters of the warnings. The excess risk in psychotropic drug poisonings was consistent with results from a previous, more rigorous interrupted time series analysis but use of the maxSPRT method allows timely detection. While we also detected signals of increased risk of completed suicide in these younger age groups, on its own it should not be taken as conclusive evidence that the policy caused the signal. A statistical signal indicates the need for further scrutiny using rigorous quasi-experimental studies to investigate the possibility of a cause-and-effect relationship.
Conclusions
This was a proof-of-concept study. Prospective, periodic evaluation of administrative healthcare data using sequential analysis can provide timely population-based signals of effects of health policies. This method may be useful to employ as new policies are introduced.
Introduction
Governments often introduce health policies in order to increase patient access to services including prescription medicines, improve safety and/or quality of care, and lower costs.1-3 Such policies include, but are not limited to, formulary restrictions, copayments, prior authorization requirements, and US Food and Drug Administration (FDA) regulatory actions.3-5
Policies can have intended and unintended consequences. Monitoring the outcomes of policies is an important public health issue. For instance, well-controlled studies demonstrated that prior authorization requirements in state Medicaid programs reduced initiation of effective medications for bipolar illness and decreased initiation of antidepressants.6, 7 While rigorous quasi-experimental approaches to policy evaluation such as interrupted time series (ITS) analysis are useful and important,1, 8 they require many pre- and post-policy data points to assess the statistical significance of a change in the rate of outcomes.1 Therefore, these approaches are not optimal for the timely detection of possible policy effects.
Methods for real-time surveillance of safety of medical products have been developed and used by the Centers for Disease Control sponsored Vaccine Safety Datalink,9-11 the Health Care Systems Research Network's (formerly HMO Research Network) Center for Education and Research on Therapeutics (CERT),12 and the FDA-sponsored Sentinel initiative.13, 14 These methods could potentially also enable prospective surveillance of policy changes and timely detection of potential intended and unintended consequences of policies for subsequent study. Routine surveillance would enable policymakers to investigate sooner the possible unintended impacts of policies as needed.
The FDA is responsible for formulating policies to improve patient safety related to use of marketed drugs.15 When safety information emerges after drug approval, the FDA systematically evaluates and responds to it. If the FDA identifies new safety concerns, it must decide how to effectively communicate the information about risks to the general public and providers.15 These communications range from minor revisions to the label of the drug to boxed warnings – FDA's strongest warning about a drug or drug class – when risks may be severe.5, 15 FDA safety communications influence prescribing and medication use behavior, especially when they are widely publicized by the media.16-18 For example, longitudinal studies found that the 2004 boxed warnings for antidepressants19, 20 and media reports21-23 led to decreases in antidepressant use,24-27 and there is evidence suggesting increases in suicide attempts among youth.27 Increased suicidality may have resulted from under-treated depression or failure to increase outpatient monitoring of young people with depression.28 Importantly, these outcomes were not reported years after the warnings.
This article describes our application of sequential analysis and the maximized sequential probability ratio test (maxSPRT)29 to detect signals of effects of health policies. We studied the consequences of the FDA's warnings cautioning that antidepressant use could increase suicidal risk in youth.19, 20 Specifically, the objective of the present study was to evaluate the utility of prospective near real-time sequential analysis for quickly assessing the need for more rigorous confirmatory analysis of policy impact.
Methods
Study design
This was a retrospective, longitudinal study using sequential analysis and the Poisson maxSPRT29 to simulate prospective surveillance. This study was part of a larger project designed to examine the effects of FDA boxed warnings and media reports regarding use of antidepressants and suicidal behavior.
Data source
This study used 2000-2010 data from 11 US healthcare organizations involved in the Mental Health Research Network (www.mhresearchnetwork.org).27, 30-32 Together the 11 healthcare systems provided care to a diverse population of 10 million people in 12 states. Members were enrolled through employer-sponsored insurance, individual insurance plans, and capitated Medicare and Medicaid programs. Members served by these systems are generally representative of each system's geographic service area. See our previously published article27 for a comparison of demographic characteristics between the study cohort and the US general population.
Data were obtained from the Health Care Systems Research Network Virtual Data Warehouse (VDW).33-35 The VDW has been developed and is maintained using a series of data standards and automated processes that guide the generation of similarly constructed data tables at each organization. In this federated data structure, common data definitions and formats facilitate sharing of de-identified data for research across multiple sites. The VDW data include demographics, health plan enrollment, inpatient and outpatient care utilization, diagnoses, procedures, and outpatient pharmacy data. At each site, source data are extracted from the health system's administrative and claims databases as well as electronic medical records. The VDW also includes date (month, year) and information on causes of death, derived from state death registries and internal health plan data. This study was approved by Institutional Review Boards at each participating site as well as from state departments of public health when required for the use of information from death certificates.
Intended and unintended consequences of policies
Intended consequences of a policy are defined by its goals. Unintended consequences of a policy, however, are difficult to classify because they are frequently side effects of the process of the policy change. Unintended consequences have been characterized by the following attributes by Rogers and Bloomrosen et al.:36, 37 (i) Desirable or undesirable effects: is the consequence positive, negative, or mixed (good in some ways and bad in other ways)? (ii) Anticipated or unanticipated effects: can the consequences be predicted or anticipated, and if so, by whom? Such consequences might include events that are easily anticipated, events that are only anticipatable by experts, and events that are completely unanticipatable by anyone (i.e., a total surprise). How anticipatable the consequences might be relates to the presence or absence of an event as well as its magnitude; (iii) Direct or indirect effects: does the policy change cause the consequence directly or is there a chain of events leading to it? It is worth noting that unanticipated, unintended consequences are frequently the result of an indirect causal chain; and (iv) Obvious or latent effects: is the consequence easily visible or does it become obvious only in a certain environment or at a later time?
Policy exposure
The intervention of interest in this study was the policy exposure. The FDA released several advisories beginning in 2003 followed by a boxed warning in late 2004 stating that use of antidepressants could increase risk of suicidality in youth and explicitly recommended monitoring of patients in the initial phase of treatment. These warnings were widely publicized by media reports.21 Many news stories emphasizing the risk of pediatric antidepressant use;21 thus, distorting the message of the well-intended safety warnings. Given the widespread media coverage, we considered the combination of FDA warnings and media reports as the policy exposure. Previous studies have found reductions in antidepressant treatment in all age groups following the warnings and no increases in use of treatment alternatives (e.g., psychotherapy, atypical antipsychotics),24, 27, 28, 38-40 suggesting an overall reduction in treatment of mood disorders. Because depression is an independent risk factor for suicidality and appropriate treatment with antidepressants is effective in reducing depressive symptoms,41-43 the falling rates of treatment of mood disorder following the policy and lack of increased outpatient monitoring of youth with mood disorder44 had the potential to increase suicide attempts and completed suicides at the population level – the two measures of unintended (indirect, latent) impacts of the policy considered in this study. Based on the messages of the warnings and management of mood disorders, Figure 1 outlines the conceptual model of the likely intended and unintended consequences of this policy exposure.
Figure 1.
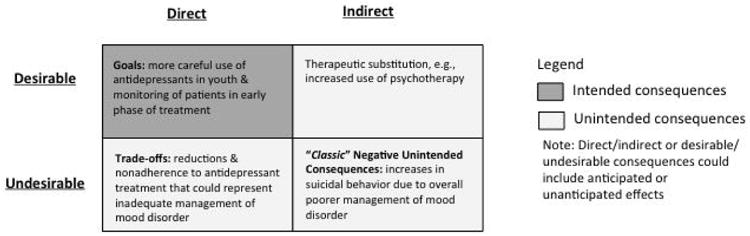
Conceptual model of likely consequences of FDA warnings and media reports regarding antidepressants
In order to evaluate the impacts of policies, one key consideration is the precise timing of the policy. Unexposed person-time was defined as time before the policy (the baseline period; the first quarter of 2000 to the third quarter of 2003). The last quarter of 2003 to the last quarter of 2004 was considered a “phase-in” period that spanned the entire period of the FDA advisories, the boxed warning, and intense media coverage to deal with the possibility of an anticipatory response to the warnings. We did not compare observed and expected event rates during the phase-in period in the main analysis. The phase-in period allowed for patients and clinicians to learn about the evidence and consider changing their patterns of antidepressant use. Thus excluding the phase-in period assessed the effects of the warnings at ‘full strength.’ Anticipatory effects of policies are common. Without accounting for anticipatory effects, the signal might have been detected sooner or later but the interpretation might be difficult. We conducted a sensitivity analysis to include the phase-in period in the post-policy period. Policy exposed time began in the first quarter of 2005 and continued through the last quarter of 2010 for the poisoning outcome and through the last quarter of 2008 for the suicide outcome (we only had death data up to 2008 for all participating sites at the time of study due to lag in availability of such data). Because of delays in obtaining the completed suicide data from one study site, which provided ∼3% of the data, and to be consistent with our previously reported study, we used completed suicide data through 2008 for the main analysis. We also conducted a sensitivity analysis using suicide data up to 2009 but including only 10 of 11 study sites.
Study population
Study cohorts included adolescents (ages 10-17), young adults (ages 18-29), and adults (ages 30-64). We used these age cut-offs because the prevalence of serious suicidal thoughts, planning, and attempts is higher among young adults aged 18–29 years than among adults aged 30 years and older.45 Adults were not targeted by the warnings and were therefore included in this study as a control cohort where no policy effect should be seen. To avoid introducing selection bias, we did not limit our cohorts to individuals with a coded depression diagnosis.
This is because previous studies showed that rates of depression diagnosis changed after the warnings,24, 38, 40 including in populations that are part of our study sample.46 Further, outpatient claims are frequently incomplete for mental health conditions like depression.47, 48
Outcome measures
While encounters for suicide attempts can be identified in administrative databases using external cause of injury codes (E-codes), they are incompletely captured in commercial plan databases.49 Our previous analysis found that E-code completeness varied across study sites, across treatment settings, and across years.30 Therefore, instead of deliberate self-harm E-codes, we used poisoning by psychotropic agents (International Classification of Diseases, 9th revision, Clinical Modification [ICD-9-CM] code: 969), a more reliable proxy for population-level suicide attempts.30, 49 Poisoning by drugs or toxic substances is the most frequent method of suicide attempt leading to hospitalization49 and emergency room treatments.50, 51 Non-fatal poisoning by psychotropic drugs (predominantly tranquilizers) has a positive predictive value of 79.7% for suicide attempts (sensitivity was 38.3% and specificity was 99.3%), outperforming other injury/poisoning types.49 In addition, ICD-9-CM coding is more stable over time as compared to E-codes and therefore can detect sudden changes in incidence rates in large populations over time. We identified deaths with suicide as a cause of death (ICD-10 codes: X60-X84, Y87.0), consistent with the algorithm used by the US Centers for Disease Control and Prevention.27
To examine changes in suicide attempts following the warnings, we identified a rolling cohort of continuous enrollees (individuals who had enrolled for the full 90 days in the quarter). We calculated the quarterly numbers of enrollees and encounters in hospitals or in emergency rooms by these enrollees for psychotropic drug poisoning.
To examine changes in completed suicides after the warnings, we identified a rolling group of individuals who had enrolled at any time in a given quarter or the month immediately prior to that quarter. We did not require continuous enrollment because health plan membership is terminated by death. We then calculated the quarterly numbers of enrollees as denominators and completed suicides as numerators.
Calculating observed and expected events
For each quarter the analysis requires information about the number of observed events during the quarter as well as the expected number, under the assumption that the null hypothesis of no excess risk following the warnings is correct.29
To calculate expected event rates, we used a Poisson regression with a linear term to model the temporal trend in the quarterly rates during the baseline and extrapolated the baseline trend to the post-warning period to calculate the expected event rates for comparison purposes for all post-warning quarters. A key assumption was that the baseline trend would have continued if the policy had not occurred.
Sequential Analyses
We performed analyses that mimicked quarterly prospective surveillance. Sequential analysis is used when there are repeated queries of the data over time, on a periodic basis (e.g., monthly, quarterly). Adjustments are made for multiple tests inherent in the repeated looks at the gradually accumulating longitudinal data. We used the Poisson maxSPRT in this study because it does not require an ‘a priori’ specification of the magnitude of the risk level under the alternative hypothesis.29 The null hypothesis is rejected if there are sufficiently more observed cases than expected in the accumulated data to date. An event signal is generated the first time the log likelihood ratio (LLR) exceeds a critical value, B (i.e., when LLR(t)>B) during the post-policy period, indicating a statistically significant association between the policy and the outcome. Relative risk (RR) is the increased relative risk associated with the policy; RR was calculated as observed events divided by expected counts.
To establish the critical value, it is necessary to specify the alpha level and a pre-specified upper limit on the length of surveillance defined in terms of the expected number of observations (events) under the null hypothesis. We chose alpha to be 0.05 so that the overall probability of rejecting the null at any time during the surveillance was 0.05. We chose the length of surveillance to be a maximum of five years, which meant specifying a maximum of 2000 expected events under the null for psychotropic drug poisonings. That is, surveillance ends if and when a cumulative number of 2000 expected events has been reached without rejecting the null. The critical value was calculated using the R Sequential package (https://www.r-project.org/), and a signal was generated when the LLR exceeded 4.42 for psychotropic drug poisoning for all age groups. For completed suicides among adolescents and young adults, we specified a maximum of 100 and 500 expected events respectively under the null for completed suicides to correspond to approximately five years of surveillance; corresponding critical values are 3.95 and 4.22. We specified a maximum of 2000 expected events under the null for completed suicides among adults; the corresponding critical value is again 4.42. The Poisson models adjusted for health plan, sex, and educational status (defined by whether the individual resided in neighborhoods with less than 25% of the population having a college degree, Y/N). We conducted separate analyses for the three age groups.
Results
We studied approximately 1.1 million adolescents, 1.4 million young adults, and 5 million adults in each quarter. Characteristics of the three age groups included in the study have been reported previously.27
Psychotropic Drug Poisoning
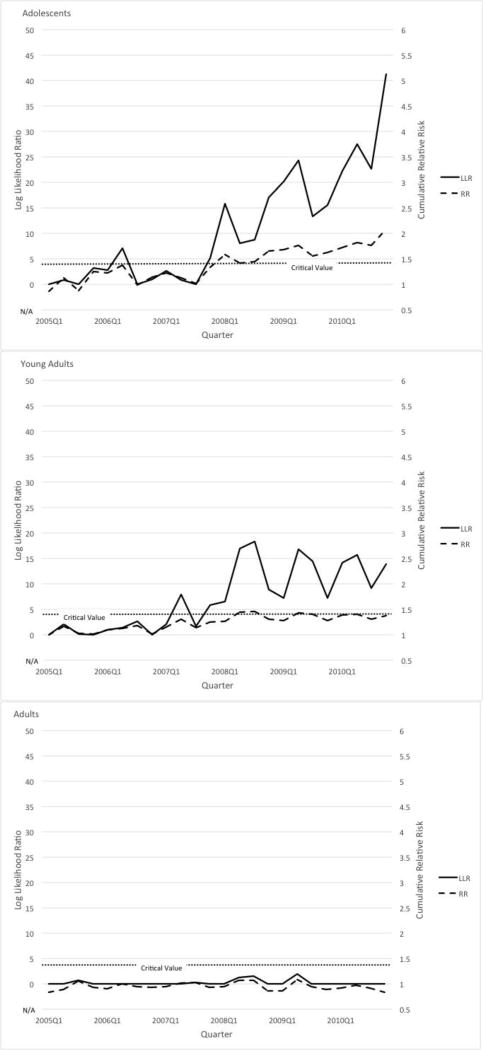
Table 1 presents the results using sequential analysis to assess the effect of the FDA warnings on psychotropic drug poisoning for all three age groups. Figure 2 displaysLLR and RR plots for psychotropic drug poisoning for the three age groups.
Table 1.
Sequential analysis results detecting signals in changes in psychotropic drug poisoning and suicide outcomes by age group
| Psychotropic drug poisoning | Suicide | |||||
|---|---|---|---|---|---|---|
| When critical value* was reached | Log likelihood | Relative risk | When critical value* was reached | Log likelihood | Relative risk | |
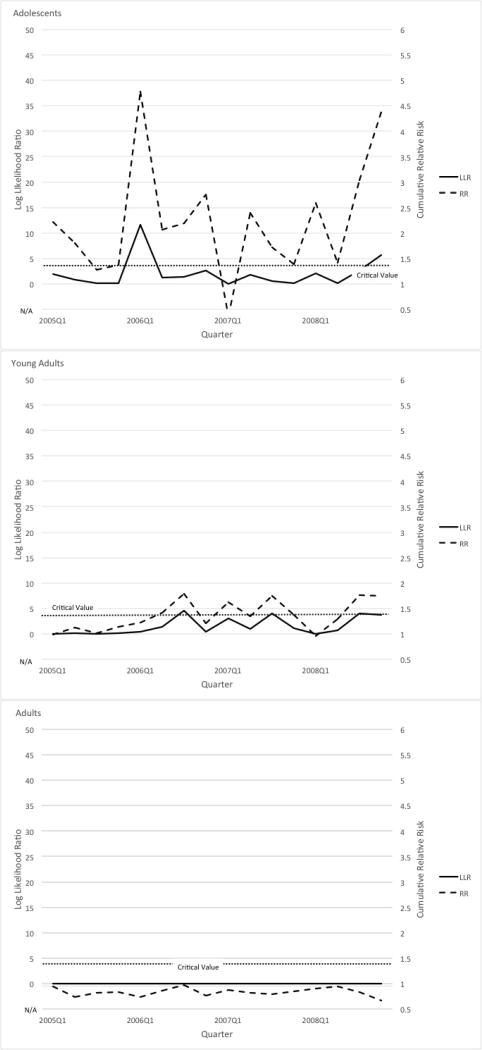
| Adolescents | 2007 Q4 | 5.12 | 1.34 | 2006 Q1 | 11.59 | 4.77 |
| Young adults | 2007 Q2 | 7.89 | 1.30 | 2006 Q3 | 4.62 | 1.79 |
| Adults | N/A | N/A | N/A | N/A | N/A | N/A |
Critical value = 4.42 based on alpha=0.05 and 2000 expected events under the null hypothesis for psychotropic drug poisonings for all age groups. Critical value = 3.95, 4.22, and 4.42 for adolescents, young adults, and adults respectively based on alpha = 0.05 and 100, 500, and 200 expected events under the null for completed suicides.
Figure 2.
Sequential analysis results on psychotropic drug poisonings among (a) adolescents, (b) young adults, and (c) adults.
The sequential analysis detected a signal in psychotropic drug poisonings in the last quarter of 2007 among adolescents and in the second quarter of 2007 among young adults. We did not detect a signal among adults for this outcome. Results remained the same in a sensitivity analysis that included the phase-in period (last quarter of 2003 through last quarter of 2004) in the post-policy period.
Completed Suicides
The results for completed suicides are shown in Table 1. Figure 3 displays LLR and RR plots for this outcome for all three age groups. Among adolescents, sequential analysis detected a signal in the first quarter of 2006 and among young adults, a signal in the third quarter of 2006. We did not detect a signal among adults for the suicide outcome. A sensitivity analysis that included the phase-in period (last quarter of 2003 through last quarter of 2004) in the post-policy period found the same results as the main analysis. Another sensitivity analysis that used data up to 2009 among 10 of 11 participating sites did not change the timing of the detected signals.
Figure 3.
Sequential analysis results on completed suicide among (a) adolescents, (b) young adults, and (c) adults.
Discussion
To our knowledge this is the first study to apply near real-time sequential analysis methods to examine potential unintended consequences of health policies. With the sequential approach, only pre-policy data are needed to estimate the expected count. Prospective, near real-time surveillance for selected outcomes can be implemented immediately after a policy change using accumulating data to rapidly assess the outcomes of policies. Using historical data, we modeled prospective surveillance for suicidality risk among youth following FDA antidepressant warnings and media reports. We assessed the timing of such signals and found that, had these innovative methods been employed prospectively, signals of increased risk of psychotropic drug poisoning (e.g., suicide attempts) – an unintended (indirect, latent) policy impact – might have been identified in adolescents and young adults within 10 quarters of the policy. Indeed, our previous study27 of the same policy using ITS analysis and data from the same organizations also found increases in psychotropic drug poisonings among adolescents and young adults following the widely publicized FDA warnings, but not among adults. Our prior study was conducted in 2012 after the 2010 data had been collected; hence, we only detected the effect eight years after the warnings were issued. An ITS analysis of data only up to the end of 2006 (two years after the warnings) did not detect increases in psychotropic drug poisonings among adolescents, demonstrating that longer follow-up data were essential using this method.
We also detected signals of increased risk of completed suicides in adolescents and young adults within 5-7 quarters of the policy. This, however, might be a false alert because our time series study27 did not detect increases in completed suicides after the policy. Sequential analysis works equally well for rare and common outcomes except for the difference in sample sizes that influences power and time to signal. On its own a signal from the sequential analysis should not be taken as conclusive evidence that the policy led to excess risk. A statistical signal indicates the need for further scrutiny using rigorous quasi-experimental studies (ITS for example) to investigate the possibility of a cause-and-effect relationship.
Near real-time sequential analysis may also be useful for other policy changes with possible intended and unintended consequences, for example, cost-containment policies (e.g., copayments, prior authorization) that are commonly used for prescription drugs. Other key benefits of the method relate to its use of routinely collected health plan encounter and dispensing data that are commonly used in health services and policy research, minimal data requirements in terms of needed data elements, the ability to simultaneously apply the method within a number of data systems and the use of a highly summarized data structures for aggregation across study sites.33, 35 Most public and private health insurers in the US have data that could support sequential analyses. Prospective surveillance of policies that affect commercially insured and/or publicly insured populations is therefore possible.
Our conceptual model presents an approach for designing policy research by examining different types of consequences. Ideally the possible consequences should be examined using data from the same database that contains sufficient observations before and after the policy; this guides the selection of databases for policy research. In our more rigorous quasi-experimental study, we examined three distinct outcomes (antidepressant use, psychotropic drug poisonings as proxy for suicide attempts, and completed suicides) using the same database and in a study population representative of the US general population.27 There are a number of databases that also contain information on rates of suicide attempts in the US.52 These data, however, would not allow examination of multiple likely consequences of this policy in the same study population and some provide insufficient data points before and after the policy to control for secular trends (history bias). They would not meet the simple inclusion criteria of international systematic reviews.53 The appropriateness of these databases for examining this policy is discussed in detail in our Counterpoint article.54
There are potential study limitations. Developing and applying robust measures that are consistent over time to examine policy consequences are important and could be challenging. We used psychotropic drug poisonings as a proxy for suicide attempts. These poisonings include both suicidal and non-suicidal overdoses but they underestimate suicide attempts.30 However, psychotropic drug poisonings were the most appropriate measure in our data for examining this policy over time because use of other metrics (self-harm E-codes and an established algorithm for suicide attempts) would introduce ascertainment bias. The limitations of this proxy measure are discussed in detail in our Counterpoint article.30, 49, 54 Our other measure – completed suicides in this policy example – derived predominantly from state death registries, is not vulnerable to similar limitations. Another challenge for policy evaluation is that there might be unanticipated consequences; thus, measures for such outcomes might not be developed and implemented a priori. Sequential analysis using automated healthcare claims data will only be useful if it has reasonable sensitivity and does not generate an unacceptable number of false positives. False-positive findings might be possible early in the study when relatively few data have accumulated and test statistics are less stable. We used quarterly data in this study because we studied rare outcomes. Many intended and unintended outcomes of policies are more frequent (e.g., therapeutic substitution); thus, prospective surveillance of policy impacts using sequential analysis could take advantage of administrative data that might be updated as frequently as weekly or monthly to allow near real-time detection of signals. This is a proof-of-concept study. This method should be tested for other FDA regulatory actions and policy changes. Further applications and accumulated experience with implementation, analysis and reporting of results would help investigators establishing methodological criteria to address issues of policy exposure, events, and setting a minimum number of observations before accepting a signal.
In summary, signals of excess risk for psychotropic drug poisonings in adolescents and young adults were detected after the warnings. These results were confirmed by our more rigorous previous study using interrupted time series analysis that can establish causal relationship.8 Our results support the continued investigation of sequential analysis as a potentially important tool for other health policy changes. Prospective, periodic evaluation of observational healthcare data can complement strong quasi-experimental studies as it holds the potential to rapidly identify early signals of intended and unintended effects of large-scale interventions on population health.
Acknowledgments
We thank all project managers and analysts at participating sites. We are grateful to Caitlin Lupton for administrative assistance. This work was conducted at the Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute.
Funding/Support: This research was supported by a cooperative agreement (U19MH092201; principal investigator, GS) with the US National Institute of Mental Health, Bethesda, MD; SBS was the study principal investigator. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The statements, findings, conclusions, views, and opinions contained an expressed herein are not necessarily those of Harvard Medical School and Harvard Pilgrim Health Care Institute or any of its affiliated or subsidiary entities.
Potential conflicts of interest: All authors report grant support from the National Institute of Mental Health during the conduct of the study. Dr. Penfold reports grants from Novartis Pharmaceuticals, grants from Bristol-Meyers Squibb and grants from Otsuka Pharmaceuticals that were outside the submitted work. Dr. Simon reports grants from Novartis Pharmaceuticals, for research regarding depression and suicidal behavior in psoriasis and grants from Otsuka Pharmaceuticals, for research regarding patterns of antidepressant augmentation in community practice, outside the submitted work. Dr. Copeland reports grants from Veterans Health Administration, VA HSR&D DHI-09-237, VA RR&D 1I21RX000809-01A1, VA HSRD HX-12-347-0, VA Office of Rural Health #N17-FY14Q3-S0-P01250, VA HSRD HX-12-358, VHA HSR&D FOP-15-464, VA HSR&D HX-13-029, grants from Scott & White Research Mentor Award, grants from Commonwealth Fund, grants from HMORN-OAIC AGING Initiative Pilot/NIA, sub award of 1R24AG045050-01A1, outside the submitted work.
Contributor Information
Christine Y Lu, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, 401 Park Drive, Suite 401 East, Boston, MA 02115.
Robert B Penfold, Group Health Research Institute and Department of Health Services Research, University of Washington, 1730 Minor Ave #1600, Seattle, WA 98101.
Sengwee Toh, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, 401 Park Drive, Suite 401 East, Boston, MA 02115.
Jessica L Sturtevant, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, 401 Park Drive, Suite 401 East, Boston, MA 02115.
Jeanne M Madden, School of Pharmacy, Northeastern University, 360 Huntington Ave., 226X, 140, The Fenway, Boston, MA 02215 and Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, 401 Park Drive, Suite 401 East, Boston, MA 02115.
Gregory Simon, Group Health Research Institute, 1730 Minor Ave #1600, Seattle, WA 98101.
Brian K Ahmedani, Center for Health Policy and Health Services Research and Behavioral Health Services, Henry Ford Health System, Detroit, 1 Ford Place, Detroit, MI 48202.
Gregory Clarke, Center for Health Research, Kaiser Permanente Northwest, 3800 N. Interstate Avenue, Portland, OR 97227.
Karen J Coleman, Research and Evaluation, Southern California Permanente Medical Group, 100 S. Los Robles Ave. Pasadena, CA 91101.
Laurel A Copeland, Center for Applied Health Research, Baylor Scott & White Health jointly with Central Texas Veterans Health Care System, 2102 Birdcreek Drive, Temple, TX 76502.
Yihe Daida, Center for Health Research, Kaiser Permanente Hawaii, 501 Alakawa Street, Suite 201 Honolulu, HI 96817.
Robert L Davis, Center for Biomedical Informatics, University of Tennessee Health Science Center, 920 Madison Ave. Memphis, TN 38163.
Enid M Hunkeler, Kaiser Permanente, Division of Research, 2000 Broadway, Oakland, CA 94612.
Ashli Owen-Smith, Health Management & Policy, Georgia State University School of Public Health, 1 Park Place, Suite 662D, Atlanta, GA 30303 and Kaiser Permanente Georgia, The Center for Clinical and Outcomes Research, 3495 Piedmont Road NE, Building 11, Suite 110, Atlanta, GA 30305.
Marsha A Raebel, Kaiser Permanente Colorado, Institute for Health Research, 10065 E. Harvard Ave. Suite 300 Denver, CO 378066.
Rebecca Rossom, HealthPartners Institute for Education and Research, 311 E. Old Shakopee Road, Bloomington, MN 55425.
Stephen B Soumerai, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, 401 Park Drive, Suite 401 East, Boston, MA 02115.
Martin Kulldorff, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Harvard Medical School and Brigham and Women's Hospital, 1620 Tremont Street, Suite 3030, Boston, MA 02120.
References
- 1.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 2.Schneeweiss S, Maclure M, Walker AM, et al. On the evaluation of drug benefits policy changes with longitudinal claims data: the policy maker's versus the clinician's perspective. Health Policy. 2001;55:97–109. doi: 10.1016/s0168-8510(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 3.Lu CY, Ross-Degnan D, Soumerai SB, et al. Interventions designed to improve the quality and efficiency of medication use in managed care: a critical review of the literature - 2001-2007. BMC Health Serv Res. 2008;8:75. doi: 10.1186/1472-6963-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff (Millwood) 2004;23:135–146. doi: 10.1377/hlthaff.23.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Dusetzina SB, Higashi AS, Dorsey ER, et al. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50:466–478. doi: 10.1097/MLR.0b013e318245a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams AS, Zhang F, LeCates RF, et al. Prior authorization for antidepressants in Medicaid: effects among disabled dual enrollees. Arch Intern Med. 2009;169:750–756. doi: 10.1001/archinternmed.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Med Care. 2010;48:4–9. doi: 10.1097/MLR.0b013e3181bd4c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2002. [Google Scholar]
- 9.Lieu TA, Kulldorff M, Davis RL, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007;45:S89–95. doi: 10.1097/MLR.0b013e3180616c0a. [DOI] [PubMed] [Google Scholar]
- 10.Yih WK, Kulldorff M, Fireman BH, et al. Active surveillance for adverse events: the experience of the Vaccine Safety Datalink project. Pediatrics. 2011;127(1):S54–64. doi: 10.1542/peds.2010-1722I. [DOI] [PubMed] [Google Scholar]
- 11.Davis RL, Kolczak M, Lewis E, et al. Active surveillance of vaccine safety: a system to detect early signs of adverse events. Epidemiology. 2005;16:336–341. doi: 10.1097/01.ede.0000155506.05636.a4. [DOI] [PubMed] [Google Scholar]
- 12.Avery TR, Kulldorff M, Vilk Y, et al. Near real-time adverse drug reaction surveillance within population-based health networks: methodology considerations for data accrual. Pharmacoepidemiol Drug Saf. 2013;22:488–495. doi: 10.1002/pds.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yih WK, Kulldorff M, Sandhu SK, et al. Prospective influenza vaccine safety surveillance using fresh data in the Sentinel System. Pharmacoepidemiol Drug Saf. 2016;25:481–492. doi: 10.1002/pds.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fireman B, Toh S, Butler MG, et al. A protocol for active surveillance of acute myocardial infarction in association with the use of a new antidiabetic pharmaceutical agent. Pharmacoepidemiol Drug Saf. 2012;21(1):282–290. doi: 10.1002/pds.2337. [DOI] [PubMed] [Google Scholar]
- 15.Dal Pan GJ. Communicating the risks of medicines: time to move forward. Med Care. 2012;50:463–465. doi: 10.1097/MLR.0b013e31825852f0. [DOI] [PubMed] [Google Scholar]
- 16.Chace MJ, Zhang F, Fullerton CA, et al. Intended and unintended consequences of the gabapentin off-label marketing lawsuit among patients with bipolar disorder. J Clin Psychiatry. 2012;73:1388–1394. doi: 10.4088/JCP.12m07794. [DOI] [PubMed] [Google Scholar]
- 17.Moynihan R, Bero L, Ross-Degnan D, et al. Coverage by the news media of the benefits and risks of medications. N Engl J Med. 2000;342:1645–1650. doi: 10.1056/NEJM200006013422206. [DOI] [PubMed] [Google Scholar]
- 18.Soumerai SB, Ross-Degnan D, Kahn JS. Effects of professional and media warnings about the association between aspirin use in children and Reye's syndrome. Milbank Q. 1992;70:155–182. [PubMed] [Google Scholar]
- 19.FDA. FDA Launches a Multi-Pronged Strategy to Strengthen Safeguards for Children TreatedWith Antidepressant Medications. [Accessed May 01, 2016];2004 Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108363.htm.
- 20.FDA. Worsening Depression and Suicidality in Patients Being Treated With Antidepressants. [Accessed May 01, 2016];2004 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161696.htm.
- 21.Barry CL, Busch SH. News coverage of FDA warnings on pediatric antidepressant use and suicidality. Pediatrics. 2010;125:88–95. doi: 10.1542/peds.2009-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris G. F.D.A links drugs to being suicidal. The New York Times. 2004 [Google Scholar]
- 23.Vedantam S. FDA confirms antidepressants raise children's suicide risk. The Washington Post. 2004 [Google Scholar]
- 24.Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry. 2007;164:884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- 25.Busch SH, Frank RG, Martin A, et al. Characterizing declines in pediatric antidepressant use after new risk disclosures. Med Care Res Rev. 2011;68:96–111. doi: 10.1177/1077558710374197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busch SH, Barry CL. Pediatric antidepressant use after the black-box warning. Health Aff (Millwood) 2009;28:724–733. doi: 10.1377/hlthaff.28.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi: 10.1136/bmj.g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch SH, Frank RG, Leslie DL, et al. Antidepressants and suicide risk: how did specific information in FDA safety warnings affect treatment patterns? Psychiatr Serv. 2010;61:11–16. doi: 10.1176/appi.ps.61.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulldorff M, Davis RL, Kolczak M, et al. A Maximized Sequential Probability Ratio Test for Drug and Vaccine Safety Surveillance. Sequential Analysis. 2011;30:58–78. [Google Scholar]
- 30.Lu CY, Stewart C, Ahmed AT, et al. How complete are E-codes in commercial plan claims databases? Pharmacoepidemiol Drug Saf. 2014;23:218–220. doi: 10.1002/pds.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmedani BK, Solberg LI, Copeland LA, et al. Psychiatric comorbidity and 30-day readmissions after hospitalization for heart failure, AMI, and pneumonia. Psychiatr Serv. 2015;66:134–140. doi: 10.1176/appi.ps.201300518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman KJ, Stewart C, Waitzfelder BE, et al. Racial-Ethnic Differences in Psychiatric Diagnoses and Treatment Across 11 Health Care Systems in the Mental Health Research Network. Psychiatr Serv. 2016 doi: 10.1176/appi.ps.201500217. appips201500217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornbrook MC, Hart G, Ellis JL, et al. Building a virtual cancer research organization. J Natl Cancer Inst Monogr. 2005:12–25. doi: 10.1093/jncimonographs/lgi033. [DOI] [PubMed] [Google Scholar]
- 34.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 35.Andrade SE, Raebel MA, Boudreau D, et al. Health Maintenance Organizations / Health Plans. In: Strom BL, Kimel SE, Hennessy S, editors. Pharmacoepidemiology. Wiley-Blackwell; 2012. pp. 163–188. [Google Scholar]
- 36.Rogers EM. Diffusion of Innovations 5th Edition. New York: Simon and Schuster; 2003. [Google Scholar]
- 37.Bloomrosen M, Starren J, Lorenzi NM, et al. Anticipating and addressing the unintended consequences of health IT and policy: a report from the AMIA 2009 Health Policy Meeting. J Am Med Inform Assoc. 2011;18:82–90. doi: 10.1136/jamia.2010.007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby AM, Orton HD, Valuck RJ. Persisting decline in depression treatment after FDA warnings. Arch Gen Psychiatry. 2009;66:633–639. doi: 10.1001/archgenpsychiatry.2009.46. [DOI] [PubMed] [Google Scholar]
- 39.Pamer CA, Hammad TA, Wu YT, et al. Changes in US antidepressant and antipsychotic prescription patterns during a period of FDA actions. Pharmacoepidemiol Drug Saf. 2010;19:158–174. doi: 10.1002/pds.1886. [DOI] [PubMed] [Google Scholar]
- 40.Valuck RJ, Libby AM, Orton HD, et al. Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry. 2007;164:1198–1205. doi: 10.1176/appi.ajp.2007.07010007. [DOI] [PubMed] [Google Scholar]
- 41.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 42.March JS, Silva S, Petrycki S, et al. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64:1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons RD, Brown CH, Hur K, et al. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:580–587. doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrato EH, Libby AM, Orton HD, et al. Frequency of provider contact after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry. 2008;165:42–50. doi: 10.1176/appi.ajp.2007.07010205. [DOI] [PubMed] [Google Scholar]
- 45.Crosby AE, Han B, Ortega LAG, et al. Suicidal thoughts and behaviors among adults aged ≥18 years-United States, 2008-2009. MMWR Surveill Summ. 2011;60(no. SS-13) [PubMed] [Google Scholar]
- 46.Clarke G, Dickerson J, Gullion CM, et al. Trends in youth antidepressant dispensing and refill limits, 2000 through 2009. J Child Adolesc Psychopharmacol. 2012;22:11–20. doi: 10.1089/cap.2011.0048. [DOI] [PubMed] [Google Scholar]
- 47.Townsend L, Walkup JT, Crystal S, et al. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(1):163–173. doi: 10.1002/pds.2310. [DOI] [PubMed] [Google Scholar]
- 48.Spettell CM, Wall TC, Allison J, et al. Identifying physician-recognized depression from administrative data: consequences for quality measurement. Health Serv Res. 2003;38:1081–1102. doi: 10.1111/1475-6773.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patrick AR, Miller M, Barber CW, et al. Identification of hospitalizations for intentional self-harm when E-codes are incompletely recorded. Pharmacoepidemiol Drug Saf. 2010;19:1263–1275. doi: 10.1002/pds.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiller HA, Appana S, Brock GN. Epidemiological trends of suicide and attempted suicide by poisoning in the US: 2000-2008. Leg Med (Tokyo) 2010;12:177–183. doi: 10.1016/j.legalmed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry. 2012;34:557–565. doi: 10.1016/j.genhosppsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber CW, Miller M. Re: Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi: 10.1136/bmj.g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cochrane Effective Practice and Organization of Care. EPOC-specific resources for review authors. [Accessed February 15, 2017];2017 Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors.
- 54.Simon LuCY, Kulldorff GM, et al. Counterpoint: Title TBD. Med Care. 2017 [Google Scholar]