Abstract
Objective To provide a comprehensive summary (systematic review) of medication adherence rates by assessment method and medication type for pediatric patients with sickle cell disease (SCD), as well as identify important correlates for future research. Methods Articles assessing medication adherence and published between 1982 and February 2015 ( n = 49) were identified using electronic databases. A meta-analysis of 14 studies examining demographic, medical, and psychosocial factors and medication adherence was conducted. Results Adherence rates ranged from 12% to 100% across all medications. Approximately 30% of studies reported associations between adherence and key demographic, medical, and psychosocial correlates. Mean effect sizes were small to moderate ( r = .02–.53). Conclusions The wide range of adherence rates reported in the literature may be because of, in part, the use of variable assessment strategies. Future studies examining pediatric SCD adherence should incorporate key correlates with the goal of replication.
Keywords: adherence, children, meta-analysis, sickle cell disease, systematic review
Sickle cell disease (SCD) is an inherited red blood cell disorder that affects approximately 1 in 400–500 African American and 1 in 1,000–1,400 Hispanic/Latino American children in the United States ( Division of Blood Diseases and Resources, National Institutes of Health, 2002 ; Hassell, 2010 ). Complications of SCD include pain crises, infections, organ damage, and other medical sequelae that result in increased health care utilization, as well as death ( Kauf, Coates, Huazhi, Mody-Patel, & Hartzema, 2009 ). Despite the significant morbidity and mortality of SCD, developments in research and clinical care have been slow relative to other potentially fatal medical conditions, prompting legislation to expand comprehensive SCD care ( Smith, Oyeku, Homer, & Zuckerman, 2006 ). However, some treatment advances have been significant in improving the course of the disease including prophylactic antibiotics to prevent septic infections and hydroxyurea to increase fetal hemoglobin. Although these treatments have potential for reducing morbidity and increasing the life span ( Rees, Williams, & Gladwin, 2010 ), there is evidence that adherence to these medications is variable ( Walsh et al., 2014 ).
Medication adherence difficulties in children with SCD can lead to additional disease complications and even death. In one study, poor adherence to prophylactic antibiotics was related to higher rates of infection and sickle cell pain crises ( Patel & Athavale, 2004 ). Less than optimal medication adherence to these treatments may increase emergency department visits and inpatient hospitalizations, thereby increasing health care costs. This is the case in other pediatric chronic conditions ( McGrady & Hommel, 2013 ), and suggests that poor medication adherence may have a significant negative financial impact, on top of contributing to detrimental health outcomes.
Relative to other common pediatric chronic illnesses, such as asthma and diabetes, there is less known about what contributes to adherence difficulties in children with SCD. The Pediatric Self-Management Model ( Modi et al., 2012 ) is a framework that emphasizes modifiable and nonmodifiable influences, and suggests that demographic, medical, and psychosocial factors at the patient, family (e.g., marital status, parental involvement), and health care system (e.g., access to care, patient-provider communication) levels may have a negative impact on adherence and health outcomes. However, there is considerable inconsistency in study findings regarding correlates of adherence in pediatric SCD. For example, some studies have found significant associations between demographic factors such as employment status ( Witherspoon & Drotar, 2006 ), insurance type ( Raphael et al., 2013 ), and family income ( Barakat, Smith-Whitley, & Ohene-Frempong, 2002 ) and adherence, while other studies have failed to demonstrate these associations ( Bitaraes et al., 2008 ; Fisak, Belkin, von Lehe, & Bansal, 2011 ). Studies examining the relation between patient and family factors and adherence have also produced conflicting results. Specifically, poorer child or parent psychosocial functioning was significantly related to medication adherence difficulties in some studies ( Barakat, Lutz, Smith-Whitley, & Ohene-Frempong, 2005 ; Witherspoon & Drotar, 2006 ), but not others ( Barakat et al., 2002 ; Raphael et al., 2013 ; Treadwell et al., 2005 ).
Because SCD affects a greater number of individuals from diverse backgrounds, there may be specific stressors, such as discrimination and systemic factors that uniquely contribute to adherence difficulties in these families. Specifically, bias surrounding race and ethnicity negatively influence providers’ perceptions of adherence, and contributes to lower quality of care, particularly when no clear standard of treatment exists ( Sabin, Rivara, & Greenwald, 2008 ) as is true with pediatric SCD. Individuals from diverse backgrounds may also experience overt and covert racism in medical settings ( Musa, Schilz, Harris, Silverman, & Thomas, 2009 ), which can contribute to the development of general mistrust of health care providers ( Blanchard & Lurie, 2004 ). This is problematic, as trust in the health care system and relationship with medical providers influence adherence to recommended medication regimens ( De Civita & Dobkin, 2004 ). Additionally, from a logistical standpoint, there may be difficulties accessing medical care and medication, as patients with SCD have a lower mean income than the national average and are more likely to receive coverage through Medicaid ( Brawley et al., 2008 ). These barriers are cumulative among children with SCD, such that higher numbers of risk factors result in even lower adherence rates ( Witherspoon & Drotar, 2006 ). In short, understanding culturally relevant or modifiable factors that influence adherence and how best to optimize adherence is critical for mitigating disparate outcomes for youth with SCD.
The first objective of this study was to conduct a systematic review to provide the most comprehensive summary of adherence rates by assessment method and medication type in pediatric SCD. Additionally, we aimed to describe characteristics of the individual studies to highlight the current state of this literature and common methodological approaches. Identifying methodological strengths and weaknesses will serve to inform future research design and maximize the extremely limited research funding for SCD. The second objective was to conduct a meta-analysis to examine the magnitude of associations between medication adherence and key demographic, medical, and psychosocial factors as suggested by the Pediatric Self-Management Model ( Modi et al., 2012 ). Few studies of medication adherence in children with SCD evaluate the association between adherence and key correlates (e.g., age, internalizing symptoms, family functioning) that have been identified in the larger pediatric literature. Although a recent review of the SCD adherence literature ( Walsh et al., 2014 ) identified potentially important correlates, including health care utilization (e.g., hospitalizations) and barriers (e.g., forgetting), the magnitude of associations was not reported in a systematic manner. This study is the first to examine associations between demographic, medical, and psychosocial factors and SCD medication adherence using meta-analytic methodology. Identifying significant modifiable correlates of adherence across existing studies is crucial for informing intervention development, which could lead to improved adherence and fewer health care disparities.
Method
Search Strategy and Study Selection
The following electronic databases were searched: PubMed (all dates); Scopus, CINAHL, PsycINFO, ERIC, Ovid, EBSCOhost, and EBMR Reviews (1982–February 2015). Additionally, reference lists from SCD medication adherence studies were checked for additional papers that were not identified in the initial electronic database searches. Key terms included, “medication adherence,” “medication compliance,” “treatment compliance,” “sickle cell disease,” “sickle cell anemia,” “infant,” “child,” and “adolescent.” Studies were included if they met the following criteria: (1) individuals in the sample were diagnosed with SCD, (2) the study included patients who were ≤21 years old, and (3) the study assessed medication (vs. clinic attendance, transcranial doppler screenings, etc.) adherence rates.
Data Extraction
Three of the authors (J.L.L., L.S., S.D.) retrieved data from identified articles using a standardized data collection form. The data retrieved included participant demographic (e.g., age, sex, family income) and medical (e.g., SCD genotype, disease severity) characteristics, adherence assessment instruments used, rates of adherence, and correlates (e.g., health care utilization, lab values, psychosocial functioning) of adherence. These correlates were selected and structured for analysis using theoretical guidance from the Pediatric Self-Management Model ( Modi et al., 2012 ). All abstractions were reviewed by the first author (K.L.) and discrepancies were resolved by consensus.
Study Quality Ratings
The studies included in the meta-analysis were evaluated using a 22-item quality rating rubric, which was developed for this study. Items were generated with guidance from other study quality rating tools used for observational studies ( DuRant, 1994 ; Fowkes & Fulton, 1991 ). Two independent coders (J.L.L., S.D.) rated each study according to the presence (1) or absence (0) of each quality metric (e.g., authors used a theoretical framework for the study; at least one validated adherence measure was used), yielding a total possible score from 0 ( poor quality ) to 22 ( excellent quality ). Cohen’s kappa was used to determine the degree of agreement between the two coders for each metric ( Cohen, 1960 ). Rater agreement (κ = .73) was substantial ( Landis & Koch, 1977 ). Disagreement regarding codes was resolved by the first author (K.L.).
Statistical Analyses
Studies that considered the association between medication adherence and a demographic, medical, or psychosocial factor of interest were included in the meta-analysis. Correlates were assigned to one of the following broader categories: Demographic (e.g., age, family income, insurance status), Medical (e.g., hospitalizations, biomarkers), Disease Knowledge, Adherence Barriers, and Psychosocial Functioning (e.g., health-related quality of life [HRQL], child behavior problems). If a correlate was assessed in two or more studies, a separate effect size (ES) was calculated. If only one study examined a particular association (e.g., adherence and self-efficacy), it was used to calculate the ES for the broader category. Comprehensive Meta-analysis V2 ( Biostat, 2005 ) was used to calculate the mean ES. Pearson’s r was used as the measure of association between a given correlate and rate of medication adherence. The random-effects model is reported for all ES. Random-effects models are used when studies have variable methodologies and to generalize the mean ES to the larger population ( Borenstein, Hedges, Higgins, & Rothstein, 2010 ).
Results
Search Results
After thorough review of abstracts and manuscript content, our searches identified 49 studies that reported rates of medication adherence among pediatric patients with SCD. Of the 49 articles that were included in the systematic review, 14 met criteria for the meta-analysis. The additional 35 articles were excluded because 9 (18.4%) did not include any measures of correlates, 13 (26.5%) did not examine associations between adherence and other factors assessed in the study (e.g., hospitalizations, % HbF), and 14 (28.6%) required additional information from authors because an association was tested, but a correlation coefficient was not reported. One author supplied the requested data. Two authors could not be reached owing to failure to find current contact information. Four authors indicated that they no longer had access to the raw data. The remaining seven authors did not respond. As a result, no additional information could be gathered, and those 13 articles were excluded from the meta-analysis. Figure 1 contains the PRISMA four-phase flow diagram depicting study selection.
Figure 1.
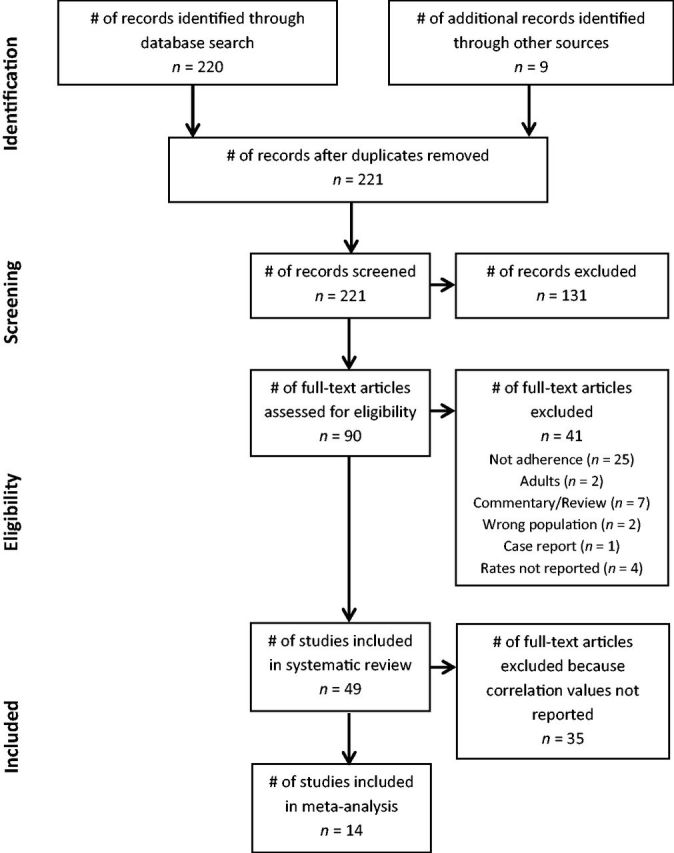
The PRISMA four-phase flow diagram detailing study selection.
Systematic Review Study Characteristics
There were 49 studies that met criteria for the systematic review. Studies were conducted on five different continents, including North America (United States, n = 36; Canada, n = 1; Jamaica, n = 1), Europe (England, n = 3; France, n = 1; Italy, n = 1), South America (Brazil, n = 1), Asia (Saudi Arabia, n = 3; India, n = 1), and Africa (Nigeria, n = 1). Study sample sizes ranged from 8 to 763 (median = 50). The mean age across participants was 8.4 years. Of note, eight studies did not report the mean age of participants. Forty-one (83.7%) studies provided information about the children’s gender. The percentage of males ranged from 39.5% to 100% ( M = 56.8%, SD = 11.4%). Only 12 (24.5%) studies reported quantifiable information about the race or ethnicity of the participants. Thirteen (6.5%) reported family income. Seven (14.3%) studies reported the participants’ insurance status. Of these studies, the average percentage of participants with public insurance was 89% (range = 66.7–100%). Thirty-five (71.4%) studies reported the participants’ SCD genotype breakdown. Of those studies, the mean percentage of patients with HbSS (the most severe and common genotype) was 86.4% (range = 48.4–100%). Fourteen (28.6%) studies reported recruitment rates. Of those studies, the mean recruitment rate was 69.4% (range = 44%–100%). The average retention rate was 79.5%; however, only two studies reported this information.
Rates of Adherence by Assessment Method
Twenty-seven (55.1%) studies used a single method to assess adherence, 21 (42.9%) studies used multiple methods (e.g., questionnaire and pharmacy refill data), while one study (2%) did not report the assessment method. Twenty-nine (59.2%) studies used at least one subjective measure (e.g., questionnaire, diary), while 33 (67.3%) studies used at least one objective (e.g., bioassay, electronic monitoring) measure (see Table I ).
Table I.
Summary of Studies Based on Medication Type
| Author, Year | N | M age or range (years) | % male | Adherence measure | Adherence % or M adherence |
|---|---|---|---|---|---|
| Prophylactic antibiotics | |||||
| Anglin et al., 1984 | 34 | 0.5–5 | NR | Bioassay (urine test) | 64% |
| Babiker, 1986a | 24 | 2–5 | NR | Bioassay (urine test) | 40% |
| Babiker, 1986b | 32 | 5.9 | 66.0 | Bioassay (urine test) | 44% |
| Berkovitch, 1998 | 45 | 3.1 | NR | Electronic monitoring (MEMS) | 65.8–82% |
| Bitaraes et al., 2008 | 108 | 2.1 | 45.0 | Self-report | 48% |
| Medical record review | 89% | ||||
| Bioassay (urine test) | 56% | ||||
| Buchanan, Siegel, Smith, & DePasse, 1982 | 38 | 1.9 | 65.8 | Bioassay (urine test) | 64% |
| Buchanan & Smith, 1986 | 88 | 0.25–4.6 | Self-report | 12.5% | |
| Colombatti et al., 2012 | 90 | 2.9 | 45.6 | Medical record review | 40–100% |
| Cummins, Heuschkel, & Davies, 1991 | 50 | <16 | NR | Self-report | 62% |
| Bioassay (urine test) | 47% | ||||
| Dalton et al., 2005 | 12 | 11.3 | 66.7 | Bioassay (urine test) | 75% |
| Day, 1992 | 8 | NR | NR | Self-report | 12.5% |
| Elliott et al., 2001 | 50 | 2.4 | 48 | Self-report | 54% |
| Pharmacy refill data | 12% | ||||
| King, Ali, Knight-Madden, MooSang, & Reid, 2011 | 78 | 2.6 | 53.8 | Medical record review | 88.5% |
| Patel and Athavale, 2004 | 325 | 7.05 | 57.5 | Urine test + pill count | 24.87% |
| Patel, Lindsey, Strunk, & DeBaun, 2010 | 93 | 7.0 | 59.1 | Medical record review | 54.9% |
| Pejaver, Ahmed, & Al Hifzi, 1997 | 42 | 0.9–12 | 53.6 | Self-report | 63.4% |
| Bioassay (urine test) | 46.3% | ||||
| Sox, Cooper, Koepsell, DiGiuseppe, & Christakis, 2003 | 261 | 1.4 | 54.8 | Pharmacy refill data | 41% |
| Teach, Lillis, & Grossi, 1998 | 125 | 8.9 | 49.6 | Self-report | 67.5% |
| Bioassay (urine test) | 43.1% | ||||
| Warren et al., 2010 | 407 | “Infants” | NR | Pharmacy refill data | 40% |
| Witherspoon & Drotar, 2006 | 30 | 2.95 | 50.0 | Self-report (barriers interview) | 56.7% |
| Medical provider rating (1–7 scale) | 50% | ||||
| Pharmacy refill data | 33.3% | ||||
| Hydroxyurea | |||||
| Creary, Gladwin, Byrne, Hildesheim, & Krishnamurti, 2014 | 14 | 13.7 | 50.0 | Self-report (Morisky) ≥ 2 | 93% |
| MPR | 75% | ||||
| Video observation | 88.6% | ||||
| Crosby et al., 2012 | 43 | 12.81 | 39.5 | Self-report | 88% |
| Medical provider rating | 40% | ||||
| Dalton, 2005 | 12 | 11.3 | 66.7 | Self-report | 87–100% |
| Bioassay (urine test) | 83% | ||||
| de Montalembert et al., 2006 | 225 | 9.2 | 60.9 | Self-report | 92.5% |
| Kinney et al., 1999 | 84 | 9.8 | 52.4 | Pill count | 74% |
| Olivieri & Vichinksy, 1998 | 17 | 12.3 | NR | Self-report (diary) | 76.3% |
| Electronic monitoring (MEMS) | 96% | ||||
| Patel, 2010 | 93 | 7.0 | 59.1 | Medical record review | 60.5% |
| Thornburg, Calatroni, Telen, & Kemper, 2010 | 75 | <18 | 53.0 | Self-report (VAS) | 59% |
| Self-report (Morisky) | 62% | ||||
| Medical provider rating | 64% | ||||
| Pharmacy refill data | 37% | ||||
| Thornburg, Rogers, et al., 2010b | 153 | 0.75–1.4 | NR | Pill count | 88.9% |
| Ware et al., 2002 | 53 | 9.5 | 60.0 | Pill count | 94.4% |
| Ware et al., 2004 | 35 | 11.9 | 65.7 | Discussion with patients + frequency of missed clinic appointments + blood counts + % HbF | 86% |
| Zimmerman et al., 2004 | 122 | 0.5–19.7 | 71.0 | Self-report + pill count + lab values | 88% |
| Zimmerman, Schultz, Burgett, Mortier, & Ware, 2007 | 37 | 6.8 | NR | Hematological parameters + clinic attendance | 84% |
| Iron chelators | |||||
| Alvarez et al., 2009 | 21 | 13.8 | 52.0 | Self-report | 71% |
| Pill count | 43% | ||||
| Jordan, Vekeman, Sengupta, Corral, Guo, & Duh, 2012 | 762 | NR | 41.0 | MPR (deferoxamine) | 41.8% |
| MPR (deferasirox) | 58.4% | ||||
| Treadwell et al., 2005 | 15 | 12.1 | 46.7 | Self-report (Morisky) | 2.0 |
| Medical provider rating | “Moderate” | ||||
| Pharmacy refill data | 60% | ||||
| Tsouana et al., 2015 | 62 | 9.2 | 45.0 | Self-report (questionnaire) | 75% |
| Clinical assessment + medical record review + interview | 89% | ||||
| Ware, Zimmerman, & Schultz, 1999 | 16 | NR | 68.75 | Not specified | 22.2% |
| Other medications | |||||
| Resar et al., 2002 | 8 | 8.6 | 87.5 | Self-report (diary) + pill count + electronic monitoring (APREX) | 97.6% |
| Williams et al., 1996 | 80 | 3.3 | 59.0 | Self-report | 94% |
| Bioassay (urine test) | 96% | ||||
| Williams et al., 2004 | 27 | 5.2–17.9 | 48.1 | Self-report (calendar) | 96% |
| Overall medication regimen | |||||
| Barakat et al., 2002 | 81 | 7.54 | 58.0 | Self-report (general information form) | 84.8% |
| Medical provider rating (1–7 scale) | 5.3 | ||||
| Barakat et al., 2005 | 64 | 10.5 | 59.6 | Self-report (general information form) | 84.2% |
| Physician rating (1–7 scale) | 5.4 | ||||
| Davis, 1998 | 519 | 2.5 | NR | Insurance claims | 81.7% |
| Fisak et al., 2011 | 78 | 11.38 | 55.1 | Self-report (SCI-SC; 18–90 range) | 75.7 |
| Hilker et al., 2006 | 99 | 10.85 | 59.6 | Self-report (SCI-SC) | 86% |
| Ikefuna & Emodi, 2007 | 71 | 7.5 | 53.5 | Medical record review | 29.6% |
| Jensen, 2005 | 97 | 10.8 | 59.8 | Self-report (SCI-SC; 1–5 scale) | 4.0 |
| Patel, 2010 | 93 | 7.0 | 59.1 | Medical record review | 58.4% |
| Raphael et al., 2013 | 150 | 1–17 | 52.7 | Self-report (SCI-SC; 1–5 scale) | 4.3 |
| Van Sciver, D’Angelo, Rappaport, & Woolf, 1995 | 22 | 11.9 | 100 | Medical provider rating (1-4 scale) | 3.0–3.6 |
| Wojciechowski, Hurtig, & Dorn, 2002 | 18 | 20.3 | 50.0 | Self-report (SCD compliance questionnaire; 0–28 scale) | 23.7 |
Note . MEMS = medication event monitoring system; SCI-SC = self-care inventory-sickle cell; MPR = medication possession ratio; VAS = visual analog scale; HbF = fetal hemoglobin; NR = not reported.
Subjective Methods
Twenty-seven (55.1%) studies assessed adherence using self-report and/or parent-proxy report. The instrument used varied considerably, with 63% of studies reporting adherence as a percentage. Most of the remaining studies reported adherence on a Likert scale, which was either validated (e.g., Self Care Inventory-Sickle Cell; Hilker, Jordan, Jensen, Elkin, & Iyer, 2006 ) or developed for the purpose of the study (e.g., Visual Analog Scale), or based on the presence or absence (yes/no) of adherence behaviors (e.g., Morisky score; Morisky, Green, & Levine, 1986 ). Adherence rates reported as percentages for self- or proxy-report ranged from 12.5% to 96% ( M = 66.5%, Mdn = 69.25%) across studies. Seven (14.3%) studies assessed adherence using medical provider rating. Of these seven studies, three (42.9%) reported adherence using a percentage, with the remaining studies using an unvalidated Likert scale. Adherence rates reported as percentages for medical provider rating ranged from 40% to 64% ( M = 51.3%, Mdn = 50%).
Objective Methods
Fourteen (28.6%) studies assessed adherence using bioassay (e.g., urine test). Adherence rates by this method ranged from 40% to 96% ( M = 59.9%, Mdn = 56%). Six (12.2%) assessed adherence using pharmacy refill records. Adherence rates by this method ranged from 12% to 60% ( M = 37.2%, Mdn = 38.5%). Ten (20.4%) studies assessed adherence using pill count. Adherence rates by this method ranged from 43% to 94.4% ( M = 66.8%, Mdn = 66.2%). Five (10.2%) studies assessed adherence using medical record review (no further details provided). Adherence rates by this method ranged from 29.6% to 100% ( M = 65.1%, Mdn = 59.4%). Three (6.1%) assessed adherence using electronic monitoring. Adherence rates by this method ranged from 65.8% to 96% ( M = 74.9%, Mdn = 69.3%). One study used electronic directly observed therapy (i.e., video recordings of medication ingestion) and the adherence rate was 88.6%. Finally, one study used insurance claims data and the adherence rate was 81.7%.
Combined Methods
Five (10.6%) studies reported adherence rates based on a combination of two or more methods (e.g., self-report, pill count, and lab values). In this case, only one rate was reported based on an aggregate of different assessment strategies. Adherence rates when a combination was used ranged from 24.87% to 97.6% ( M = 76.1%, Mdn = 86%).
Rates of Adherence by Medication Type
Prophylactic Antibiotics
There were 20 (40.8%) studies that assessed adherence to antibiotics (i.e., penicillin). Adherence rates for this medication type ranged from 12% to 100% ( M = 54.6%, Mdn = 55.5%).
Hydroxyurea
There were 13 (26.5%) studies that assessed adherence to hydroxyurea. Adherence rates for this type ranged from 12% to 100% ( M = 73.7%, Mdn = 79.7%).
Iron Chelators
There were five (10.2%) studies that assessed adherence to deferasirox ( n = 2), desferoxamine ( n = 2), or both ( n = 1). Adherence rates for this medication ranged from 22.2% to 89% ( M = 56.1%, Mdn = 54.6%).
Other Medications
There were three (6.1%) studies that assessed adherence to other medications, including sodium phenylbutyrate ( Resar et al., 2002 ), nonprophylactic antibiotics ( Williams et al., 1996 ), and glutamine supplements ( Williams et al., 2004 ). Adherence rates for these medications ranged from 94% to 97.6% ( M = 95.9%, Mdn = 96%).
Overall Medication Regimen
There were 11 (22.4%) studies that assessed the overall medication regimen. Of these, six (54.5%) reported adherence using a percentage. Adherence rates reported as percentages for the overall medication regimen ranged from 29.6% to 86% ( M = 70.8%, Mdn = 82.95%).
Meta-Analysis Results
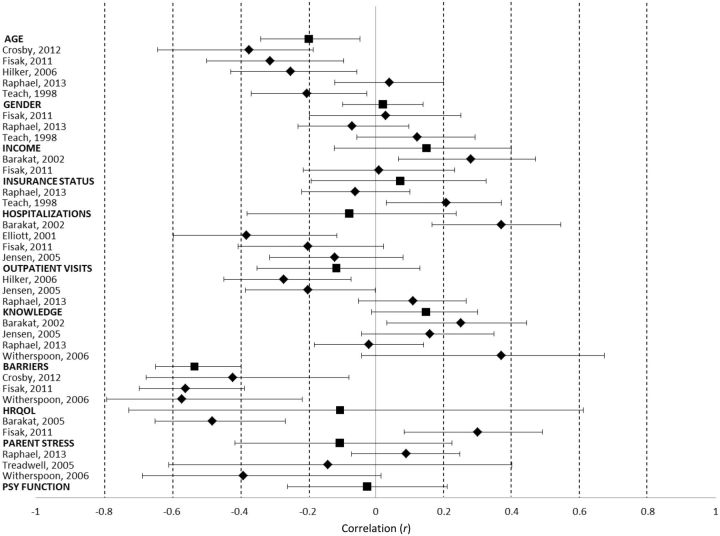
There were 14 studies that qualified to be included in the meta-analysis. The total sample size across these studies was 921. On average, participants were 9.4 years old and 56% male. Studies in the meta-analysis examined adherence to the overall medication regimen (50%), prophylactic antibiotics (21.4%), hydroxyurea (14.3%), iron chelators (7.1%), and sodium phenylbutyrate (7.1%). A majority (92.9%) of studies used self- or parent-proxy report of adherence. Other adherence assessment methods included medical provider rating (50%), pharmacy refill data (28.6%), pill count (14.3%), bioassay (7.1%), and electronic monitoring (7.1%). Adherence rates collected from these studies were evaluated in relation to various demographic, medical, and psychosocial correlates. These correlates were assessed using a variety of established and nonvalidated measures. All factors were examined in relation to adherence, such that a greater value of the factor was associated with higher adherence. Mean ES and 95% confidence intervals are presented in Table II and graphically in Figure 2 .
Table II.
Meta-Analysis Results
| Factor | n studies | Mean ES | 95% CI |
|---|---|---|---|
| Demographic | 12 | −0.055 | −0.21, 0.11 |
| Age | 5 | −0.197** | −0.34, −0.05 |
| Gender | 3 | 0.021 | −0.10, 0.14 |
| Income | 2 | 0.149 | −0.12, 0.40 |
| Insurance status | 2 | 0.072 | −0.19, 0.33 |
| Medical | 12 | −0.068 | −.28, 0.15 |
| Biomarkers | 4 | −0.292 * | −0.54, 0.00 |
| Hospitalizations | 4 | −0.078 | −0.38, 0.24 |
| Outpatient visits | 3 | −0.166 | −0.35, 0.13 |
| Disease knowledge | 4 | 0.148 | −0.01, 0.30 |
| Adherence barriers | 3 | −0.533** | −0.65, −0.40 |
| Psychosocial functioning | 11 | −0.025 | −0.26, 0.21 |
| Child HRQOL | 2 | −0.105 | −0.73, 0.61 |
| Parent stress | 3 | −0.106 | −0.41, 0.22 |
Note . ES = effect size; CI = confidence interval; HRQOL = health-related quality of life.
* p < .05, ** p < .01.
Figure 2.
Forest plot of correlations and 95% confidence intervals. Broader domains are displayed in bolded font.
Demographic Factors
The following patient and family demographics were examined in relation to medication adherence: age, gender, insurance status, and income. There was a small mean ES across all factors. However, there was a significant effect for age, with older age being associated with poorer adherence.
Medical Factors
The following medical factors were evaluated in relation to medication adherence: genotype (e.g., HbSS, HbSC), hospitalizations, pain crisis frequency, health status, transfusion status, SCD complications, urgent care visits, routine care visits, number of phone calls to the hematology clinic, and biomarkers. The mean ES across medical factors was small. There was a negative correlation between adherence and hospitalizations in three of four studies and adherence and outpatient visits in two of three studies, such that better adherence was associated with decreased health care utilization. The following biomarkers were examined in relation to medication adherence: fetal hemoglobin (HbF), hemoglobin level, white blood count, and % F-reticulocyte. The mean ES for biomarkers was moderate.
Disease Knowledge
This construct was assessed in relation to adherence in 28.6% of the 14 studies. Mean ES for knowledge was small.
Adherence Barriers
Three studies assessed the association between adherence and barriers (e.g., forgetting, caregiver is too busy, medication ran out). The mean ES was moderate, with fewer barriers being associated with better adherence.
Psychosocial Factors
The following psychosocial functioning factors across family members were examined in relation to adherence: HRQOL, parent coping, parent problem solving, parent behavior control, parent perceived stress, parent social support, parent health motivation, health optimism, self-efficacy, child behavior problems, parent beliefs about medication. There were small mean ES across these domains.
Study Quality
Studies included in the meta-analysis had a mean quality rating of 15.7 ( SD = 3.0; range = 9–20; maximum = 22). Of the studies rated, 8 (57%) had clearly stated hypotheses, 10 (71%) described inclusion/exclusion criteria, 11 (78%) used at least one validated tool to assess adherence correlates (e.g., barriers, knowledge), and only 1 (7%) included a power analysis.
Discussion
This study aimed to comprehensively identify and summarize research to date examining medication adherence rates among pediatric patients with SCD. Forty-nine studies were identified that reported medication adherence rates as either a primary or secondary aim since the early 1980s, which is more than double the number of studies that have been reviewed previously ( Walsh et al., 2014 ). There was a wide variation in adherence rates reported based on type of adherence method used and the medication being measured. Specifically, the mean adherence rate from subjective methods was higher than the mean from objective measures, a trend that has been established in other pediatric chronic medical conditions ( Hommel, Davis, & Baldassano, 2009 ). Adherence was lowest for prophylactic antibiotics (e.g., penicillin) and iron chelators compared with hydroxyurea and other medications. Additionally, less than a third of studies evaluated adherence in relation to demographic, medical, or psychosocial factors, indicating that the literature regarding correlates is underdeveloped.
Among the correlates tested in the meta-analysis, medication barriers and biomarker levels had the strongest associations with adherence. Medication barriers represent a modifiable factor that has also been related to adherence in other pediatric chronic illness populations ( Ingerski, Baldassano, Denson, & Hommel, 2010 ; MacDonell, Naar-King, Huszti, & Belzer, 2013 ; Simons, McCormick, Devine, & Blount, 2010 ). Small mean ESs were found for other factors, including demographic characteristics, health care utilization, and HRQL. This was unexpected given that these associations have been documented in past pediatric adherence research ( Rapoff, 2010 ). The small ESs were likely owing to the limited number of studies examining these correlates, as well as inconsistent direction of effects. For example, some studies reported an inverse association between adherence and hospitalizations ( Elliott, Morgan, Day, Mollerup, & Wang, 2001 ; Fisak et al., 2011 ; Jensen et al., 2005 ), while one study found a positive correlation ( Barakat et al., 2002 ). This trend was also found in studies of the relation between adherence and HRQL. More studies need to be conducted to gain a clearer understanding of how these factors relate to adherence in pediatric SCD.
When making conclusions about the body of pediatric SCD adherence literature, it is important to consider potential limitations regarding the design of individual studies. First, a wide range of assessment strategies were used to measure adherence. The most frequent method of assessment was subjective, with self-report (53% of studies) being the most common across all studies in the review. Additionally, 57% of studies used only a single measure of adherence. This is in contrast to recommendations in the literature ( Hommel et al., 2009 ; Quittner, Espelage, Ievers-Landis, & Drotar, 2000 ), which advocate for combining multiple methods of adherence assessment (given the limitations inherent in currently available measures). Second, many articles had an unclear definition of adherence. For example, several authors reported that a certain percentage of participants were classified as “nonadherent,” but did not provide the parameters they used to make that determination. Even when definitions were provided, there was extreme variability in terms of the criteria for a patient to be classified as adherent or nonadherent. Additionally, adherence was assessed over a variable time frame (yesterday vs. past week vs. no period specified), which makes comparisons across studies tenuous. Further, it is concerning that statements about medication efficacy and positive impact on clinical outcomes (e.g., hospitalizations) were made based on pharmaceutical trials that did not assess or report adherence rates ( Aygun et al., 2013 ; Nottage et al., 2013 ; Scott, Hillery, Brown, Misiewicz, & Labotka, 1996 ), particularly given that existing literature shows a wide range in adherence. In contrast, among drug trials that did report adherence rates ( Kinney et al., 1999 ; Thornburg, Rogers, et al., 2010 ; Ware et al., 2002 ), there may be issues related to validity, including lack of evidence-based assessment strategies and demand characteristics. Finally, many of the studies that evaluated associations between adherence and other factors did not provide information about the magnitude of the relation.
The results of the current systematic review and meta-analysis can directly inform future research in SCD to optimize the limited funding that is available for these studies. First, consistent with recommendations from the broader pediatric literature, studies should use evidence-based adherence assessment tools (e.g., electronic monitoring), and, ideally, combine subjective and objective methods ( Hommel et al., 2009 ). Second, results from the current meta-analysis revealed that barriers to the medication regimen and biomarkers (e.g., HbF) had the strongest associations with adherence. Despite the strong ESs for these constructs, there were few studies that examined adherence in relation to barriers ( n = 3) or biomarkers ( n = 4). Third, there are factors that have been consistently linked to poor adherence in other pediatric chronic illnesses, including single parent household, less disease knowledge, greater family conflict, and low perceived social support ( Modi et al., 2012 ). It is not known at this time whether these associations generalize to children with SCD, particularly given the unique aspects of SCD, including that it predominantly affects African Americans in the United States. Fourth, the association between adherence and health outcomes (e.g., pain crises, stroke, health care utilization) should be further examined. This will help clarify how adherence is or is not linked to relevant health outcomes. Fifth, there were only a handful of studies that assessed adherence over time. Longitudinal research is a crucial step in identifying predictors and health outcomes of adherence behaviors. Sixth, only 2 ( Berkovitch et al., 1998 ; Day, Brunson, & Wang, 1992 ) of the 49 studies described interventions to improve adherence among children with SCD. Although there is a critical need for adherence-promotion interventions, additional research is needed to identify which modifiable correlates (e.g., knowledge, allocation of responsibility, family functioning) should be targeted in these treatment programs. Finally, future research should examine adherence to other existing and emerging therapies for SCD, including chronic blood transfusions and hematopoietic stem cell transplantation as rates may differ from medications. Adequate funding must be available to conduct high-quality research in the future. In combination, there is almost nine times the amount of money spent per person with cystic fibrosis compared with SCD, despite the fact that it is a third of the prevalence in the United States ( Smith et al., 2006 ). Resources are needed to support studies that identify best practices for overcoming historical and logistical barriers (e.g., transportation, reimbursement) to improve enrollment and retention of patients with SCD.
Study findings also have implications for the clinical care of pediatric patients with SCD. First, clinicians should be aware that estimates of adherence based on self-report will likely be higher than when other methods are used. The use of objective or multiple forms of adherence assessment should be implemented when possible. Second, clinicians should pay particular attention to children who are prescribed penicillin and iron chelators, as adherence rates to this medication were lower than other medications. Third, as adolescence is a nonmodifiable risk factor for adherence difficulties, patients in this developmental stage should receive close monitoring. Lastly, clinicians should routinely assess and provide intervention (e.g., problem-solving) around barriers to the medication regimen, as they have a strong association with adherence.
Findings from the current investigation should be considered within the context of methodological limitations. First, only results published in peer-reviewed journals are represented in this systematic review and meta-analysis. There may be other studies that have examined medication adherence in pediatric SCD. Second, there were a small number of studies included in the meta-analysis owing to most studies not examining medication adherence in relation to demographic, medical, or psychosocial factors. Finally, there was heterogeneity in the method of adherence assessment used, as well as how correlates were assessed. Stronger correlations may have been detected with the use of validated measures to assess adherence and correlates.
In sum, there have been 49 adherence studies published since the early 1980s, approximately half of which were within the past 10 years. This body of literature is less than that of children with chronic conditions that are not as prevalent, such as solid organ transplant recipients, which has 61 studies in a shorter time frame (1989–2008; Dew et al., 2009 ). Although this review provides some initial insight into potential correlates of adherence, many questions remain about predictors of adherence, as well as the impact of poor adherence on health outcomes. Additionally, it is critical that this information is translated into the development of interventions to improve adherence among children with SCD, particularly as medications such as hydroxyurea become the standard of care for treatment.
Funding
This work was supported by a training grant from the National Institutes of Health (T32HD068223) to K.L. and L.S. and a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health (K07HL108720) to L.E.C.
Conflicts of interest : None declared.
References
- * = References marked with an asterisk indicate studies included in the systematic review only.
- ** = References marked with a double asterisk indicate studies included in the systematic review and meta-analysis.
- Alvarez O., Rodriguez-Cortes H., Robinson N., Lewis N., Sang C. D. P., Lopez-Mitnik G., Paley C. ( 2009. ). Adherence to deferasirox in children and adolescents with sickle cell disease during 1-year of therapy . Journal of Pediatric Hematology/Oncology , 31 , 739 – 744 . doi: 10.1097/mph.0b013e3181b53363* [DOI] [PubMed] [Google Scholar]
- Anglin D. L., Siegel J. D., Pacini D. L., Smith S. J., Adams G., Buchanan G. R. ( 1984. ). Effect of penicillin prophylaxis on nasopharyngeal colonization with Streptococcus pneumoniae in children with sickle cell anemia . The Journal of Pediatrics , 104 , 18 – 22 . doi: 10.1016/s0022-3476(84)80582-x* [DOI] [PubMed] [Google Scholar]
- Aygun B., Mortier N. A., Smeltzer M. P., Shulkin B. L., Hankins J. S., Ware R. E. ( 2013. ). Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia . American Journal of Hematology , 88 , 116 – 119 . doi: 10.1002/ajh.23365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker M. A. ( 1986a. ). Compliance with penicillin prophylaxis by children with impaired splenic function . Tropical and Geographical Medicine , 38 , 119 – 122 . * [PubMed] [Google Scholar]
- Babiker M. A. ( 1986b. ). Prophylaxis of pneumococcal infection in sickle-cell disease . Annals of Tropical Paediatrics , 6 , 179 – 181 . Retrieved from http://ingentaconnect.com/content/0272-4936* [DOI] [PubMed] [Google Scholar]
- Barakat L. P., Lutz M., Smith-Whitley K., Ohene-Frempong K. ( 2005. ). Is treatment adherence associated with better quality of life in children with sickle cell disease? Quality of Life Research , 14 , 407 – 414 . doi: 10.1007/s11136-004-5328-0** [DOI] [PubMed] [Google Scholar]
- Barakat L. P., Smith-Whitley K., Ohene-Frempong K. ( 2002. ). Treatment adherence in children with sickle cell disease: Disease-related risk and psychosocial resistance factors . Journal of Clinical Psychology in Medical Settings , 9 , 201 – 209 . doi: 10.1023/A:1016047210623** [Google Scholar]
- Berkovitch M., Papadouris D., Shaw D., Onuaha N., Dias C., Olivieri N. F. ( 1998. ). Trying to improve compliance with prophylactic penicillin therapy in children with sickle cell disease . British Journal of Clinical Pharmacology , 45 , 605 – 607 . doi: 10.1046/j.1365-2125.1998.00730.x* [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitaraes E. L., de Oliveira B. M., Viana M. B. ( 2008. ). Compliance with antibiotic prophylaxis in children with sickle cell anemia: A prospective study . Journal de Pediatria , 84 , 316 – 322 . doi: 10.2223/JPED.1819* [DOI] [PubMed] [Google Scholar]
- Biostat . ( 2005. ). Comprehensive meta-analysis . Englewood, NJ: : Biostat; . [Google Scholar]
- Blanchard J., Lurie N. ( 2004. ). R-E-S-P-E-C-T: Patient reports of disrespect in the health care setting and its impact on care . Journal of Family Practice , 53 , 721 – 730 . [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. R. ( 2010. ). A basic introduction to fixed-effect and random-effects models for meta-analysis . Research Synthesis Methods , 1 , 97 – 111 . doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Brawley O. W., Cornelius L. J., Edwards L. R., Northington Gamble V., Green B. L., Inturrisi C., James A. H., Laraque D., Mendez M., Montoya C. J., Pollock B. H., Robinson L., Scholnik A. P., Schori M. ( 2008. ). National Institutes of Health consensus development conference statement: Hydroxyurea treatment for sickle cell disease . Annals of Internal Medicine , 148 , 932 – 938 . doi: 10.7326/0003-4819-148-12-200806170-00220 [DOI] [PubMed] [Google Scholar]
- Buchanan G. R., Siegel J. D., Smith S. J., DePasse B. M. ( 1982. ). Oral penicillin prophylaxis in children with impaired splenic function: A study of compliance . Pediatrics , 70 , 926 – 930 . doi: 10.1203/00006450-198104001-00049* [PubMed] [Google Scholar]
- Buchanan G. R., Smith S. J. ( 1986. ). Pneumococcal septicemia despite pneumococcal vaccine and prescription of penicillin prophylaxis in children with sickle cell anemia . American Journal of Diseases of Children , 140 , 428 – 432 . doi: 10.1001/archpedi.1986.02140190038020* [DOI] [PubMed] [Google Scholar]
- Cohen J. A. ( 1960. ). A coefficient of agreement for nominal scales . Educational and Psychological Measurement , 20 , 37 – 46 . doi: 10.1177/001316446002000104 [Google Scholar]
- Colombatti R., Montanaro M., Guasti F., Rampazzo P., Meneghetti G., Giordan M., Basso G., Sainati L. ( 2012. ). Comprehensive care for sickle cell disease immigrant patients: A reproducible model achieving high adherence to minimum standards of care . Pediatric Blood and Cancer , 59 , 1275 – 1279 . doi: 10.1002/pbc.24110* [DOI] [PubMed] [Google Scholar]
- Creary S. E., Gladwin M. T., Byrne M., Hildesheim M., Krishnamurti L. ( 2014. ). A pilot study of directly observed therapy to improve hydroxyurea adherence in pediatric patients with sickle-cell disease . Pediatric Blood and Cancer , 61 , 1068 – 1073 . doi: 10.1002/pbc.24931* [DOI] [PubMed] [Google Scholar]
- Crosby L. E., Barach I., McGrady M. E., Kalinyak K. A., Eastin A. R., Mitchell M. J. ( 2012. ). Integrating interactive web-based technology to assess adherence and clinical outcomes in pediatric sickle cell disease . Anemia , 2012 , 1 – 8 . doi: 10.1155/2012/492428** [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins D., Heuschkel R., Davies S. C. ( 1991. ). Penicillin prophylaxis in children with sickle cell disease in Brent . British Medical Journal , 302 , 989 – 990 . doi: 10.1136/bmj.302.6783.989* [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton R. N., Turner C., Dick M., Height S. E., Awogbade M., Inusa B., Okpala I., O'Driscoll S., Thein S. L., Rees D. C. ( 2005. ). The measurement of urinary hydroxyurea in sickle cell anaemia . British Journal of Haematology , 130 , 138 – 144 . doi: 10.1111/j.1365-2141.2005.05583.x* [DOI] [PubMed] [Google Scholar]
- Davis H. ( 1998. ). Use of computerized health claims data to monitor compliance with antibiotic prophylaxis in sickle cell disease . Pharmacoepidemiology and Drug Safety , 7 , 107 – 112 . Retrieved from http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291099-1557* [DOI] [PubMed] [Google Scholar]
- Day S., Brunson G., Wang W. ( 1992. ). A successful education program for parents of infants with newly diagnosed sickle cell disease . Journal of Pediatric Nursing , 7 , 52 – 58 . Retrieved from http://www.pediatricnursing.org/* [PubMed] [Google Scholar]
- De Civita M., Dobkin P. L. ( 2004. ). Pediatric adherence as a multidimensional and dynamic construct, involving a triadic partnership . Journal of Pediatric Psychology , 29 , 157 – 169 . doi: 10.1093/jpepsy/jsh018 [DOI] [PubMed] [Google Scholar]
- de Montalembert M., Brousse V., Elie C., Bernaudin F., Shi J., Landais P. ( 2006. ). Long-term hydroxyurea treatment in children with sickle cell disease: Tolerance and clinical outcomes . Haematologica , 91 , 125 – 128 . Retrieved from www.haematologica.org* [PubMed] [Google Scholar]
- Dew M. A., Dabbs A. D., Myaskovsky L., Shyu S., Shellmer D. A., DiMartini A. F., Steel J., Unruh M., Switzer G. E., Shapiro R., Greenhouse J. B. ( 2009. ). Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation . Transplantation , 88 , 736 – 746 . doi: 10.1097/TP.0b013e3181b2a0e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division of Blood Diseases and Resources , National Institutes of Health , National Heart , Lung , and Blood Institute . ( 2002. ). The management of sickle cell disease (NIH Publication No. 02-2117) . Retrieved from http://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf [Google Scholar]
- DuRant R. H. ( 1994. ). Checklist for the evaluation of research articles . Journal of Adolescent Health , 15 , 4 – 8 . doi: 10.1016/1054-139x(94)90381-6 [DOI] [PubMed] [Google Scholar]
- Elliott V., Morgan S., Day S., Mollerup L. S., Wang W. ( 2001. ). Parental health beliefs and compliance with prophylactic penicillin administration in children with sickle cell disease . Journal of Pediatric Hematology/Oncology , 23 , 112 – 116 . doi: 10.1097/00043426-200102000-00009** [DOI] [PubMed] [Google Scholar]
- Fisak B., Belkin M. H., von Lehe A. C., Bansal M. M. ( 2011. ). The relation between health-related quality of life, treatment adherence and disease severity in a paediatric sickle cell disease sample . Child: Care, Health and Development , 38 , 204 – 210 . doi: 10.1111/j.1365-2214.2011.01223.x** [DOI] [PubMed] [Google Scholar]
- Fowkes F. G. R., Fulton P. M. ( 1991. ). Critical appraisal of published research: Introductory guidelines . British Medical Journal , 302 , 1136 – 1140 . doi: 10.1136/bmj.302.6785.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell K. L. ( 2010. ). Population estimates of sickle cell disease in the U.S . American Journal of Preventive Medicine , 38 , S512 – S521 . doi: 10.1016/j.amepre.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Hilker K. A., Jordan S. S., Jensen S., Elkin T. D., Iyer R. ( 2006. ). Development of a screening instrument of adherence in pediatric sickle cell disease . Children’s Health Care , 35 , 235 – 246 . doi: 10.1207/s15326888chc3503_3** [Google Scholar]
- Hommel K. A., Davis C. M., Baldassano R. N. ( 2009. ). Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease . Inflammatory Bowel Disease , 15 , 589 – 593 . doi: 10.1002/ibd.20798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikefuna A. N., Emodi I. J. ( 2007. ). Hospital admission of patients with sickle cell anaemia pattern and outcome in Enugu area of Nigeria . Nigerian Journal of Clinical Practice , 10 , 24 – 29 . Retrieved from http://www.njcponline.com/* [PubMed] [Google Scholar]
- Ingerski L. M., Baldassano R. N., Denson L. A., Hommel K. A. ( 2010. ). Barriers to oral medication adherence for adolescents with inflammatory bowel disease . Journal of Pediatric Psychology , 35 , 683 – 691 . doi: 10.1093/jpepsy/jsp085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. A., Elkin T. D., Hilker K., Jordan S., Iyer R., Smith M. G. ( 2005. ). Caregiver knowledge and adherence in children with sickle cell disease: Knowing is not doing . Journal of Clinical Psychology in Medical Settings , 12 , 333 – 337 . doi: 10.1007/s10880-005-7819-8** [Google Scholar]
- Jordan L. B., Vekeman F., Sengupta A., Corral M., Guo A., Duh M. S. ( 2012. ). Persistence and compliance of deferoxamine versus deferasirox in Medicaid patients with sickle-cell disease . Journal of Clinical Pharmacy and Therapeutics , 37 , 173 – 181 . doi: 10.1111/j.1365-2710.2011.01276.x* [DOI] [PubMed] [Google Scholar]
- Kauf T. L., Coates T. D., Huazhi L., Mody-Patel N., Hartzema A. G. ( 2009. ). The cost of health care for children and adults with sickle cell disease . American Journal of Hematology , 84 , 323 – 327 . doi: 10.1002/ajh.21408 [DOI] [PubMed] [Google Scholar]
- King L., Ali S., Knight-Madden J., MooSang M., Reid M. ( 2011. ). Compliance with intramuscular penicillin prophylaxis in children with sickle cell disease in Jamaica . West Indian Medical Journal , 60 , 177 – 180 . Retrieved from http://caribbean.scielo.org/* [PubMed] [Google Scholar]
- Kinney T. R., Helms R. W., O’Branski E. E., Ohene-Frempong K., Wang W., Daeschner C., Vichinsky E., Redding-Lallinger R., Gee B., Platt O. S., Ware R. E. ( 1999. ). Safety of hydroxyurea in children with sickle cell anemia: Results of the HUG-KIDS study, a phase I/II trial . Blood , 94 , 1550 – 1554 . Retrieved from http://www.bloodjournal.org/* [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. ( 1977. ). The measurement of observer agreement for categorical data . Biometrika , 33 , 159 – 174 . doi: 10.2307/2529310 [PubMed] [Google Scholar]
- MacDonell K., Naar-King S., Huszti H., Belzer M. ( 2013. ). Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV . AIDS and Behavior , 17 , 86 – 93 . doi: 10.1007/s10461-012-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady M. E., Hommel K. A. ( 2013. ). Medication adherence and health care utilization in pediatric chronic illness: A systematic review . Pediatrics , 132 , 730 – 740 . doi: 10.1542/peds.2013-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., lfoyle S. M., Gray W. N., Drotar D. ( 2012. ). Pediatric self-management: A framework for research, practice, and policy . Pediatrics , 129 , e473 – e485 . doi: 10.1542/peds.2011-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisky D. E., Green L. W., Levine D. M. ( 1986. ). Concurrent and predictive validity of a self-reported measure of medication adherence . Medical Care , 24 . 67 – 74 . Retrieved from http://journals.lww.com/lww-medicalcare/Pages/default.aspx [DOI] [PubMed] [Google Scholar]
- Musa D., Schilz R., Harris R., Silverman M., Thomas S. B. ( 2009. ). Trust in the health care system and the use of preventive health services by older Black and White adults . American Journal of Public Health , 99 , 1293 – 1299 . doi: 10.2105/AJPH.2007.123927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottage K. A., Hankins J. S., Smeltzer M., Mzayek F., Wang W. C., Aygun B., Gurney J. G. ( 2013. ). Hydroxyurea use and hospitalization trends in a comprehensive pediatric sickle cell program . PLoS ONE , 8 , e72077 . doi: 10.1371/journal.pone.0072077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri N. F., Vichinksy E. P. ( 1998. ). Hydroxyurea in children with sickle cell disease: Impact on splenic function and compliance with therapy . Journal of Pediatric Hematology/Oncology , 20 , 26 – 31 . doi: 10.1097/00043426-199801000-00004* [DOI] [PubMed] [Google Scholar]
- Patel A. B., Athavale A. M. ( 2004. ). Sickle cell disease in central India . Indian Journal of Pediatrics , 71 , 789 – 793 . doi: 10.1007/bf02730713* [DOI] [PubMed] [Google Scholar]
- Patel N. G., Lindsey T., Strunk R. C., DeBaun M. R. ( 2010. ). Prevalence of daily medication adherence among children with sickle cell disease: A one-year retrospective cohort analysis . Pediatric Blood and Cancer , 55 , 554 – 556 . doi: 10.1002/pbc.22605* [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejaver R. K., Ahmed F. E., Al Hifzi I. ( 1997. ). Compliance to penicillin prophylaxis amongst Saudi children . Journal of the Irish Colleges of Physicians and Surgeons , 26 , 268 – 270 . * [Google Scholar]
- Quittner A. L., Espelage D. L., Ievers-Landis C., Drotar D. ( 2000. ). Measuring adherence to medical treatments in childhood chronic illness: Considering multiple methods and sources of information . Journal of Clinical Psychology in Medical Settings , 7 , 41 – 54 . doi: 10.1023/A:1009545319673 [Google Scholar]
- Raphael J. L., Butler A. M., Rattler T. L., Kowalkowski M. A., Mueller B. U., Giordano T. P. ( 2013. ). Parental information, motivation, and adherence behaviors among children with sickle cell disease . Pediatric Blood and Cancer , 60 , 1204 – 1210 . doi: 10.1002/pbc.24466** [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff M. A. ( 2010. ). Adherence to pediatric medical regimens ( 2nd ed .). New York, NY: : Springer; . [Google Scholar]
- Rees D. C., Williams T. N., Gladwin M. T. ( 2010. ). Sickle-cell disease . The Lancet , 376 , 2018 – 2031 . doi: 10.1016/S0140-6736(10)61029-X [DOI] [PubMed] [Google Scholar]
- Resar L. M. S., Segal J. B., Fitzpatric L. K., Friedmann A., Brusilow S. W., Dover G. J. ( 2002. ). Induction of fetal hemoglobin synthesis in children with sickle cell anemia on low-dose oral sodium phenylbutyrate therapy . Journal of Pediatric Hematology/ Oncology , 24 , 737 – 741 . doi: 10.1097/00043426-200212000-00011** [DOI] [PubMed] [Google Scholar]
- Sabin J. A., Rivara F. P., Greenwald A. G. ( 2008. ). Physician implicit attitudes and stereotypes about race and quality of medical care . Medical Care , 46 , 678 – 685 . doi: 10.1097/MLR.0b013e3181653d58 [DOI] [PubMed] [Google Scholar]
- Scott J. P., Hillery C. A., Brown E. R., Misiewicz V., Labotka R. J. ( 1996. ). Hydroxyurea therapy in children severely affected with sickle cell disease . The Journal of Pediatrics , 128 , 820 – 828 . doi: 10.1016/s0022-3476(96)70335-9 [DOI] [PubMed] [Google Scholar]
- Simons L. E., McCormick M. L., Devine K., Blount R. L. ( 2010. ). Medication barriers predict adolescent transplant recipients’ adherence and clinical outcomes at 18-month follow-up . Journal of Pediatric Psychology , 35 , 1038 – 1048 . doi: 10.1093/jpepsy/jsq025 [DOI] [PubMed] [Google Scholar]
- Smith L. A., Oyeku S. O., Homer C., Zuckerman B. ( 2006. ). Sickle cell disease: A question of equity and quality . Pediatrics , 117 , 1763 – 1770 . doi: 10.1542/peds.2005-1611 [DOI] [PubMed] [Google Scholar]
- Sox C. M., Cooper W. O., Koepsell T. D., DiGiuseppe D. L., Christakis D. A. ( 2003. ). Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease . JAMA , 290 , 1057 – 1061 . doi: 10.1001/jama.290.8.1057* [DOI] [PubMed] [Google Scholar]
- Teach S. J., Lillis K. A., Grossi M. ( 1998. ). Compliance with penicillin prophylaxis in patients with sickle cell disease . Archives of Pediatric Medicine , 152 , 274 – 278 . doi: 10.1001/archpedi.152.3.274** [DOI] [PubMed] [Google Scholar]
- Thornburg C. D., Calatroni A., Telen M., Kemper A. R. ( 2010. ). Adherence to hydroxyurea therapy in children with sickle cell anemia . Journal of Pediatrics , 156 , 415 – 419 . doi: 10.1016/j.peds.2009.09.044* [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg C. D., Rogers Z. R., Jeng M. R., Rana S. R., Iyer R. V., Faughnan L., Hassen L., Marshall J., McDonald R. P., Wang W. C., Huang X., Rees R. C. ( 2010. ). Adherence to study medication and visits: Data from the BABY HUG trial . Pediatric Blood and Cancer , 54 , 260 – 264 . doi: 10.1002/pbc.22324** [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell M. J., Law A. W., Sung J., Hackney-Stephens E., Quirolo K., Murray E., Glendenning G. A., Vichinsky E. ( 2005. ). Barriers to adherence to deferoxamine usage in sickle cell disease . Pediatric Blood and Cancer , 44 , 500 – 507 . doi: 10.1002/pbc.20290** [DOI] [PubMed] [Google Scholar]
- Tsouana E., Kaya B., Gadong N., Hemmaway C., Newell H., Simmons A., Whitmarsh S., Telfer P. ( 2015. ). Deferasirox for iron chelation in multitransfused children with sickle cell disease; long-term experience in the East London clinical haemoglobinopathy network . European Journal of Haematology . 94 , 336 – 342 . doi: 10.1111/ejh.12345* [DOI] [PubMed] [Google Scholar]
- Van Sciver M. M., D’Angelo E. J., Rappaport L., Woolf A. D. ( 1995. ). Pediatric compliance and the roles of distinct treatment characteristics, treatment attitudes, and family stress: A preliminary report . Journal of Developmental and Behavioral Pediatrics , 16 , 350 – 358 . doi: 0196-206X/95/1605-0350$03.00/0** [PubMed] [Google Scholar]
- Walsh K. E., Cutrona S. L., Kavanah P. L., Crosby L. E., Malone C., Lobner K., Bundy D. G. ( 2014. ). Medication adherence among pediatric patients with sickle cell disease: A systematic review . Pediatrics , 134 , 1175 – 1183 . doi: 10.1542/peds.2014-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware R. E., Eggleston B., Redding-Lallinger R., Wang W. C., Smith-Whitley K., Daeschner C., Gee B., Styles L. A., Helms R. W., Kinney T. R., Ohene-Frempong K. ( 2002. ). Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy . Blood , 99 , 10 – 14 . doi: 10.1182/blood.V99.1.10* [DOI] [PubMed] [Google Scholar]
- Ware R. E., Zimmerman S. A., Schultz W. H. ( 1999. ). Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease . Blood , 94 , 3022 – 3026 . Retrieved from http://www.bloodjournal.org/* [PubMed] [Google Scholar]
- Ware R. E., Zimmerman S. A., Sylvestre P. B., Mortier N. A., Davis J. S., Treem W. R., Schultz W. H. ( 2004. ). Prevention of secondary stroke and resolution of transfusional iron overload in children with sickle cell anemia using hydroxyurea and phlebotomy . Journal of Pediatrics , 145 , 346 – 352 . doi: 10.1016/j.peds.2001.04.058 . * [DOI] [PubMed] [Google Scholar]
- Warren M. D., Arbogast P. G., Dudley J. A., Kaltenbach L., Ray W. A., Wang W. C., Cooper W. O. ( 2010. ). Adherence to prophylactic antibiotic guidelines among Medicaid infants with sickle cell disease . Archives of Pediatric and Adolescent Medicine , 164 , 298 – 299 . doi: 10.1001/archpediatrics.2009.286* [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. L., Wilimas J. A., Harris S. C., Day S. W., Dancy R. M., Wang W. C. ( 1996. ). Outpatient therapy with ceftriaxone and oral cefixime for selected febrile children with sickle cell disease . Journal of Pediatric Hematology/Oncology , 18 , 257 – 261 . doi: 10.1097/00043426-199608000-00004* [DOI] [PubMed] [Google Scholar]
- Williams R., Olivi S., Chin-Shang L., Storm M., Cremer L., Mackert P., Wang W. ( 2004. ). Oral glutamine supplementation decreases resting energy expenditure in children and adolescents with sickle cell anemia . Journal of Pediatric Hematology/Oncology , 26 , 619 – 625 . doi: 10.1097/01.mph.0000140651.65591.b8* [PubMed] [Google Scholar]
- Witherspoon D., Drotar D. ( 2006. ). Correlates of adherence to prophylactic penicillin therapy in children with sickle cell disease . Children’s Health Care , 35 , 281 – 296 . doi: 10.1207/s15326888chc3504_1** [Google Scholar]
- Wojciechowski E. A., Hurtig A., Dorn L. ( 2002. ). A natural history study of adolescents and young adults with sickle cell disease as they transfer to adult care: A need for case management services . Journal of Pediatric Nursing , 17 , 18 – 27 . doi: 10.1053/jpdn.2002.30930* [DOI] [PubMed] [Google Scholar]
- Zimmerman S. A., Schultz W. H., Burgett S., Mortier N. A., Ware R. E. ( 2007. ). Hydroxyurea therapy lowers transcranial doppler flow velocities in children with sickle cell anemia . Blood , 11 , 1043 – 1047 . doi: 10.1182/blood-2006-11-057893* [DOI] [PubMed] [Google Scholar]
- Zimmerman S. A., Schultz W. H., Davis J. S., Pickens C. V., Mortier N. A., Howard T. A., Ware R. E. ( 2004. ). Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease . Blood , 103 , 2039 – 2045 . doi: 10.1182/blood-2003-07-2475* [DOI] [PubMed] [Google Scholar]