Abstract
Introduction:
The purpose of this electronic daily diary study was to examine the relation of pain to smoking and quit attempts among 34 cancer patients with pain enrolled in a smoking cessation program.
Methods:
Electronic daily diary assessments of pain and smoking were collected at the end of each day for a 2-week period during smoking cessation treatment. Pain experienced throughout the day was measured on a scale from 1 to 5, from “no pain” to “pain as bad as you can imagine.” Smoking was defined as the number of cigarettes smoked per day.
Results:
Linear multilevel modeling was used in examining associations between pain and smoking. A within-person pain and smoking association was found, such that greater daily pain was linked to greater daily smoking within individuals, controlling for baseline symptoms, nicotine dependence, smoking urge, age, and gender. No between-person pain and smoking association was observed. Additionally, cancer patients with higher average pain across the 2-week assessment period were less likely to make a quit attempt (defined as a day on which participants smoked no cigarettes) during the study period.
Conclusions:
The findings of this study add to a nascent literature on pain and smoking by providing initial evidence that pain may be a barrier to quitting among cancer patients who smoke and have pain. Future research examining the effectiveness of integrated pain and smoking cessation treatment in this population may be warranted.
Introduction
It is well established that cigarette smoking is a major risk factor for the development of many types of cancer.1,2 Among cancer patients, continued smoking after a cancer diagnosis has been consistently linked to increased likelihood of experiencing cancer recurrence or developing a second primary cancer and greater risk for experiencing cancer treatment-related toxicities.3 Despite the strong evidence linking smoking to poorer cancer outcome, many smokers continue smoking after cancer diagnosis.4,5 For example, research suggests that up to 50% of lung and head and neck cancer patients are smokers at the time of cancer diagnosis and that over half of these smokers will continue smoking postdiagnosis.6,7 These findings highlight the need for a better understanding of the factors that relate to smoking and quitting among cancer patients.
One variable that may be important in smoking among cancer patients is pain.8,9 It is estimated that over half of cancer patients experience cancer-related pain as a result of the disease or its treatment.10–12 In fact, pain is often described as one the most troubling aspects of cancer and has a large impact on quality of life among cancer patients.13 Among cancer patients, greater pain has been linked to smoking status. Findings from two cross-sectional surveys of cancer patients demonstrated that current smokers reported more pain than nonsmokers.8,9
The reciprocal model of pain and smoking proposed by Ditre and colleagues provides a framework for understanding this association between pain and smoking. This model proposes that the relationship between pain and smoking is characterized by bidirectional influences, such that pain can influence smoking and smoking can lead to an exacerbation of painful conditions overtime. In describing the effect of pain on smoking (the focus on the current study), the reciprocal model posits that some people with pain may use smoking as a way to cope with and manage pain.14 Support for this pain-smoking link has been found in noncancer populations. For example, an experimental study of current smokers found that pain applied in a laboratory setting increased smoking urge and decreased latency to smoke.15 Additionally, a qualitative study found that smokers with chronic, noncancer pain may use smoking as a means to cope with their pain.16
Although this research suggests that some smokers may use smoking as a means of coping with pain, little is known about how pain relates to smoking and quitting among cancer patients with painful conditions who are trying to quit. Previous research on pain and smoking among cancer patients has found between-person differences in pain levels by smoking status (eg, smokers report more pain than nonsmokers).8,9 However, these studies cannot address the question of whether or not painful events co-vary with smoking among cancer patients with pain who are trying to quit (eg, on a day in which an individual has higher pain, he/she smokes more cigarettes). In this study, we seek to expand on previous research by examining the day-to-day experiences of pain and smoking among cancer patients trying to quit. It is important to understand how pain may relate to quitting among cancer patients with pain as we seek to design more effective, tailored interventions for smoking cessation in this population.
This observational study utilizes an electronic daily diary approach to examine the association between pain and smoking among cancer patients with pain enrolled in a smoking cessation treatment program. Daily diary studies have been widely used in the measurement of both pain17–19 and smoking20,21 and are demonstrated to be a valid method of capturing variation in both constructs.22–24 The design of this study allowed us to examine between-person associations (ie, Do participants who have greater pain smoke more?) and within-person associations (ie, Within each individual, is daily smoking greater on days when individuals report more pain?). We hypothesized that greater daily pain would be associated with greater daily smoking (within-person association), controlling for baseline pain, nicotine dependence, smoking urge, and demographic factors. Secondly, we hypothesized that smokers with higher overall pain relative to other smokers would smoke more (between-person association), controlling for all model variables. Lastly, as smokers in this study were trying to quit, we also examined quit attempts as an outcome. We hypothesized that smokers with higher levels of average pain across the 2-week assessment period will be less likely to make a quit attempt, controlling for nicotine dependence, smoking urge, and demographic factors.
Method
Participants
Participants included 34 cancer patients who were treatment-seeking smokers. Participants were recruited from the Tobacco Treatment Program (TTP) at The University of Texas MD Anderson Cancer Center during their baseline visit to the TTP. Participants had an average age of 52 (SD = 10.3). Fifty-five percent of the participants were women. The majority of participants were Caucasian (88%). The most common cancer types were breast (38.2%), lung (29.4%), and head and neck (32.4%; Table 1). Over half (56%) of the participants were undergoing cancer treatment at the time of the study. Among those participants receiving cancer treatment during the study 2-week assessment period, chemotherapy was the most common treatment received (36.8%) followed by hormone therapy (21.1%), radiation therapy (15.8%), and other therapies, including multiple therapies (26.3%).
Table 1.
Baseline Characteristics (N = 34)
| Baseline characteristics | Mean or % |
|---|---|
| Age | 51.94 (SD = 10.30) |
| Gender (female) | 55.9% (n = 19) |
| Race | |
| White | 88.2% (n = 30) |
| Black | 8.8% (n = 3) |
| Hispanic | 2.9% (n = 1) |
| Cancer site | |
| Breast | 38.2% (n = 13) |
| Head and neck | 32.4% (n = 11) |
| Lung | 29.4% (n = 10) |
| Cancer treatmenta (n = 19) | |
| Chemotherapy | 36.8% (n = 7) |
| Hormone therapy | 21.1% (n = 4) |
| Radiation therapy | 15.8% (n = 3) |
| Multiple therapies | 26.3% (n = 5) |
aIndicates those participants who were undergoing active cancer treatment during the 2-week study period.
Eligibility and Recruitment
This article presents the daily diary results from a larger ecological momentary assessment study examining immediate precipitants of smoking behavior among cancer patients enrolled in cessation treatment, and was supported by an American Cancer Society MRSG-09-002-01-CPHPS grant. This article presents the daily diary results on pain and smoking from the larger study and includes all participants from the larger study (ie, not a subset of participants). Only men and women with a diagnosis of head and neck, breast, or lung cancer, who were 18 years or older, current smokers, motivated to quit within the next 30 days, fluent in English, and scheduled to begin their cancer treatment within the next 30 days were included in the study. Individuals were excluded if they regularly used tobacco products other than cigarettes or smoking-cessation treatments other than those supplied by the TTP, if they experienced no pain, if they were pregnant or lactating, or if another household member was enrolled in the study. Of the 325 patients initially screened, 66 patients were found to be ineligible. An additional 142 patients did not attend their initial TTP appointment. Of the 117 eligible patients who were approached about the study, 77 declined to participate, 40 were enrolled, and 34 completed the study. Of the 77 participants who declined to participate, most cited not being interested in participating as the primary reason for not enrolling (80.5%), followed by conflicts with work or cancer treatment schedule (14.3%). Three (3.9%) participants stated that they did not want to use, or did not think they would be able to use, the handheld electronic device. The six participants who enrolled, but did not complete the study failed to return any completed daily diary assessments and were not included in the analysis.
Procedures
Prospective participants were approached about the study during their first (baseline) visit to the TTP. For patients who expressed interest in participating, study personnel provided a detailed description of the study, answered questions, and obtained written informed consent. Participants then completed a battery of questionnaires including the Center for Epidemiologic Studies Depression Scale (CESD) and the MD Anderson Symptom Inventory (MDASI).
During their baseline TTP visit, participants were also given a palmtop personal computer (PPC) and trained in its use. The PPC uses a user-friendly, pen-based touch-screen system (pens were used to mark the appropriate answer on the computer screen). Competency in PPC use was assessed by asking participants to complete a list of tasks (eg, turning the PPC on and off, using the pen-based touch screen system to indicate desire to smoke, using the pen-based touch screen system to initiate and complete an assessment). Participants did not need computer skills or typing skills in order to use this device. We used 20 HP iPAQ H1945 PPCs that ran on Window Mobile 5 operating system and a software program that has been developed specifically for ecological momentary assessment. All assessments were date and time stamped for temporal analyses.
Participants were instructed to start using the PPC for monitoring on the day following their baseline visit to the TTP. Daily diary assessments were completed during participants’ first 2 weeks of treatment in the TTP. The TTP is a full-service program that offers free smoking-cessation treatment to cancer patients. It is staffed by a physician, advanced practice nurses, and doctoral- and master-level smoking-cessation counselors. Patients in the TTP receive: (1) counseling that includes cognitive-behavioral strategies such as building abstinence skills and motivational interviewing techniques to promote change and (2) tobacco cessation pharmacological treatment, including non-nicotine based medications and nicotine replacement therapy. The counseling is delivered either over the phone or in clinic, with the first counseling session occurring 1 week following the initial baseline evaluation.
Electronic Daily Diary
Participants completed a daily diary assessment 1 hour before their bedtime each day during the 2-week assessment period. The PPC administered assessments of pain, cigarette smoking, negative affect, and smoking urge, following a method of assessment used in other electronic daily diary studies.25–28 To assess pain, participants were asked to report the intensity of pain they experienced throughout the day on a 5-point rating scale, from 1, “No pain,” to 5, “Pain as bad as you can imagine.” To assess sad mood, anxious mood, and urge, smokers were asked to respond to two items summarizing how much they felt of each throughout the day (“I felt sad,” “I felt anxious,” “I have had an urge to smoke”) on a 5-point rating scale with the following anchors: “Strongly disagree,” “Disagree,” “Neutral,” “Agree,” and “Strongly agree.” Cigarette use was assessed as the number of cigarettes smoked per day, using the following scale: “0” representing no cigarettes, “1” for one cigarette, “2” for two cigarettes, “3” for three cigarettes, “4” for four cigarettes, “5” for 5–10 cigarettes, “6” for 11–20 cigarettes, and “7” for more than 20 cigarettes. Lastly, we assessed daily usage of over-the-counter and prescription pain medication by asking participants to indicate how many pills of each they took each day (eg, “Overall, how many prescription pills have you taken today?”).
Nondiary Assessments
Participants also completed a battery of questionnaires at the time of their baseline visit to the TTP. The battery included several questionnaires that assessed aspects of mood and symptom-reporting. Here, we describe those measures which were examined in the present study.
Center for Epidemiologic Studies Depression Scale
The CES-D is a 20-item self-report measure developed to assess depressive symptoms in community (nonclinical) populations.29 Scores range from 0 to 60, with higher scores indicating greater depression symptoms. A total score of depressive symptomatology is calculated by summing the scores of the 20 items (items 4, 8, 12, and 16 are reverse scored), with higher scores representing greater depressive symptomatology. The CES-D has been demonstrated to be a reliable and valid measure of depressive symptomatology in cancer patients, with alpha coefficients > 0.85 and moderate correlations with other measures of distress and symptom reporting in cancer patients.30 In a study examining test-retest reliability of the CES-D among cancer patients, mean scores were found to be 10.9 (SD = 8.9) and 12.8 (SD = 10.2) at a 2 ½ week follow-up.
MD Anderson Symptom Inventory
The MDASI is a 19-item questionnaire that assesses the effect and severity of pain and other cancer-related symptoms including fatigue, nausea, shortness of breath, poor appetite, and disturbed sleep.31 Symptoms are assessed on a scale from 0 (symptom not present) to 10 (symptom is as bad as can be imagined). The MDASI is scored by computing two subscales. The MDASI Symptom Severity scale, an average of the 13 cancer symptom severity items, assesses the severity of various symptoms related to cancer disease and treatment. The MDASI Symptom Interference scale, an average of the six interference items, assesses the extent to which cancer symptoms interfere with a patient’s day-to-day life (eg, “How much have your symptoms interfered with your general activity?”). Higher scores indicate greater interference in functioning. Among head and neck cancer patients, mean symptom severity and interference scores have been found to be 1.72 and 3.59, respectively, for those with less severe disease and 1.68 and 4.55, respectively, for those patients with more severe disease. The MDASI shows a high level of reliability on both subscales (alpha ranges from 0.87 to 0.94) and excellent sensitivity in distinguishing cancer patients according to their disease severity and treatment status.31
Fagerstrom Test for Nicotine Dependence
The Fagerstrom Test for Nicotine Dependence (FTND) is a 6-item questionnaire that measures nicotine dependence by assessing various components of smoking behavior such as daily intake, difficulty in refraining from smoking, and time to first cigarette of the day.32 An example of one FTND item is: “How soon after you wake up do you smoke your first cigarette?,” rated from 0 (“after 60 minutes”) to 3 (“within 5 minutes”). A total FTND score is calculating by averaging the scores on the 6 items, with higher scores on the FTND indicating higher nicotine dependence. In some studies, the scale has been found to correlate with cotinine level33 and to predict the outcome of smoking treatment.34 Average FTND scores among smokers seeking cessation help have been reported as 5.63.35
Data Analytic Approach
The data collected in this study have an inherent nested structure. There are 14 daily pain and smoking assessments nested within each participant, making these data ideally suited to a multilevel modeling (MLM) approach. MLM accounts for dependence between observations resulting from clustering of the data by participant and day of observation. Multilevel model analyses were conducted using SAS PROC MIXED. All MLM analyses were run with a random intercepts model (which was the best fitting model in each case) using an autoregressive order 1 model of the covariance structure.
The data analytic approach used in this article allows us to decompose effects within and between-person in our nested data structure. Given that a significant parameter estimate for pain would not provide us with specific information on the nature of this relationship (ie, whether it represents between-person effects, within-person effects, or both), we created level-specific predictors to parse apart between- and within-effects. The between-person overall pain predictor was calculated by taking the difference between each participant’s mean pain score and the mean pain score of all participants (participant level predictor). The within-person daily pain predictor was calculated by taking the difference between the observed pain score at each assessment day and each individual’s mean pain score (assessment level predictor). This method of computing group-mean centered participant level predictors and person-mean centered assessment level predictors is well-documented36,37 and has been used in other daily diary studies with similar nested data structures.20
Using a multilevel model, we regressed cigarettes smoked during the day on the within-person (assessment level) predictors of urge to smoke and daily pain and between-person (participant level) predictors of overall pain, age, gender, nicotine dependence (FTND) and baseline cancer pain, as assessed on the MDASI (Table 3). The multilevel analyses allowed us to examine within-person effects (eg, examining associations between daily pain and daily smoking within individuals), and between-person effects (eg, examining differences in smoking between individuals by overall pain). In addition to the multilevel analysis, we used logistic regression to test our second hypothesis that smokers with higher pain would be less likely to make a quit attempt during the study period, controlling for nicotine dependence, demographic factors, and smoking urge. This analysis allowed us to examine whether individuals with greater pain would have greater difficulty in making a quit attempt. In the logistic regression analysis, pain represented each participant’s average pain across the 2-week assessment period.
Table 3.
Results of Multilevel Model Examining Predictors of Smoking Throughout 2-Week Assessment Period
| Variable | Estimate | SE | P |
|---|---|---|---|
| Assessment level pain (within-person association) | 0.52 | 0.18 | <.01** |
| Participant level pain (between-person association) | 0.70 | 0.40 | .09 |
| Gender (female is reference group) | −0.75 | 0.54 | .18 |
| Age | −0.02 | 0.03 | .39 |
| Smoking urge | 0.49 | 0.17 | <.01** |
| Baseline pain | 0.01 | 0.14 | .93 |
| FTND (baseline) | 0.20 | 0.12 | .09 |
FTND = Fagerstrom Test for Nicotine Dependence; SE = standard error.
*Significance at the P < .05 level.
**Significance at the P < .01 level.
Results
Descriptive Statistics and Data Completion
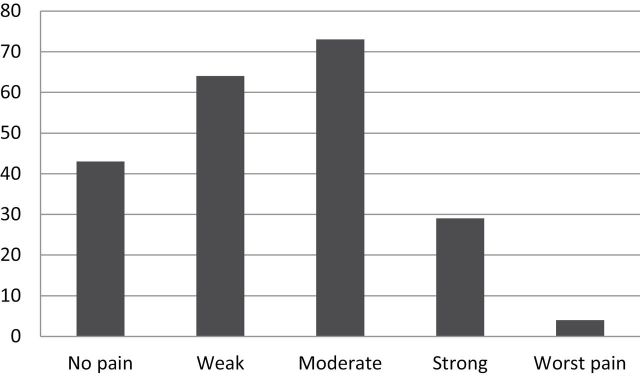
Mean MDASI Symptom Severity and MDASI Symptom Interference scores at baseline were 3.39 (SD = 1.67) and 4.0 (SD = 2.57), respectively (Table 2). Participants reported mean depressive symptomatology on the CES-D of 20.12 (SD = 13.12) at the baseline assessment. Higher scores on the CES-D at baseline were associated with greater cancer symptom reporting on the MDASI Symptom Severity scale (r = .44, P < .01) and MDASI Symptom Interference (r = .52, P < .01). Higher average daily diary pain scores (averaged across the 2-week assessment period) were associated with higher baseline MDASI Symptom Severity scores (r = .36, P = .04) and higher baseline MDASI Symptom Interference scores (r = .46, P < .01). Thus, those individuals who scored higher on the MDASI Symptom Severity and Interference scales tended to have higher average daily diary pain ratings during our 2-week assessment period. Of the 213 daily diary pain assessments collected over the 2-week assessment period, the most frequently endorsed daily pain rating was “Moderate” pain (34%; Figure 1). Cigarettes smoked per day ranged from 0 to more than 20, with the modal response being 5–10 cigarettes/d.
Table 2.
Mean and Standard Deviation Values for Baseline Assessments
| Range | Mean | Standard deviation | |
|---|---|---|---|
| CESD total score | 0–51 | 20.12 | 13.12 |
| FTND total | 0–10 | 4.18 | 2.42 |
| MDASI symptom severity subscale | 0.62–6.69 | 3.39 | 1.67 |
| MDASI symptom interference subscale | 0–9 | 4 | 2.57 |
CESD = Center for Epidemiologic Studies Depression Scale; FTND = Fagerstrom Test for Nicotine Dependence; MDASI = MD Anderson Symptom Inventory.
Figure 1.
Frequency of daily diary pain intensity scores over 2-week assessment period (N = 213).
We analyzed daily reports of pain medication usage by coding participants as having used neither prescription nor over-the-counter pain medication, only prescription pain medication, only over-the-counter pain medication, or both types of medication. Participants who reported using at least one pill at any point during the 14-day assessment period were coded having used that type of medication. Five participants (14.7%) reported using neither prescription nor over-the counter pain medication at any point during the 14-day assessment period. Most participants (n = 14, 41.2%) reported using both, whereas 12 participants (35.3%) reported using only prescription medication and three (8.8%) reported using only over-the-counter pain medication during the 14-day assessment period.
Participants completed a range of 1–14 assessments during the 2-week assessment period. The mean number of assessments completed per participant was 6.2 (SD = 3.7). A total of 286 daily diary assessments were collected over the 2-week assessment period from the 34 participants. The majority of the assessments (208, or 73%) had complete data for all daily diary items. The remaining assessments included partially completed assessments (6, or 2%) and assessments for which participants accessed the PPC, but failed to complete any items (72, or 25%). Of the six “partially completed” assessments, one assessment was missing all primary study variables but one and was not included in the analysis. The remaining five assessments had complete data on the primary study variables of daily cigarettes, pain, urge, and sad mood. These five assessments were flagged as “incomplete” by the electronic handheld device because they were missing a few items from other variables assessed in the larger study, but not in the present study. These five assessments were retained, resulting in a total of 213 assessments (representing 45% completion). The total number of daily diary assessments completed by each participant was not related to baseline MDASI Symptom Severity (r = .04, P = .98), baseline MDASI Symptom Interference (r = .08, P = .67), or baseline Center for Epidemiologic Studies Depression Scale scores (r = −.03, P = .99).
Treatment Information
All participants in this observational study received pharmacological medication and counseling for smoking cessation through the TTP during the 2-week study period. Thirty-two of the 34 (94%) participants in this study completed the counseling session at the week 1 follow-up, with 21 participants (66%) completing by phone and 11 participants (34%) completing the session in the TTP clinic. Two participants missed their appointment for the week 1 follow-up counseling session. Participants also received pharmacological medication as part of their regular TTP treatment, with 19 participants receiving varenicline (55.9%), nine using nicotine replacement therapy (26.5%), five receiving bupropion (14.7%), and one participant using two medications (2.9%).
Linear Mixed Model Results
Linear mixed modeling was used to examine the associations of smoking with pain and other model variables. We first ran four separate multilevel models, each including only one predictor, in order to examine how daily smoking related to model variables univariately. The models reported here include only one predictor and allow us to examine how each primary study variable relates univariately to daily smoking. In examining within-person associations between pain and smoking using MLM, daily pain was found to be positively associated with daily smoking (β = 0.54, P < .01). Examining between-person associations in pain and smoking using MLM, between-person overall pain was found to be positively associated with daily smoking (β = 0.82, P < .03). Daily urge (β = 0.60, P < .01) was related to greater daily smoking but neither daily sad mood (β = 0.15, P = .36) nor daily anxiety (β = 0.14, P = .37) were found to be significantly associated with daily smoking. Because sad mood and anxiety were not found to relate to daily smoking, we did not include these variables in the full MLM.
Secondly, a full multilevel model was run to examine the association between pain and smoking, controlling for baseline pain, nicotine dependence, smoking urge, age, and sex. In the full MLM model, within-person daily pain (β = 0.52, P < .01) and daily smoking urge (β = 0.49, P <.01) were found to be associated with number of cigarettes smoked during the day. Between-person overall pain (β = 0.70, P = .09) was not related to number of cigarette smoked during the day.
Quit Attempt Results
Lastly, we examined the relationship between pain and quit attempts. An average pain score was computed for each participant that represented the average of the participant’s daily pain ratings across the 2-week assessment period. A quit attempt was defined as a day on which participants reported smoking no cigarettes. Of the 213 daily diary smoking assessments, 14% (or 30 assessments) represented quit attempts. Given the low number of quit attempts across all assessments, participants who had a quit attempt at any point during the 2-week assessment period were coded as “1” and those with no quit attempts during the 2-week period were coded as “0.” A logistic regression model was run using the binary “quit attempt” variable as the outcome and average pain intensity as the predictor; the model controlld for nicotine dependence, smoking urge, daily diary completion, gender, and age. Average pain across the 2-week assessment period significantly predicted quit attempts over the 2-week period (odds ratio [OR] = 0.19, P < .01), indicating that for every one unit increase in average pain experienced during the 2-week assessment period, the odds of experiencing at least one quit attempt during the study period were reduced by more than half. Additionally, men were less likely to make a quit attempt than women (OR = 0.12, P < .01), and participants who reported greater smoking urge across the 2-week assessment period were less likely to make a quit attempt (OR = 0.53, P < .02).
Discussion
This study examined how pain related to smoking and quit attempts among cancer patients with pain who were trying to quit. Results demonstrated that cancer patients who reported greater average pain during the first 2 weeks of smoking cessation treatment were less likely to make a quit attempt during the study period. Moreover, we found a within-person pain-smoking association, such that daily pain ratings were positively associated with daily cigarettes smoked within individuals, controlling for baseline cancer pain and other model variables. On days that participants had higher pain, they smoked more cigarettes than on days when they had lower pain.
Previous research that has observed greater prevalence of painful conditions among smokers, compared with nonsmokers, has been unable to capture this naturalistic, day-to-day variation in pain and smoking.8,9 This study expanded on past findings by demonstrating that daily painful events are accompanied by greater daily smoking among cancer patients trying to quit. The significant within-person pain-smoking effect (and null between-person effect) found in this study suggests that variation in pain rating at the individual level may be more important in explaining daily smoking than overall pain levels among cancer patients trying to quit.
These findings have important clinical implications. The results of this study suggest that interventions to help cancer patients who smoke to better manage pain, especially on days when pain intensity is highest, may be an important addition to smoking cessation treatment in this population. Many psychological and pharmacological interventions have been found to be effective for cancer pain management,38–40 yet to our knowledge, no research has examined the effectiveness of smoking cessation treatment for cancer patients with pain which integrates aspects of pain management, highlighting an important area for future research. Cancer patients who smoke and have pain represent a group of patients with high symptom burden, and one that may benefit from more comprehensive care.
This study has some limitations. First, the completion rate was 45% for daily diary assessments. Completion rates for daily diary studies have been found to vary widely across different participant populations and different daily diary methodologies.23,41 Although a completion rate of 45% appears low at first glance, this is in line with other completion rates we have observed in studies that used daily diary measurement among cancer patients at MD Anderson.42 Given that we do not have daily diary assessment for each day for all participants, it is possible that the assessments collected do not represent the full experience of pain and smoking during our 2-week assessment period (eg, perhaps participants were more likely to complete diary assessments during times of high, or low, pain). Secondly, our sample was small and the participation rate in this study was fairly low. Most patients who declined to participate cited lack of interest or time constraints as the primary reasons for declining. Given the time commitment involved for this study (2 weeks of daily assessments) it is understandable that cancer patients with pain and symptom burden may not have had interest in participating. Thus, caution should be used in interpreting these findings, as results may not be generalizable to all cancer patients with pain who are trying to quit.
As an additional limitation, the observational design of the study did not allow for examination of causal pathways or mechanisms in the pain-smoking relation, as study variables were assessed at the same time on each day. Previous research suggests that the pain-smoking relationship may be characterized by bidirectional influences.14 Specifically, pain may motivate smoking and smoking may lead to the onset or exacerbation of painful conditions overtime. Thus, it is difficult to ascertain from the current design whether the observed effects represent the influence of pain on smoking, or of smoking on pain.
A closer examination of the reciprocal model proposed by Ditre et al.14 can help to shed some light on the observed findings. The effect of smoking on pain is largely characterized as an effect that develops over time with chronic exposure to tobacco smoking.43,44 Conversely, the influence of pain on smoking can manifest as a more immediate effort to cope with spikes in pain level and thus, would likely be reflected in day-to-day fluctuations in pain intensity and cigarette smoking. We argue that the observed within-person associations in the current study between pain and smoking, controlling for baseline pain and symptoms, likely reflect patient efforts to cope with pain on days when pain is higher. Certainly more research is needed on smoking among cancer patients with painful conditions in order to better understand the causal pathways which underlie this relation.
Future research may expand on the findings of the present study by examining how smokers with painful conditions respond to spikes in pain using real-time assessment. Real-time assessment which captures moment to moment variation in constructs can help us to tease apart the temporal effects of pain and smoking. Future research may also examine the effectiveness of integrating pain management interventions with smoking cessation treatment for patients with pain. For some patients with pain, interventions which help to increase the patient’s self-efficacy for coping with pain,45 in addition to standard smoking cessation treatment, may help to improve cessation outcomes. Currently, pain is not routinely addressed in coordination with tobacco cessation treatment. These findings suggest that pain may be an overlooked, but important, symptom in tobacco cessation treatment, particularly in cancer settings where pain is a common complaint. Continued research on pain and smoking has the potential to yield important information for better tailoring smoking cessation treatment for smokers with pain.
Funding
This research was supported by an American Cancer Society, MRSG-09-002-01-CPHPS grant for CYL. The research was also supported by a Cancer Prevention Fellowship for CJA, supported by the National Cancer Institute grant R25T CA57730, Shine Chang, PhD, Principal Investigator, and by the National Institutes of Health MD Anderson Cancer Center Support grant CA016672.
Declaration of Interests
None declared.
References
- 1. Newcomb P, Carbone P. The health consequences of smoking: cancer. Med Clin North Am. 1992;76(2):305–331. http://europepmc.org/abstract/med/1548964 Accessed August 1, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Sasco A, Secretan M, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiogical evidence. Lung Cancer. 2004;45(suppl 2):S3–S9. doi:10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 3. The health consequences of smoking—50 years of progress. US Department of Health and Human Services, Office of the Surgeon General 2014 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/ Accessed August 1, 2014.
- 4. Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with Stage I non-small cell lung cancer. Cancer Causes Control. 1991;2(2):105–112. http://link.springer.com/article/10.1007/BF00053129 Accessed August 1, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Ostroff J, Garland J, Moadel A, et al. Cigarette smoking patterns in patients after treatment of bladder cancer. J Cancer Educ. 2000;15(2): 86–90. doi:10.1080/08858190009528663. [DOI] [PubMed] [Google Scholar]
- 6. Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98(3):632–644. doi:10.1002/cncr.11538. [DOI] [PubMed] [Google Scholar]
- 7. Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2370–2376. doi:10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 8. Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–65. doi:10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daniel M, Keefe FJ, Lyna P, et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10(3):323–328. doi:10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest. 2005;23(2):182–190. doi:10.1081/CNV-50482. [PubMed] [Google Scholar]
- 11. Petzke F, Radbruch L, Zech D, Loick G, Grond S. Temporal presentation of chronic cancer pain: transitory pains on admission to a multidisciplinary pain clinic. J Pain Symptom Manage. 1999;17(6):391–401. doi:10.1016/S0885-3924(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 12. Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93(3):247–257. doi:10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 13. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi:10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 14. Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–1093. doi:10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117(2):467–472. doi:10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hooten WM, Vickers KS, Shi Y, et al. Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Pract. 2011;11(6):552–563. doi:10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peters M, Sorbi M, Kruise D, Kerssens J, Verhaak P, Bensing J. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84(2–3):181–192. www.ncbi.nlm.nih.gov/pubmed/10666523 Accessed August 1, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain. 2001;91(3):277–285. doi:10.1016/S0304-3959(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 19. Roelofs J, Peters ML, Patijn J, Schouten EG, Vlaeyen JW. Electronic diary assessment of pain-related fear, attention to pain, and pain intensity in chronic low back pain patients. Pain. 2004;112(3):335–342. doi:10.1016/j.pain.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20. Todd M. Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychol Addict Behav. 2004;18(1):31–39. doi:org/10.1037/0893-164X.18.1.31. [DOI] [PubMed] [Google Scholar]
- 21. McCarthy D, Piasecki T, Fiore M, Baker T. Life before and after quitting smoking: An electronic diary study. J Abnorm Psychol. 2006;115(3):454–466. doi:10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 22. Leigh B. Using daily reports to measure drinking and drinking patterns. J Subst Abuse. 2000;12(1–2):51–65. doi:10.1016/S0899-3289(00)00040-7. [DOI] [PubMed] [Google Scholar]
- 23. Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104 (1–2):343–351. doi:10.1016/S0304-3959(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 24. Aaron LA, Turner JA, Mancl L, Brister H, Sawchuk CN. Electronic diary assessment of pain-related variables: is reactivity a problem? J Pain. 2005;6(2):107–115. doi:10.1016/j.jpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25. Businelle MS, Lam CY, Kendzor DE, et al. Alcohol consumption and urges to smoke among women during a smoking cessation attempt. Exp Clin Psychopharmacol. 2013;21(1):29–37. doi:10.1037/a0031009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam CY, Businelle MS, Aigner CJ, et al. Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse. Nicotine Tob Res. 2014;16(5):569–575. doi:10.1093/ntr/ntt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lam CY, Businelle MS, Cofta-Woerpel L, McClure JB, Cinciripini PM, Wetter DW. Positive smoking outcome expectancies mediate the relation between alcohol consumption and smoking urge among women during a quit attempt. Psychol Addict Behav. 2014;28(1):163–172. doi:10.1037/a0034816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiftman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004;113(1):166–171. doi:10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- 29. Ross C, Mirowsky J. Components of depressed mood in married men and women. The Center for Epidemiologic Studies’ Depression Scale. Am. J. Epidemiol. 1984;119(6):997–1004. http://aje.oxfordjournals.org/content/119/6/997.short Accessed August 1, 2015. [DOI] [PubMed] [Google Scholar]
- 30. Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res. 1999;46(5):437–443. doi:10.1016/S0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 31. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89: 7<1634::AID-CNCR29>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 32. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 33. Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: a comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addict Behav. 1994;19(3):307–317. doi:10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 34. Pinto RP, Abrams DB, Monti PM, Jacobus SI. Nicotine dependence and likelihood of quitting smoking. Addict Behav. 1987;12(4):371–374. doi:10.1016/0306-4603(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 35. Fagerström KO, Kunze M, Schoberberger R, et al. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tob Control. 1996;5(1):52–56. doi:10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curran D, Bauer P. Introduction to multilevel modeling. Paper presented at: Introduction to Multilevel Modeling Workshop 2013 2013; Chapel Hill, NC. [Google Scholar]
- 37. Heck R, Thomas S, Tabata L. Mulilevel and Longitudinal Modeling With ABM SPSS. New York, NY: Routledge Taylor and Francis Group; 2014. [Google Scholar]
- 38. Johannsen M, Farver I, Beck N, Zachariae R. The efficacy of psychosocial intervention for pain in breast cancer patients and survivors: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138(3):675–690. doi:10.1007/s10549-013-2503-4. [DOI] [PubMed] [Google Scholar]
- 39. Sheinfeld Gorin S, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30(5):539–547. doi:10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puetz TW, Morley CA, Herring MP. The challenge of definition and moving creative arts therapy research forward. JAMA Intern Med. 2013;173(11):2094–2095. doi:10.1001/jamainternmed.2013.836. [DOI] [PubMed] [Google Scholar]
- 41. Fraser SC, Ramirez AJ, Ebbs SR, et al. A daily diary study for quality of life measurement in advanced breast cancer trials. Br J Cancer. 1993;67(2):341–346. www.ncbi.nlm.nih.gov/pmc/articles/PMC1968179/ Accessed August 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ratcliff CG, Lam CY, Arun B, Valero V, Cohen L. Ecological momentary assessment of sleep, symptoms, and mood during chemotherapy for breast cancer. Psychooncology. 2014;23(11):1220–1228. doi:10.1002/pon.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akmal M, Kesani A, Anand B, Singh A, Wiseman M, Goodship A. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine. 2004;29(5):568–575. doi:10.1097/01.BRS.0000101422.36419.D8. [DOI] [PubMed] [Google Scholar]
- 44. Waldie KE, McGee R, Reeder AI, Poulton R. Associations between frequent headaches, persistent smoking, and attempts to quit. Headache. 2008;48(4):545–552. doi:10.1037/a0032515. [DOI] [PubMed] [Google Scholar]
- 45. Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. J Abnorm Psychol. 2010;119(3):524–533. doi:10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]