Abstract
Introduction:
Electronic cigarettes (E-Cigs) are popular alternatives to conventional tobacco cigarettes. Disposable E-Cigs are single-use devices that emit aerosols from a nicotine-containing solution (e-liquid) by activating a heating coil during puffing. However, due to lack of regulations and standards, it is unclear how product claims are aligning with actual content and performance. Some analytical methods for characterizing E-Cigs are still in an exploratory phase.
Methods:
Five products of disposable E-Cigs (purchased March–April, 2014 from a local smoke shop and an on-line US distributor) were studied for nicotine content, number of puffs obtained before depletion, portion of nicotine delivered via aerosolization, and e-liquid pH. Protocols were developed to consistently extract e-liquid from puffed and unpuffed E-Cigs. An in-house mechanical puffing machine was used to consistently puff E-Cig aerosols onto filter pads. A gas chromatography-mass spectrometry method was developed that produced sensitive and repeatable nicotine determinations.
Results:
Under our experimental parameters, results showed a disparity between nicotine content and number of puffs achieved relative to what was claimed on product packaging. The portion of nicotine delivered to filter pads was often less than half that which was available, indicating much of the nicotine may be left in the E-Cig upon depletion.
Conclusions:
Analyses of unpuffed E-Cigs by gas chromatography-mass spectrometry indicate the nicotine content of these products can be considerably different from manufacture’s labeling. Furthermore, a large portion of the nicotine in E-Cigs may not be transferred to the user, and that which is transferred, may often be in the less bioavailable form.
Introduction
Electronic cigarettes (E-Cigs) are designed to deliver tobacco-free nicotine in an aerosolized form as a potentially safer alternative to smoking conventional cigarettes. 1–5 Considering the recent popularity of E-Cigs, there is a need for independent research to assess the validity of manufacture claims. 6–14 Metrics such as actual nicotine content, nicotine transfer efficiency, and e-liquid pH are of interest.
There are numerous E-Cigs on the market in an array of styles, flavors, configurations, and nicotine content. Aerosol emissions derive from a solution within E-Cigs (often referred to as the “e-liquid”) typically composed of propylene glycol, glycerol, water, nicotine, and various flavorings. E-Cigs have in common: a battery power source, an atomizer assembly that produces the aerosol (often referred to as a clearomizer or cartomizer), and an electronic control system. Typically, the atomizer consists of an e-liquid filled reservoir, wicking system to meter e-liquid delivery, and heating coil (powered by the battery and control system). The many styles of available E-Cigs can be grouped into four general E-Cig categories: (1) disposable—single use E-Cig, typically powered by a nonrechargeable lithium battery, and prefilled with e-liquid that is disposed of upon depletion; (2) rechargeable—E-Cigs with rechargeable batteries and replaceable prefilled cartridges; (3) refillable—E-Cigs with rechargeable batteries and clearomizers that are refilled by the user; and (4) rebuildable—advanced versions of refillable E-Cigs having adjustable air flow, coil size, and coil wattage. Unlike refillable and rebuildable models, the e-liquid in disposable E-Cigs is typically contained in a thick absorbent polymer fiber pad with a thin cotton-like fabric layer that transfers e-liquid to the wick. Common to both disposable and refillable E-Cigs, a wick of flame and heat resistant cord (to which the coil is wrapped around) operates as the delivery path of e-liquid to the coil.
Studies have reported nicotine content in e-liquid solutions and cartridges of rechargeable E-Cigs by various methods. 12 , 15–19 In addition, emission studies for E-Cigs have been performed by several groups of researchers using a variety of analytical techniques and puffing regimes. 15 , 18–24 And a few groups have specifically reported nicotine concentrations in the emissions of E-Cigs. 15 , 18–20 However, the culmination of these studies have published emissions data from only a limited number of E-Cigs among the almost 500 products available on the market, 25 necessitating the need for broader testing. And while nicotine has been measured by multiple methods, despite its utility as an analytical tool, gas chromatography-mass spectrometry (GC-MS) has not been thoroughly investigated as a technique for directly quantifying nicotine in e-liquid or E-Cig emissions. GC-MS has the advantage over GC and high-performance liquid chromatography in that other species in the e-liquid/emissions do not readily interfere with the measurement. Further, sample preparation, specifically for analysis of nicotine in E-Cigs, is relatively straight-forward.
Stepanov and Fujioka 26 have published pH data on a selection of e-liquids and have endorsed the importance of such measurements on E-Cigs for experimental design/interpretation and regulation discussions. Therefore, pH measurements of the e-liquids from the disposable E-Cigs in the current study are presented. However, detailed research on the pKa of nicotine in E-Cig aerosols, including inherent temperature and solution matrix effects throughout the vaping process for products with varying proprietary mixtures of e-liquids, are needed in the field in order to relate pH data to the portion of nicotine delivered in the free-base form. The present study also reports nicotine content in both unpuffed E-Cigs and aerosol emissions (collected using an in-house puffing machine) measured by GC-MS, while quantifying nicotine transfer efficiency for each of five disposable E-Cig products.
Methods
Choice of E-Cig Products
The five products chosen for this study represent brands locally and nationally popular, and frequently mentioned in online vaping forums. The selection was made qualitatively by interviewing local smoke shops to identify which brands they sold and which were most popular among them. Twelve smoke shops were interviewed by phone in five major cities across the United States (New York, Los Angeles, Houston, Philadelphia, and Chicago) seeking the same information. Furthermore, several online vaping forums were viewed to determine frequently discussed brands. Four of the E-Cigs brands chosen for this study were purchased from a local smoke shop in Rochester, NY and the fourth brand, White Cloud, was purchased from an online US distributor because it was not sold at the local smoke shop. Four of the five brands were manufactured in China according to packaging, with the only US brand being Swisher. The nicotine levels selected generally represented the highest available for each product at the time of the study. All products were purchased between March and April, 2014.
Sample Selection and Puffing Topography
Each of the five products of disposable E-Cigs (listed in Table 1 ), with five replicates of each, were evaluated for the number of puffs before depletion, total nicotine content of the e-liquid, residual nicotine left in the reservoir upon depletion, e-liquid pH, and portion of nicotine delivered to filter pads via aerosolization. Depletion was defined as the point in which the electronic controller within the E-Cig detected a low battery level and either presented a low battery indicator (such as a flashing LED) or no longer activated the coil during a puff. Note that stated comparisons in this article refer to the specific products tested within each brand, and not the brands as a whole. Within each product, all samples were selected from the same batch/lot number. E-Cigs were orientated horizontally during puffing events. Not being privy to the puffing topography used by the various manufacturers for the claimed number of puffs, a fixed puffing topography was used that would allow comparisons between brands. The puffing topography employed a bell-shaped flow profile with a maximum air flow rate of 30mL/s, puff duration of 5 seconds, and a 30-second interval between puffs. The total puff volume was approximately 90mL/puff and the average puff flow rate was 18mL/s. The topography parameters chosen in the current study are within the range of the limited data available on E-Cig puff topography. Video observations report puff durations of 4.2±0.7 seconds 27 and 4.3±1.5 seconds 28 (no volume or flow rate was reported in these studies). Laboratory environment topography studies report puff durations (and puff volumes) of 3 seconds (118.2mL), 29 2.65±0.98 seconds (51±21mL), 30 and 1.8±0.9 seconds (70±68mL). 18 E-Cig topography measurements recorded over a 24-hour period in the natural environment indicated that puff durations can range from 0.9 to 6.9 seconds and puff volumes can range from 29 to 388mL. 31 Early emission studies typically used puff durations and puff volumes of 1.8 seconds and 70mL 18 , 19 , 32 , 33 , 2 seconds and 100mL, 15 2.2 seconds (no reported puff volume) 23 , 24 or 100mL puff volumes (no reported puff duration). 20 A more recent study applied puff durations of 2, 4, and 8 seconds with flow rates of 17mL/s and 33mL/s. 34
Table 1.
Comparison Between Manufacture’s Claims and Study Measurements for Number of Puffs
| Product (battery capacity) | Claimed number of puffs | Actual number of puffs achieved in this study ( SE ) | % puffs achieved | E-liquid extracted from unpuffed E-cig a (mg/E-Cig) ( SE ) |
|---|---|---|---|---|
| Criss Cross Regular (0.33 wH) | 200 | 56 (1.7) | 28 | 563.3 (10.0) |
| Swisher Natural (0.63 wH) | 400 | 131.6 (14.4) | 33 | 612.9 (46.6) |
| Blu Magnificent Menthol (1.00 wH) | 400 | 184.6 (2.3) | 46 | 1013.8 (7.7) |
| White Cloud Fling (1.04 wH) | 400 | 239.6 (4.1) | 60 | 1498.0 (9.3) |
| Encore Summer Punch (1.04 wH) | 500 | 219.0 (6.9) | 44 | 1570.1 (9.7) |
E-Cig = electronic cigarette; SE = standard error.
a Determined gravimetrically.
Puffing Machine
E-Cig performance was studied using a new in-house mechanical puffing machine designed for use with E-Cigs. It utilizes an evacuated chamber, at 60” H 2 O (Vacuum), to provide suction to the E-Cig. Puff flow rate through the E-Cig was regulated by a fast acting proportioning valve under the control of Proportional-Integral-Derivative controller. Air flow rate was monitored by a calibrated Alicat Scientific flow meter (Model # M-50SLPM-D-30PSIA/5M) to provide feedback to the Proportional-Integral-Derivative controller. The mouthpiece of an E-Cig was inserted into the inlet of a Cambridge filter assembly, which was attached to the puffing machine. The puffing topography (flow profiles, air flow rates, puff intervals, etc.) and all other aspects of machine operation were under the control of National Instruments’ LabView software and USB-6008 Multifunction IO. During a puffing event, emissions were collected on a 44mm silica Cambridge filter pad in the filter holder.
Many commercially available smoking machines use a piston-in-cylinder mechanism to generate puffs from tobacco cigarettes and E-Cigs. Our machine has the advantages of being relatively simple (with few moving parts), has an easily programmable puff topography, has Proportional-Integral-Derivative compensation for differences in air flow resistance among E-Cig products, and is capable of using different aerosol collection methods. The fast acting proportioning valve permits rapid changes in flow rate to more closely reproduce complex puff profiles. The machine also permits recording of actual puff flow rates throughout a puffing event.
Filter Pad Loading
In order to prevent overloading the filter pad with aerosolized e-liquid, a series of filter pads were used for collection during a puffing experiment. The goal was to limit sample collection to less than 150mg per filter pad to be consistent with loading limits defined by ISO protocol for conventional cigarettes. 35 Preliminary testing showed the delivery rate of e-liquid from E-Cigs declined with use. Therefore, a protocol was developed that exposed the first filter pad to the first 40 puffs, followed by the next 80 puffs to the second filter pad, and then the next 100 puffs to all subsequent filter pads within a given trial. All filter pads for a given trial were pooled and analyzed together by GC-MS to record the total nicotine content of each E-Cig.
Depletion Determination
Each experimental puffing event began with a new/unpuffed disposable E-Cig. As the E-Cig was machine-puffed, the indicator light on the tip of the E-Cig would illuminate with each puff. As the battery moved to depletion, the indicator light would rapidly flash. In the case of the Blu E-Cig, the indicator light ceased to light altogether. The puff number of the first indicator light flashing (or ceasing of the indicator light) was recorded as the depletion point.
E-liquid Extraction
E-liquid was extracted from unpuffed disposable E-Cigs for pH measurement and nicotine quantitation by GC-MS. The extraction process involved cutting open the e-liquid containing portion of the E-Cig and removing all e-liquid wetted parts. The wick/sponge material was immediately pressed against a perforated metal plate, resulting in 2–3 drops of e-liquid being collected onto a glass microscope slide. A buffer-calibrated combination micro-pH electrode (Thermo Scientific 9863BN) was positioned with the pH sensing membrane immersed in the e-liquid along with a cover being placed over the slide to minimize evaporation. Stepanov and Fujioka 26 also measured the pH of e-liquids by bringing them to volume with ultrapure water and measuring with a pH meter. Their range of pH results agree well with ours, while our use of the microelectrode allowed for direct pH measurement of the e-liquid, without dilution, and could potentially be employed in situ in some e-liquid samples/products.
After pH measurement, the e-liquid was then transferred to a glass beaker by rinsing the microscope slide and pH probe with methanol (Fisher Scientific, high-performance liquid chromatography grade). All of the remaining wetted parts were rinsed with methanol (and the wick/sponge material was rinsed and wrung out multiple times) into the glass beaker. The collected solution was passed through a 0.45 µm regenerated cellulose syringe filter, the syringe filter was rinsed with methanol, and the solution was brought to volume with methanol in a 50mL volumetric flask for GC-MS analysis. The methanol-rinsed parts and the remaining parts of the E-Cig were dried and the total weight of the parts was compared to the unpuffed E-Cig weight. This weight difference represents the quantity of e-liquid placed into the E-Cig during manufacturing. For extraction of remaining e-liquid from the reservoirs of puffed E-Cigs, the same process was followed except pH measurements were omitted and the computed weight difference was for the remaining e-liquid in puffed E-Cigs.
Analysis by GC-MS
E-liquid samples from unpuffed E-Cigs were spiked with a quinolone (Acros, 99%) surrogate and diluted with methanol (Fisher, high-performance liquid chromatography grade) prior to analysis. The filter pad samples that were collected from puffed E-Cigs were also spiked with quinolone and extracted with methanol. Loaded filter pads/methanol were shaken on a wrist shaker for 20 minutes and then on an orbital shaker for 24 hours, filtered through a 0.45 µm cellulose filter to remove particulate matter from the disintegrated filter pads, and an aliquot was placed into a 1.5-mL GC vial for analysis. In parallel, seven standard solutions of nicotine (Pfaltz & Bauer, 98%) and quinolone surrogates were prepared in methanol and sequentially spiked onto unused filter pads to produce calibration curves for nicotine quantification. Standard concentrations on the pads ranged from 0.01 to 1mg/mL and were prepared using the same procedure as that of the E-Cig emission samples. Nicotine/quinolone in methanol standards were also made in this range for calibration curves for the e-liquid samples from unpuffed E-Cigs. All calibration curves were run in triplicate and linear correlations of R2 > 0.999 were observed.
A Perkin Elmer Clarus 500 gas chromatograph coupled to a quadrupole mass spectrometer (run in the electron ionization mode) was used in this study. In triplicate, 1 µL samples and standards were sequentially injected into the GC and passed through a 5% phenyl–95% dimethylpolysiloxane capillary column (30 m × 0.25mm I.D. × 0.25 µm, Agilent, Santa Clara, CA). Helium carrier gas was run through a split injector (50:1 split) at a temperature of 230°C. The oven temperature was increased by 20°C/min from 60°C to 200°C and then held for 3 minutes. The MS source and transfer line were kept at 180°C and 280°C, respectively, and the MS was run in the single-ion-monitoring mode at m/z values of 84 (confirmation), 133 (quantitation), and 162 (confirmation) for nicotine and 102 (quantitation), 129 (confirmation), and 161 (confirmation) for quinolone. Peaks were integrated and the ratio of nicotine’s integration to its corresponding surrogate’s integration was used to establish calibration curves and determine nicotine concentrations in the samples. The limit of quantitation for this method was 0.09 µg/mL, and its recovery and precision were 96% and 17%, respectively. A detailed investigation of the GC-MS analytical method as a protocol for nicotine analysis of E-Cig products is currently underway.
Statistical Methods
Due to the selection of E-Cigs with varying starting levels of nicotine, data expectedly deviated from normality. Therefore, Kruskall–Wallis tests were used to compare products in regard to the concentration of nicotine in unpuffed samples, as well as nicotine delivered as calculated by two methods: nicotine content collected on filter pads and nicotine remaining in puffed reservoirs relative to their unpuffed e-liquid content. Nicotine delivery results using the two methods were comparable, and we present the results as measured by the delivery to the pads in this study. Kruskall-Wallis tests were also used to compare the percentage transfer efficiency of nicotine to the filter pads among products. Specific pairwise differences between products for each measurement were assessed using a follow-up Dunn’s tests. All statistical tests were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Accuracy of Manufacture’s Claims
Table 1 shows the difference between manufactures’ claims and actual puffs achieved. With consistent puff topography across all E-Cig products, a proportional relationship between number of puffs achieved before depletion and the battery capacity would be expected. Noting the capacity printed on the batteries (in watt hours, wH) of the studied E-Cig products, this work found the average number of puffs achieved was indeed substantially proportional to the battery capacity and any deviations are likely attributable to variations in coil current requirements, battery freshness, etc. This information indicates each E-Cig product was puffed to a consistent depletion point relative to its battery capacity.
Table 2 shows a comparison of nicotine content based on manufactures’ claims for the products tested in this study. The manufacturers’ packaging does not always clearly communicate the actual nicotine concentration of the product. For example, White Cloud is labeled as “Full Strength” and Criss Cross is labeled as “High.” Therefore, the nicotine contents based on manufacturer labels for White Cloud and Criss Cross were not ranked. Furthermore, unlike e-liquid used with refillable E-Cigs, which is generally in units of mg/mL nicotine, units are not typically indicated for disposable E-Cigs. It is assumed for the purposes of this study, the claimed nicotine concentration in disposable E-Cigs is total mg nicotine/E-Cig.
Table 2.
Comparison Between Manufacture’s Claims and Study Measurements a for Nicotine Content
| Product | Manufacturer label | Measured total nicotine in unpuffed E-cig (mg/E-Cig) ( SE ) | Delivered to filter pad b (mg/E-Cig) ( SE ) | Nicotine transfer efficiency (%) ( SE ) | Comparative rank of nicotine content (1 = highest) | ||
|---|---|---|---|---|---|---|---|
| Based on manufacturer label c | Based on measurement in unpuffed E-cig d | Based on measurement delivered to filter pad d | |||||
| White Cloud Fling | Full strength, 2.4% | 24.6 (0.71) | 5.41 (0.22) | 22.0 (0.9) | unknown | 1 | 1 |
| Criss Cross Regular | High | 6.2 (0.02) | 1.09 (0.25) | 17.6 (4.0) | unknown | 4 | 5 |
| Blu Magnificent Menthol | 24 | 14.0 (0.11) | 2.02 (0.10) | 14.4 (0.7) | highest | 3 | 4 |
| Swisher Natural | 18 | 5.2 (0.67) | 2.99 (0.52) | 57.5 (10.2) | middle | 5 | 3 |
| Encore Summer Punch | 16 | 19.3 (0.40) | 5.03 (0.16) | 26.0 (0.8) | lowest | 2 | 2 |
E-Cig = electronic cigarette; GC-MS = gas chromatography-mass spectrometry; SE = standard error.
a From GC-MS analyses.
b Total puffs used to make nicotine delivery measurements (as dictated by E-Cig depletion) are shown in Table 1 .
c For the three products that stated quantifiable nicotine content.
d Rankings based on averaged measurements of nicotine concentration.
The actual GC-MS measured nicotine contents in unpuffed reservoirs are also shown in Table 2 . Kruskall–Wallis tests confirmed statistically significant differences between the measured nicotine content of the unpuffed reservoirs of the products ( H = 21.93, df = 4, P = .0002), with White Cloud being higher than Criss Cross and Swisher, and Blu being higher than Swisher. Relative rankings of nicotine measurements in unpuffed reservoirs were compared to qualitative rankings based on product packaging claims, and indeed the two sets of rankings differed. Based on the product label, Blu had the highest nicotine content of the products tested and Encore had the lowest (when the two products that do not explicitly state nicotine content are removed from the ranking). However, based only on averaged measured nicotine concentrations in the unpuffed reservoir, Encore was higher than both Blu and Swisher (the lowest). Likewise, White Cloud was measured to contain the most average nicotine in the unpuffed reservoir of the five products tested and Criss Cross ranked fourth. Interestingly, the packaging for both White Cloud and Criss Cross might imply they would both rank among the strongest in nicotine content.
Table 2 also lists the amount of nicotine delivered to the filter pads, determined by GC-MS, for each product when machine-puffed. Kruskall–Wallis tests confirmed statistically significant differences between the measured nicotine content delivered to filter pads of the products ( H = 19.62, df = 4, P = .0006), with White Cloud and Encore being higher than Criss Cross. And based on averaged measurements only, relative rankings of nicotine delivered to filter pads place White Cloud highest, followed by Encore, Swisher, Blu, and then Criss Cross. However, when these relative rankings were compared to those based only on average nicotine measurements in unpuffed reservoirs, the rankings shifted such that Swisher ranked lowest.
The relative rankings related to nicotine delivery to filter pads show the importance of transfer efficiency on nicotine emissions. Table 2 shows E-Cig transfer efficiencies, which are defined as the percentage of nicotine delivered to filter pads after puffing relative to the nicotine available in unpuffed E-Cigs. A small device transfer efficiency would indicate a significant amount of nicotine remained in the E-Cig reservoir without being delivered to the filter pad. Dixon’s Q test revealed that two data points were outliers (nicotine delivered to the filter pad and percentage transfer efficiency for a single Blu E-Cig trial). These two data points were removed from all subsequent statistical analyses. The outliers were not in the analytical detection method, as the measurements of multiple injections were in agreement. Even though the statistical tests for outliers cannot distinguish between collection method and variation in this product, the possibility of product variation exists. This may be potentially due to a lack of regulation and quality control, and more testing on multiple batches is needed. There were significant differences among products in the percentage transfer efficiency of nicotine ( H = 14.53, df = 4, P = .0055), with Swisher being significantly higher than Blu. Of the five products tested, Swisher had the lowest unpuffed nicotine content when relative rankings were based on averaged measurements only, but the highest nicotine transfer efficiency, resulting in it ranking third based on averaged measurements of nicotine delivery to pads.
pH Measurements
Average pH measurements varied across the products tested. The highest average pH of 8.37 (±0.01 standard error [ SE ]) was found for White Cloud, followed by 8.22 (±0.03 SE ) for Swisher, 7.56 (±0.02 SE ) for Encore, 6.53 (±0.07 SE ) for Criss Cross, and 6.47 (±0.06 SE ) for Blu.
Discussion
Under our experimental conditions, the actual number of puffs from all E-Cig products fell short of the claimed puffs printed on the packaging. While this was not unexpected given that we likely used a longer puff duration compared to that used to make the marketing claims, using a consistent puff topography, allows us to examine the longevity between products. Here, the various E-Cigs are not consistent in longevity relative to claimed number of puffs to depletion, with percentages of puffs achieved per puffs claimed ranging from 28% to 60%. Such a disparity suggests a consistent testing protocol among E-Cig manufactures for determining the expected number of puffs is not being employed.
Several studies have shown there can be inconsistencies between the claimed nicotine content on the packaging of unpuffed E-Cigs and what is measured in the laboratory. 12 , 15–19 Our results show that the claimed nicotine content was indeed statistically different than the measured nicotine concentration for all three of the tested products for which we had unambiguous package labeling (one-sample t tests: P = .000, .000, and .001 for Swisher, Blu, and Encore, respectively). In the case of the Encore sample, the nicotine content was 121% of what was claimed. Conversely, for the Swisher and Blu samples, the measured nicotine content was only 29% and 59% of what was claimed, respectively. In this study, samples were taken from the same batch for each brand, but as Goniewicz et al. 19 showed, nicotine concentrations between batches can vary up to ~30%. A percent difference between claimed and measured nicotine concentration could not be calculated for White Cloud and the Criss Cross E-Cigs because a numerical indication of nicotine content was not printed on the packaging. The Criss Cross packaging indicated a “High” nicotine level, suggesting the nicotine content would be among the higher levels available for E-Cigs. Yet, at 6.2mg of measured nicotine per E-Cig, it was among the lowest studied. White Cloud packaging indicated the nicotine content to be “Full Strength, 2.4%,” and like the Criss Cross product claim, this designation might suggest the nicotine content to be among the higher levels available for E-Cigs. The GC-MS measurements (24.6mg nicotine per E-Cig) indeed rank the White Cloud product at the highest nicotine level in unpuffed E-Cigs among the products tested.
Cambridge filter pad capture of E-Cig emissions had the advantage of not requiring the use of large amounts of volatile solvent and avoiding logistical issues with flow rates and getting aerosols into solution (as could be experienced with impinger set-ups). As stated, in addition to measuring nicotine delivery by analyzing filter pads, we also examined nicotine remaining in puffed reservoirs relative to their unpuffed e-liquid concentrations. Results of the two methods were comparable, with the filter pad method differing from those of the reservoir method by about the same as Goniewicz et al. 18 , 19 showed in comparing their gas wash bottle/sparger collection method to the same reservoir method. The filter pad method captures e-liquid droplets in the filters, with the nicotine ultimately being measured in the liquid phase. Since some nicotine could also be present in the gas phase, the filter pad method could underestimate delivered nicotine concentrations, while impinger methods could be capturing both phases of nicotine. A better understanding of the liquid-gas partitioning of aerosols produced by E-Cigs is needed in the field, and would work to quantitate nicotine losses by the filter pad method.
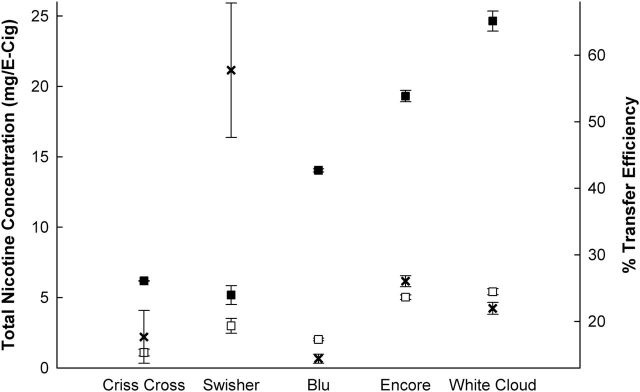
As shown in Figure 1 , the device transfer efficiencies of nicotine to filter pads after puffing are often under 30%, indicating the majority of the nicotine contained in the unpuffed E-Cig is often not delivered to the filter pad. The Swisher product delivered greater than 50% of its nicotine to the filter pad, yet the nicotine content in the unpuffed product is low to begin with, resulting in a net small amount of nicotine actually delivered. The White Cloud product delivered approximately 20% of its available nicotine to the filter pad, yet its high unpuffed/starting nicotine content allows for the greatest net nicotine delivery to the filter pad. Our results on disposable E-Cigs resonate with Goniewicz et al., 18 who tested rechargeable/refillable E-Cigs and refill solutions, and found a relatively large range of aerosol delivery (between 0.5 and 15.4mg of nicotine) and reported an average of 50%–60% of nicotine from reservoirs was aerosolized. Under a consistent puffing regime, variations in nicotine transfer efficiencies are likely caused by the chemical make-up of the e-liquid and the engineering characteristics of the E-Cig devices. And whereas the amount of nicotine delivered is, in part, a function of the starting concentration in the e-liquid, being able to directly compare the transfer efficiency of nicotine delivery between products with varying starting nicotine availability is important. To this end, our results of percentage transfer efficiency represent the amount of nicotine delivered to the filter pads relative to the starting concentration of nicotine in the unpuffed e-liquid.
Figure 1.
Measured nicotine concentration in unpuffed electronic cigarettes (E-Cigs; solid squares), nicotine content delivered to filter pads (open squares), and percentage nicotine transfer efficiency (thick “X,” right axis) of five E-Cig products. Symbols represent mean ± standard error.
In line with the call by Stepanov and Fujioka 26 related to the importance of incorporating pH measurements into E-Cig studies, pH values of the disposable products in this study range from ~6.5 to 8.4. And though complicated by temperature and matrix effects of the e-liquid on the pKa of nicotine, which further changes contemporaneously under different puffing regimes and differently with respect to the proprietary chemical make-up of product e-liquids, E-Cigs can vary the delivery of nicotine in the free-base form. True pKa values are not known across the solvent matrices and temperature gradients of the e-liquids and aerosols throughout the puffing procedure used on the products in this study, so it is not possible to extrapolate the portion of free-base nicotine delivered to the pads. In fact, there is currently very little information on actual nicotine pKa profiles of aerosolized e-liquids during the vaping process, but we believe that this is an important area for future research in the field. The bioavailability of nicotine can depend on its fraction present in the free-base form, as protonated nicotine must be absorbed from aerosol particles deposited on the lungs while unprotonated nicotine is available in the gas phase. 36 Further, particle deposition efficiency on the airway wall is affected by the aerosol size distribution. 37–39 The relative importance of nicotine absorption by direct particle deposition and diffusion has been of interest in recent studies. 40 Pankow et al. 41 , 42 suggested high pH levels in conventional cigarettes promote nicotine partitioning in the free-base form. Despite the limited pKa data, several recent clinical studies have reported on user exposure to nicotine from E-Cigs. 6 , 43–47
Misleading package labeling may lead to confusion for E-Cig users who are anticipating the indicated nicotine level, and not employing a consistent labeling system for nicotine content inhibits the user’s ability to make product selections. Varied nicotine transfer efficiency of the devices likely further complicates users’ experiences by making it challenging to select an E-Cig product with predictable results. Results from this study are for a select group of products available in the United States, and therefore, should not be generalized to products in other countries. It is assumed that users will perceive product quality partially based on their satisfaction of the experience that is likely associated with the nicotine dose and form delivered.
Several new techniques were presented in this study that enabled the efficient characterization of multiple parameters of E-Cigs. The in-house puffing machine was able to treat all E-Cigs with consistent puffs, while the Proportional-Integral-Derivative controlled proportioning valve was able to compensate for variations in air flow restriction between brands. The use of filter pads made capturing the aerosol easier and generated less organic waste. The e-liquid extraction technique allowed for quick and complete extraction of e-liquid from disposable E-Cigs using only methanol as the solvent. The pH measurements were easily incorporated into the extraction process using the microelectrode. The GC-MS technique for measuring nicotine in both the unpuffed e-liquid and the emissions delivered to filter pads shows promise in being reliable, repeatable, and relatively easy to process. Further work is warranted on the full validation of the analytical methodologies of this study, including the GC-MS technique, as potential standard protocols for nicotine analysis of E-Cigs. Assessments via the statistical methods employed can be useful in comparing the total dose of nicotine delivered to filter pads by different E-Cig products even when starting concentrations listed on manufacturing labels are misleading, ambiguous, or missing altogether. Though the relatively small number of disposable E-Cig products sampled potentially limits the application of generalizations from this early investigation, the work resulted in a better understanding of the performance of disposable E-Cigs, and particularly, how it relates to nicotine delivery. Given their popularity among consumers and the plethora of new products available, this work provides an important step in developing a protocol for evaluating manufacture claims, performance, user exposure, and potential health risks of E-Cigs.
Funding
Research reported in this publication was supported by the Office of the Director, National Institutes of Health, National Institute on Drug Abuse, and by the FDA Center for Tobacco Products (CTP) under award number R21DA036057-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Declaration of Interests
None declared .
References
- 1. Goniewicz ML, Zielinska-Danch W . Electronic cigarette use among teenagers and young adults in Poland . Pediatrics . 2012. ; 130 ( 4 ): e879 – 885 . doi: 10.1542/peds.2011-3448 [DOI] [PubMed] [Google Scholar]
- 2. Goniewicz ML, Lingas EO, Hajek P . Patterns of electronic cigarette use and user beliefs about their safety and benefits: an Internet survey . Drug Alcohol Rev . 2013. ; 32 ( 2 ): 133 – 140 . doi: 10.1111/j.1465-3362.2012.00512.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caponnetto P, Campagna D, Papale G, Russo C, Polosa R . The emerging phenomenon of electronic cigarettes . Expert Rev Respir Med . 2012. ; 6 ( 1 ): 63 – 74 . doi: 10.1586/ers.11.92 . [DOI] [PubMed] [Google Scholar]
- 4. Cho JH, Shin E, Moon SS . Electronic-cigarette smoking experience among adolescents . J Adolesc Health . 2011. ; 49 ( 5 ): 542 – 546 . doi: 10.1016/j.jadohealth.2011.08.001 . [DOI] [PubMed] [Google Scholar]
- 5. Grana RA, Ling PM . “Smoking Revolution” A content analysis of electronic cigarette retail websites . Am J Prev Med . 2014. ; 46 ( 4 ): 395 – 403 . doi: 10.1016/j.amepre.2013.12.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V . Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices . Sci Rep . 2014. ; 4 ( 4133 ): 1 – 7 . doi: 10.1038/srep04133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajek P, Etter J-F, Benowitz N, Eissenberg T, McRobbie H . Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit . Addiction . 2014. ; 109 ( 11 ): 1801 – 1810 . doi: 10.1111/add.12659 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng T . Chemical evaluation of electronic cigarettes . Tob Control . 2014. ; 23 (suppl 2): ii11 – 17 . doi: 10.1136/tobaccocontrol-2013-051482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen IL . FDA summary of adverse events on electronic cigarettes . Nicotine Tob Res . 2013. ; 15 ( 2 ): 615 – 616 . doi: 10.1093/ntr/nts145 . [DOI] [PubMed] [Google Scholar]
- 10. Cervellati F, Muresan X, Sticozzi C, et al. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells . Toxicol in Vitro . 2014. ; 28 ( 5 ): 999 – 1005 . doi: 10.1016/j.tiv.2014.04.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P . Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids . Toxicol in Vitro . 2013. ; 28 ( 2 ): 198 – 208 . doi: 10.1016/j.tiv.2013.10.006 . [DOI] [PubMed] [Google Scholar]
- 12. Etter J-F, Zather E, Svensson S . Analysis of refill liquids for electronic cigarettes . Addiction . 2013. ; 108 ( 9 ): 1671 – 1679 . doi: 10.1111/add.12235 . [DOI] [PubMed] [Google Scholar]
- 13. Grana R, Benowitz N, Glantz SA . E-cigarettes: a scientific review . Circulation . 2014. ; 129 ( 19 ): 1972 – 1986 . doi: 10.1161/circulationaha.114.007667 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palazzolo DL . Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review . Front Public Health . 2013. ; 1 ( 56 ): 1 – 20 . doi: 10.3389/fpubh.2013.00056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities . J Liq Chromatogr Relat Technol . 2011. ; 34 ( 14 ): 1442 – 1458 . doi: 10.1080/10826076.2011.572213 . [Google Scholar]
- 16. Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM . Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions . Tob Control . 2013. ; 23 ( 1 ): 1468 – 3318 . doi: 10.1136/tobaccocontrol-2012-050604 . [DOI] [PubMed] [Google Scholar]
- 17. Cheah NP, Chong NWL, Tan J, Morsed FA, Yee SK . Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective . Tob Control . 2014. ; 23 ( 2 ): 119 – 125 . doi: 10.1136/tobaccocontrol-2012-050483 . [DOI] [PubMed] [Google Scholar]
- 18. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L . Nicotine levels in electronic cigarettes . Nicotine Tob Res . 2013. ; 15 ( 1 ): 158 – 166 . doi: 10.1093/ntr/nts103 . [DOI] [PubMed] [Google Scholar]
- 19. Goniewicz ML, Hajek P, McRobbie H . Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications . Addiction . 2014. ; 109 ( 3 ): 500 – 507 . doi: 10.1111/add.12410 . [DOI] [PubMed] [Google Scholar]
- 20. Westenberger BJ . Evaluation of E-cigarettes . St. Louis, MO: : Food and Drug Administration: Department of Health and Human Services; ; 2009. . www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf . Accessed December 16, 2014 . [Google Scholar]
- 21. McAuley TR, Hopke PK, Zhao J, Babaian S . Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality . Inhal Toxicol . 2012. ; 24 ( 12 ): 850 – 857 . doi: 10.3109/08958378.2012.724728 . [DOI] [PubMed] [Google Scholar]
- 22. Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A . Secondhand exposure to vapors from electronic cigarettes . Nicotine Tob Res . 2013. ; 16 ( 6 ): 655 – 662 . doi: 10.1093/ntr/ntt203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams M, Talbot P . Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production . Nicotine Tob Res . 2011. ; 13 ( 12 ): 1276 – 1283 . doi: 10.1093/ntr/ntr164 . [DOI] [PubMed] [Google Scholar]
- 24. Trtchounian A, Williams M, Talbot P . Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics . Nicotine Tob Res . 2010. ; 12 ( 9 ): 905 – 912 . doi: 10.1093/ntr/ntq114 . [DOI] [PubMed] [Google Scholar]
- 25. Zhu S-HZ, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation . Tob Control . 2014. ; 23 ( suppl 3 ): iii3 – 9 . doi: 10.1136/tobaccocontrol-2014-051670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stepanov I, Fujioka N . Bringing attention to e-cigarette pH as an important element for research and regulation . Tob Control . 2014. ; 0 ( 0 ): 1 – 2 . doi: 10.1136/tobaccocontrol-2014–051540 . [DOI] [PubMed] [Google Scholar]
- 27. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V . Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation . Int J Environ Res Public Health . 2013. ; 10 ( 6 ): 2500 – 2514 . doi: 10.3390/ijerph10062500 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hua M, Yip H, Talbot P . Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos . Tob Control . 2013. ; 22 ( 2 ): 103 – 106 . doi: 10.1136/tobaccocontrol-2011-050226 . [DOI] [PubMed] [Google Scholar]
- 29. Norton KJ, June KM, O’Connor RJ . Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes . Tob Induc Dis . 2014. ; 12 : 1 – 8 . doi: 10.1186/1617-9625-12-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behar ZR, Hua M, Talbot P . Puffing topography and nicotine intake of electronic cigarette users . PLoS ONE . 2015. ; 10 ( 2 ): 1 – 18 . doi: 10.1371/journal.pone.0117222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson RJ, Hensel EC, Morabito PN, Roundtree KA . Electronic cigarette topography in the natural environment . PLoS ONE . 2014. ; 10 ( 6 ): 1 – 14 . doi:10.1371/journal.pone.012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes . Tob Control . 2014. ; 23 ( 2 ): 133 – 139 . doi: 10.1136/tobaccocontrol-2012-050859 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage . Nicotine Tob Res . 2014. ; 16 ( 10 ): 1319 – 1326 . doi: 10.1093/ntr/ntu078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions . Nicotine Tob Res . 2015. ; 17 ( 2 ): 150 – 157 . doi: 10.1093/ntr/ntu174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. ISO 4387 . Cigarettes-Determination of Total and Nicotine Free Dry Particulate Matter Using a Routine Analytical Smoking Machine. Amended 2008 . Geneva, Switzerland: : International Organization for Standardization; ; 2000. . www.iso.org/iso/home/store/catalogue_tc/catalogue_detail.htm?csnumber=28323 . Accessed December 16, 2014 . [Google Scholar]
- 36. Pankow JF, Tavakoli AD, Luo WT, Isabelle LM . Percent free base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes . Chem Res Toxicol . 2003. ; 16 ( 8 ): 1014 – 1018 . doi: 10.1021/tx0340596 . [DOI] [PubMed] [Google Scholar]
- 37. Robinson RJ . Trumpet Analytical Deposition Model . 2000. . http://people.rit.edu/rjreme/research_Trumpet.htm . Accessed November 11, 2014 .
- 38. Robinson RJ, Yu CP . Theoretical analysis of hygroscopic growth rate of mainstream and sidestream cigarette smoke particles in the human respiratory tract . Aerosol Sci Technol . 1998. ; 28 ( 1 ): 21 – 32 . doi: 10.1080/02786829808965509 . [Google Scholar]
- 39. Harding EMJ, Berg EJ, Robinson RJ . Diffusion in replica healthy and emphysematous alveolar models using computational fluid dynamics . ISRN Biomedical Engineering . 2013. ; 2013 : 1 – 9 . doi: 10.1155/2013/919802 . [Google Scholar]
- 40. Gowadia N, Dunn-Rankin D . A transport model for nicotine in the tracheobronchial and pulmonary region of the lung . Inhal Toxicol . 2010. ; 22 ( 1 ): 42 – 48 . doi: 10.3109/08958370902862442 . [DOI] [PubMed] [Google Scholar]
- 41. Pankow JF, Mader BT, Isabelle LM, Luo WT, Pavlick A, Liang CK . Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia . Environ Sci Technol . 1997. ; 31 ( 8 ): 2428 – 2433 . doi: 10.1021/es970402f . [Google Scholar]
- 42. Pankow JF . A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract . Chem Res Toxicol . 2001. ; 14 ( 11 ): 1465 – 1481 . doi: 10.1021/tx0100901 . [DOI] [PubMed] [Google Scholar]
- 43. Yan XS, D’Ruiz C . Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes . Regul Toxicol Pharm . 2015. ; 71 (1): 24 – 34 . doi: 10.1016/j.yrtph.2014.11.004 . [DOI] [PubMed] [Google Scholar]
- 44. Nides MA, Leischow SJ, Bhatter M, Simmons M . Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system . Am J Health Behav . 2014. ; 38 ( 2 ): 265 – 274 . doi: 10.5993/ajhb.38.2.12 . [DOI] [PubMed] [Google Scholar]
- 45. Dawkins L, Corcoran O . Acute electronic cigarette use: nicotine delivery and subjective effects in regular users . Psychopharmacology . 2014. ; 231 ( 2 ): 401 – 407 . doi: 10.1007/s00213-013-3249-8 . [DOI] [PubMed] [Google Scholar]
- 46. Vansickel AR, Eissenberg T . Electronic cigarettes: effective nicotine delivery after acute administration . Nicotine Tob Res . 2013. ; 15 ( 1 ): 267 – 270 . doi: 10.1093/ntr/ntr316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H . Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use . Nicotine Tob Res . 2015. ; 17 ( 2 ): 175 – 179 . doi: 10.1093/ntr/ntu153 . [DOI] [PMC free article] [PubMed] [Google Scholar]