Abstract
Introduction:
Electronic cigarettes (ECIGs) aerosolize a liquid that usually contains propylene glycol and/or vegetable glycerin, flavorants, and the dependence-producing drug nicotine in various concentrations. This study examined the extent to which ECIG liquid nicotine concentration is related to user plasma nicotine concentration in ECIG-naïve tobacco cigarette smokers.
Methods:
Sixteen ECIG-naïve cigarette smokers completed four laboratory sessions that differed by the nicotine concentration of the liquid (0, 8, 18, or 36mg/ml) that was placed into a 1.5 Ohm, dual coil “cartomizer” powered by a 3.3V battery. In each session, participants completed two, 10-puff ECIG use bouts with a 30-second inter-puff interval; bouts were separated by 60 minutes. Venous blood was sampled before and after bouts for later analysis of plasma nicotine concentration; puff duration, volume, and average flow rate were measured during each bout.
Results:
In bout 1, relative to the 0mg/ml nicotine condition (mean = 3.8ng/ml, SD = 3.3), plasma nicotine concentration increased significantly immediately after the bout for the 8 (mean = 8.8ng/ml, SD = 6.3), 18 (mean = 13.2ng/ml, SD = 13.2), and 36mg/ml (mean = 17.0ng/ml, SD = 17.9) liquid concentration. A similar pattern was observed after bout 2. Average puff duration in the 36mg/ml condition was significantly shorter compared to the 0mg/ml nicotine condition. Puff volume increased during the second bout for 8 and 18mg/ml conditions.
Conclusions:
For a given ECIG device, nicotine delivery may be directly related to liquid concentration. ECIG-naïve cigarette smokers can, from their first use bout, attain cigarette-like nicotine delivery profiles with some currently available ECIG products.
Implications:
Liquid nicotine concentration can influence plasma nicotine concentration in ECIG-naïve cigarette smokers, and, at some concentrations, the nicotine delivery profile of a 3.3V ECIG with a dual coil, 1.5-Ohm cartomizer approaches that of a combustible tobacco cigarette in this population. Finding a product that delivers nicotine as effectively as a tobacco cigarette, as we report here, may be essential for smokers who want to replace completely their combustible tobacco cigarettes with ECIGs.
Introduction
Electronic cigarettes (ECIGs) heat a liquid solution so that the resulting aerosol can be inhaled by the user. ECIG devices vary considerably, but generally consist of a power source (typically a battery) and a heating element (commonly called an “atomizer”) that is either integrated into a liquid-containing cartridge (called a “cartomizer”) or reservoir (called a “tank”). The cartomizer or tank simultaneously stores the liquid solution and brings it into contact with the heating element. 1 The liquid solutions usually contain propylene glycol and/or vegetable glycerin, flavorants, and other additives, as well as the dependence-producing drug nicotine in concentrations as high as 48mg/ml. 1 , 2
Some commentators have suggested that ECIGs may substitute completely for tobacco cigarettes for most smokers, 3 , 4 though recent results from a year-long longitudinal study suggest that this substitution effect does not happen for the majority of smokers. 5 This substitution effect, if it is to occur, may be related to the ability of an ECIG to match a tobacco cigarette’s nicotine delivery profile, which means increasing plasma nicotine concentration by approximately 15–20ng/ml after approximately 10 puffs taken over about 5 minutes. 6–7 However, early model ECIGs may not have delivered nicotine reliably and only recently have some ECIGs been shown to provide tobacco-cigarette like plasma nicotine delivery, and then only in the hands of experienced ECIG users who used nicotine liquids that varied by individual. 6 , 8 , 9 The purpose of this study was to determine the extent to which ECIG nicotine delivery profile in ECIG-naïve tobacco cigarette smokers is influenced by ECIG liquid nicotine concentration, one of the factors known to influence ECIG nicotine emissions. 10
Methods
In this preliminary report, 16 ECIG-naïve cigarette smokers completed this Institutional Review Board-approved clinical laboratory study. Participants were eligible if they reported that they were healthy, aged 18–55, used at least 15 cigarettes daily, and used an ECIG less than 5 instances in their lifetime. Individuals were ineligible if they reported a history of chronic disease or psychiatric condition, regular use of a prescription medication (except vitamins and/or birth control), marijuana use more than 10 days and/or alcohol use more than 25 days in the past 30, and use of other illicit drugs (eg, cocaine, opioids, benzodiazepines, and methamphetamine) in the past 30 days. Women were excluded if they tested positive for pregnancy by urinalysis at screening.
The methodology reported here is similar to the methodology of a parallel study examining experienced ECIG users. 11 All participants completed four randomly ordered sessions preceded by at least 12 hours of combustible tobacco abstinence (verified with expired air carbon monoxide < 10 ppm) and were separated by at least 48 hours. In each session, participants were provided with an eGo 3.3V, 1000 mAh battery with a 1.5-Ohm, dual-coil, 510-style cartomizer (SmokTech; Shenzhen, China). Participants were informed how to activate the ECIG but received no other ECIG-related instructions. The cartomizer was pre-loaded (by staff with no participant contact) with 1ml of a flavored (tobacco or menthol), 70% propylene glycol/30% vegetable glycerin liquid (AVAIL Vapor, Richmond, VA) and sessions differed by the nicotine concentration of that liquid: 0, 8, 18, or 36mg/ml (liquid nicotine concentration was verified prior to use). Participants and research staff were blind to nicotine concentration. In each session, participants completed two, 10-puff ECIG use bouts with a 30-second inter-puff interval. A research assistant told participants when to puff and observed and counted each puff. The two bouts were separated by 60 minutes. A forearm vein catheter was used to sample blood (~7ml) 10 times in each session for later analysis of plasma nicotine concentration using methods described previously. 6 , 9 , 12 Puff duration, volume, and average flow were measured by an automated device designed specifically to measure ECIG puff topography. 9 Other outcomes (eg, heart rate, subjective effects) were also assessed but are not the focus of this preliminary report and thus are not discussed further.
For plasma nicotine data, to maintain statistical power in this preliminary report while also limiting Type-I error, we conducted a set of a priori comparisons at each measurement time point using dependent samples t tests in which the mean plasma nicotine concentration for the 0mg/ml condition was compared to the corresponding mean of the 8, 18, and 36mg/ml conditions. Because these comparisons were non-orthogonal at each time point, a Bonferroni correction was used (ie, initial α < 0.05/3 comparisons = α < 0.017 for each comparison). 13 For topography data, we used the same analytic strategy within each bout (ie, for each measure, comparing 0 to 8, 18, and 36mg/ml, with a Bonferroni correction) but compared across bouts within each dose using uncorrected dependent samples t tests for these orthogonal comparisons. 13
Results
Participant Characteristics
A total of 27 individuals consented to participate in this study, of whom five withdrew before beginning any session, four were discontinued (two for poor venous access, one for noncompliance with pre-session abstinence, and one due to elevated blood pressure) and two failed to attend scheduled sessions. Of the 16 ECIG-naïve cigarette smokers who completed the study, 11 were males and seven self-identified as black or African American. Mean ( SD ) age was 32.2 (11.0) years, participants smoked 16.4 (4.8) cigarettes/d on average, and had been smoking for an average of 10.0 (11.0) years. Participants reported ever use of an ECIG for an average of 1.6 (1.0) times, indicating that they were indeed naïve to ECIG use. At screening (ie, before sessions were scheduled and without any study-related tobacco abstinence, average Fagerström Test for Nicotine Dependence score was 5.3 (1.6) indicating moderate nicotine dependence, 14 and average expired air carbon monoxide concentration was 20.3 (5.6) ppm.
Plasma Nicotine
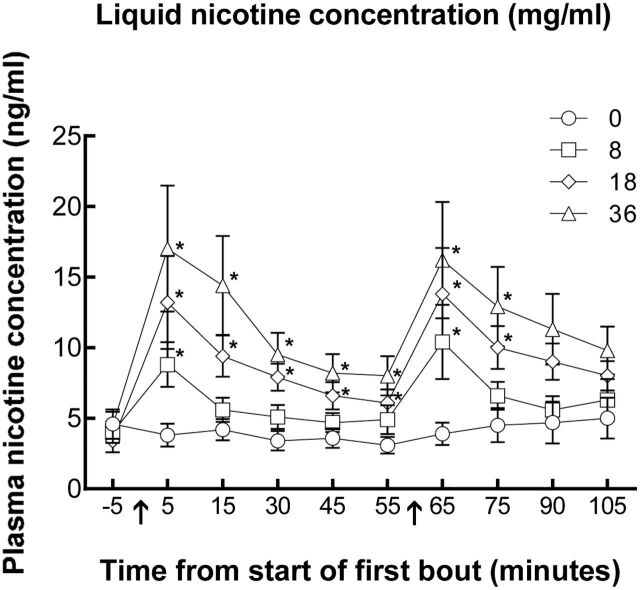
Figure 1 shows the time course of plasma nicotine concentration by each liquid nicotine concentration. Paired-samples t tests indicated significant differences between 0 and 8mg/ml at 5min, immediately after the 10th puff of bout 1, and at 65 minutes, immediately after the 10th puff of bout 2 [ t s(15) < −2.91]. There were also significant differences between the 0 and 18mg/ml nicotine liquid concentration conditions at time points 5, 15, 30, 45, 55, 65, and 75 [ t s(15) < −2.78]. Finally, there were significant differences between 0 and 36mg/ml at time point 5, 15, 30, 45, 55, 65, and 75 [ t s(15) < −2.77]. Immediately after bout 1, mean ( SD ) plasma nicotine concentration for the 0mg/ml liquid nicotine concentration was 3.8 (3.3), for 8mg/ml was 8.8 (6.3), for 18mg/ml was 13.2 (13.2), and for 36mg/ml was 17.0 (17.9). Immediately after bout 2, mean (SD) plasma nicotine concentration for the 0mg/ml liquid nicotine concentration was 3.9 (3.2), for 8mg/ml was 10.4 (10.6), for 18mg/ml was 13.8 (13.1), and for 36mg/ml was 16.2 (16.5). In order to explore the reliability of these results further, we examined post-pre difference scores for all participants in each condition for each bout. For bout 1, two participants showed an increase in plasma nicotine concentration in the 0mg/ml condition, 13 showed an increase at 8mg/ml, all 16 showed an increase in the 18mg/ml condition, and 15 showed an increase in the 36mg/ml condition. For bout 2, three participants showed an increase in plasma nicotine concentration in the 0mg/ml condition, 15 showed an increase at 8mg/ml, 14 showed an increase in the 18mg/ml condition, and 15 showed an increase in the 36mg/ml condition. No adverse events occurred in any session.
Figure 1.
Time course of plasma nicotine concentration as a function of electronic cigarette (ECIG) nicotine concentration. Mean (±SEM) plasma nicotine values for 16 ECIG-naïve cigarette smokers using ECIGs that varied by liquid nicotine concentration. Arrows indicate bouts where participants used the ECIG for 10 puffs with a 30-second inter-puff interval. * indicates a significant difference from 0mg/ml.
Topography
Topography results for puff duration, volume, and flow rate for each nicotine concentration are shown in Table 1 . Paired-samples t tests indicate no significant differences between 0mg/ml liquid nicotine concentration conditions and all other concentrations for puff volume during either bout. There were significant difference between bouts for 8mg/ml [ t (15) = −3.00, P < .01] and 18mg/ml [ t (15) = −2.32, P < .05], with greater puff volumes observed during bout 2. Regarding puff duration, there were significant differences between 0mg/ml and 36mg/ml for bout 1 and bout 2 [ t s(15) > 3.60], with the 36mg/ml leading to shorter puff durations. The only significant finding observed for average flow rate was between bouts 1 and 2 for 8mg/ml [ t (15) = −2.27, P < .05], with greater flow rates observed in bout 2.
Table 1.
Mean ( SD ) Puff Topography for Each Concentration of Liquid Nicotine
| Liquid nicotine concentration (mg/ml) | ||||
|---|---|---|---|---|
| 0 | 8 | 18 | 36 | |
| Volume (ml) | ||||
| Bout 1 | 83.2 (62.6) | 80.3 (53.8) | 70.2 (28.8) | 66.7 (55.9) |
| Bout 2 | 97.0 (63.1) | 93.0 b (59.7) | 79.3 b (38.9) | 63.0 (44.2) |
| Puff duration (s) | ||||
| Bout 1 | 3.00 (1.38) | 2.80 (1.41) | 2.85 (1.49) | 2.27 a (0.99) |
| Bout 2 | 3.21 (1.63) | 2.89 (1.42) | 3.00 (1.61) | 2.29 a (1.10) |
| Average flow rate (ml/s) | ||||
| Bout 1 | 30.0 (25.7) | 30.9 (20.1) | 27.1 (13.1) | 31.8 (33.1) |
| Bout 2 | 32.2 (22.1) | 33.6 b (21.8) | 29.0 (13.3) | 30.4 (28.5) |
a Nonorthogonal paired-samples t test difference from 0mg/ml with a Bonferroni correction of α < 0.017. 10
b Orthogonal paired-samples t test difference at that dose between bout 1 and bout 2. 10
Discussion
The purpose of this study was to determine the extent to which ECIG plasma nicotine concentration is influenced by ECIG liquid nicotine concentration in ECIG-naïve tobacco cigarette smokers. Results clearly demonstrate that higher liquid nicotine concentrations increase plasma nicotine concentration, with the highest concentration of liquid nicotine used in this study (ie, 36mg/ml) producing the highest concentrations of plasma nicotine in both 10-puff bouts. In fact, using that highest concentration, participants in this study were able to obtain cigarette-like plasma nicotine concentrations (ie, a mean nicotine “boost” of 12.5ng/ml, SD = 14.1) after 10 puffs from a 3.3V device with a 1.5-Ohm cartomizer, and, for each bout, the peak concentration was observed in the sample taken immediately after the 10th puff in that bout. Thus, in the ECIG-naïve participants who completed this study, we observed a nicotine delivery profile that was very close to that observed when tobacco cigarette smokers take 10 puffs from their usual brand of combustible cigarettes (eg, a nicotine boost of ~16ng/ml). 6 These results were observed with puffing profiles that are consistent with tobacco cigarette smokers, and not those seen in experienced ECIG users who have likely learned how to extract nicotine effectively from their preferred device (ie, puff durations of between 2–3 seconds and not the 4+ seconds observed in ECIG-experienced users). 9
Liquid nicotine concentration also influenced puff duration, with participants taking significantly shorter puffs in the 36mg/ml condition as compared with 0mg/ml. Because this study did not include a combustible cigarette condition, determining the extent to which participants were taking longer puffs to compensate for the lack of nicotine in the 0mg/ml condition or taking shorter puffs to compensate for the relatively high nicotine content of the 36mg/ml condition is challenging. While these preliminary analyses showed no effects of liquid nicotine concentration on puff volume, there were differences between bouts with two of the concentrations (ie, 8 and 18mg/ml), in which participants inhaled a significantly greater volume of ECIG aerosol during the second bout. The extent to which these differences reflect an attempt by participants to self-administer more nicotine in the second bout is unclear. To our knowledge, this is the first study to examine the potential influence of liquid nicotine concentration on puff topography among cigarette smokers naïve to ECIGs, so these results should be regarded as preliminary and await replication.
Study limitations include the absence of a combustible tobacco control condition that would have allowed characterization of “usual brand” topography and nicotine delivery, as well as the controlled puffing procedures used (ie, 10 puffs with a 30-sec inter-puff interval). Future work would benefit from detailed study of ECIG-naïve tobacco cigarette smokers under ad libitum puffing conditions, and could also examine the extent to which nicotine delivery profile and/or topography change with experience within and across days. Also, although we measured subjective effects in this study, we do not examine the results of those measures in this preliminary report due to a lack of statistical power.
In summary, liquid nicotine concentration can influence plasma nicotine concentration in ECIG-naïve cigarette smokers, and, at some concentrations, the nicotine delivery profile of a 3.3V ECIG with a dual coil, 1.5-Ohm cartomizer approaches that of a combustible tobacco cigarette in this population. These results may be relevant to recently reported results regarding ECIG use among cigarette smokers. 5 That is, one potential explanation for ECIGs failing to help combustible tobacco cigarette smokers quit smoking after a year of trying is that they may be using products that do not deliver nicotine effectively. Finding a product that delivers nicotine as effectively as a tobacco cigarette, as we report here, may be essential for smokers who want to replace completely their combustible tobacco cigarettes with ECIGs. 15 , 16
Funding
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Declaration of Interests
None declared .
Acknowledgments
The authors thank Janet Austin, Barbara Kilgalen, Kendall Pettaway, and Kathleen Osei for their help with data collection.
References
- 1. Etter J-F . The Electronic Cigarette: An Alternative to Tobacco? Geneva, Switzerland: : Jean-Francois Etter; ; 2012. . [Google Scholar]
- 2. Vaporzone, Inc. [Vaperzone’s website for e-cigarettes and e-liquids] . www.vaporzone.com . Accessed November 13, 2014 .
- 3. Hajek P . Electronic cigarettes have a potential for huge public health benefit . BMC Med . 2014. ; 12 : 225 . doi: 10.1186/s12916-014-0225-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Etter JF . Commentary on Goniewicz et al . (2014): if wisely regulated, electronic cigarettes can make cigarettes obsolete . Addiction . 2014. ; 109 ( 3 ): 508 – 509 . doi: 10.1111/add.12473 [DOI] [PubMed] [Google Scholar]
- 5. Brose LS, Hitchman SC, Brown J, West R, McNeill A . Is use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up . Addiction . 2015. ; 110 ( 7 ): 1160 – 1168 . doi:10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE . A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects . Cancer Epidemiol Biomarkers Prev . 2010. ; 19 ( 8 ): 1945 – 1953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan XS, D’Ruiz C . Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes . Regul Toxicol Pharmacol . 2015. ; 71 ( 1 ): 24 – 34 . [DOI] [PubMed] [Google Scholar]
- 8. Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M . Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial . Tob Control . 2010. ; 19 ( 2 ): 98 – 103 . doi: 10.1136/tc.2009.031567 . [DOI] [PubMed] [Google Scholar]
- 9. Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T . Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects . Nicotine Tob Res . 2015. ; 17 ( 2 ): 142 – 149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talih S, Balhas Z, Eissenberg T, et al. . Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions . Nicotine Tob Res . 2015. ; 17 ( 2 ): 150 – 157 . doi: 10.1093/ntr/ntu174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramôa CP, Hiler MM, Spindle T, et al. . Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in electronic cigarette users: a preliminary report . Tob Control . Online first. doi:10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breland AB, Kleykamp BA, Eissenberg T . Clinical laboratory evaluation of potential reduced exposure products for smokers . Nicotine Tob Res . 2006. ; 8 ( 6 ): 727 – 738 . doi: 10.1080/14622200600789585 . [DOI] [PubMed] [Google Scholar]
- 13. Keppel G . Design and Analysis, a Researcher’s Handbook . Englewood Cliffs, NJ: : Prentice Hall; ; 1991. . [Google Scholar]
- 14. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO . The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire . Br J Addict . 1991. ; 86 ( 9 ): 1119 – 1127 . [DOI] [PubMed] [Google Scholar]
- 15. Blank M, Eissenberg T . Protecting individual and public health by regulating electronic cigarette nicotine delivery . Addiction . 2015. ; 110 ( 7 ): 1169 - 70 . doi:10.1111/add.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cobb CO, Hendricks PS, Eissenberg T . Electronic cigarettes and nicotine dependence: evolving products, evolving problems . BMC Medicine . 2015. ; 13 : 119 . doi:10.1186/s12916-015-0355-y . [DOI] [PMC free article] [PubMed] [Google Scholar]