Abstract
Introduction:
Perceived health risk (PHR) of a tobacco product may influence both uptake and continued use. In this study, we examined PHRs of snus and medicinal nicotine using the PHR scale and the relationship of PHR responses to use of these products in smokers seeking an alternative to smoking.
Methods:
Smokers were randomly assigned to snus or to medicinal nicotine for a period of 12 weeks and asked to only use the assigned product. The PHR scale involves rating the extent of perceived risk of a product for different diseases and was given at baseline and weeks 4 and 12 during treatment. Relationships between PHR scale scores and study attrition, compliance with only using the product, and continued use of the product after treatment were determined.
Results:
Response to the PHR scale showed no significant differences between the snus and medicinal nicotine for perceived risks for lung cancer, emphysema, and bronchitis. However, significant differences were observed for other cancers, heart disease, stroke and risk for addiction, particularly after product use, with higher scores among those assigned to snus. Scores on the PHR scale were not related to any of the trial outcome variables.
Conclusions:
Among smokers seeking an alternative to smoking in a clinic setting, PHR of a product changes after product use but may not be related to product use patterns.
Implications:
PHRs of snus or medicinal nicotine in smokers assigned to these products become more accurate after product use. PHR does not appear to be associated with patterns of product use; rather satisfaction with a product is a better indicator as to whether a smoker is compliant with only using the product or continues to use the product.
Introduction
Oral noncombustible products have been considered to potentially reduce individual risk because compared with cigarettes these products do not expose consumers to combustion toxicants. 1 Reduced exposure to toxicants has been observed if snus (smokeless tobacco with reduced tobacco specific nitrosamines) is used to completely substitute for usual brand cigarette smoking. 1–3 Epidemiological studies in Scandinavian countries show that an increase in snus use with concomitant decrease in smoking has led to a substantial reduction in smoking-related mortality and morbidity. 4 , 5 This substitution of snus for cigarettes in Norway has been associated with the perceived relative safety of this product compared with cigarettes. 6 However, little research in the United States has been conducted on how consumers perceive the health risk of products such as snus compared with cigarettes (high potential for harm) and medicinal nicotine (low potential for harm). Furthermore, few studies have examined the relationship between health risk perceptions to how the product is used. The goals of this study were to examine differences in perceived health risks (PHRs) between snus and medicinal nicotine, between cigarettes and these products and the extent to which PHRs are associated with product use.
Methods
Data for this analysis was obtained from a randomized clinical trial that was conducted to examine the effects of medicinal nicotine versus snus on pattern of use, toxicant exposure and subjective responses. 7 Smokers, interested in completely switching to snus or nicotine gum, were recruited from Minneapolis/St. Paul (MSP), Minnesota and Eugene (EUG), Oregon. Eligible participants were between the ages of 18 to 70, smoked at least 10 cigarettes per day for the past year, were in good or stable physical and mental health, had no contraindications for nicotine replacement therapies, did not use other tobacco or smoking cessation medicines, and were not pregnant or nursing. After 1-week of baseline smoking assessments, subjects were randomized to 12 weeks of either Camel Snus (Winterchill and Robust, 4.59 and 5.09mg/g dry weight free nicotine, respectively, distributed by Reynolds American Inc., n = 196) or medicinal nicotine (4mg Nicorette distributed by GlaxoSmithKline, n = 195). Participants were asked to stop smoking completely while using these products and were provided behavioral counseling to help them in this effort. Participants were encouraged to use at least 6 to 8 pieces per day and beginning at week 7, participants underwent a product-tapering period.
Participants were only asked about the product to which they were assigned. The PHR scale was administered at baseline prior to product assignment and weeks 4 and 12. This scale is a modified version of a prior scale used in assessing the risk associated with medicinal nicotine use. 8 The PHR scale involves rating perceived disease risk on a 1–10 visual analogue scale with 1 anchored at very low risk for disease and 10 anchored at very high risk (See Figure 1 for examples of items). In addition, we administered a Relative Health Risk scale, which compared toxicity and specific disease risks (cancer, heart disease, lung disease, nicotine addiction) of study products with usual brand of cigarettes ( Table 1 ). At the end of the treatment, the participants were asked if they were concerned about becoming addicted to study product, the safety of the study product, and whether they considered the study product higher or lower risk than cigarettes. Participants were also asked to complete a Product Evaluation Scale (PES) after 1 week of product use. This scale assesses product satisfaction, psychological reward, mouth sensation and aversiveness. 9 Product use and cigarette smoking were recorded on a daily basis using an Interactive Voice Response system during treatment. After treatment, continued product use was assessed using timeline follow-back.
Figure 1.
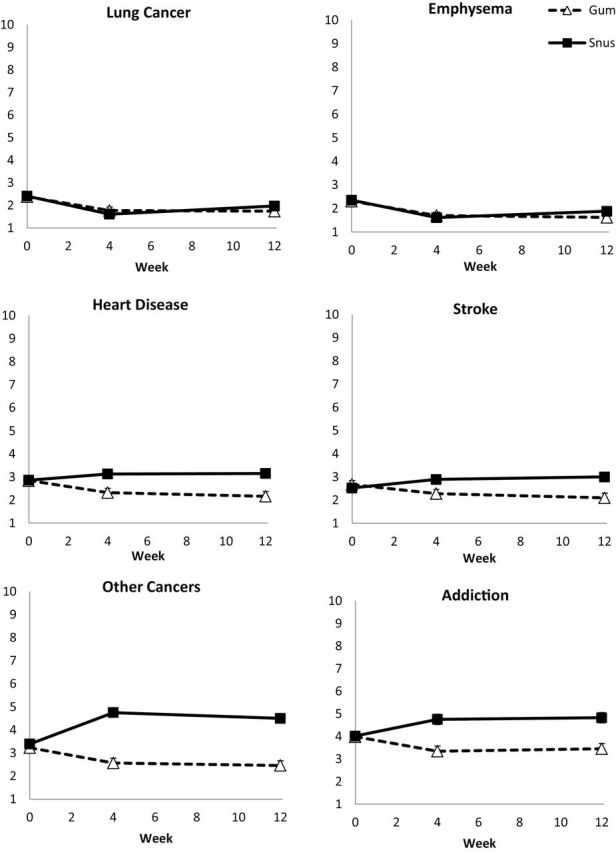
Responses to the Perceived Health Risk Scale at baseline prior to and at 4 and 12 weeks of product use for nicotine gum and snus.
Table 1.
Comparison of Relative Risk of Nicotine Gum (NG) and Snus Products With Usual Brand Cigarettes at Baseline (Prior to Study Product Assignment) and Weeks 4 and 12 of Use
| Variables | Much or somewhat lower | Neither higher or lower | Somewhat or much higher | Don’t know | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| NG (%) | Snus (%) | NG (%) | Snus (%) | NG (%) | Snus (%) | NG (%) | Snus (%) | ||
| Toxin level | |||||||||
| Baseline | 50.5 | 48.7 | 2.1 | 5.2 | 1.1 | 1.0 | 46.3 | 45.1 | .456 |
| Week 4 | 84.8 | 66.5 | 1.3 | 5.8 | 1.3 | 1.9 | 12.7 | 25.8 | .0008 |
| Week 12 | 87.8 | 68.3 | 2.0 | 7.6 | 0.7 | 3.5 | 9.5 | 20.7 | .0004 |
| Risk for cancer | |||||||||
| Baseline | 73.9 | 70.0 | 5.9 | 6.7 | 1.6 | 2.6 | 18.6 | 20.7 | .808 |
| Week 4 | 94.3 | 69.7 | 1.9 | 19.4 | 0.6 | 4.5 | 3.2 | 6.5 | <.0001 |
| Week 12 | 90.5 | 66.2 | 6.1 | 26.2 | 0.7 | 0.7 | 2.7 | 6.9 | .0001 |
| Risk for heart disease | |||||||||
| Baseline | 74.5 | 70.5 | 6.4 | 6.2 | 1.1 | 1.6 | 18.1 | 21.8 | .788 |
| Week 4 | 92.4 | 77.4 | 3.8 | 11.6 | 0.6 | 1.9 | 3.2 | 9.0 | .002 |
| Week 12 | 91.2 | 69.7 | 4.7 | 23.5 | 1.4 | 0.7 | 2.7 | 6.2 | <.0001 |
| Risk for lung disease | |||||||||
| Baseline | 80.9 | 76.7 | 1.6 | 3.6 | 1.1 | 1.6 | 16.5 | 18.1 | .604 |
| Week 4 | 94.9 | 89.7 | 1.3 | 3.2 | 0.6 | 0.7 | 3.2 | 6.5 | .314 |
| Week 12 | 96.6 | 91.0 | 0.7 | 5.5 | 0.7 | 0.7 | 2.0 | 2.8 | .060 |
| Risk for nicotine addiction | |||||||||
| Baseline | 48.9 | 48.7 | 28.2 | 26.9 | 3.7 | 3.6 | 19.2 | 20.7 | .982 |
| Week 4 | 70.3 | 49.7 | 20.9 | 38.1 | 5.1 | 7.7 | 3.8 | 4.5 | .002 |
| Week 12 | 72.3 | 44.1 | 18.9 | 47.6 | 4.7 | 4.8 | 4.1 | 3.5 | <.0001 |
Statistical differences are between perceived relative risk between NG and snus.
Statistical Methods
The focus of this secondary exploratory analysis was to describe and compare participant reported PHR by randomization group. Frequencies, percentages, and means ± SD are reported unless otherwise noted. All subjects were included in the analysis whether or not they reported use of the assigned product; almost all subjects reported some use of the product during the course of treatment (99% for snus and 100% for nicotine gum). PHR over the treatment period was analyzed with available data from for all participants using linear mixed models with fixed effects for site, product, week, interaction between product and week, and a random effect for subject. Items measuring the relative risk of the study product compared with usual brand cigarettes were summarized at each time point for participants who had not dropped out prior to the visit and compared by randomization group using chi-squared and Fisher’s exact tests as appropriate. Univariate logistic regression models were used to assess the relationship between demographic variables and PES and PHR scale with three outcomes for all participants: study dropout at week 12, study compliance at week 4 (Interactive Voice Response reported study product use and no cigarette use with confirmation using timeline follow-back and verified using CO < 6 ppm; those who dropped out prior to week 4 were considered noncompliant), and continued use of study product 1 week beyond the treatment period (those who dropped out prior to week 12 were considered to have stopped study product use). For each outcome, variables with P < .10 in univariate analyses were included in final multivariate models and odds ratios ( OR s) and 95% confidence intervals (CIs) are presented. Data were analyzed using SAS version 9.3 (Cary, NC) and P values less than .05 were considered statistically significant. As this was an exploratory analysis, P values were not adjusted for multiple comparisons.
Results
Characteristics of the Sample
Of the 391 subjects recruited into the study, 195 were randomly assigned to the nicotine and 196 to snus. The mean ± SD age of the participants was 43.9±12.5, 47.1% were females, and 81.8% were non-Hispanic whites. The mean ± SD cigarettes per day was 18.0±6.5 and Fagerstrom Test for Nicotine Dependence (FTND) 10 score was 5.1±2.0. No significant differences were observed between the two experimental conditions. Among the 391 subjects, 322 (82.4%) completed week 4 and 279 (71.4%) completed week 12 measurements.
Pre-Use Versus Post-Use Perception of Snus Versus Nicotine Gum
Figure 1 shows the results from the analysis of the PHR scale, in which health risk of the study product was assessed prior to and after product use. Significant reductions were observed in PHR after use of both gum and snus at weeks 4 and 12 compared with before use for lung cancer (all P < .05), emphysema (all P < .05) and bronchitis (all P < .05; not shown in figure). No significant differences were observed between snus versus nicotine gum groups prior to or during product use. PHR for other cancer was similar prior to product use for snus and nicotine but showed significant product ( F1,585 = 49.30; P < .0001) and product by week interaction effects ( F2,585 = 25.67; P < .0001). The nicotine gum group reported lower PHR at weeks 4 and 12 compared with the snus group (both P < .0001). The snus group reported increased risk for other cancers at weeks 4 and 12 compared with baseline prior to product use (both P < .0001) whereas those in the gum group reported lower risk after use at both weeks 4 and 12 (both P = .003 and .0008, respectively). Perceived risk for heart disease and stroke had significant product ( F1,585 = 8.74; P = .003 and F1,585 = 6.10; P = .014, respectively) and product by week interaction effects ( F2,585 = 6.00; P = .003 and F2,585 = 6.66; P = .001, respectively) with no differences in PHR between products at baseline but a lower rating for gum compared with snus at week 4 ( P = .003 and P = .017, respectively) and 12 ( P = .0004 and P = .001, respectively). For heart disease, a decrease in PHR was observed for nicotine gum at weeks 4 and 12 compared with baseline ( P = .013 and P = .001, respectively) but no significant differences from baseline were observed with snus. For stroke, at week 12 the perceived risk decreased for nicotine gum ( P = .008) and increased for snus ( P = .024) compared with baseline; a similar trend was seen at week 4 though neither was quite statistically significant. Perceived risk for addiction followed the same pattern as perceived risk for other cancers, that is both product ( F1,585 = 15.99; P < .0001) and product by week interaction ( F2,585 = 9.46; P < .0001) effects with higher PHR reported for snus at both weeks 4 and 12 ( P < .0001 for both). Perceived risk increased in weeks 4 and 12 among snus users compared with baseline ( P = .004 and P = .002) whereas it decreased in weeks 4 and 12 among gum users ( P = .013 and .042, respectively).
Convergence of Scales
Table 1 shows responses to relative risk of the study product as compared usual brand cigarettes assessed at baseline as well as during product use. At baseline, about half of the participants endorsed that the study product has much or somewhat lower in toxicants and risk for nicotine addiction than cigarettes. The majority of participants (70% and over) endorsed much or somewhat lower risk for cancer, heart disease and lung disease. No significant differences were observed across study products during baseline. However, during product use, significant differences were observed for perception of risk between the two products, where significantly more people endorsed lower relative risks for the nicotine gum compared with snus at both weeks 4 and 12 on all these variables, except for than lung disease. There was a by week effect ( P < .001) for both nicotine gum and snus, with increases in the proportion who reported lower risks of these products than cigarettes and fewer don’t know responses.
At the end of the treatment period, participants completed a questionnaire that assessed their perception of the assigned product. The majority of participants believed that their assigned study product was of lower risk than cigarettes (98.6% for gum and 96.5% for snus). However, more participants assigned to snus were concerned about the safety of the product than those assigned to the gum (39.6% vs. 10.9%, respectively, P < .0001) and likewise were concerned about becoming addicted to the study product (31.3% vs. 20.4%, respectively, P = .035).
There was significant convergent validity between the responses to the end of treatment product questionnaire and the PHR scales. Those who endorsed yes (as opposed to no) for the item regarding concern about the safety of the study product reported higher mean combined score for PHR (excluding addiction) assessed at week 12 for gum (yes: 3.14±1.66 vs. no: 1.82±1.48, P = .001) and for snus (yes: 3.34±1.70 vs. no: 2.36±1.74, P = .001). Similarly, those who endorsed yes to the item regarding being concerned about becoming addicted to the study product reported higher mean scores for the perceived risk for addiction as opposed to those who endorsed no for gum (yes: 6.40±2.74 vs. no: 2.75±2.36, respectively, P < .0001) and for snus (yes: 6.56±2.61 vs. no: 4.01±2.68, respectively, P < .0001).
Prediction of Dropping Out, Compliance to Product Use and Continued Product Use After Treatment
Scores averaged across PHR scales related to disease (excluding addiction) and addiction assessed at week 4 of product use were used to determine their effects on the extent of dropout from the study by week 12, the extent of compliance to only product use at week 4 (23.5% for snus an 27.7% for nicotine gum), and extent of continued use of the product after treatment (23.5% for snus and 13.3% for nicotine gum). In the univariate analysis, dropping out of the study was associated with site (21.5% EUG vs. 35.6% MSP [95% CI] = 0.50 [0.32–0.78], P = .002) and lower PES scores at week 1 for Satisfaction ( OR [95% CI] = 0.69 [0.52–0.91], P = .010) and Psychological Reward ( OR [95% CI] = 0.74 [0.58–0.94], P = .015) but not to PHR scores. Following simultaneous adjustment for these three variables, participants from EUG ( P = .001) and those with higher Satisfaction scores at week 1 were less likely to dropout ( P = .044; Table 2 ).
Table 2.
Multiple Logistic Regression Analysis for Dropping Out by End of Treatment (Week 12), Compliance to Use of Only Study Product (Week 4), and Continued Use of Assigned Product After Treatment
| Dependent variable = dropping out by week 12 ( N = 341) | ||
|---|---|---|
| Variables | Odds ratio (95% CI) | P |
| Study site | .001 | |
| MSP | 1.00 | |
| EUG | 0.41 (0.24–0.71) | |
| PES satisfaction | 0.69 (0.48–0.99) | .044 |
| PES psychological reward | 0.85 (0.63–1.14) | .277 |
| Dependent variable = compliance to study product only at week 4 ( N = 340) | ||
| Variables | Odds ratio (95% CI) | P |
| Study site | .007 | |
| MSP | 1.00 | |
| EUG | 0.47 (0.27–0.81) | |
| Age (per 1 year) | 1.02 (1.00–1.04) | .100 |
| Gender | .004 | |
| Male | 1.00 | |
| Female | 0.45 (0.26–0.78) | |
| FTND score (per 1 point) | 0.76 (0.66–0.87) | <.0001 |
| PES satisfaction | 1.76 (1.23–2.52) | .002 |
| PES psychological reward | 1.05 (0.78–1.43) | .735 |
| PES aversion | 1.26 (0.92–1.72) | .144 |
| Dependent variable = continued use of study product after treatment ( N = 317) | ||
| Variables | Odds ratio (95% CI) | P |
| Randomization group | .012 | |
| Gum | 1.00 | |
| Snus | 2.21 (1.19–4.11) | |
| Study site | <.0001 | |
| MSP | 1.00 | |
| EUG | 0.15 (0.08–0.31) | |
| Age (per 1 year) | 1.00 (0.98–1.03) | .834 |
| FTND score (per 1 point) | 1.15 (0.98–1.34) | .081 |
| PES psychological reward | 1.02 (0.76–1.36) | .911 |
| PES aversion | 1.44 (1.01–2.05) | .043 |
| Baseline mean PHR | 1.08 (0.96–1.22) | .210 |
CI = confidence interval; EUG = Eugene; FTND = Fagerstrom Test for Nicotine Dependence; MSP = Minneapolis/St. Paul; PES = Product Evaluation Scale; PHR = perceived health risk. The final model for each outcome includes all the covariates from the univariate analysis that were significant with P value < .10. The bolded numbers are the significance values.
In the univariate analyses, compliance at week 4 was associated with site (18.0% EUG vs. 33.5% MSP [95% CI] = 0.44 [0.27–0.70], P = .0005), age ( OR [95% CI] = 1.03 [1.01–1.05], P = .007), gender (19.0% females vs. 31.4% males, OR [95% CI] = 0.51 [0.32–0.82], P = .005), FTND score ( OR [95% CI] = 0.81 [0.72–0.91], P = .0004), and week 1 PES scores for Satisfaction ( OR [95% CI] = 2.04 [1.55–2.69], P < .0001), Psychological Reward ( OR [95% CI] = 1.47 [1.19–1.82], P = .0004) and Aversion ( OR [95% CI] = 1.34 [1.04–1.72], P = .025), but again not PHR scores. Following simultaneous adjustment for these variables, participants who were from EUG ( P = .007), female ( P = .004), had higher FTND scores ( P < .0001) and lower PES Satisfaction scores ( P = .002) were less likely to be compliant ( Table 2 ).
Finally, in the univariate analyses for continued use of the product after treatment, assignment to snus as opposed to gum ( OR [95% CI] = 1.99 [1.18–3.38], P = .011), study site (EUG 7.5% vs. MSP 29.8%; OR [95% CI] = 0.19 [0.10–0.35], P < .0001), age ( OR [95% CI] = 1.02 [1.00–1.04], P = .057), FTND score ( OR [95% CI] = 1.17 [1.02–1.35], P = .026), PES scores at week 1 for Psychological Reward ( OR [95% CI] = 1.23 [0.98–1.54], P = .078) and for Aversion ( OR [95% CI] = 1.38 [1.05–1.81], P = .021) and baseline PHR score ( OR [95% CI] = 1.12 [1.01–1.25], P = .029) were significantly or near significantly associated with continued post-study product use. After multivariate adjustment, participants who were assigned to snus ( P = .012), from MSP ( P < .0001), and had higher PES Aversion scores ( P = .043) were more likely to continue using the study product ( Table 2 ).
Discussion
Several important findings emerged from this study. First, on the PHR scale perception of risk about the product changed over the course of using the product. The direction of change depended on the product. Second, participants assigned to snus reported similar health risk ratings as those participants assigned to medicinal nicotine for diseases associated with the lung, however, higher health risks were associated with cardiovascular disease, cancers other than lung and addiction. The results from the PHR scale are buoyed by the concordance of the different measures for PHRs of a product. Third, smokers perceived noncombusted products to result in lower risk for diseases compared with cigarettes, although a significant number reported poor knowledge of the relative risks of cigarettes prior to use. Fourth, there was no association between PHR with dropping out of the study, compliance with study product and not smoking, or continued product use after treatment; however, other variables were associated with these outcomes.
The differences in PHR between the two products, snus versus medicinal nicotine, occurred after product use and the divergence on perceived risk occurred for diseases that were not related to respiratory diseases. In general, these perceptions are likely to be accurate. Snus is expected to be associated with greater risks for esophageal or oropharyngeal and pancreatic cancers 11–13 and perhaps fatal cardiovascular disease 14 , 15 than medicinal nicotine due to higher exposures to toxicants, 7 but expected not lead to greater risk for lung cancer or other respiratory diseases 5 compared with medicinal nicotine; although no scientific studies exist that have made this comparison. Interestingly, snus users as a group perceived a higher risk for addiction to the product than medicinal nicotine users, even though total nicotine equivalent concentrations in participants were similar across the two products. 7 The results from this perception is concordant with a greater rate of snus users who continued to use their assigned product after treatment compared with those assigned to medicinal nicotine users. It is possible that constituents other than nicotine may contribute to the appeal or addictiveness of snus 16 , 17 or the fact that snus is a tobacco product and not a medicine might affect the perception of addiction risk. On the other hand, subjective reports for higher risk for addiction were not correlated with continued use of the assigned product. Post hoc analysis that examined only snus users showed no relationship between perceived risk for addiction at week 4 and continued product use beyond the treatment period. It is possible that for some individuals, risk for addiction is a deterrent for continued use, while in others a cause for continued use.
Another interesting observation from our study is that the ratings on the PHR scale tended to be stable over time for both types of products. It appears that once a decision is made on perception of risk after use of a product, no significant changes in this perception occur over time.
The initial misperceptions or lack of knowledge of risk of the noncombusted products are in agreement with other survey studies that show misperceptions of the risk of medicinal nicotine 18–22 and smokeless tobacco or snus 21–30 compared with cigarettes. However, with use of these products, the accuracy of relative risk improved. This finding is concordant with some of the results from prior studies. For example, in one study, ever users of nicotine medications had more favorable attitudes towards them compared with never users. 31 Similarly, in another study, former or current smokers who had a history of or were currently using snus, perceived snus as being less risky than daily cigarette smoking compared with cigarette smokers who did not have a history of using snus. 6 In a general population survey, respondents who perceived snus to be less harmful and addictive than cigarettes were more likely to have used snus. 32 It is possible that those who used these products may have had a more favorable attitude or belief towards them prior to use (see below), but the current study suggests that use of the product may also lead to more accurate perceptions of the product.
PHR of the product, however, did not predict dropping out of the study, compliance with only study product use, or continued use of the product. The variables that were consistently associated with all these outcomes were site of study, which suggests that subject characteristic and the cultural context in which the study is conducted might affect the results that are observed. Other variables that were associated with dropping out of the study or being compliant with using only the product included the users’ satisfaction with the product. Prior studies have also shown that product satisfaction is associated with choice of an oral tobacco product and the amount of product used. 9 These results indicate the importance of measuring satisfaction with the product as part of evaluating tobacco products. This measure in particular may be predictive of uptake, amount of use and ability to completely substitute for cigarettes. Other important measures to consider for product evaluation would be the extent of dependence on cigarettes, which could predict the extent to which a participant would engage in dual use of a product. Gender also played a role in an individual’s ability to completely substitute a product for cigarettes. In this study, females experienced more difficulty refraining from cigarettes. This finding coincides with studies that demonstrate that females are less sensitive to nicotine and also less responsive to medicinal nicotine, but more responsive to sensory aspects of smoking. 33 , 34 Finally and interestingly, aversion was positively associated with continued use of study product, which is contrary to expectations. It is possible that this result was spurious, but also could have been a demonstration of strong conditioned associations between an aversive cue and pharmacological reinforcement leading to continued use, or that those who used the product despite aversive effects had greater interest in continuing its use. It is also possible that aversiveness is a misnomer. In a post hoc analysis, this scale had a weakly positive association with product satisfaction ( r = 0.18, P = .001) and moderately positive association with psychological reward ( r = 0.39, P < .001). This analysis suggests that the items on this scale (eg, dizzy, nauseous) may be experienced more positively than negatively (especially for the sensation of dizziness).
The results from our study would suggest that PHR may not play a role in persistence or success in using a product as much as other variables. The lack of this relationship may in part be explained by the nature of the study, which involved short-term use of the product, whereas health risks may have been perceived for the long-term. These results do not negate the possibility that PHR would play a role in the uptake of a product in a general population, current smokers or nontobacco users. For example, a prior survey study found that those respondents who believed that medicinal nicotine was just as harmful as cigarettes or did not know whether this statement was true were less likely to have used medicinal nicotine or consider using medicinal nicotine in future quit attempts. 20 Furthermore, of those who used nicotine gum in the recent past and were concerned about the safety of the product used fewer pieces of the gum and for a short duration of time. 20 Other studies have shown that potential trial of snus or willingness to try snus in future quit attempts was more likely in those smokers who believed that this product had less health risks than cigarettes. 6 , 35 , 36
One of the major limitations of this study is the institutional review board requirement to inform subjects of the risks of each of the tobacco products. The information that was provided to subjects may have influenced their perception of risk even though differences in risks between products were not described. Despite having to provide this information at the beginning of the study, there was less discrepancy in PHRs between the two products initially and greater divergence only after product use. The consent form also informed participants of the likelihood of greater toxicants and health risks (not specified) associated with cigarettes compared with study products, yet lack of knowledge of relative harm was reported earlier in the study in a notable number of participants. An additional limitation includes the generalizabilty of the findings to only a population of smokers who are interested in switching from cigarettes and who attend a clinic for visits. And finally, the samples sizes that comprised those participants who were compliant and continued product use were relatively small.
In summary, our study results showed that cigarette smokers who were interested in complete switching tend to perceive the risks of smoked and noncombusted oral products accurately, particularly after use of the product. That is, experience with product use appeared to shape more accurate perceptions of them, although actual causation cannot be determined because of the nature of this study. Snus users have a higher perception or risk for some nonrespiratory disease than medicinal nicotine products. In our study population, the extent of PHR had no impact on use patterns.
Funding
National Cancer Institute (R01CA135884 and U19CA157345).
Declaration of Interests
None declared.
Acknowledgments
We would like to acknowledge Amanda Anderson and Berry Broadbent for their role in conducting the study and the Clinical and Translational Science Institute, UL1TR000114.
References
- 1. Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS . Changing smokeless tobacco products new tobacco-delivery systems . Am J Prev Med . 2007. ; 33 ( suppl 6 ): S368 – 378 . doi: 10.1016/j.amepre.2007.09.005 . [DOI] [PubMed] [Google Scholar]
- 2. Breland AB, Acosta MC, Eissenberg T . Tobacco specific nitrosamines and potential reduced exposure products for smokers: a preliminary evaluation of Advance . Tob Control . 2003. ; 12 ( 3 ): 317 – 321 . doi:10.1136/tc.12.3.317. www.ncbi.nlm.nih.gov/pubmed/12958395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kotlyar M, Hertsgaard LA, Lindgren BR, et al. . Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study . Cancer Epidemiol Biomarkers Prev . 2011. ; 20 ( 1 ): 91 – 100 . doi: 10.1158/1055-9965.EPI-10-0349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramstrom L, Wikmans T . Mortality attributable to tobacco among men in Sweden and other European countries: an analysis of data in a WHO report . Tob Induc Dis . 2014. ; 12 ( 1 ): 14 . doi: 10.1186/1617-9625-12-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foulds J, Ramstrom L, Burke M, Fagerstrom K . Effect of smokeless tobacco (snus) on smoking and public health in Sweden . Tob Control . 2003. ; 12 ( 4 ): 349 – 359 . doi:10.1136/tc.12.4.349. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14660766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lund KE . Association between willingness to use snus to quit smoking and perception of relative risk between snus and cigarettes . Nicotine Tob Res . 2012. ; 14 ( 10 ): 1221 – 1228 . doi: 10.1093/ntr/nts077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatsukami DK, Severson H, Anderson A, et al. . Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching [published online ahead of print May 19, 2015] . Tob Control . 2015. . doi: 10.1136/tobaccocontrol-2014-052080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mooney ME, Leventhal AM, Hatsukami DK . Attitudes and knowledge about nicotine and nicotine replacement therapy . Nicotine Tob Res . 2006. ; 8 ( 3 ): 435 – 446 . doi: 10.1080/14622200600670397 . [DOI] [PubMed] [Google Scholar]
- 9. Hatsukami DK, Zhang Y, O’Connor RJ, Severson HH . Subjective responses to oral tobacco products: scale validation . Nicotine Tob Res . 2013. ; 15 ( 7 ): 1259 – 1264 . doi: 10.1093/ntr/nts265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO . The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire . Br J Addict . 1991. ; 86 ( 9 ): 1119 – 1127 . www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1932883 . Accessed March 30, 2015 . [DOI] [PubMed] [Google Scholar]
- 11. International Agency for Research on Cancer . Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans . Vol 89 . Lyon, France: : IARC; ; 2007. : 41 – 583 . [PMC free article] [PubMed] [Google Scholar]
- 12. Boffetta P, Hecht S, Gray N, Gupta P, Straif K . Smokeless tobacco and cancer . Lancet Oncol . 2008. ; 9 ( 7 ): 667 – 675 . doi: 10.1016/S1470-2045(08)70173-6 . [DOI] [PubMed] [Google Scholar]
- 13. Roosaar A, Johansson AL, Sandborgh-Englund G, Axell T, Nyren O . Cancer and mortality among users and nonusers of snus . Int J Cancer . 2008. ; 123 ( 1 ): 168 – 173 . doi: 10.1002/ijc.23469 . [DOI] [PubMed] [Google Scholar]
- 14. Boffetta P, Straif K . Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis . BMJ . 2009. ; 339 : b3060 . doi: 10.1136/bmj.b3060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee PN . Circulatory disease and smokeless tobacco in Western populations: a review of the evidence . Int J Epidemiol . 2007. ; 36 ( 4 ): 789 – 804 . doi: 10.1093/ije/dym039 . [DOI] [PubMed] [Google Scholar]
- 16. Henningfield JE, Hatsukami DK, Zeller M, Peters E . Conference on abuse liability and appeal of tobacco products: conclusions and recommendations . Drug Alcohol Depend . 2011. ; 116 ( 1–3 ): 1 – 7 . doi: 10.1016/j.drugalcdep.2010.12.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK . Abuse liability assessment of tobacco products including potential reduced exposure products . Cancer Epidemiol Biomarkers Prev . 2009. ; 18 ( 12 ): 3241 – 3262 . doi: 10.1158/1055-9965.EPI-09-0948 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bansal MA, Cummings KM, Hyland A, Giovino GA . Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res . 2004. ; 6 ( suppl 3 ): S303 – 310 . doi:10.1080/ 14622200412331320707. www.ncbi.nlm.nih.gov/pubmed/15799593 [DOI] [PubMed] [Google Scholar]
- 19. Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA . Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res . 2004. ; 6 ( suppl 3 ): S333 – 340 : doi: 10.1080/14622200412331320734. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15799596 [DOI] [PubMed] [Google Scholar]
- 20. Shiffman S, Ferguson SG, Rohay J, Gitchell JG . Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: relationship with use and compliance . Addiction . 2008. ; 103 ( 8 ): 1371 – 1378 . doi: 10.1111/j.1360-0443.2008.02268.x . [DOI] [PubMed] [Google Scholar]
- 21. Smith SY, Curbow B, Stillman FA . Harm perception of nicotine products in college freshmen . Nicotine Tob Res . 2007. ; 9 ( 9 ): 977 – 982 . doi: 10.1080/14622200701540796 . [DOI] [PubMed] [Google Scholar]
- 22. Wikmans T, Ramstrom L . Harm perception among Swedish daily smokers regarding nicotine, NRT-products and Swedish Snus . Tob Induc Dis . 2010. ; 8 : 9 . doi: 10.1186/1617-9625-8-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borland R, Cooper J, McNeill A, O’Connor R, Cummings KM . Trends in beliefs about the harmfulness and use of stop-smoking medications and smokeless tobacco products among cigarettes smokers: findings from the ITC four-country survey . Harm Reduct J . 2011. ; 8 : 21 . doi: 10.1186/1477-7517-8-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heavner KK, Rosenberg Z, Phillips CV . Survey of smokers’ reasons for not switching to safer sources of nicotine and their willingness to do so in the future . Harm Reduct J . 2009. ; 6 : 14 . doi: 10.1186/1477-7517-6-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McClave-Regan AK, Berkowitz J . Smokers who are also using smokeless tobacco products in the US: a national assessment of characteristics, behaviours and beliefs of ‘dual users’ . Tob Control . 2011. ; 20 ( 3 ): 239 – 242 . doi: 10.1136/tc.2010.039115 . [DOI] [PubMed] [Google Scholar]
- 26. O’Connor RJ, Hyland A, Giovino GA, Fong GT, Cummings KM . Smoker awareness of and beliefs about supposedly less-harmful tobacco products . Am J Prev Med . 2005. ; 29 ( 2 ): 85 – 90 . doi: 10.1016/j.amepre.2005.04.013 . [DOI] [PubMed] [Google Scholar]
- 27. O’Connor RJ, McNeill A, Borland R, et al. . Smokers’ beliefs about the relative safety of other tobacco products: findings from the ITC Collaboration . Nicotine Tob Res . 2007. ; 9 ( 10 ): 1033 – 1042 . doi: 10.1080/14622200701591583 . [DOI] [PubMed] [Google Scholar]
- 28. Overland S, Hetland J, Aaro LE . Relative harm of snus and cigarettes: what do Norwegian adolescents say? Tob Control . 2008. ; 17 ( 6 ): 422 – 425 . doi: 10.1136/tc.2008.026997 . [DOI] [PubMed] [Google Scholar]
- 29. Tomar SL, Hatsukami DK . Perceived risk of harm from cigarettes or smokeless tobacco among U.S. high school seniors . Nicotine Tob Res . 2007. ; 9 ( 11 ): 1191 – 1196 . doi: 10.1080/14622200701648417 . [DOI] [PubMed] [Google Scholar]
- 30. Wilson N, Borland R, Weerasekera D, Edwards R, Russell M . Smoker interest in lower harm alternatives to cigarettes: national survey data . Nicotine Tob Res . 2009. ; 11 ( 12 ): 1467 – 1473 . doi: 10.1093/ntr/ntp152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Etter JF, Perneger TV . Attitudes toward nicotine replacement therapy in smokers and ex-smokers in the general public . Clin Pharmacol Ther . 2001. ; 69 ( 3 ): 175 – 183 . doi: 10.1067/mcp.2001.113722 . [DOI] [PubMed] [Google Scholar]
- 32. Kaufman AR, Mays D, Koblitz AR, Portnoy DB . Judgments, awareness, and the use of snus among adults in the United States . Nicotine Tob Res . 2014. ; 16 ( 10 ): 1404 – 1408 . doi: 10.1093/ntr/ntu116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vogel RI, Hertsgaard LA, Dermody SS, et al. . Sex differences in response to reduced nicotine content cigarettes . Addict Behav . 2014. ; 39 ( 7 ): 1197 – 1204 . doi: 10.1016/j.addbeh.2014.03.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perkins KA . Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking . In: Bevins R, Caggiula AR , eds. The Motivational Impact of Nicotine and Its Role in Tobacco Use . New York, NY: : Springer-Verlag; ; 2009. : 143 – 169 . [DOI] [PubMed] [Google Scholar]
- 35. Biener L, Bogen K . Receptivity to Taboka and Camel Snus in a U.S. test market . Nicotine Tob Res . 2009. ; 11 ( 10 ): 1154 – 1159 . doi: 10.1093/ntr/ntp113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gartner CE, Jimenez-Soto EV, Borland R, O’Connor RJ, Hall WD . Are Australian smokers interested in using low-nitrosamine smokeless tobacco for harm reduction? Tob Control . 2010. ; 19 ( 6 ): 451 – 456 . doi: 10.1136/tc.2009.033670 . [DOI] [PubMed] [Google Scholar]


