Abstract
Introduction:
Tobacco chippers are light smokers with stable patterns of smoking that exhibit lower nicotine dependence severity than heavy smokers. Chippers may provide valuable information about the factors influencing drug dependence. Impulsivity and stress are two factors known to influence smoking. By comparing nondependent smokers (tobacco chippers, n = 25) to dependent smokers (heavy smokers, n = 23) and nonsmokers ( n = 25), this study examines the relationship between nicotine dependence, impulsivity, chronic stress, and stress reactivity.
Methods:
A total of 73 adult participants completed a study visit that included questionnaires to measure nicotine dependence, chronic stress, personality, affect, withdrawal, and craving. Impulsivity was measured with the delay discounting task and the flanker task. Stress reactivity was assessed by monitoring respiration, heart rate, and salivary cortisol during performance of a titrated Stroop task. Effects of acute stress on affect and craving were examined.
Results:
Tobacco chippers were as impulsive as heavy smokers on the delay discounting task but no different from nonsmokers on the flanker task. Heavy smokers reported higher perceived stress than chippers and nonsmokers. Perceived stress was a significant predictor of discounting only in heavy smokers. Acute stress induced changes in respiration, heart rate, and heart rate variability. Craving and negative affect increased after stress in both smoking groups, but craving was associated with affect only in chippers.
Conclusions:
Tobacco chippers do not differ from heavy smokers in impulsivity, but do differ in perceived stress. One’s perception and experience of stress might be associated to nicotine dependence resistance and could inform smoking cessation treatments.
Implications:
By examining impulsivity, chronic stress, and stress reactivity in nondependent smokers (tobacco chippers) compared to dependent smokers and nonsmokers, this study contributes to the understanding of nicotine addiction and informs smoking cessation programs.
Introduction
Occasional tobacco smoking often progresses to heavy regular smoking and nicotine dependence. However, a fraction of smokers remain as occasional smokers with stable patterns of daily or nondaily light smoking and lower nicotine dependence severity than heavy smokers. 1 These smokers, also known as tobacco chippers, do not differ in nicotine absorption or metabolism from regular heavy smokers. 2 Identifying physiological and/or psychological differences between chippers and heavy smokers might provide information about the factors that influence drug dependence, and hence, inform and improve smoking cessation treatments. 2
Impulsivity and stress are two factors known to influence smoking behavior. 3–5 The stress-vulnerability model hypothesizes that stress increases risk for addiction by decreasing behavioral control (increasing impulsivity). 6 Impulsivity is a multidimensional concept that has been defined as an inability to wait, a tendency to act without forethought, insensitivity to consequences, and an inability to inhibit inappropriate behaviors. 7 Studies suggest that impulsive decision-making (ie, choosing small immediate rewards over delayed larger ones) and impulsive disinhibition (ie, responding prematurely or failing to inhibit responding) are distinct facets of impulsivity. 8–10 A high level of impulsivity has been equated with preferences for immediate gratification, risky activities, novel sensations, and easier routes to self-gratification, as well as inability to persist at a task and shorter reaction times. 9 , 11–13 Regular smokers have been found to be more impulsive than nonsmokers. 9 , 14 However, there is limited knowledge about impulsivity in nondependent smokers. 15–17 Studies are needed to determine if chippers are less impulsive than heavy smokers and, as a result, less dependent and more able to “choose” when and how much to smoke.
In contrast to the trait-like influence of impulsivity on smoking, stress is a state-like condition known to affect smoking maintenance and failure to quit. 18 , 19 Physiologically, a stress response involves the activation of the hypothalamic-pituitary-adrenal axis (HPA) and consequent secretion of cortisol, as well as the stimulation of the sympathetic branch of the autonomic nervous system which results, amongst others, in an increase in cardiovascular load and breath rate. 20 , 21 As with other psychoactive drugs, most smokers report smoking as a coping mechanism during stressful situations. In regular smokers stress triggers increases in craving, smoking amount, and smoking intensity. 22–24 In addition, an individual’s smoking behavior is influenced by their subjective arousal state, presmoking baseline stress level/tolerance and the environment and nature of the stressor. 25 , 26 However, the role of stress in maintaining a pattern of light smoking is still unclear. The lower dependence on nicotine exhibited by tobacco chippers might be in part explained by differences in the experience, perception, and/or response to stress. More research is needed to examine this hypothesis and better understand the relationship between stress and nicotine dependence.
Accordingly, to help understand drug dependence and identify potential factors of resistance to dependence progression, this article characterizes nondependent smokers in terms of impulsivity, stress perception, and acute stress reactivity, and investigates if they differ and how from dependent smokers and nonsmokers. In addition, we examine if facets of personality such as neuroticism, previously associated with chronic stress, 27 and sensation-seeking, 28 which has been predictive of drug use, are associated differentially in our study groups. Furthermore, following previous observations in our laboratory suggesting that the nonjudgment factor of the mindfulness trait scale is inversely associated to stress, 27 , 29 we explore its association to nicotine dependence. Individual experiences of affect, craving and withdrawal are also examined.
Methods
Participants
Using online advertising, we recruited participants from the Portland, Oregon area between 25 and 55 years of age. Each one was classified to one of the following groups:
Nonsmokers (NS). No history of regular daily tobacco smoking, with less than 100 cigarettes smoked in their lifetime, and a current breath carbon monoxide concentration under 5 ppm.
Nondependent smokers (chippers [CH]). Smoke 1–5 cigarettes/d at least 2d/wk for at least 2 years and no history of heavy smoking. 30 Nondependent with a Fagerstrom Test for Nicotine Dependence score of 4 or less. 31
Dependent smokers (heavy smokers [HS]). Smoke at least 15 cigarettes/d and breath carbon monoxide over 10 ppm at the outset of the study. 30 Classified as nicotine dependent using the Fagerstrom Test for Nicotine Dependence (score of 5 or above). 31
Exclusion criteria included: (1) unstable significant medical problems; (2) evidence suggesting significant neurologic disease; (3) a history of substance use disorder (excluding Nicotine Dependence); and (4) a history of serious psychiatric disorder.
Procedure
All participants provided informed consent. The visits were scheduled at 8.30 AM and were approximately 3 hours long. Participants were asked to smoke as usual preceding their study visit. They were given the option to smoke once during the visit (60 minutes before stress induction) to avoid confounding due to withdrawal from smoking. Among heavy smokers, 82.61% chose to smoke compared to 16% of chippers. Measures were collected before (baseline) and after (outcome) performance of a stress-inducing titrated Stroop task. 32 Once outcome measures were completed, participants were compensated and dismissed.
Measures
A summary of the measures used in this study can be found in Table 1 .
Table 1.
Measures Used in the Present Study
| Construct | Instrument | Baseline | Outcome | Cronbach’s alpha | Reference |
|---|---|---|---|---|---|
| Nicotine dependence | Fagerstrom Test for Nicotine Dependence (FTND) | x | 0.829 | Ref. 31 | |
| Chronic stress | Perceived Stress Scale (PSS) | x | 0.886 | Ref. 42 | |
| Personality traits | Neuroticism Extroversion Openness- Five Factor Inventory (NEO-FFI) | x | 0.881 | Ref. 43 | |
| Sensation-Seeking Scale (SSS) | x | 0.876 | Ref. 44 | ||
| Five-facet Mindfulness Questionnaire (FFMQ), nonjudgment factor | x | 0.866 | Ref. 45 | ||
| Withdrawal | Minnesota Nicotine Withdrawal Scale (MNWS) | x | 0.688 | Ref. 47 | |
| Craving | Questionnaire on Smoking Urges (QSU) | x | x | 0.934 | Ref. 48 |
| Affect | Positive and Negative Affect Scale (PANAS) | x | x | 0.812 | Ref. 46 |
| Impulsivity | Delay Discounting Task | x | Ref. 9 | ||
| Flanker task | x | Ref. 33 | |||
| Physiological measures of stress | Respiration | x | x | ||
| Heart rate | x | x | |||
| Heart rate variability | x | x | |||
| Salivary cortisol | x | x |
Internal consistency (Cronbach’s alpha) measured in our sample at baseline is shown.
Self-Report
Participants completed self-report questionnaires to measure nicotine dependence (Fagerstrom Test for Nicotine Dependence, FTND), 31 chronic stress (Perceived Stress Scale, PSS), 42 personality traits (Neuroticism Extroversion Openness-Five Factor Inventory, NEO-FFI 43 ; Sensation-Seeking Scale, SSS 44 ; Five Factor Mindfulness Questionnaire, Nonjudgment factor, FFMQ-NJ 45 ), affect (Positive and Negative Affect Scale, PANAS), 46 withdrawal symptoms (Minnesota Nicotine Withdrawal Scale, MNWS), 47 and cigarette craving (Questionnaire on Smoking Urges, QSU). 48
Objective Measures
Participants performed two tasks that assessed two different facets of impulsivity: impulsive decision-making (delay discounting task) and response inhibition (flanker task). To assess stress, we monitored heart rate and respiration, and obtained saliva samples for cortisol assay.
1. Delay discounting task. Impulsivity was assessed based on a self-paced computerized task. 9 Participants chose between a varying amount of hypothetical money now or a hypothetical $10.00 after a varying delay. The immediate money varied from $0.00 to $10.50 in $0.50 increments. The delayed money was available after 0, 7, 30, 90, 180, or 365 days. Each delayed alternative was paired with each immediate alternative, and presented in a random sequence. Two main measures were determined: the “indifference point,” the amount of immediate money at which participants are indifferent between the immediate reward or the delayed amount for each delay period; and the “rate of discounting” using the following hyperbolic equation V = M / (1 + k × D ), where V = subjective value of delayed item, M = value of delayed item, D = delay length, and k = gradient of discounting function (rate of discounting). 9
2. Flanker task. This task measures a cognitive form of response inhibition in which participants resolve conflicting responses due to interfering stimulus that, if not inhibited, lead to errors. 49 , 50 A computerized version of the Eriksen flanker task was used. 33 Participants pushed a left or right button depending on the orientation of the central of the 5 chevrons presented at variable intervals. Stimuli were preceded half the time by a nonspecific cue. The direction of the 4 flanker chevrons was 50% congruent and 50% incongruent with the central chevron. For each condition, median reaction time and accuracy were used as outcome measures. Analysis focused on the incongruent condition, which required more cognitive inhibition processing. Higher reaction times and lower accuracy indicated less efficient inhibition.
3. Heart rate and respiration. Heart rate monitoring was performed using ECG electrodes placed bilaterally and subclavicular. Respiration was recorded using an elastic piezoelectric strap (Ambu-Sleepmate, Maryland). ECG and respiration were recorded during an auditory vigilance task (baseline) and during the Stroop task (outcome) using a Biosemi data acquisition system (Biosemi, Amsterdam, The Netherlands) collected at 1024 Hz. Data processing used Brain Vision Analyzer 2.0 (Brain Products GmbH, Munich, Germany), Matlab r2007a, and Kubios HRV v 2.0 (University of Kuopio, Kuopio, Finland). We computed time-domain measures of HRV because they have been shown to be more reliable, 51 and present data on pNN50 (the proportion of NN50 divided by total number of NNs).
4. Salivary cortisol. Saliva was collected in Sarstedt Salivette tubes (Sarstedt, Germany) at baseline (10.30 AM to 11.00 AM) and 20 minutes after the end of the stress-inducing task (11.00 AM to 11.30 AM). Due to the circadian rhythm of cortisol concentration and for consistency across samples, all procedures were performed in the morning. 34 Participants chewed on a cotton swab for 2 minutes. Cortisol values were quantified by the Oregon Clinical and Translational Research Institute lab in duplicate with enzyme-linked immunoassay (Salimetrics, State College, PA).
Stress Induction
A titrated version of the Stroop task was used to induce acute stress. 32 Laboratory events that have novelty, unpredictability, threat to one’s ego, or sense of loss of control (NUTS) such as public speaking, cognitive testing, problem-solving, or emotionally demanding social interactions, are capable of inducing a stress response. 21 , 35–37 , 40 When applied carefully, mental stress testing induces consistent physiological responses with good test-retest reliability. 38 , 39 A titrated version of the Stroop color-word task includes all of the NUTS features and is considered a nonspecific stressor. 35 , 38 Participants rated how stressed they felt during performance of the titrated Stroop using a 5-point Likert scale.
Data Analysis
Means, medians, and standard deviations were calculated for each variable and values examined for outliers and normality of distribution. A log transformation was used when necessary for statistical analysis. To investigate group differences at baseline, variables were compared between groups using analysis of variance (ANOVA). Nonparametric analyses (Kruskal-Wallis ANOVA) were performed for categorical data. To analyze the effect of stress induction on affect, craving, heart rate, heart rate variability, respiration, and salivary cortisol, a repeated measures ANOVA with group as a between-subjects factor and time as a repeated within-subjects factor was applied. Bonferroni correction was applied for post hoc testing. To investigate the relationship between baseline measures and changes in outcome measures after stress, regression analyses were performed.
Missing data, due to poor signal or technological malfunctions, were randomly distributed and were handled by excluding participants from specific analyses. Thus, some analyses had a different n . All statistical analyses were performed with Stata version 11 (STATA Corp., Texas)
Results
Baseline Measures
Demographics and Personality Traits
After an initial online screening, 178 subjects were eligible. From these, 73 were interested in the study visit, scheduled an appointment, were confirmed eligible, and after informed consent, completed participation. A summary of the demographics of the study sample is shown in Table 2 . The majority of participants were of Caucasian origin (84.9%). The average age was 33 and there were slightly more females than males. No significant differences between groups were found for age, years of education, race and ethnicity, and marital status. There were significant differences in baseline smoking measures and no differences in baseline measures of personality (NEO-FFI, the sensation seeking scale, or the nonjudgment factor of the mindfulness scale).
Table 2.
Baseline Characteristics of the Sample Population
| Nonsmokers (NS) | Chippers (CH) | Heavy smokers (HS) | P | Post hoc | |
|---|---|---|---|---|---|
| Sample size (female) | 25 (14) | 25 (14) | 23 (14) | ||
| Demographics | |||||
| Age | 33.68±1.61 | 31.38±1.39 | 34.75±1.67 | .308 | |
| Education (% >16 years) | 40.0 | 32.0 | 18.2 | .148 | |
| Race/ethnicity (%) | |||||
| African American | 4.0 | 12.5 | 4.2 | .896 | |
| Caucasian | 92.0 | 75.0 | 87.5 | ||
| Hispanic | 0 | 0 | 4.2 | ||
| Other | 4.0 | 16.7 | 0 | ||
| Marital status (%) | |||||
| Never married | 73.9 | 68.0 | 40.9 | .777 | |
| Married | 17.4 | 16.0 | 27.3 | ||
| Separated/divorced | 8.7 | 16.0 | 31.8 | ||
| BMI | 27.48±1.41 | 29.78±1.75 | 31.69±2.38 | .288 | |
| Alcohol (drinks/wk) | 2.20±0.63 | 6.04±1.38 | 2.39±0.63 | .008 | CH>HS, CH>NS |
| Smoking measures | |||||
| Cig/day | 0.00 | 2.61±0.39 | 17.21±1.00 | .000 | HS>CH>NS |
| FTND | 0.00 | 0.63±0.28 | 5.04±0.36 | .000 | HS>CH, HS>NS |
| BCO | 0.00 | 4.27±0.66 | 18.27±2.25 | .000 | HS>NS, HS>CH |
| Cigarette craving | 44±2.12 | 84.08±5.88 | 137.91±6.05 | .000 | HS>CH>NS |
| Withdrawal symptoms | 13.36±0.66 | 15.88±1.11 | 16.52±0.76 | .030 | HS>NS |
| Stress measures | |||||
| Perceived Stress | 14.08±1.25 | 14.48±1.31 | 19.04±1.48 | .022 | HS>NS |
| Respiration | 14.36±0.83 | 14.97±0.78 | 15.91±0.63 | .237 | |
| Heart rate | 66.19±1.67 | 70.94±1.72 | 76.37±2.45 | .001 | HS>NS |
| Heart rate variability | 21.61±3.75 | 27.06±3.90 | 8.20±2.25 | .003 | CH>HS |
BCO = breath carbon monoxide (ppm); BMI = body mass index; Cig/day = average number of cigarettes smoked in a day; FTND = Fagerstrom Test for Nicotine Dependence (score ranges between 0 for no dependence and 10 for high dependence); Heart rate (beats/min); HRV = heart rate variability (pNN50); Respiration (breaths/min). Differences between groups are represented by P values ( P ) calculated using analysis of variance (ANOVA) and Kruskal-Wallis test for categorical variables (race/ethnicity and education). P values < .05 are shown in bold. All measures represent mean ± standard error unless otherwise specified.
Impulsivity
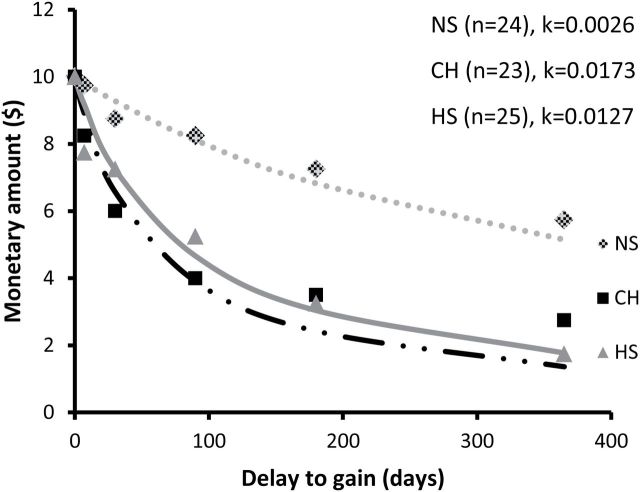
Performance of the delay discounting task confirmed that smokers behave more impulsively than nonsmokers ( Figure 1 ). ANOVA with discounting rate (lnk) as dependent variable identified a significant effect of group [ F (2, 69) = 9.11, P < .001; NS = 25, CH = 24, HS = 23]. Bonferroni post hoc analyses showed that both heavy smokers and chippers discounted significantly more than nonsmokers ( P < .01). There was no significant effect of age or gender in our sample. In contrast, analysis of inhibition (as another measure of impulsivity) using the flanker task revealed no significant baseline differences between study groups in median reaction time [ F (2, 64) = 1.26, P > .05] or accuracy [ F (2, 64) = 0.56, P > .05] during the incongruent condition (NS = 22, CH = 22, HS = 23).
Figure 1.
Fitted hyperbolic functions and median indifference points for each study group as a result of performance of the delay discounting task ( k , discounting rate). One-way analysis of variance detected significant differences in average rate of discounting (lnk, natural logarithm of k ) between groups (NS, −5.887±0.32; CH, −4.076±0.39; HS, −4.036±0.35; F (2,69) = 9.11, P < .001). NS = nonsmokers; CH = tobacco chippers; HS = heavy smokers.
Stress
Significant group differences were detected in baseline self-reported chronic stress perception [ F (2, 68) = 4.06, P < .05] with heavy smokers scoring significantly higher than nonsmokers (NS = 24, CH = 25, HS = 22). In contrast, tobacco chippers’ perception of chronic stress did not differ from that of nonsmokers. ANOVA using respiration rate (breaths/min) revealed no differences in baseline respiration between study groups [ F (2, 66) = 1.01, P > .05; NS = 24, CH = 22, HS = 23]. However, significant differences were detected in average heart rate [ F (2, 62) = 6.50, P < 0.01; NS = 21, CH = 22, HS = 22]. Post hoc analysis identified a significantly higher baseline average heart rate in heavy smokers ( Table 2 ).
Response to Stress
On average participants responded that they felt moderately stressed during the titrated Stroop task, with heavy smokers expressing the most stress ( Table 3 ).
Table 3.
Effects of Acute Stress on Craving, Affect, and Stress-Associated Physiological Measures in Nonsmokers (NS), Chippers (CH), and Heavy Smokers (HS)
| Baseline (mean ± SE ) | Acute stress (mean ± SE ) | P , group | P , stress | P , group × stress | ||
|---|---|---|---|---|---|---|
| Smoking urges | NS | 44.00±2.12 | 41.40±2.52 | <.001 a | .002 a | .693 a |
| CH | 84.08±5.88 | 102.92±8.29 | ||||
| HS | 137.91±6.05 | 162.13±5.55 | ||||
| Positive affect | NS | 32.48±1.57 | 29.88±1.55 | .051 | .003 | .784 |
| CH | 33±1.31 | 29.56±1.74 | ||||
| HS | 30.39±1.06 | 25.74±1.37 | ||||
| Negative affect | NS | 12.56±0.56 | 13.2±0.57 | .008 | .028 | .612 |
| CH | 14.68±1.29 | 16.92±1.36 | ||||
| HS | 13.69±0.64 | 16.00±0.90 | ||||
| Self-rated acute stress | NS | n/a | 2.72±0.17 | <.001 | n/a | n/a |
| CH | n/a | 3.28±0.17 | ||||
| HS | n/a | 3.74±0.15 | ||||
| Respiration (breaths/ min) | NS | 14.36±0.83 | 20.53±1.11 | .146 | <.001 | .519 |
| CH | 14.97±0.78 | 19.27±0.57 | ||||
| HS | 15.91±0.63 | 21.50±0.99 | ||||
| Heart rate (beats/ min) | NS | 66.19±1.67 | 70.13±1.64 | <.001 | .009 | .889 |
| CH | 70.94±1.72 | 74.89±1.70 | ||||
| HS | 76.37±2.45 | 82.08±2.97 | ||||
| Heart rate variability (pNN50) | NS | 21.61±3.75 | 15.43±3.39 | <.001 | .013 | .521 |
| CH | 27.06±3.90 | 17.03±3.02 | ||||
| HS | 8.20±2.25 | 5.25±1.87 | ||||
| Salivary cortisol (µg/dl) | NS | 0.30±0.03 | 0.27±0.03 | .020 | .594 | .239 |
| CH | 0.29±0.03 | 0.36±0.04 | ||||
| HS | 0.24±0.02 | 0.25±0.03 |
SE = standard error. P values < .05 are shown in bold.
a Only smoking groups comparison.
Smoking Urges
We limited the analysis of smoking urges to the smoking groups. Craving was assessed before and after acute stress induction. Because time since last cigarette may differentially impact heavy smokers and chippers, we recorded if participants smoked or not during the visit. We used this discrete variable as covariate in our analyses. A two-factor repeated measures ANOVA with smoking urges as dependent variable and time and group as independent variables indicated that there was a significant effect of time [ F (1, 86) = 9.81, P < .01] and group [ F (1, 86) = 41.85, P < .0001; CH = 25; HS = 22]. There was no significant interaction, indicating that the effect of stress on smoking urges was the same for heavy smokers as for chippers ( Table 3 ). However, additional analyses showed a significant increase in craving after stress only for heavy smokers [ F (1, 42) = 9.15, P < .01] but not for chippers [ F (1, 48) = 3.43, P > .05].
Affect
Positive and negative affect were evaluated before and after stress. In this case analysis includes all three study groups (NS = 25; CH = 25; HS = 23). Using a two-factor repeated measures ANOVA an effect of time [ F (1, 140) = 8.88, P < .01] was identified on positive affect. There was no significant effect of group [ F (2, 140) = 3.03, P > .05] and no interaction. Post hoc analyses using ANOVA with positive affect as the dependent variable and time as the independent factor showed that only heavy smokers significantly decreased positive affect after experimental stress [ F (1, 44) = 7.21, P < .05]. An effect of group [ F (2, 140) = 4.99, P < .01] and time [ F (1, 140) = 4.91, P < .05] were identified for negative affect. As before, post hoc analysis showed that heavy smokers were the only group in which stress significantly increased negative affect [ F (1, 44) = 4.37, P < .05]. No significant interaction between group and stress effect was found ( Table 3 ).
Respiration Rate
A repeated measures ANOVA identified an effect of stress on respiration rate [ F (1, 129) = 60.59, P < .0001] but no group differences [ F (2, 129) = 1.95, P > .05; NS = 24; CH = 22; HS = 23]. Post hoc analyses showed a significant increase in respiration rate for all three groups [CH, F (1, 42) = 19.77, P < .001; HS, F (1,42) = 23.60, P < .0001; NS, F (1, 45) = 19.88, P < .001; Table 3 ].
Heart Rate
An effect of group [ F (2, 126) = 13.96, P < .0001] and stress [ F (1, 126) = 7.04, P < .01] was observed on heart rate (NS = 22; CH = 23; HS = 22; Table 3 ). However, there was no interaction between group and stress. The effect of stress was not significant when analyzing groups separately (all P s > .05). However, one-way ANOVA using group as independent factor indicated that heavy smokers had significant higher heart rate than nonsmokers at baseline and this difference remained constant throughout stress induction ( P < .01).
Heart Rate Variability
A repeated measures ANOVA showed a significant effect of group and stress on heart rate variability measured using pNN50 ([ F (2, 126) = 13.37, P < .0001] and [ F (1, 126) = 6.32, P < .05], respectively; NS = 22; CH = 23; HS = 22). No interaction between group and stress was observed. At baseline and during stress, heavy smokers showed significantly lower heart rate variability than chippers and nonsmokers ( P < .05). Post hoc analysis of the effect of stress by study group showed a statistically significant effect on chippers ( P < .05) but not on heavy smokers or nonsmokers ( Table 3 ).
Salivary Cortisol
We applied a repeated measures ANOVA using salivary cortisol as the dependent variable (NS = 25; CH = 25; HS = 23). Although the model was not significant ( F (5, 140) = 2.24, P = .053), there was an effect of group [ F (2, 140) = 4.02, P < .05]. There was no effect of stress or any interaction. Post hoc analyses using a one-way ANOVA confirmed the group effect with a significant difference in post stress salivary cortisol between chippers and heavy smokers [ F (2, 70) = 3.41, P < .05].
Variables Associated With Stress and Smoking
We performed pairwise correlation analyses using baseline one-time measures. As expected, there was a significant correlation between perceived stress and neuroticism in the whole sample, r (69) = 0.5885, P < .0001. This correlation was significant for heavy smokers [ r (19) = 0.634, P < .05] and chippers [ r (23) = 0.6247, P < .01] but not for nonsmokers. In the heavy smoker group, perceived stress was positively correlated with nicotine dependence [ r (20) = 0.6469, P < .05] and negatively correlated with mindfulness trait (nonjudgment facet) [ r (20) = −0.6344, P < .05]. None of these correlations were significant for chippers or nonsmokers.
We explored if perceived stress would predict performance on the delay discounting task. Linear regression analysis identified the score on the perceived stress scale as a significant predictor of rate of discounting in the heavy smoker group [ b = 0.15, t (19) = 3.30, P < .01], suggesting that chronically stressed heavy smokers tend to discount more.
Baseline variables were also used in a regression model to identify predictors of stress effects on affect, smoking urges, heart rate and respiration. In our sample, neuroticism was positively associated with increase in smoking urges after stress in heavy smokers [ b = 2.75, t (15) = 2.18, P < .05] and the number of cigarettes smoked each day was a significant predictor of increase in urges in chippers [ b = 7.52, t (19) = 2.28, P < .05]. Pairwise correlation analysis was conducted among outcome variables (change after stress). In chippers, change in positive affect was inversely correlated to change in smoking urges [ r (23) = −0.57, P < .05], and change in negative affect positively correlated to change in urges [ r (23) = 0.63, P < .05]. No significant association was found for heavy smokers.
Discussion
To examine further the differences between low-level smoking and heavy smoking, our study characterized and compared nicotine dependent smokers (heavy smokers), nondependent smokers (chippers), and nonsmokers in terms of impulsivity, stress perception, and stress response. There is extensive literature about the relationship between impulsivity and nicotine addiction, and stress and smoking. However, the majority refers to dependent smokers as defined by the number of cigarettes smoked per day and high dependence scores. 31 Less is known about nondependent smokers. Our aim was to contribute to the understanding of this group of smokers hypothesizing that differences in impulsivity, personality traits, stress perception, and stress reactivity might be associated to nicotine dependence resistance.
Impulsivity and Personality Traits
Similar to previous studies, 9 , 41 , 52 , 53 heavy smokers in our sample showed greater discounting of delayed money than nonsmokers. Literature on delay discounting in nondependent smokers is scarce. Previous studies have described a significant difference in rate of discounting between dependent smokers and nondependent ones and a relationship between degree of nicotine dependence and discounting of delayed rewards. 15 , 17 Our study found no significant difference in rate of discounting between chippers and heavy smokers, as well as no significant correlation with nicotine dependence or cigarettes smoked daily, suggesting that nondependent smokers are as impulsive as dependent smokers. Several studies have documented the relationship between drinking alcohol and smoking in light smokers. 54–57 In our sample alcohol consumption was significantly higher in chippers and could account for higher discounting ( Table 2 ). However, after controlling for amount of alcohol, there still was no difference in rate of discounting between chippers and heavy smokers. No differences were found in response inhibition with performance on the flanker task. Delay discounting and response inhibition load onto different factors to account for variance in smoking behavior. 58 , 59 Although some studies find response inhibition differences between smokers and nonsmokers, 60 , 61 studies comparing different impulsivity measures report that delay discounting discriminates controls from addicts better than response inhibition. 58 , 62 , 63 Our results might reflect that there are truly no differences in this facet of impulsivity between chippers and heavy smokers. However, alternatively, response inhibition tasks such as the flanker task might not be sensitive enough to detect differences in impulsivity in our population. If this is the case, response inhibition might still be associated with chippers’ ability to maintain a pattern of long-term low level smoking.
We did not find significant differences in self-reported personality traits. Previously, smokers have been characterized as high on facets related to impulsivity and neuroticism, low on agreeableness and conscientiousness, 64–66 and in some older studies smokers scored high on extraversion. 64 , 67 , 68 Sensation seeking has also been reported as higher in nicotine dependent smokers than in nonsmokers. 9 , 69–71 In our sample dependent smokers were not significantly different from chippers or nonsmokers in any of those traits. This discrepancy might be explained by: (1) Differences in the average age of the sample, since our population averaged 33 years old while recent studies have assessed personality traits and smoking in college and adolescence. 69 , 71 (2) Changes in the profile of heavy smokers associated with cultural and environmental differences across geography and/or time. For example, research in Europe and Japan found extraversion associated with cigarette smoking. 67 , 72 Additionally, studies using a similar age sample to ours tend to be older studies that precede the impact of smoking restrictions on the profile of current smokers. 9 , 70
Consistent with what has been published, we confirmed the relationship between smoking and chronic stress in nicotine dependent smokers. 23 , 24 Heavy smokers scored high in perceived stress, while chippers were undistinguishable from nonsmokers. Mindfulness was negatively correlated with stress only in dependent smokers. This association, in addition to the association between nicotine dependence and stress, suggests that nicotine dependence treatments could be improved by addressing stress, and that mindfulness based approaches might provide smokers with the useful skills to quit successfully. 73 , 74
Neuroticism has been associated to smoking and stress. 75–77 In our study, neuroticism was associated with higher perceived stress in both smoking groups but not in nonsmokers. This observation is consistent with the hypothesis that increased neuroticism is associated with self-medication of negative affect with nicotine and that decreased serotonergic activity, the neurobiological substrate of neuroticism, is associated with increased likelihood of smoking. 76
The stress vulnerability model suggests that stress influences substance abuse through maladaptive response to the environment. 78–80 Perceived stress was significantly higher in our sample of heavy smokers compared to nonsmokers. Furthermore, perceived stress was a significant predictor of performance in the delay discounting task, suggesting that higher levels of stress result in a shift to a more immediate-oriented mindset and, subsequently, an increased probability of relapse. Accordingly, a recent meta-analysis of the literature on impulsivity and stress found a moderate to large effect of stress on impulsive decision making. 81
Acute Stress Effects
Our results indicate that nicotine-dependent and nondependent smokers respond to acute stress with a similar increase in smoking urges. Analyses of associated variables differed between smoking groups. Only in chippers were increases in craving associated with decreases in positive affect and increases in negative affect suggesting that smoking behavior is more linked to emotional state in nondependent smokers than in heavy regular smokers. Only heavy smokers showed a significant change in affect after stress. This, however, wasn’t associated to increases in craving, indicating that heavy smokers might be more prone to smoke regardless of context, as reported previously in nicotine deprived dependent smokers. 82
Acute stress in all three groups increased breathing rate equivalently. Thus, breathing rate might be a good physiological marker of acute stress. However, breathing rate does not seem to be a marker for chronic stress based on our sample or the literature. 83 Similarly, stress increased heart rate in the sample as a whole. As expected, 84 heavy smokers showed elevated resting heart rate at baseline compared to nonsmokers, and chippers were in between. Heart rate variability was also significantly lower in heavy smokers. Our observations agree with the well-established relationship between nicotine consumption and cardiovascular symptoms, even in occasional or light smokers. 85–87
Limitations
While heavy smoking is relatively easy to define (usually >15 cigarettes/d), 30 there is little consensus when defining low-levels of cigarette smoking. 88 A number of operational definitions of low-level smoking can be found in the literature. 89–92 We based our distinction between tobacco chippers and heavy smokers on the Fagerstrom Test for Nicotine Dependence score. This score relies heavily on amount of cigarettes smoked, which, as smoking restrictions increase, might not be an accurate measure of dependence anymore. 93 Future research might examine alternative methods of classifying smokers such as the measure of autonomy over smoking, 94 , 95 the Diagnostic and Statistical Manual-IV ( DSM-IV ) nicotine dependence criteria, 96 or the Nicotine Dependence Syndrome Scale (NDSS). 97
Regarding our salivary cortisol results, we cannot exclude the possibility of Type II error. Cortisol’s circadian rhythm and rapid morning decline might have masked some of the acute stress effects. 73 , 74 In addition, our sample size, which was sufficient to detect significant effects of stress on respiration, heart rate and heart rate variability, might have been too small to detect changes in salivary cortisol concentration. Finally, it might be worth enhancing some aspects of the titrated Stroop (ie, performance feedback) to maximize the physiological response.
Conclusions
Tobacco chippers are as impulsive as heavy smokers. However, chippers report less stress than heavy smokers. Smoking urges were associated to emotional state more in chippers than heavy smokers. Autonomic activity differed between heavy smokers and controls, but chippers trended between nonsmokers and heavy smokers. Nicotine dependence treatments could benefit from an emphasis on stress reduction stress coping strategies.
Funding
This work was supported by the National Institutes of Health (grants R21DA035877 to LC-T, T32AT002688, and K24AT005121 to BSO, and UL1TR000128). NIH had no further role in study design, collection, analysis and interpretation of data, manuscript writing, or in the decision to submit for publication.
Declaration of Interests
None declared.
Acknowledgments
We are grateful to Roger Ellingson for adapting the titrated Stroop task and to Vanessa Wilson for valuable help with recruitment and support throughout the study. We thank all study participants for their participation.
References
- 1. Shiffman S, Ferguson SG, Dunbar MS, Scholl SM . Tobacco dependence among intermittent smokers . Nicotine Tob Res . 2012. ; 14 ( 11 ): 1372 – 1381 . doi: 10.1093/ntr/nts097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiffman S, Zettler-Segal M, Kassel J, Paty J, Benowitz NL, O’Brien G . Nicotine elimination and tolerance in non-dependent cigarette smokers . Psychopharmacology (Berl) . 1992. ; 109 ( 4 ): 449 – 456 . doi: 10.1007/BF02247722 . [DOI] [PubMed] [Google Scholar]
- 3. Gilbert DG, Gilbert BO . Personality, psychopathology, and nicotine response as mediators of the genetics of smoking . Behav Genet . 1995. ; 25 ( 2 ): 133 – 147 . doi: 10.1007/BF02196923 . [DOI] [PubMed] [Google Scholar]
- 4. Kassel JD, Stroud LR, Paronis CA . Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking . Psychol Bull . 2003. ; 129 ( 2 ): 270 – 304 . doi: 10.1037/0033-2909.129.2.270 . [DOI] [PubMed] [Google Scholar]
- 5. Nichter M, Vuckovic N, Quintero G, Ritenbaugh C . Smoking experimentation and initiation among adolescent girls: qualitative and quantitative findings . Tob Control . 1997. ; 6 ( 4 ): 285 – 295 . doi: 10.1136/tc.6.4.285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sinha R . Chronic stress, drug use, and vulnerability to addiction . Ann NY Acad Sci . 2008. ; 1141 ( 1 ): 105 – 130 . doi: 10.1196/annals.1441.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ainslie G . Specious reward: a behavioral theory of impulsiveness and impulse control . Psychol Bull . 1975. ; 82 ( 4 ): 463 – 496 . www.ncbi.nlm.nih.gov/pubmed/1099599 . Accessed May 20, 2015 . [DOI] [PubMed] [Google Scholar]
- 8. Reynolds B, Ortengren A, Richards JB, de Wit H . Dimensions of impulsive behavior: personality and behavioral measures . Pers Individ Dif . 2006. ; 40 ( 2 ): 305 – 315 . doi: 10.1016/j.paid.2005.03.024 . [Google Scholar]
- 9. Mitchell SH . Measures of impulsivity in cigarette smokers and non-smokers . Psychopharmacology (Berl) . 1999. ; 146 ( 4 ): 455 – 464 . doi: 10.1007/PL00005491 . [DOI] [PubMed] [Google Scholar]
- 10. Bari A, Robbins TW . Inhibition and impulsivity: behavioral and neural basis of response control . Prog Neurobiol . 2013. ; 108 : 44 – 79 . doi: 10.1016/j.pneurobio.2013.06.005 . [DOI] [PubMed] [Google Scholar]
- 11. Zuckerman M, Kuhlman DM . Personality and risk-taking: common biosocial factors . J Pers . 2000. ; 68 ( 6 ): 999 – 1029 . doi: 10.1111/1467-6494.00124 . [DOI] [PubMed] [Google Scholar]
- 12. Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW . Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits . Biol Psychiatry . 2010. ; 68 ( 8 ): 770 – 773 . doi: 10.1016/j.biopsych.2010.06.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enticott PG, Ogloff JRP, Bradshaw JL . Associations between laboratory measures of executive inhibitory control and self-reported impulsivity . Pers Individ Dif . 2006. ; 41 ( 2 ): 285 – 294 . [Google Scholar]
- 14. Luijten M, Littel M, Franken IH . Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials . PLoS One . 2011. ; 6 ( 4 ): e18898 . doi: 10.1371/journal.pone.0018898 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heyman GM, Gibb SP . Delay discounting in college cigarette chippers . Behav Pharmacol . 2006. ; 17 ( 8 ): 669 – 679 . doi: 10.1097/FBP.0b013e3280116cfe . [DOI] [PubMed] [Google Scholar]
- 16. Johnson MW, Bickel WK, Baker F . Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers . Exp Clin Psychopharmacol . 2007. ; 15 ( 2 ): 187 – 194 . doi: 10.1037/1064-1297.15.2.187 . [DOI] [PubMed] [Google Scholar]
- 17. Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB . Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day . Nicotine Tob Res . 2008. ; 10 ( 10 ): 1571 – 1575 . doi: 10.1080/14622200802323274 . [DOI] [PubMed] [Google Scholar]
- 18. McFarlane A, Clark CR, Bryant RA, et al. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects . J Integr Neurosci . 2005. ; 4 ( 1 ): 27 – 40 . doi: 10.1142/S0219635205000689 . [DOI] [PubMed] [Google Scholar]
- 19. Norton R, Brown K, Howard R . Smoking, nicotine dose and the lateralisation of electrocortical activity . Psychopharmacology (Berl) . 1992. ; 108 ( 4 ): 473 – 479 . doi: 10.1007/BF02247424 . [DOI] [PubMed] [Google Scholar]
- 20. Seeman TE, McEwen BS, Rowe JW, Singer BH . Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging . Proc Natl Acad Sci USA . 2001. ; 98 ( 8 ): 4770 – 4775 . doi: 10.1073/pnas.081072698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oken BS, Chamine I, Wakeland W . A systems approach to stress, stressors and resilience in humans . Behav Brain Res . 2015. ; 282 : 144 – 154 . doi: 10.1016/j.bbr.2014.12.047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siqueira L, Diab M, Bodian C, Rolnitzky L . Adolescents becoming smokers: the roles of stress and coping methods . J Adolesc Health . 2000. ; 27 ( 6 ): 399 – 408 . doi: 10.1016/S1054-139X(00)00167-1 . [DOI] [PubMed] [Google Scholar]
- 23. Parrott AC . Stress modulation over the day in cigarette smokers . Addiction . 1995. ; 90 ( 2 ): 233 – 244 . doi: 10.1111/j.1360-0443.1995.tb01041.x . [DOI] [PubMed] [Google Scholar]
- 24. Pomerleau OF, Pomerleau CS . Cortisol response to a psychological stressor and/or nicotine . Pharmacol Biochem Behav . 1990. ; 36 ( 1 ): 211 – 213 . doi: 10.1016/0091-3057(90)90153-9 . [DOI] [PubMed] [Google Scholar]
- 25. Fleming SE, Lombardo TW . Effects of cigarette smoking on phobic anxiety . Addict Behav . 1987. ; 12 ( 2 ): 195 – 198 . doi: 10.1016/0306-4603(87)90027-x . [DOI] [PubMed] [Google Scholar]
- 26. Perkins KA, Grobe JE . Increased desire to smoke during acute stress . Br J Addict . 1992. ; 87 ( 7 ): 1037 – 1040 . doi: 10.1111/j.1360-0443.1992.tb03121.x . [DOI] [PubMed] [Google Scholar]
- 27. Oken BS, Fonareva I, Wahbeh H . Stress-related cognitive dysfunction in dementia caregivers . J Geriatr Psychiatry Neurol . 2011. ; 24 ( 4 ): 191 – 198 . doi: 10.1177/0891988711422524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carton S, Jouvent R, Widlocher D . Sensation seeking, nicotine dependence, and smoking motivation in female and male smokers . Addict Behav . 1994. ; 19 ( 3 ): 219 – 227 . doi: 10.1016/0306-4603(94)90026-4 . [DOI] [PubMed] [Google Scholar]
- 29. Wahbeh H, Lu M, Oken B . Mindful awareness and non-judging in relation to posttraumatic stress disorder symptoms . Mindfulness (NY) . 2011. ; 2 ( 4 ): 219 – 227 . doi: 10.1007/s12671-011-0064-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M . Smoking typology profiles of chippers and regular smokers . J Subst Abuse . 1994. ; 6 ( 1 ): 21 – 35 . www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8081107 . Accessed June 29, 2015 . [DOI] [PubMed] [Google Scholar]
- 31. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO . The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire . Br J Addict . 1991. ; 86 ( 9 ): 1119 – 1127 . doi: 10.1111/j.1360-0443.1991.tb01879.x . [DOI] [PubMed] [Google Scholar]
- 32. Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR . Anterior cingulate activity correlates with blood pressure during stress . Psychophysiology . 2005. ; 42 ( 6 ): 627 – 635 . doi: 10.1111/j.1469-8986.2005.00366.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI . Testing the efficiency and independence of attentional networks . J Cogn Neurosci . 2002. ; 14 ( 3 ): 340 – 347 . doi: 10.1162/089892902317361886 . [DOI] [PubMed] [Google Scholar]
- 34. Edwards S, Clow A, Evans P, Hucklebridge F . Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity . Life Sci . 2001. ; 68 ( 18 ): 2093 – 2103 . doi: 10.1016/S0024-3205(01)00996-1 . [DOI] [PubMed] [Google Scholar]
- 35. Dickerson SS, Kemeny ME . Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research . Psychol Bull . 2004. ; 130 ( 3 ): 355 – 391 . doi: 10.1037/0033-2909.130.3.355 . [DOI] [PubMed] [Google Scholar]
- 36. Kirschbaum C, Pirke KM, Hellhammer DH . The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting . Neuropsychobiology . 1993. ; 28 ( 1–2 ): 76 – 81 . doi: 119004 . [DOI] [PubMed] [Google Scholar]
- 37. Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC . The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain . J Psychiatry Neurosci . 2005. ; 30 ( 5 ): 319 – 325 . www.ncbi.nlm.nih.gov/pubmed/16151536 . Accessed May 15, 2015 . [PMC free article] [PubMed] [Google Scholar]
- 38. Chida Y, Hamer M . Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations . Psychol Bull . 2008. ; 134 ( 6 ): 829 – 885 . doi: 10.1037/a0016852 . [DOI] [PubMed] [Google Scholar]
- 39. Bellingrath S, Weigl T, Kudielka BM . Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers . Stress . 2009. ; 12 ( 1 ): 37 – 48 . doi: 10.1080/10253890802042041 . [DOI] [PubMed] [Google Scholar]
- 40. Lupien S . Well Stressed : How You Can Manage Stress Before It Turns Toxic . Mississauga, ON: John Wiley & Sons Canada, Ltd; ; 2012. . [Google Scholar]
- 41. Imhoff S, Harris M, Weiser J, Reynolds B . Delay discounting by depressed and non-depressed adolescent smokers and non-smokers . Drug Alcohol Depend . 2014. ; 135 : 152 – 155 . doi: 10.1016/j.drugalcdep.2013.11.014 . [DOI] [PubMed] [Google Scholar]
- 42. Cohen S, Kamarck T, Mermelstein R . A global measure of perceived stress . J Health Soc Behav . 1983. ; 24 ( 4 ): 385 – 396 . doi: 10.2307/2136404 . [PubMed] [Google Scholar]
- 43. Costa PT, Jr, McCrae RR . Domains and facets: hierarchical personality assessment using the revised NEO personality inventory . J Pers Assess . 1995. ; 64 ( 1 ): 21 – 50 . doi: 10.1207/s15327752jpa6401_2 . [DOI] [PubMed] [Google Scholar]
- 44. Zuckerman M, Link K . Construct validity for the sensation-seeking scale . J Consult Clin Psychol . 1968. ; 32 ( 4 ): 420 – 426 . doi: 10.1037/h0026047 . [DOI] [PubMed] [Google Scholar]
- 45. Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples . Assessment . 2008. ; 15 ( 3 ): 329 – 342 . doi: 10.1177/1073191107313003 . [DOI] [PubMed] [Google Scholar]
- 46. Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales . J Pers Soc Psychol . 1988. ; 54 ( 6 ): 1063 – 1070 . doi: 10.1037/0022-3514.54.6.1063 . [DOI] [PubMed] [Google Scholar]
- 47. Hughes JR, Hatsukami D . Signs and symptoms of tobacco withdrawal . Arch Gen Psychiatry . 1986. ; 43 ( 3 ): 289 – 294 . doi: 10.1001/archpsyc.1986.01800030107013 . [DOI] [PubMed] [Google Scholar]
- 48. Tiffany ST, Drobes DJ . The development and initial validation of a questionnaire on smoking urges . Br J Addict . 1991. ; 86 ( 11 ): 1467 – 1476 . doi: 10.1111/j.1360-0443.1991.tb01732.x . [DOI] [PubMed] [Google Scholar]
- 49. Wostmann NM, Aichert DS, Costa A, Rubia K, Moller HJ, Ettinger U . Reliability and plasticity of response inhibition and interference control . Brain Cogn . 2013. ; 81 ( 1 ): 82 – 94 . doi: 10.1016/j.bandc.2012.09.010 . [DOI] [PubMed] [Google Scholar]
- 50. Casaletto KB, Umlauf A, Beaumont J, et al. Demographically Corrected Normative Standards for the English Version of the NIH Toolbox Cognition Battery . J Int Neuropsychol Soc . 2015. ; 21 ( 5 ): 378 – 391 . doi: 10.1017/S1355617715000351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mukherjee S, Yadav R, Yung I, Zajdel DP, Oken BS . Sensitivity to mental effort and test-retest reliability of heart rate variability measures in healthy seniors . Clin Neurophysiol . 2011. ; 122 ( 10 ): 2059 – 2066 . doi: 10.1016/j.clinph.2011.02.032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reynolds B, Fields S . Delay discounting by adolescents experimenting with cigarette smoking . Addiction . 2012. ; 107 ( 2 ): 417 – 424 . doi: 10.1111/j.1360-0443.2011.03644.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedel JE, DeHart WB, Madden GJ, Odum AL . Impulsivity and cigarette smoking: discounting of monetary and consumable outcomes in current and non-smokers . Psychopharmacology (Berl) . 2014. ; 231 ( 23 ): 4517 – 4526 . doi: 10.1007/s00213-014-3597-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. King AC, Epstein AM . Alcohol dose-dependent increases in smoking urge in light smokers . Alcohol Clin Exp Res . 2005. ; 29 ( 4 ): 547 – 552 . doi: 10.1097/01.ALC.0000158839.65251.FE . [DOI] [PubMed] [Google Scholar]
- 55. Harrison EL, McKee SA . Non-daily smoking predicts hazardous drinking and alcohol use disorders in young adults in a longitudinal U.S. sample . Drug Alcohol Depend . 2011. ; 118 ( 1 ): 78 – 82 . doi: 10.1016/j.drugalcdep.2011.02.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ray LA, Miranda R, Jr, Kahler CW, et al. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study . Psychopharmacology (Berl) . 2007. ; 193 ( 4 ): 449 – 456 . doi: 10.1007/s00213-007-0794-z . [DOI] [PubMed] [Google Scholar]
- 57. Shiffman S, Dunbar MS, Li X, et al. Smoking patterns and stimulus control in intermittent and daily smokers . PLoS One . 2014. ; 9 ( 3 ): e89911 . doi: 10.1371/journal.pone.0089911 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Courtney KE, Arellano R, Barkley-Levenson E, et al. The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach . Alcohol Clin Exp Res . 2012. ; 36 ( 6 ): 923 – 931 . doi: 10.1111/j.1530-0277.2011.01635.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christiansen P, Cole JC, Goudie AJ, Field M . Components of behavioural impulsivity and automatic cue approach predict unique variance in hazardous drinking . Psychopharmacology . 2012. ; 219 ( 2 ): 501 – 510 . doi: 10.1007/s00213-011-2396-z . [DOI] [PubMed] [Google Scholar]
- 60. Yakir A, Rigbi A, Kanyas K, et al. Why do young women smoke? III. Attention and impulsivity as neurocognitive predisposing factors . Eur Neuropsychopharmacol . 2007. ; 17 ( 5 ): 339 – 351 . doi: 10.1016/j.euroneuro.2006.09.004 . [DOI] [PubMed] [Google Scholar]
- 61. Spinella M . Correlations between orbitofrontal dysfunction and tobacco smoking . Addict Biol . 2002. ; 7 ( 4 ): 381 – 384 . doi: 10.1080/1355621021000005964 . [DOI] [PubMed] [Google Scholar]
- 62. Torres A, Catena A, Megias A, et al. Emotional and non-emotional pathways to impulsive behavior and addiction . Front Hum Neurosci . 2013. ; 7 : 43 . doi: 10.3389/fnhum.2013.00043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ledgerwood DM, Alessi SM, Phoenix N, Petry NM . Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls . Drug Alcohol Depend . 2009. ; 105 ( 1–2 ): 89 – 96 . doi: 10.1016/j.drugalcdep.2009.06.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Malouff JM, Thorsteinsson EB, Schutte NS . The five-factor model of personality and smoking: a meta-analysis . J Drug Educ . 2006. ; 36 ( 1 ): 47 – 58 . doi: 10.2190/9EP8-17P8-EKG7-66AD . [DOI] [PubMed] [Google Scholar]
- 65. Paunonen SV, Ashton MC . Big five factors and facets and the prediction of behavior . J Pers Soc Psychol . 2001. ; 81 ( 3 ): 524 – 539 . doi: 10.1037/0022-3514.81.3.524 . [PubMed] [Google Scholar]
- 66. Terracciano A, Costa PT . Smoking and the Five-Factor Model of personality . Addiction . 2004. ; 99 ( 4 ): 472 – 481 . doi: 10.1111/j.1360-0443.2004.00687.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arai Y, Hosokawa T, Fukao A, Izumi Y, Hisamichi S . Smoking behaviour and personality: a population-based study in Japan . Addiction . 1997. ; 92 ( 8 ): 1023 – 1033 . doi: 10.1111/j.1360-0443.1997.tb02982.x . [PubMed] [Google Scholar]
- 68. Munafò MR, Black S . Personality and smoking status: a longitudinal analysis . Nicotine Tob Res . 2007. ; 9 ( 3 ): 397 – 404 . doi: 10.1080/14622200701188869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Balevich EC, Wein ND, Flory JD . Cigarette smoking and measures of impulsivity in a college sample . Subst Abus . 2013. ; 34 ( 3 ): 256 – 262 . doi: 10.1080/08897077.2012.763082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Harmsen H, Bischof G, Brooks A, Hohagen F, Rumpf HJ . The relationship between impaired decision-making, sensation seeking and readiness to change in cigarette smokers . Addict Behav . 2006. ; 31 ( 4 ): 581 – 592 . doi: 10.1016/j.addbeh.2005.05.038 . [DOI] [PubMed] [Google Scholar]
- 71. Rezvanfard M, Ekhtiari H, Mokri A, Djavid GE, Kaviani H . Psychological and behavioral traits in smokers and their relationship with nicotine dependence level . Arch Iran Med . 2010. ; 13 ( 5 ): 395 – 405 . doi: 010135/AIM.006 . [PubMed] [Google Scholar]
- 72. Harakeh Z, Scholte RHJ, de Vries H, Engels RCME . Association between personality and adolescent smoking . Addict Behav . 2006. ; 31 ( 2 ): 232 – 245 . doi: 10.1016/j.addbeh.2005.05.003 . [DOI] [PubMed] [Google Scholar]
- 73. Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles . J Clin Endocrinol Metab . 2009. ; 94 ( 5 ): 1548 – 1554 . doi: 10.1210/jc.2008-2380 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krieger DT, Allen W, Rizzo F, Krieger HP . Characterization of the normal temporal pattern of plasma corticosteroid levels . J Clin Endocrinol Metab . 1971. ; 32 ( 2 ): 266 – 284 . doi: 10.1210/jcem-32-2-266 . [DOI] [PubMed] [Google Scholar]
- 75. Oken BS, Fonareva I, Wahbeh H . Stress-related cognitive dysfunction in dementia caregivers . J Geriatr Psychiatry Neurol . 2011. ; 24 ( 4 ): 191 – 198 . doi: 10.1177/0891988711422524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Munafo MR, Zetteler JI, Clark TG . Personality and smoking status: a meta-analysis . Nicotine Tob Res . 2007. ; 9 ( 3 ): 405 – 413 . doi: 10.1080/14622200701188851 . [DOI] [PubMed] [Google Scholar]
- 77. Lahey BB . Public health significance of neuroticism . Am Psychol . 2009. ; 64 ( 4 ): 241 – 256 . doi: 10.1037/a0015309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sinha R . Modeling stress and drug craving in the laboratory: implications for addiction treatment development . Addict Biol . 2009. ; 14 ( 1 ): 84 – 98 . doi: 10.1111/j.1369-1600.2008.00134.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward . J Psychopharmacol . 2011. ; 25 ( 4 ): 490 – 502 . doi: 10.1177/0269881110376694 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ansell EB, Gu P, Tuit K, Sinha R . Effects of cumulative stress and impulsivity on smoking status . Hum Psychopharmacol . 2012. ; 27 ( 2 ): 200 – 208 . doi: 10.1002/hup.1269 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fields SA, Lange K, Ramos A, Thamotharan S, Rassu F . The relationship between stress and delay discounting: a meta-analytic review . Behav Pharmacol . 2014. ; 25 ( 5–6 ): 434 – 444 . doi: 10.1097/FBP.0000000000000044 . [DOI] [PubMed] [Google Scholar]
- 82. Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA . A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers . Addiction . 2001. ; 96 ( 10 ): 1419 – 1432 . doi: 10.1080/09652140120075152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wahbeh H, Oken BS . Peak high-frequency HRV and peak alpha frequency higher in PTSD . Appl Psychophysiol Biofeedback . 2013. ; 38 ( 1 ): 57 – 69 . doi: 10.1007/s10484-012-9208-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Winniford MD . Smoking and cardiovascular function . J Hypertens Suppl . 1990. ; 8 ( 5 ): S17 – 23 . www.ncbi.nlm.nih.gov/pubmed/2286853 . Accessed May 20, 2015 . [PubMed] [Google Scholar]
- 85. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States . N Engl J Med . 2013. ; 368 ( 4 ): 351 – 364 . doi: 10.1056/NEJMsa1211127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States . N Engl J Med . 2013. ; 368 ( 4 ): 341 – 350 . doi: 10.1056/NEJMsa1211128 . [DOI] [PubMed] [Google Scholar]
- 87. Luoto R, Uutela A, Puska P . Occasional smoking increases total and cardiovascular mortality among men . Nicotine Tob Res . 2000. ; 2 ( 2 ): 133 – 139 . doi: 10.1080/713688127 . [DOI] [PubMed] [Google Scholar]
- 88. WHO . WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package . 2008. . www.who.int/tobacco/mpower/2008/en/ . Accessed May 20, 2015 .
- 89. Wortley PM, Husten CG, Trosclair A, Chrismon J, Pederson LL . Nondaily smokers: a descriptive analysis . Nicotine Tob Res . 2003. ; 5 ( 5 ): 755 – 759 . doi: 10.1080/1462220031000158753 . [DOI] [PubMed] [Google Scholar]
- 90. The 2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary . Respir Care . 2008. ; 53 ( 9 ): 1217 – 1222 . www.ncbi.nlm.nih.gov/pubmed/18807274 . Accessed June 29, 2015 . [PubMed] [Google Scholar]
- 91. Biener L, Albers AB . Young adults: vulnerable new targets of tobacco marketing . Am J Public Health . 2004. ; 94 ( 2 ): 326 – 330 . www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1448251&tool=pmcentrez&rendertype=abstract . Accessed June 29, 2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, Feldner MT . Anxiety sensitivity and early relapse to smoking: a test among Mexican daily, low-level smokers . Nicotine Tob Res . 2007. ; 9 ( 4 ): 483 – 491 . doi: 10.1080/14622200701239621 . [DOI] [PubMed] [Google Scholar]
- 93. Donny EC, Griffin KM, Shiffman S, Sayette MA . The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers . Nicotine Tob Res . 2008. ; 10 ( 5 ): 934 – 942 . doi: 10.1080/14622200802133681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wellman RJ, DiFranza JR, Wood C . Tobacco chippers report diminished autonomy over smoking . Addict Behav . 2006. ; 31 ( 4 ): 717 – 721 . doi: 10.1016/j.addbeh.2005.05.043 . [DOI] [PubMed] [Google Scholar]
- 95. Difranza JR, Savageau JA, Wellman RJ . A comparison of the autonomy over tobacco scale and the Fagerstrom test for nicotine dependence . Addict Behav . 2012. ; 37 ( 7 ): 856 – 861 . doi: 10.1016/j.addbeh.2012.03.013 . [DOI] [PubMed] [Google Scholar]
- 96. Sledjeski EM, Dierker LC, Costello D, et al. Predictive validity of four nicotine dependence measures in a college sample . Drug Alcohol Depend . 2007. ; 87 ( 1 ): 10 – 19 . doi: 10.1016/j.drugalcdep.2006.07.005 . [DOI] [PubMed] [Google Scholar]
- 97. Shiffman S, Sayette MA . Validation of the nicotine dependence syndrome scale (NDSS): a criterion-group design contrasting chippers and regular smokers . Drug Alcohol Depend . 2005. ; 79 ( 1 ): 45 – 52 . doi: 10.1016/j.drugalcdep.2004.12.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]