Abstract
Introduction:
The goal of this study was to identify maternal patterns of prenatal and postnatal cigarette smoking associated with adolescent smoking. We hypothesized that maternal use at multiple time points, especially at later assessments when the offspring were adolescents, would predict offspring use.
Methods:
Pregnant women ( N = 456: ages 13–42) were recruited from a prenatal clinic and interviewed during pregnancy and at delivery, providing data on cigarette use (any/none) for the first and third trimesters. Mothers were re-assessed at 6, 10, 14, and 16 years postpartum. Offspring reported cigarette use at age 16. Covariates included maternal race, age, education, family income, child age, parenting behavior, and other maternal and child substance use.
Results:
A growth mixture model revealed five patterns of tobacco use: infrequent/nonuse (39%), postpartum quitters (5%), later quitters (7%), increasing likelihood of being smokers (17%), and chronic users (32%). Offspring of postpartum quitters and the increasing likelihood of being smokers groups were more likely to use cigarettes, compared to adolescents of mothers from the infrequent/nonuse group, controlling for significant covariates.
Conclusions:
This is the first study to examine trajectories of maternal cigarette use from pregnancy to 16 years postpartum, linking prenatal and postnatal patterns of maternal use to use in adolescent offspring. Our findings highlight the risk associated with prenatal exposure, because mothers who used during pregnancy but quit by 6 years postpartum still had offspring who were 3.5 times more likely to smoke than non/infrequent users.
Implications:
This is the first study to examine trajectories of maternal cigarette use from the prenatal period to 16 years postpartum, and to link prenatal and postnatal patterns of use to use in adolescent offspring. We identified two long-term patterns of maternal cigarette use that were associated with offspring smoking at age 16, including one where offspring were exposed prenatally, but much less likely to be exposed to maternal cigarette use postpartum. Our findings highlight the risk associated with prenatal exposures for cigarette use in offspring, even if mothers quit in the postpartum.
Introduction
Although girls begin regular smoking earlier than boys and are more likely to be smokers from adolescence to young adulthood, they are also more likely to mature out of smoking by their late 20s, when many transition to a parenting role. 1 According to the Pregnancy Risk Assessment and Monitoring System (PRAMS), 20% of mothers smoke prior to pregnancy, and 12% smoke during pregnancy, 2 with 10% smoking during the last trimester. 3 Of the women who smoked prior to pregnancy, 55% quit during pregnancy, but among these, 40% relapsed within 6 months after delivery. Thus, smoking remains common during pregnancy among American women, and many who manage to quit during pregnancy relapse in the postpartum.
Cigarette smoking harms not only the user, but also the offspring’s growth and neurobehavioral development, according to epidemiological and animal studies. 4 , 5 Adolescents whose parents smoke are more likely to initiate smoking, and this is especially true for maternal smoking. 6 , 7 Several studies show associations between prenatal cigarette smoke exposure and offspring tobacco use. 8–13 For example, a study using a large birth cohort from New Zealand demonstrated a link between maternal smoking during pregnancy and nicotine dependence and withdrawal at age 21. 14 Most of these studies, however, focus on maternal smoking during the prenatal or postnatal periods, and few have data on both. Even fewer follow maternal smoking patterns well beyond the perinatal period or control for many possible confounds of the association between maternal and child smoking, such as demographic and psychological factors. No study has examined trajectories of maternal prenatal and postnatal smoking on smoking in offspring.
Methods
Participants
Study data are from three birth cohorts from a consortium of studies on the effects of prenatal substance use on physical and neurobehavioral development. Participants were from two studies of pregnant adults (AA06390; DA03874: PI—NLD) and one study of pregnant adolescents (DA09275: PI—MDC). All women were enrolled at their fourth prenatal month clinic visit and were seen again at delivery. Follow-up visits were conducted with mothers and their children when offspring were ages 6, 10, 14, and 16. A new dataset was created by combining the birth cohorts for an integrative data analysis. 15 We avoid most potential sources of between-subject heterogeneity common to integrative data analysis because (1) all participants were drawn from the same prenatal clinic, (2) the same measures and personnel were used in all birth cohorts, and (3) we had the same follow-up time periods.
At birth, the combined sample size was 768 mothers. By 16 years postpartum, a total of 85 mothers were lost to follow-up, 38 refused participation, nine children had died, seven were adopted or in foster care, and 33 mothers had moved out of the area. The attrition rate for the combined sample at the last wave of assessment was 23%. This represents an excellent retention rate for prospective studies spanning two decades. To examine patterns of maternal substance use over time using trajectory analysis, we excluded all mothers who did not complete the substance use assessment from two or more phases, resulting in a sample of 456 mothers for the current analyses ( Table 1 —Sample Characteristics). Participants retained in the study ( n = 456) were significantly more likely to be black and less likely to smoke during pregnancy than those not included in the analyses ( n = 312). There were no significant differences between these groups in maternal age, socioeconomics, or prenatal alcohol and marijuana use.
Table 1.
Sample Characteristics
| Mean ( SD ) | Range | Percentage | |
|---|---|---|---|
| Maternal characteristics | |||
| Age at Time 1 | 19.58 (4.74) | 13–42 years | |
| Black/white | 64/36 | ||
| Married at Time 1 | 19.1 | ||
| Education by last assessment | 12.63 (1.93) | 6–18 years | |
| Monthly family income at last assessment | $2254 (1816) | $0–18 000 | |
| First trimester tobacco use | 39.9 | ||
| Third trimester tobacco use | 46.3 | ||
| Tobacco use 6 years postpartum | 50.4 | ||
| Tobacco use 10 years postpartum | 50.9 | ||
| Tobacco use 14 years postpartum | 46.7 | ||
| Tobacco use 16 years postpartum | 48.5 | ||
| Cigarettes per day | 5.86 (8.43) | 0–45 | |
| Heavier alcohol use 16 years postpartum | 16.2 | ||
| Cannabis use 16 years postpartum | 11.0 | ||
| Child characteristics | |||
| Male/female | 51.5/48.5 | ||
| Age at last assessment | 16.56 (0.56) | 15.93–18.91 | |
| Tobacco use at last assessment | 26.8 | ||
| Cigarettes per day | 1.24 (3.42) | 0–20 | |
| Alcohol use at last assessment | 39.7 | ||
| Cannabis use at last assessment | 35.0 | ||
Procedure
Participants were recruited during their fourth month of pregnancy and interviewed about tobacco, alcohol, cannabis, and other drug use during the first trimester. Mothers were interviewed again 24–36 hours after delivery and queried about tobacco, alcohol, cannabis, and other drug use during their third trimester of pregnancy. At the 6-, 10-, 14-, and 16-year visits, mothers provided information about their substance use (current, past year), demographic, and psychological status.
Measures
Maternal Tobacco Use
Mothers were interviewed in a private setting by interviewers who were comfortable discussing alcohol and drug use and trained to instrument reliability and accurately identifying and quantifying drug use. Information on maternal tobacco use included prenatal use, and use at 6-, 10- 14-, and 16-year postpartum time periods. Mothers were queried about the average number of cigarettes smoked on a typical day. Smoking was dichotomized to use/no use, consistent with other prospective studies of maternal smoking. 8 , 12–14
Other Maternal Substance Use
Dichotomous variables were created to characterize maternal heavy alcohol use and maternal marijuana use over time. Heavy alcohol use was defined as more than 7 drinks per week, which is higher than the moderate level of use recommended for women by the National Institute on Alcohol and Alcoholism (NIAAA). A summary score was created for chronic maternal heavy alcohol use, with a point for any heavy use at each wave of testing (0 = no heavy use, 1 = heavy alcohol use). Similarly, a summary score was also created for marijuana use, with a point assigned for any use at each wave of testing (0 = no use, 1 = any marijuana use).
Demographic Characteristics
During the first visit, mothers reported date of birth and race. For socioeconomic status, monthly family income and maternal educational attainment reported at the 16-year assessment was used (highest level obtained). Due to the range of child age at the last assessment, child age was included as a covariate.
Parenting Style
Parenting style was measured at the 14-year follow-up with the “My Parents” questionnaire, 16 a 27-item instrument measuring three dimensions of parenting: acceptance-involvement, autonomy-granting, and strictness-supervision.
Substance Use in Offspring
The offspring self-reported substance use at age 16, which included cigarette, alcohol, and marijuana use. Dichotomous variables were created. Ever cigarette use was defined as more than just a puff; ever alcohol use was defined as more than just a sip of alcohol; and ever marijuana use was defined as ever having tried marijuana.
Statistical Analysis
Data imputation was used for missing data, but we conservatively did not impute for more than one missing postpartum phase of maternal tobacco use. A growth mixture model (GMM) was applied to maternal tobacco use at different phases to explore trajectories of use over time. 17 Cubic growth curves were fitted, creating trajectory classes of maternal cigarette use. The number of classes that best fit the data was determined using Bayesian Information Criteria 18 and the Lo-Mendell-Rubin likelihood ratio test. 19 Logistic regression was used to test the association between maternal tobacco use trajectories (non/infrequent users—reference group) and adolescent cigarette use. To adjust for sample loss, we repeated all of the analyses to reflect differential loss by maternal race and first trimester cigarette use. Weights were constructed using the inverse of the probability of response for each racial and prenatal tobacco exposure group. The results were similar to the original data. We present the unweighted data for ease of interpretation.
Results
Trajectories of Maternal Cigarette Use
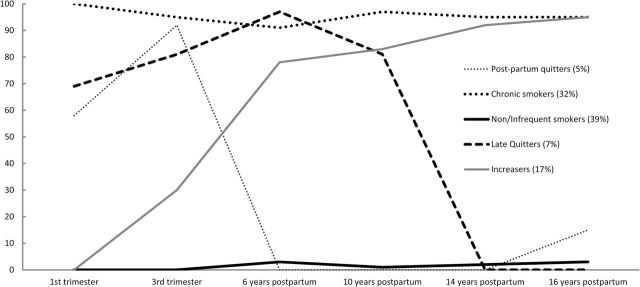
Five patterns of maternal tobacco use best fit the data. This model had the lowest Bayesian Information Criteria (compared to models with four or six classes) and the Lo-Mendell-Rubin likelihood ratio test was significant (entropy = 0.95). The five classes included: non/infrequent users at any time point (39%), this class was least likely to use during pregnancy and in later phases; postpartum quitters (5%) only used during pregnancy but quit by the first follow-up phase; late quitters (7%), this class did not smoke 14 or 16 years postpartum; increasers (17%) were more likely to use postpartum; and chronic users (32%), this class smoked at almost all time points. Over 90% of the mothers in the increasing likelihood group used cigarettes 14 and 16 years postpartum. In contrast, none of the mothers in the postpartum quitters group used cigarettes 14 years later, and only 15% used 16 years later. The observed frequencies of maternal tobacco use at each time point for the five classes are depicted in Figure 1 . The mean posterior probabilities for the five classes were 0.99, 0.97, 0.95, 0.97, and 0.99, respectively. The posterior probabilities for classification of participants into their respective classes were all above 0.6, showing no ambiguity in delineation of classes.
Figure 1.
Percent of mothers using cigarettes in each trajectory class.
Multivariate Analyses Predicting Adolescent Cigarette Use
The variable for maternal tobacco use trajectory group membership was used as a predictor in a regression on adolescent cigarette use, controlling for maternal age, race, educational attainment, family income, parenting style, other maternal substance use, and offspring alcohol and marijuana use. Adolescents whose mothers were in the postpartum quitters group (odds ratio [ OR ] = 3.51, confidence interval [CI] = 1.19–10.4) and the increasers group ( OR = 2.61, CI =1.23–5.54) were more likely to use cigarettes themselves, compared to adolescents whose mothers were from the infrequent/nonuser group. Using weights to adjust for sample loss, the odds ratios for the postpartum quitters and the increasers group were 1.75 ( P = .01) and 3.03 ( P = .01), respectively. The other groups were not significantly different from the infrequent/nonuser group. Maternal age (older: OR = 1.13, CI = 1.08–1.20) and race (white vs. black: OR = 2.33, CI = 1.34–4.04), adolescent’s own alcohol ( OR = 3.05, CI = 1.75–5.31) and marijuana use ( OR = 3.96, CI = 2.25–6.98) were also predictors of adolescent cigarette use in this model.
Discussion
These results provide converging evidence for the role of maternal smoking in risk for adolescent smoking, consistent with other studies on parental smoking. 6 , 7 We identified two long-term patterns of maternal cigarette use that were associated with offspring smoking at age 16, including one where offspring were exposed prenatally, but much less likely to be exposed to maternal cigarette use postpartum. Our findings highlight the risk associated with prenatal exposures for cigarette use in offspring, because mothers who used during pregnancy but quit by 6 years postpartum still had offspring who were 3.5 times more likely to smoke than non/infrequent users. Other researchers have found long-term effects of prenatal cigarette exposure on tobacco outcomes and other drug use in offspring. 8 , 11–14 However, they were not able to also account for maternal postnatal smoking patterns. Surprisingly, the adolescent offspring of chronic smokers were not significantly more likely to be smokers. Chronic maternal cigarette use was highly correlated with white race, alcohol and marijuana use in offspring and once these covariates were entered into the multivariate model, the chronic maternal cigarette user class only marginally predicted offspring smoking.
In this study, we included many possible confounders of the association between gestational exposure to cigarettes and adolescent smoking, such as maternal age, race, socioeconomic status, and parenting style. We also controlled for other adolescent substance use, in case prenatal exposure conferred a more general risk for substance use in offspring. Nonetheless, there are other differences between mothers who do and do not smoke during pregnancy that were not considered in these analyses, such as prior history of conduct disorder and nicotine dependence. 20 Future work will include mediating models examining differences in maternal psychological status and exposed offspring’s childhood attention and behavior problems. More work is clearly needed to elucidate the mechanisms linking prenatal exposure to cigarette use during adolescence.
Our measurement of maternal smoking was prospective, unlike many other studies of maternal smoking that include retrospective data on smoking during pregnancy. However, our data remain subject to recall bias because women were asked to remember past-year smoking at each of the postnatal visits. Smoking during pregnancy was measured for first and third trimesters, another strength of the study. However, other investigations of maternal smoking should focus on attempted quit patterns, because many smokers decrease use and make multiple quit attempts, especially during pregnancy. 21–23 We examined maternal and not paternal smoking, because the literature highlights the primacy of maternal smoking, and fathers were less often the primary caregiver in this sample. Nonetheless, it is also important to consider other smokers in the environment. Both maternal and offspring substance use were self-report, capturing a wider window of use than is possible using biomechanical confirmation of use. Nonetheless, social desirability may bias results based on self-report. Another limitation is that these results may not generalize to mothers from rural areas or from racial/ethnic backgrounds, such as Asian-American, Hispanic, and Native American mothers. However, black mothers were well represented, which is crucial because black communities have been targeted by tobacco manufacturers, and black Americans are more likely to suffer disease and to die from smoking-related causes. 24
Funding
This study was funded by the National Institutes of Health (NIH). The original data collection was funded by the National Institute on Alcohol Abuse and Alcoholism (AA06390—PI: NLD; AA08284—PI: MDC) and the National Institute on Drug Abuse (DA03874—PI: NLD; DA09275—PI: MDC). The secondary data analysis presented in the manuscript was funded by the National Institute on Drug Abuse (DA037209—PI: NMDG).
Declaration of Interests
The authors have no conflicts of interest to disclose. This manuscript was not reviewed by the sponsor prior to submission. This manuscript is not and will not be under review by another publication while it is being considered by Nicotine & Tobacco Research.
References
- 1. White HR, Pandina RJ, Chen PH . Developmental trajectories of cigarette use from early adolescence into young adulthood . Drug Alcohol Depend . 2002. ; 265 ( 2 ): 167 – 178 . doi: 10.1016/S0376-8716(01)00159-4 . [DOI] [PubMed] [Google Scholar]
- 2. Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—pregnancy risk assessment monitoring system, United States, 40 sites, 2000–2010 . Morb Mortal Wkly Rep (MMWR) . 2013. ; 62 ( SS06 ): 1 – 19 . [PubMed] [Google Scholar]
- 3. Center for Disease Control . Pregnancy Risk Assessment and Monitoring System—2011, Division of Reproductive Health . Atlanta, GA: : National Center for Chronic Disease Prevention and Health Promotion; ; 2015. . [Google Scholar]
- 4. Cornelius M, Day N . Developmental consequences of prenatal tobacco exposure . Curr Opin Neurol . 2009. ; 22 ( 2 ): 121 – 125 . doi: 10.1097/WCO.0b013e328326f6dc . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slotkin T . If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol . 2008. ; 30 ( 1 ): 1 – 19 . doi: 10.1016/j.ntt.2007.03.039 . [DOI] [PubMed] [Google Scholar]
- 6. Escario JJ, Wilkinson AV . The intergenerational transmission of smoking across three cohabitant generations: a count data approach . J Community Health . 2015. ; 40 ( 5 ): 912 – 919 . doi: 10.1007/s10900-015-0013-5 . [DOI] [PubMed] [Google Scholar]
- 7. Leonardi-Bee J, Jere ML, Britton J . Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis . Thorax . 2011. ; 66 ( 10 ): 847 – 855 . doi: 10.1136/thx.2010.153379 . [DOI] [PubMed] [Google Scholar]
- 8. Buka SL, Shenassa ED, Niaura R . Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study . Am J Psychiatry . 2003. ; 160 ( 11 ): 1978 – 1984 . [DOI] [PubMed] [Google Scholar]
- 9. Cornelius M, Leech S, Goldschmidt L, Day N . Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res . 2000. ; 2 ( 1 ): 45 – 52 . doi: 10.1080/14622200050011295 . [DOI] [PubMed] [Google Scholar]
- 10. Cornelius M, Leech S, Goldschmidt L, Day N . Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study . Neurotoxicol Teratol . 2005. ; 4 ( 4 ): 667 – 676 . doi: 10.1016/j.ntt.2005.05.006 . [DOI] [PubMed] [Google Scholar]
- 11. Lotfipour S, Ferguson E, Leonard G, et al. Maternal cigarette smoking during pregnancy predicts drug use via externalizing behavior in two community-based samples of adolescents . Addiction . 2014. ; 109 ( 10 ): 1718 – 1729 . doi: 10.1111/add.12665 . [DOI] [PubMed] [Google Scholar]
- 12. O’Brien J, Hill S . Effects of prenatal alcohol and cigarette exposure on offspring substance use in multiplex, alcohol-dependent families . Alcohol Clin Exp Res . 2014. ; 8 ( 12 ): 2952 – 2961 . doi: 10.1111/acer.12569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rydell M, Magnusson C, Cnattingius S, Granath F, Svensson A, Galanti M . Exposure to maternal smoking during pregnancy as a risk factor for tobacco use in adult offspring . Am J Epidemiol . 2014. ; 179 ( 12 ): 1409 – 1141 . doi: 10.1093/aje/kwu074 . [DOI] [PubMed] [Google Scholar]
- 14. O’Callaghan FV, Mamun A, O’Callaghan et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults-a birth cohort study . Aust N Z J Public Health . 2009. ; 33 ( 4 ): 371 – 377 . doi: 10.1111/j.1753-6405.2009.00410.x . [DOI] [PubMed] [Google Scholar]
- 15. Curran PJ, Hussong AM . Integrative data analysis: the simultaneous analysis of multiple data sets . Psychol Methods . 2009. ; 14 ( 2 ): 81 – 100 . doi: 10.1037/a0015914 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinberg L, Lamborn S, Dornbusch S, Darling N . Impact of parenting practices on adolescent achievement: authoritative parenting, school involvement, and encouragement to succeed . Child Dev . 1992. ; 63 ( 5 ): 1266 – 1281 . doi: 10.2307/1131532 . [DOI] [PubMed] [Google Scholar]
- 17. Muthén B, Muthén LK . Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes . Alcohol Clin Exp Res . 2000. ; 24 ( 6 ): 882 – 891 . doi: 10.1111/j.1530-0277.2000.tb02070.x [PubMed] [Google Scholar]
- 18. Nylund KL, Asparouhov T, Muthén B . Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study . Structural Equation Modeling . 2007. ; 14 ( 4 ): 535 – 569 . [Google Scholar]
- 19. Lo Y, Mendell NR, Rubin DB . Testing the number of components in a normal mixture . Biometrika . 2001. ; 8 ( 3 ): 767 – 778 . doi: 10.1093/biomet/88.3.767 [Google Scholar]
- 20. Massey SH, Compton MT . Psychological differences between smokers who spontaneously quit during pregnancy and those who do not: a review of observational studies and directions for future research . Nicotine Tob Res . 2013. ; 15 ( 2 ): 307 – 319 . doi: 10.1093/ntr/nts142 . [DOI] [PubMed] [Google Scholar]
- 21. Eiden RD, Homish GG, Colder CR, et al. Changes in smoking patterns during pregnancy . Subst Use Misuse . 2013. ; 48 ( 7 ): 513 – 533 . doi: 10.3109/10826084.2013.787091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pickett KE, Wakschlag LS, Dai L, Leventhal BL . Fluctuations of maternal smoking during pregnancy . Obstet Gynecol . 2003. ; 101 ( 1 ): 140 – 147 . [DOI] [PubMed] [Google Scholar]
- 23. Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R . Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy . Paediatr Perinat Epidemiol . 2005. ; 19 ( 5 ): 368 – 376 . doi: 10.1111/j.1365-3016.2005.00660.x . [DOI] [PubMed] [Google Scholar]
- 24. US Department of Health and Human Services . Tobacco Use Among U.S. Racial/Ethnic Minority Groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General . Washington, DC: Department of Health and Human Services; 1998. . [PubMed] [Google Scholar]