Abstract
Physical activity plays an important role in preventing muscle atrophy and chronic diseases in adults and in the elderly. Calcium (Ca2+) cycling and activation of specific molecular pathways are essential in contraction-induced muscle adaptation. This study attains human muscle sections and total homogenates prepared from biopsies obtained before (control) and after 9 weeks of training by electrical stimulation (ES) on a group of volunteers. The aim of the study was to investigate about the molecular mechanisms that support functional muscle improvement by ES. Evidences of kinase/phosphatase pathways activation after ES were obtained. Moreover, expression of Sarcalumenin, Calsequestrin and sarco/endoplasmic reticulum Ca2+-ATPase (Serca) isoforms was regulated by training. In conclusion, this work shows that neuromuscular ES applied to vastus lateralis muscle of sedentary seniors combines fiber remodeling with activation of Ca2+-Calmodulin molecular pathways and modulation of key Ca2+-handling proteins.
Keywords: aging, muscle remodeling, electrical stimulation, Ca2+-handling proteins, NFAT
Introduction
Established effects of aging on skeletal muscle are reduction in muscle fiber size, fiber loss, denervation and impaired reinnervation, and excitation–contraction uncoupling. These features are hallmarks of muscle functional decline, possibly but not only related to unloading due to progressive disuse (Cruz-Jentoft et al., 2010; Payne & Delbono, 2004; Scicchitano, Rizzuto, & Musarò, 2009; Vinciguerra, Musarò, & Rosenthal, 2010). It is still debated whether low muscle activity due to age-related pathological conditions contributes to sarcopenia. Another factor affecting muscle performance in elderly is early fatigue development because of muscle weakness as muscles have to work near to maximal capacity eliciting delayed relaxation. Modulation of these aging processes is possible by physical exercise (Paffenbarger et al., 1994; Sakuma & Yamaguchi, 2010) that affects both at muscle and neural levels. Neuromuscular control and adaptation following prolonged inactivity is counteracted by specific exercise (Salanova et al., 2011; Salanova et al., 2013). In seniors, physical exercise also preserves muscle morphology, guarantees a greater maximal isometric force and function, and modulates the expression of genes related to autophagy and reactive oxygen species detoxification (Mosole et al., 2014; Zampieri et al., 2015). Lifelong physical activity and exercise training are also beneficial for insulin resistance and glucose regulation in humans (Biensø et al., 2015; Bunprajun, Henriksen, Scheele, Pedersen, & Green, 2013). On the contrary, pathologic conditions and aging per se limit the feasibility of physical training. Electrical stimulation (ES) is an alternative to improve muscle performance (Strasser et al., 2009). ES has been used for rehabilitation purposes to counteract neuromuscular disabilities ranging from age-related neuromuscular junction (NMJ) degeneration to paraplegic patients (Kern et al., 2010; Maddocks, Gao, Higginson, & Wilcock, 2013). Recently it was demonstrated that ES improves muscle performance and mimics some beneficial effects of regular physical exercise in muscle of aging people increasing the size and number of fast muscle fibers, stimulating satellite cells, and modulating mitochondrial apparatus leading to improvement of physical abilities (Kern et al., 2014; Zampieri et al., 2016).
During aging, deterioration of the mechanism that converts motoneuron firing to muscle contraction in skeletal muscle (excitation–contraction coupling [EC-coupling]) contributes to force decline and leads to utilization of external Calcium (Ca2+) as an alternative source (Boncompagni, d’Amelio, Fulle, Fanò, & Protasi, 2006; Carnio et al., 2014; Payne & Delbono, 2004). At molecular level, Ca2+ plays an essential role in metabolic and structural adaptation of skeletal muscle via activation of signaling pathways which decode a specific Ca2+ signal. Both Ca/Calmodulin(CaM)-dependent kinase and phosphatase pathways are involved in muscle adaptation to exercise and neuromuscular activity, mainly through fiber-type maintenance or switching, mitochondria plasticity, and metabolic modifications (Chin, 2005; Flück, Waxham, Hamilton, & Booth, 2000). These adaptation mechanisms are key targets of an effective training protocol. Phosphorylation of CamKII is a marker of Ca/Calmodulin(CaM)-dependent kinase pathway activation especially in muscle (Flück et al., 2000). Different isoforms of CamKII have been reported in human skeletal muscle (Rose & Hargreaves, 2003; Rose, Kiens, & Richter, 2006) with different Ca2+ sensitivity and subcellular localization. Both CamKII γδ and βM isoforms have been associated to sarcoplasmic reticulum (SR) membranes by αKaps specific anchors (Nori et al., 2003).
The Calcineurin-NFAT (part of CaM-dependent phosphatase signaling) is an important pathway involved in excitation–transcription coupling (Rana, Gundersen, & Buonanno, 2009), modification of fiber type (Allen & Leinwand, 2002; Ehlers, Celona, & Black, 2014; Hudson & Price, 2013; Serrano et al., 2001), increase of muscle mass (Musarò, McCullagh, Naya, Olson, & Rosenthal, 1999; Vechetti et al., 2013), and NMJ maintenance (Angus et al., 2005; T. V. Cohen & Randall, 2004). Activation of this pathway starts with phosphorylation-dependent cytoplasm to nuclei translocation of NFAT (nuclear factor of activated T cells) transcription factors. NFATc1 is a key transcription factor in muscle adaptation to different stimuli. Over expression in vitro and in vivo of NFATc1 point out that nuclear translocation is promoted both in insulin/insulin-like growth factor (IGF-1) induced muscle hypertrophy (Musarò et al., 1999; Salanova et al., 2013), and switch from fast to slow phenotype (Liu, Cseresnyés, Randall, & Schneider, 2001; Tothova et al., 2006). NFATc1 is expressed in fast and slow fibers, coexpressed with MyoD, and maintains fiber type composition by repressing MyoD-dependent gene activation (Ehlers et al., 2014). In absence of NFATc1, mice muscles do not undergo fiber type switching in response to exercise (Ehlers et al., 2014).
The aim of this study is to extend the knowledge on the molecular pathways triggered by ES exploring the activated downstream targets, which ameliorate muscle quality.
Method
Samples: Muscle Biopsies Used in the Study
Biopsies obtained from 15 subjects (eight males and seven females, mean age 71.8 ± 3.5 years) were randomly selected from a group described in a previous study (Kern et al., 2014). Briefly, all the subjects were sedentary people (they did not perform recreational physical activity), who signed an informed consent and received information about the functional test protocols, the trainings, and muscle biopsies. All subjects included were healthy, declared not to have any physical/disease issue, and were instructed to maintain their normal daily activities during the training period. Samples were obtained from the vastus lateralis (VL) of subjects trained with ES as described (Kern et al., 2014; Zampieri et al., 2016). The biopsies before training were taken 10 days after the initial assessment and ES started 14 days later. A peculiar aspect of this study is that posttraining biopsies were taken 7 days after the last training session to reduce effects due to acute responses. Approval from the national committee for medical ethics was obtained at the beginning of the study (EK08-102-0608) ClinicalTryals. govNCT01679977.
ES Training
Subjects were exposed to neuromuscular ES seated over the edge of a table with the trunk upright and lower legs freely swinging. Stimulation started when the knee was at 90°C. The training was performed for a total of 24 sessions (3×10 min each session) in 9 weeks as described (Kern et al., 2014). Additional ankle weights were also used starting from the third week onward, increasing the weight from 1 to 2.5 kg. A two-channel custom-built battery-powered stimulator specifically designed for elderly people with impaired cognitive and functional abilities was used with large rubber electrodes (each 9 × 14 cm; 126 cm2) covered by wet sponge and placed to the skin on each anterior thigh (Krenn et al., 2011). Constant voltage stimulation was applied (Kern et al., 2014); the mean stimulation current was 128 ± 16 mA and voltage 39 ± 14 V. The intensity of the ES training was about 40% of the maximal voluntary contraction (Sarabon, Loefler, Cvecka, Sedliak, & Kern, 2013). With this intensity, all of the subjects achieved full knee extension, even with loaded legs. The discussed system was intended for evoked muscle training of the anterior and posterior thigh.
Immunofluorescence Analysis
For each sample, serial cryosections (8 µm thick) were mounted on glass slides, rehydrated in phosphate-buffered saline (PBS), permeabilized with a 0.1% Triton in PBS for 15 min, blocked with blocking buffer (10% fetal bovine serum in PBS) for 30 min to avoid nonspecific staining and finally incubated 1 hr at room temperature (RT) with anti-Myosin Heavy Chain Fast (pan MHC Fast) mouse monoclonal antibody (1:50 AbCam, Cambridge, UK) and anti-Serca2 (sarco/endoplasmic reticulum Ca2+-ATPase) goat polyclonal antibody (1:200 Santa Cruz, Dallas, TX, USA) mixed together in PBS. Sections were then incubated for 1 hr at RT with Alexa Fluor®594 and Alexa Fluor®488 dye conjugated antibodies against mouse or goat IgG (1:200, ThermoFisher Scientific, Waltham, MA, USA). Sections were then mounted with a cover glass using ProLong Gold antifade reagent with 4’, 6-diamidin-2-fenilindolo (DAPI) (ThermoFisher Scientific, Waltham, MA, USA). Photos were taken on a Leica DMR Epifluorescence microscope (Wetzlar, Germany) and assembled to obtain the total coverage of the biopsy section (about 10 pictures at 20× magnification per sample were sufficient to cover the section). For quantitative analysis, two replicas were performed, and the average was calculated for each sample.
For NFATc1 staining (1:200 ThermoFisher Scientific, Waltham, MA, USA), the same protocol was used. Five randomly selected pictures (40× magnification around 100 fibers total) were analyzed for each sample.
Homogenates
Whole homogenates were prepared from 10 cryosections (20 μm each) for each sample. Tissues were homogenized with a Teflon pestle equipped Potter-Elvehjem Tissue grinder in the presence of a medium containing 3% (wt/v) sodium dodecyl sulfate (SDS), 0.1 mm EGTA, pH 7.0 in the presence of 0.2 mM PMSF (phenylmethanesulfonyl fluoride, Sigma-Aldrich, Saint Louis, MO, USA) and 0.8 mM benzamidine (Sigma-Aldrich, Saint Louis, MO, USA), until the sections were completely dissolved. Homogenates were then boiled for 5 min and clarified at 15,000 g for 10 min. Supernatants were used as whole protein extracts.
Protein Analysis
Protein concentration was determined by bicinchoninic acid protein assay (ThermoFisher Scientific, Waltham, MA, USA) using bovine serum albumin as standard. Tissue homogenates (5-10 μg/lane) were separated on 7.5% or 10% Sodium dodecyl sulphate - polyacrylamide gel electrophoresis and then electroblotted onto nitrocellulose membrane. In each gel, prestained protein markers (Prestained SDS-PAGE Standards, dual color; Bio-Rad, Hercules, CA, USA) were also loaded. The integrity of the western blot was analyzed by Red Ponceau staining (Sigma-Aldrich, Saint Louis, MO, USA). Destained membranes were blocked with 10% (v/v) skimmed milk in PBS-Tween 0.05% for 3 hours and primary antibodies in PBS-Tween 0.05% containing 2% (v/v) skimmed milk for 2 hr at 20°C. Immunostained proteins were detected with a horseradish peroxidase–labeled secondary antibody and visualized by Pierce™ ECL Western Blotting Substrate (ThermoFisher Scientific, Waltham, MA, USA) for CamKII or with an alkaline phosphatase–labeled secondary antibody and visualized by using the 5-bromo-4-chloro-3-indolyl phosphate/nitro-blue tetrazolium system for other proteins. Apparent molecular weight (MW) of the proteins were calculated from a graph of relative mobility versus log MW of standard proteins. Densitometric analysis was performed from six gels containing all samples. Actin signal was used to normalize protein loading, and normalization among gels was made by loading a common sample in all gels analyzed.
Working dilution of primary antibodies was anti-pCamKII (1:1000 Cell Signaling Technology, Leiden, The Netherlands), Serca2 (1:1500 Santa Cruz, Dallas, TX, USA), Sarcalumenin (SRL; 1:200 Santa Cruz, Dallas, TX, USA), and Calsequestrin 2 (1:2000 ThermoFisher Scientific, Waltham, MA, USA).
Serca2, SRL,, and Actin were detected on the same gel by cutting the nitrocellulose membranes at proper MW, as well as Calsequestrin 1 (Casq1) and Actin on a second gel. To analyze all the samples, eight mini gels were loaded. Normalization among gels was made by loading a common sample for all gels analyzed.
RNA Analysis
RNA was isolated from cryostatic sections of VL muscles biopsies and transcribed in complementary DNA (cDNA) as previously reported (Chiarello, Bortoloso, Carpi, Furlan, & Volpe, 2013). Specific primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH ), sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (ATP2A2), SRL, and Casq1 cDNAs were designed with the Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA, USA; http://frodo.wi.mit.edu/), and their thermodynamic specificity was determined using basic local alignment search tool sequence alignments (U.S. National Center for Biotechnology Information [NCBI], Bethesda, MD, USA) and Vector NTI® Software (ThermoFisher Scientific, Waltham, MA, USA). All primer sets spanned an exon–exon junction to avoid errors due to contaminating genomic DNA. Quantitative PCR was performed in duplicate in a iQ5 Thermal Cycler (Bio-Rad, Hercules, CA, USA) using SYBR Green chemistry.
All samples were run simultaneously with RNA- and RT-negative controls. Normalization was performed by the delta CT method using GAPDH as reference gene. Data are expressed as means ± standard error of the mean.
Primers sequences were as follows:
ATP2A2 FW 5′-ACAGCTATGACTGGCGATGG-3′
RV 5′-AGCGGATGAACTGTTTCATGT-3′
SRL FW 5′-AGCTGATTGGCATTGAGGTT-3′
RV 5′-GACCCACATCCAGCTTTGTT-3′
Casq1 FW 5′-GGAGCACAGGAGATCAACCC-3′
RV 5′-GGGTCAATCCAGATGATGCT-3′
GAPDH FW 5′-CACCATCTTCCAGGAGCGAG-3′
RV 5′-TTCACACCCATGACGAACAT-3′
Statistical Analysis
Quantitative evaluation of NFATc1-positive nuclei, mixed MHCII/Serca2 fibers, and expression of pCamKII was performed by one-way analysis of variance (ANOVA) test with Bonferroni correction (values of p < .05 were considered significant).
For protein expression, nonparametric matched Wilcoxon test (W significative * for p< .05) was performed except for SRL which satisfied the criteria for paired Student t test (δ = p < .05).
Results
ES Triggers Calcineurin-NFAT and CaMKII Pathways in Muscle
Kinase and phosphatase intracellular pathways are essential for muscle adaptation to multiple contractile stimuli. We asked if ES trigger NFATc1 and CaMKII pathways that act as nerve-activity sensors or Ca2+ decoders. Translocation of NFATc1 (also known as NFATc or NFAT2) and phosphorylation of CamKII were chosen as indicators of pathway activation.
Endogenous expression of NFATc1 in pre trained muscle sections
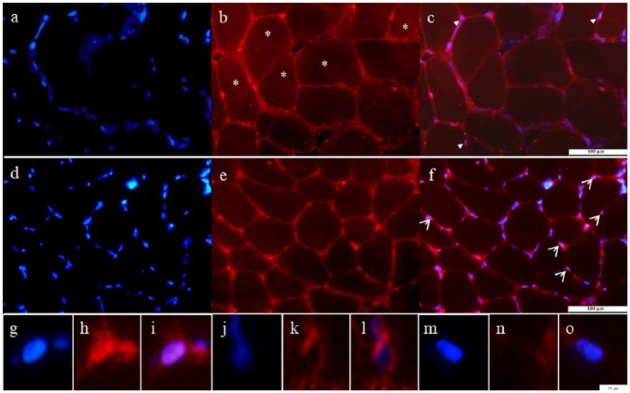
In Figure 1, representative sections of human biopsies obtained from VL muscles pre (control, Panels a-c) and post (Panels d-f) training are shown. In pretrained muscles, NFATc1 was localized at sarcolemmal level. Few fibers show focal regions that stain intensely for NFATc1 (Panel b). Rare foci colocalize with nuclei identified by DAPI staining (blue); for the quantitative analyses, these nuclei were classified as NFATc1 positive (arrowhead in Panel c and for more details see also Panels g-i). In some areas, sarcolemmal red staining surrounds nuclei instead of colocalize with DAPI, and other nuclei are completely negative; in both forms, nuclei were classified as negative (see also negative nuclei at high magnification in Panels j-o).
Figure 1.
Endogenous expression of NFATc1 in pre- and posttrained muscles.
Note. Representative transversal sections of pretrained (a-c) and posttrained (d-f) muscles stained by anti-NFATc1 (red) and counterstained by 4’,6-diamidino-2-phenylindole (DAPI blue) are shown. Arrowheads indicate examples of NFATc1 nuclear localization. Bar 100 µm. Panels g to o are examples of one positive (g-i) and two negative (j-o) nuclei at higher magnification. Bar 10 μm. NFAT = nuclear factor of activated T cells.
*points to fibers with faint cytoplasmic staining.
Nuclear translocation of NFATc1
After ES (Figure 1, Panels d-f), the staining at sarcolemmal level changed. The most visible features were the increase of peripheral foci at sarcolemmal level, as shown in Panel e, which frequently colocalize with nuclei (some representative examples are shown by arrowheads in merged Panel f). These observations indicate that NFATc1 increases at periphery of fibers and that many nuclei positive for NFATc1 were easily detectable after training. The quantitative evaluation was performed calculating the fraction of positive NFATc1 over total nuclei counted in five randomly selected pictures for each muscle section. One section for each sample was analyzed and about 300 nuclei per sample were counted. As shown in Table 1, after ES, significant increase (on average from 2% to more than 50%) of NFATc1-positive nuclei was detectable. Taken together, these data show a significant increase of NFATc1-positive nuclei after trainings and strongly suggest that ES induces nuclear translocation of NFATc1 indicating that calcineurin (CaN)-NFAT pathway is triggered by training.
Table 1.
Evaluation of NFATc1 Nuclear Translocation.
| Positive nuclei | Negative nuclei | Total nuclei | % Nuclei NFATc1 positive | |
|---|---|---|---|---|
| Pre (n = 15) | 5.0 ± 0.3 | 264.7 ± 3.6 | 269.7 ± 3.7 | 1.9 ± 0.5 |
| Post (n = 15) | 151.7 ± 3.0 | 127.3 ± 1.9 | 279.0 ± 3.7 | 54.4 ± 2.7** |
Note. Quantitative evaluation of NFATc1-positive and NFATc1-negative nuclei counted in transversal sections of pre- and posttraining muscles. Nuclei were counted from 15 subjects; five randomly selected pictures were analyzed for each transversal section. Statistical comparison was performed by one-way analysis of variance test with Bonferroni correction. Note the highly significant increase of positive nuclei after ES trainings (**p < .001). Data are presented as means ± SE. NFAT = nuclear factor of activated T cells; ES = electrical stimulation.
Activation of CamKII
Phosphor CamKII (pCamKII) content was tested in total homogenates obtained from biopsies of pre- and posttraining muscles by western blotting. Protein profile of crude homogenates is shown in Figure 2a. Qualitative comparison among lanes shows that protein patterns are identical. The anti pCamK antibody recognizes a single band around 50 kDa (Figure 2b). The densitometric quantitative analysis (Figure 2c) shows a significant increase of pCamKII in homogenates obtained after ES. As the activation of CamK occurs via phosphorylation, these findings indicate that ES activates the kinase pathway.
Figure 2.
Quantitative evaluation of pCamkII expression in total homogenates of pre- and posttrained muscles.
Note. (a) Red Ponceau stained protein profile of total homogenates (10 μg per lane) from a representative subject before and after training. (b) Immunoblot with anti-pCamkII of the same gel in the area between 75 and 45 kDa. (c) Mean ratio pCamkII/ Red Ponceau stained Actin values and respective standard errors are plotted. Pretreated values were set to one. Significant differences (***p < .001, analysis of variance test with Bonferroni correction) were observed between pretreated and posttreated muscles. pCamkII = Ca2+/calmodulin-dependent protein kinase. ES = electrical stimulation.
Protein analysis
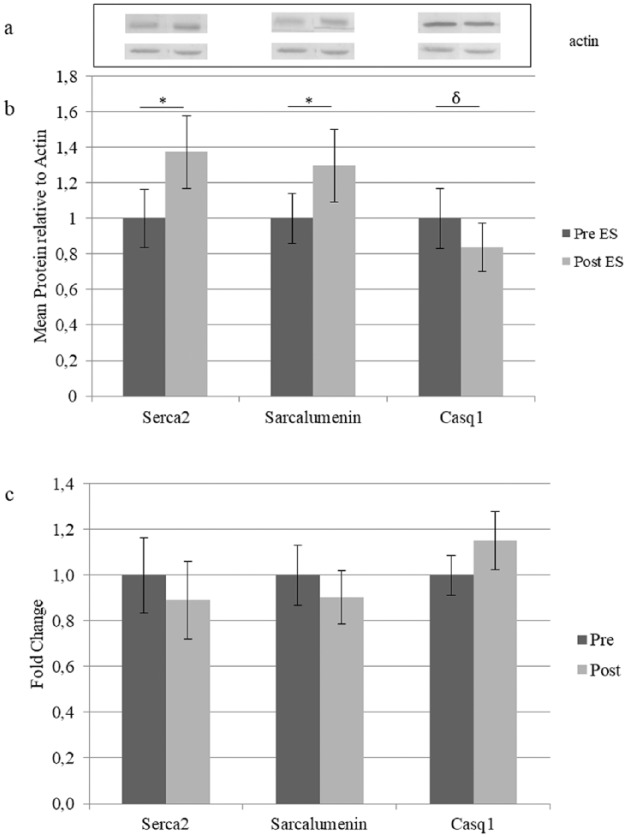
Quantitative comparison between pre- and post-training samples shows a significant increase of Serca2 and SRL and a decrease of Casq1 after ES (Figure 3b). Two other probed proteins, Casq2 and Serca1, did not change (not shown). Quantitative PCR was performed to clarify whether the RNA level of SRL Serca2 and Casq1 are regulated upon ES and shows that none of the genes was up- or down-regulated significantly by ES training (Figure 3c).
Figure 3.
Expression of SR Ca2+ handling proteins.
Note. (a) Upper part representative immunoblots obtained by antibodies specified in Figure 3b and lower part by Actin. Number of gels analyzed and replica are described in methods section. Pretreated values were set to one. Comparison between pre- and posttrained samples was performed by the nonparametric matched Wilcoxon test (W significative * for p < .05) except for Sarcalumenin which satisfied the criteria for paired Student’ T test (δ p < .05). (c) RNA analysis. Comparison between pre- and posttraining expression of indicated genes. Values are expressed as mean (n = 15) and vertical error bars represent SE; messenger RNA levels were normalized to GAPDH by the delta Ct method and then normalized to pre-ES samples which were set to one. SR = sarcoplasmic reticulum; ES = electrical stimulation.
Muscle Remodeling Upon ES: Increase of MHCII/ Serca2 Mixed Fibers
In human VL muscle, Serca2 is expressed not only in slow-twitch fibers, but also in fast oxidative fibers (Lamboley, Murphy, McKenna, & Lamb, 2014). To test if Serca2 increase is fiber-type specific, the extent of coexpression of MHCII and Serca2 was studied by immunofluorescence in muscle cross sections obtained before and after ES.
The two cytoplasmic signals (Serca2 and MHCII) segregate separately in roughly 50% of the fibers both before and after ES, while the sarcolemmal green signal (laminin) is detectable in all fibers, as expected. In Figure 4, a representative panel of muscle sections triple stained by anti-Serca2 (green), laminin (green) and MHCII (red) antibodies is shown. Occasionally fibers costained in red and green were observed (see for example Panels c, f asterisks). This observation confirms that the majority of fast-twitch fibers (red) do not express Serca2, green (see for example Panels h, k) and that a small population of mixed (MHCII/Serca2) fibers is present both before and after ES. The quantitative analysis of mixed fibers was performed in whole sections (Table 2). Two independent operators with similar results manually counted about 800 fibers for each section. In muscles before ES training, mixed MHCII/ Serca2 fibers were detected (1.6%) in agreement with single fiber biochemical analysis performed by others by which a small percentage (less than 5%) of mixed MHCII/MHCI/Serca1/Serca2 fibers has been described (Lamboley et al., 2014). After ES, the number of mixed MHCII/Serca2 fibers tripled. Morphology of mixed fibers was heterogeneous, some were small and angular (Figure 4, Panels a-c) others were large rounded (Figure 4, Panels d-f). Mixed fibers were not centronucleated as indicated by DAPI counterstaining of nuclei (not shown).
Figure 4.
Identification and morphology of mixed MHCII/Serca2 fibers.
Note. Transversal sections of muscle biopsies from post-electrostimulated muscles triple stained by anti-MHCII (red), anti-Serca2 (green), and anti-laminin (green). Note two mixed fibers of different morphology labeled with asterisks in merge Panels c and f. Δ indicates empty areas of the sections. MHC = Myosin Heavy Chain; Serca = sarco/endoplasmic reticulum Ca2+-ATPase.
Table 2.
Evaluation of Mixed Fibers.
| Total fibers | Fast fibers (MHCII) | MHCII/ Serca2 mixed fibers | % mixed/fast | % mixed/total | |
|---|---|---|---|---|---|
| Pre- (n = 15) | 751.2±87.7 | 433.8±54.8 | 12.2±3.3 | 2.8±0.6 | 1.6±0.3 |
| Post- (n = 15) | 864.5±84.5 | 523.5±71.2 | 44.7±10.4 | 8.8±1.5* | 5.2±1.4* |
Note. Quantitative evaluation of mixed MHCII/Serca2 fibers was performed by evaluation of one intact transversal section for each subject pre- and post-training into two replica. Comparison among the four groups was performed by analysis of variance test with Bonferroni correction. Note the significant increase (*p < .05) of mixed fibers after ES. Data are presented as means ± SE. MHC = Myosin Heavy Chain; ES = electrical stimulation. Serca = sarco/endoplasmic reticulum Ca2+-ATPase.
Taken together, these data show that a population of fast-twitch fibers, which express Serca2, increases after 9 weeks of ES. Another observation on the heterogeneous nature of the mixed fibers is that some of them present lower fluorescence intensity with anti-MHCII than the surrounding fast fibers, (see for example the central fiber of Figure 4, Panel d). Comparing the MHCII fluorescence intensity among fast-twitch fibers of pre- and posttraining whole sections, we found that 1.4% and 2.9% of fast-twitch fibers present a lower MHCII signal in pre and post ES sections, respectively (fibers presenting less than 50% of the MHCII signal were included); about 50% of those fibers were also Serca2 positive.
Discussion
This article shows the ability of ES protocol to trigger Ca2+-calmodulin molecular pathways involved in muscle adaptation and their effect on expression of Ca2+ handling proteins and activation of molecular pathways involved in muscle adaptation.
One major finding is the massive significant increase of NFATc1-positive nuclei and increase of pCamKII after ES. These cellular responses are clear signs of activation of two pathways involved in muscle adaptation and remodeling, Calcineurin-NFAT and Ca2+/calmodulin-dependent protein kinase (CamKII), respectively, both triggered upon 9 weeks of ES training.
Benefits From Calcineurin-NFAT Pathway Activation by ES
It is known that in mouse nuclear translocation of NFATc1 promotes muscle hypertrophy and fiber type switching after exercise (Ehlers et al., 2014). In human Soleus and VL muscles, NFATc1 nuclear translocation is induced after resistive/vibration exercise performed 3 times a week during 60 days of bed rest (Salanova et al., 2011). In the present work, we found that muscles of sedentary seniors, before training, show few (< 3%) NFATc1-positive nuclei, a strong evidence that VL muscle is very poorly activated in these subjects as an effect of their sedentary lifestyle. On the contrary, massive (>50%) increase of NFATc1-positive nuclei occurred after ES, as a demonstration that the ES training protocol is suitable to activate muscle responses similar to those of free volitional exercise.
Of notice is that muscle biopsies were collected 7 days after discontinuation of the ES last boots, revealing that activation of NFATc1 pathway was not a transient event strictly related to muscle tension induced by muscle contraction, but was stabilized days after sustained contraction induced by ES. This is to our knowledge the first observation of a long-lasting NFATc1 activation in human muscle. This finding meets up with the significant increase of IGF-1 expression, recently found in the same muscles (Kern et al., 2010; Zampieri et al., 2016) which indicates activation of IGF-1/Akt pathway known to inhibit NFATc nuclear export to cytoplasm (Crabtree & Schreiber, 2009; Shen, Cseresnyés, Liu, Randall, & Schneider, 2007).
CamKII Pathway
Increase of pCamKII after ES is marker of activation of a strategic pathway involved in muscle growth and plasticity. For example, pCamKII activity remains elevated and Ca2+ sensitive after prolonged contraction induced by exercise (Flück et al., 2000). CamKII is activated in humans by exercise (Rose & Hargreaves, 2003) promoting histone modifications and increase of GLUT4 messenger RNA (McGee, Fairlie, Garnham, & Hargreaves, 2009; Richter & Hargreaves, 2013). CamKII is a component of Neuro Muscular Junction (T. J. Cohen et al., 2007) and a primary decoder of neuromuscular activity (Tavi & Westerblad, 2011) that in response to nerve activity phosphorylates HDAC4 and promotes regulation of the myogenic transcription factor Mef2 (T. J. Cohen et al., 2009). These data show that activation of CamKII by ES protocol mimics exercise and comes to be another benefit for inactive old muscles.
Adaptation of SR Ca2+ Handling Proteins by ES
A recent study (Gueugneau et al., 2014) compares the proteome of active postmenopausal adult and old women VL muscle. Among differentially expressed Ca2+ handling proteins, these authors found that Serca2 and SRL were downregulated, whereas Casq1 was upregulated in old muscles. Interestingly we found a significant opposite trend after ES. Both modifications of SR proteins, upregulation of Serca2/SRL and downregulation of Casq1, demonstrate that ES counteracts some of the muscle aging-related protein shifts. The effect of ES on Serca2 and SRL expression mimics voluntary exercise training. In particular, Serca2a is upregulated in endurance and aerobic training of mouse and rat (Ferreira et al., 2010; Kinnunen & Mänttäri, 2012; Morissette et al., 2014; Thomas, Vigna, Betik, Tupling, & Hepple, 2010). In human VL Serca2 is downregulated in old sedentary versus young men, whereas in old trained is not different from young muscles (Klitgaard, Ausoni, & Damiani, 1989). We also show that, after ES, Serca2 and SRL RNA are unchanged indicating a posttranscriptional regulation of protein synthesis. This finding is consistent with downregulation of MuRF1 and Atrogin1, two genes of the ubiquitin–proteasome system, observed in the same muscles (Kern et al., 2014). Interestingly, the direct effect of ES on skeletal muscle protein synthesis rates was also demonstrated in elderly type 2 diabetic men with (Wall et al., 2012).
The functional advantage in upregulation of Serca2 lies on the lower SR-Ca2+ leak and higher Ca2+ sensitivity of Ca2+ uptake (Lamboley, Murphy, McKenna, & Lamb, 2013; Lamboley et al., 2014) conferred to muscles by this isoform. Moreover, Serca2 increase can also account for a fully relaxation in mixed fibers despite the high sensitivity of the slow contractile proteins to Ca2+, as proposed by the same authors.
Increase of SRL observed after ES is related to the chaperone function for Serca2 and confirms that this is a physiological response of the muscles with the final effect of improving SR Ca2+ handling (Manring, Abreu, Brotto, Weisleder, & Brotto, 2014).
The notion that ES can mimic voluntary exercise is further sustained (and confirmed) by this work at level of SR Ca2+ handling proteins against aging and inactivity, which induce their downregulation.
ES Provokes Muscle Remodeling
Increase of mixed MHCII/Serca2 fibers is an index of fiber type switching after ES. The extreme heterogeneity of fiber type with respect to contractile myosin isoforms and SR proteins of VL does not allow identification of the original phenotype of these fibers. Considering that hypertrophy and increase of fast fibers have been observed in these subjects (Kern et al., 2014), a plausible interpretation is that mixed MHCII/Serca2 fibers are fast oxidative 2A type which increase in humans after endurance training (Klitgaard et al., 1990). The increase of fast oxidative fibers would be advantageous for muscle performance as they exhibit peculiar characteristics: They are fast fibers, but present the slowest shortening speed and utilize oxidative metabolism; therefore, they are relatively strong and fatigue resistant. We cannot exclude additional effects of mechanical load on muscle remodeling due ankle weights used during training as described in Methods section. This possibility rises from recent data by Eftestøl et al. (2016) that compared anesthetized rats subjected to high-load isometric versus high-velocity low-load eccentric training protocols. Their results show an increased hypertrophic response with the high-load isometric protocol of training. On the contrary, the authors demonstrate that major changes in fiber type distribution was load independent, suggesting that electrical signaling, rather than mechanosignaling, controls fiber type.
In conclusion, neuromodulation by ES delivers beneficial effects on muscle quality potentiating Ca2+ handling proteins and by increasing mixed fibers with characteristics potentially suitable to counteract muscle fatigue and weakness which impair daily living activities in healthy and potentially in chronic pathological conditions of old people.
Acknowledgments
The authors thank the IRRCS Fondazione Ospedale San Camillo, Venice, Italy for hospitality and scientific support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research funds from the University of Padova (ex 60%) and by the European Regional Development Fund—Cross Border Cooperation Program Slovakia—Austria 2007–2013 (Interreg-IVa), project Mobilität im Alter, MOBIL, N_00033 (partners: Ludwig Boltzmann Institute of Electrical Stimulation and Physical Rehabilitation, Austria, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria, and Faculty of Physical Education and Sports, Comenius University in Bratislava, Slovakia); Austrian national cofinancing of the Austrian Federal Ministry of Science and Research; Ludwig Boltzmann Society (Vienna, Austria).
ORCID iD: Alessandra Nori  https://orcid.org/0000-0003-3968-9450
https://orcid.org/0000-0003-3968-9450
References
- Allen D. L., Leinwand L. A. (2002). Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. Journal of Biological Chemistry, 277, 45323-45330. [DOI] [PubMed] [Google Scholar]
- Angus L. M., Chakkalakal J. V., Mejat A., Eibl J. K., Bélanger G., Megeney L. A., . . . Jasmin B. J. (2005). Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1a, drives utrophin gene expression at the neuromuscular junction. American Journal of Physiology Cell Physiology, 289, C908-C917. [DOI] [PubMed] [Google Scholar]
- Biensø R. S., Olesen J., Gliemann L., Schmidt J. F., Matzen M. S., Wojtaszewski J. F., . . . Pilegaard H. (2015). Effects of exercise training on regulation of skeletal muscle glucose metabolism in elderly men. Journal of Gerontology, Series A: Biological Sciences & Medical Sciences, 70, 866-872. [DOI] [PubMed] [Google Scholar]
- Boncompagni S., d’Amelio L., Fulle S., Fanò G., Protasi F. (2006). Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. Journal of Gerontology, Series A: Biological Sciences & Medical Sciences, 61, 995-1008. [DOI] [PubMed] [Google Scholar]
- Bunprajun T., Henriksen T. I., Scheele C., Pedersen B. K., Green C. J. (2013). Lifelong physical activity prevents aging-associated insulin resistance in human skeletal muscle myotubes via increased glucose transporter expression. PLoS ONE, 8(6), e66628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnio S., LoVerso F., Baraibar M. A., Longa E., Khan M. M., Maffei M., . . . Sandri M. (2014). Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Reports, 8, 1509-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C., Bortoloso E., Carpi A., Furlan S., Volpe P. (2013). Negative feedback regulation of Homer 1a on norepinephrine-dependent cardiac hypertrophy. Experimental Cell Research, 319, 1804-1814. [DOI] [PubMed] [Google Scholar]
- Chin E. R. (2005). Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. Journal of Applied Physiology, 99, 414-423. [DOI] [PubMed] [Google Scholar]
- Cohen T. J., Barrientos T., Hartman Z. C., Garvey S. M., Cox G. A., Yao T. P. (2009). The deacetylase HDAC4 controls myocyte enhancing factor-2-dependent structural gene expression in response to neural activity. FASEB Journal, 23, 99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T. J., Waddell D. S., Barrientos T., Lu Z., Feng G., Cox G. A., . . . Yao T. P. (2007). The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. Journal of Biological Chemistry, 282, 33752-33759. [DOI] [PubMed] [Google Scholar]
- Cohen T. V., Randall W. R. (2004). NFATc1 activates the acetylcholinesterase promoter in rat muscle. Journal of Neurochemistry, 90, 1059-1067. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Schreiber S. L. (2009). SnapShot: Ca2+-calcineurin-NFAT signaling. Cell, 138, 210-210.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft A. J., Baeyens J. P., Bauer J. M., Boirie Y., Cederholm T., Landi F., . . . Zamboni M. (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and Ageing, 39, 412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftestøl E., Egner I. M., Lunde I. G., Ellefsen S., Andersen T., Sjåland C., . . . Bruusgaard J. C. (2016). Increased hypertrophic response with increased mechanical load in skeletal muscles receiving identical activity patterns. American Journal of Physiology Cell Physiology, 311(4), C616-C629. [DOI] [PubMed] [Google Scholar]
- Ehlers M. L., Celona B., Black B. L. (2014). NFATc1 controls skeletal muscle fiber type and is a negative regulator of MyoD activity. Cell Reports, 8, 1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J. C. B., Bacurau A. V., Bueno C. R., Jr., Cunha T. C., Tanaka L. Y., Jardim M. A., . . . Brum P. C. (2010). Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice. Experimental Biology and Medicine, 235, 497-505. [DOI] [PubMed] [Google Scholar]
- Flück M., Waxham M. N., Hamilton M. T., Booth F. W. (2000). Skeletal muscle Ca2+-independent kinase activity increases during either hypertrophy or running. Journal of Applied Physiology, 88, 352-358. [DOI] [PubMed] [Google Scholar]
- Gueugneau M., Coudy-Gandilhon C., Gourbeyre O., Chambon C., Combaret L., Polge C., . . . Béchet D. (2014). Proteomics of muscle chronological ageing in post-menopausal women. BMC Genomics, 15, Article 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. B., Price S. R. (2013). Calcineurin: A poorly understood regulator of muscle mass. The International Journal of Biochemistry & Cell Biology, 45, 2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern H., Barberi L., Löfler S., Sbardella S., Burggraf S., Fruhmann H., . . . Musarò A. (2014). Electrical stimulation counteracts muscle decline in seniors. Frontiers in Aging Neuroscience, 6, 189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern H., Carraro U., Adami N., Biral D., Hofer C., Forstner C., . . . Zampieri S. (2010). Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabilitation & Neural Repair, 24, 709-721. [DOI] [PubMed] [Google Scholar]
- Kinnunen S., Mänttäri S. (2012). Specific effects of endurance and sprint training on protein expression of calsequestrin and SERCA in mouse skeletal muscle. Journal of Muscle Research and Cell Motility, 33, 123-130. [DOI] [PubMed] [Google Scholar]
- Klitgaard H., Ausoni S., Damiani E. (1989). Sarcoplasmic reticulum of human skeletal muscle: Age-related changes and effect of training. Acta Physiologica Scandinavica, 137, 23-31. [DOI] [PubMed] [Google Scholar]
- Klitgaard H., Bergman O., Betto R., Salviati G., Schiaffino S., Clausen T., Saltin B. (1990). Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflügers Archiv, 416, 470-472. [DOI] [PubMed] [Google Scholar]
- Krenn M., Haller M., Bijak M., Unger E., Hofer C., Kern H., Mayr W. (2011). Safe neuromuscular electrical stimulator designed for the elderly. Artificial Organs, 35, 253-256. [DOI] [PubMed] [Google Scholar]
- Lamboley C. R., Murphy R. M., McKenna M. J., Lamb G. D. (2013). Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. Journal of Physiology, 591, 6053-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley C. R., Murphy R. M., McKenna M. J., Lamb G. D. (2014). Sarcoplasmic reticulum Ca2+ uptake and leak properties, and SERCA isoform expression, in type I and type II fibres of human skeletal muscle. Journal of Physiology, 592, 1381-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Cseresnyés Z., Randall W. R., Schneider M. F. (2001). Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. Journal of Cell Biology, 155, 27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks M., Gao W., Higginson I. J., Wilcock A. (2013). Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Systematic Reviews, (1), Article CD009419. [DOI] [PubMed] [Google Scholar]
- Manring H., Abreu E., Brotto L., Weisleder N., Brotto M. (2014). Novel excitation-contraction coupling related genes reveal aspects of muscle weakness beyond atrophy-new hopes for treatment of musculoskeletal diseases. Frontiers in Physiology, 5, Article 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee S. L., Fairlie E., Garnham A. P., Hargreaves M. (2009). Exercise-induced histone modifications in human skeletal muscle. Journal of Physiology, 587(Pt. 24), 5951-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M. P., Susser S. E., Stammers A. N., O’Hara K. A., Gardiner P. F., Sheppard P., . . . Duhamel T. A. (2014). Differential regulation of the fiber type-specific gene expression of the sarcoplasmic reticulum calcium-ATPase isoforms induced by exercise training. Applied Physiology, 117, 544-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosole S., Carraro U., Kern H., Loefler S., Fruhmann H., Vogelauer M., . . . Zampieri S. (2014). Long-term high-level exercise promotes muscle reinnervation with age. Journal of Neuropathology & Experimental Neurology, 73, 284-294. [DOI] [PubMed] [Google Scholar]
- Musarò A., McCullagh K. J., Naya F. J., Olson E. N., Rosenthal N. (1999). IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature, 400(6744), 581-585. [DOI] [PubMed] [Google Scholar]
- Nori A., Lin P. J., Cassetti A., Villa A., Bayer K. U., Volpe P. (2003). Targeting of alpha-kinase-anchoring protein (alpha KAP) to sarcoplasmic reticulum and nuclei of skeletal muscle. Biochemical Journal, 370(Pt. 3), 873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger R. S., Jr., Kampert J. B., Lee I. M., Hyde R. T., Leung R. W., Wing A. L. (1994). Changes in physical activity and other lifeway patterns influencing longevity. Medicine & Science in Sports & Exercise, 26, 857-865. [PubMed] [Google Scholar]
- Payne A. M., Delbono O. (2004). Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exercise and Sport Sciences Reviews, 32, 36-40. [DOI] [PubMed] [Google Scholar]
- Rana Z. A., Gundersen K., Buonanno A. (2009). The ups and downs of gene regulation by electrical activity in skeletal muscles. Journal of Muscle Research and Cell Motility, 30, 255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Hargreaves M. (2013). Exercise, GLUT4, and skeletal muscle glucose uptake. Physiological Reviews, 93, 993-1017. [DOI] [PubMed] [Google Scholar]
- Rose A. J., Hargreaves M. (2003). Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. The Journal of Physiology, 553(Pt. 1), 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. J., Kiens B., Richter E. A. (2006). Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. The Journal of Physiology, 574(Pt. 3), 889-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K., Yamaguchi A. (2010). Molecular mechanisms in aging and current strategies to counteract sarcopenia. Current Aging Science, 3, 90-101. [DOI] [PubMed] [Google Scholar]
- Salanova M., Bortoloso E., Schiffl G., Gutsmann M., Belavy D. L., Felsenberg D., . . . Blottner D. (2011). Expression and regulation of Homer in human skeletal muscle during neuromuscular junction adaptation to disuse and exercise. FASEB Journal, 25, 4312-4325. [DOI] [PubMed] [Google Scholar]
- Salanova M., Schiffl G., Gutsmann M., Felsenberg D., Furlan S., Volpe P., . . . Blottner D. (2013). Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biology, 1, 514-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabon N., Loefler S., Cvecka J., Sedliak M., Kern H. (2013). Strength training in elderly people improves static balance: A randomized controlled trial. European Journal of Translational Myology, 23, 85-89. [Google Scholar]
- Scicchitano B. M., Rizzuto E., Musarò A. (2009). Counteracting muscle wasting in aging and neuromuscular diseases: The critical role of IGF-1. Aging, 1, 451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A. L., Murgia M., Pallafacchina G., Calabria E., Coniglio P., Lomo T., Schiaffino S. (2001). Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proceedings of the National Academy of Sciences, 98, 13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T., Cseresnyés Z., Liu Y., Randall W. R., Schneider M. F. (2007). Regulation of the nuclear export of the transcription factor NFATc1 by protein kinases after slow fibre type electrical stimulation of adult mouse skeletal muscle fibres. The Journal of Physiology, 579(Pt. 2), 535-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser E. M., Stättner S., Karner J., Klimpfinger M., Freynhofer M., Zaller V., . . . Quittan M. (2009). Neuromuscular electrical stimulation reduces skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner: A randomized, controlled clinical trial in major abdominal surgical patients. Annals of Surgery, 249, 738-743. [DOI] [PubMed] [Google Scholar]
- Tavi P., Westerblad H. (2011). The role of in vivo Ca2+ signals acting on Ca2+–calmodulin-dependent proteins for skeletal muscle plasticity. The Journal of Physiology, 589, 5021-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. M., Vigna C., Betik A. C., Tupling A. R., Hepple R. T. (2010). Initiating treadmill training in late middle age offers modest adaptations in Ca2+ handling but enhances oxidative damage in senescent rat skeletal muscle. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 298, R1269-R1278. [DOI] [PubMed] [Google Scholar]
- Tothova J., Blaauw B., Pallafacchina G., Rudolf R., Argentini C., Reggiani C., Schiaffino S. (2006). NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. Journal of Cell Science, 119(Pt. 8), 1604-1611. [DOI] [PubMed] [Google Scholar]
- Vechetti I. J., Jr., Aguiar A. F., de Souza R. W., Almeida F. L., de Almeida Dias H. B., de Aguiar Silva M. A., . . . Dal-Pai-Silva M. (2013). NFAT isoforms regulate muscle fiber type transition without altering CaN during aerobic training. International Journal of Sports Medicine, 34, 861-867. [DOI] [PubMed] [Google Scholar]
- Vinciguerra M., Musarò A., Rosenthal N. (2010). Regulation of muscle atrophy in aging and disease. Advances in Experimental Medicine and Biology, 694, 211-233. [DOI] [PubMed] [Google Scholar]
- Wall B. T., Dirks M. L., Verdijk L. B., Snijders T., Hansen D., Vranckx P., . . . Loon L. J. (2012). Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. American Journal of Physiology Endocrinology and Metabolism, 303, E614-E623. [DOI] [PubMed] [Google Scholar]
- Zampieri S., Mammucari C., Romanello V., Barberi L., Pietrangelo L., Fusella A., . . . Rizzuto R. (2016). Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiological Reports, 4(24), e13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri S., Pietrangelo L., Loefler S., Fruhmann H., Vogelauer M., Burggraf S., . . . Kern H. (2015). Lifelong physical exercise delays age-associated skeletal muscle decline. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 70, 163-173. [DOI] [PubMed] [Google Scholar]