Abstract
Background:
Use of administrative data for outcomes assessment in living kidney donors is increasing given the rarity of complications and challenges with loss to follow-up.
Objective:
To assess the validity of living donor nephrectomy in health care administrative databases compared with the reference standard of manual chart review.
Design:
Retrospective cohort study.
Setting:
5 major transplant centers in Ontario, Canada.
Patients:
Living kidney donors between 2003 and 2010.
Measurements:
Sensitivity and positive predictive value (PPV).
Methods:
Using administrative databases, we conducted a retrospective study to determine the validity of diagnostic and procedural codes for living donor nephrectomies. The reference standard was living donor nephrectomies identified through the province’s tissue and organ procurement agency, with verification by manual chart review. Operating characteristics (sensitivity and PPV) of various algorithms using diagnostic, procedural, and physician billing codes were calculated.
Results:
During the study period, there were a total of 1199 living donor nephrectomies. Overall, the best algorithm for identifying living kidney donors was the presence of 1 diagnostic code for kidney donor (ICD-10 Z52.4) and 1 procedural code for kidney procurement/excision (1PC58, 1PC89, 1PC91). Compared with the reference standard, this algorithm had a sensitivity of 97% and a PPV of 90%. The diagnostic and procedural codes performed better than the physician billing codes (sensitivity 60%, PPV 78%).
Limitations:
The donor chart review and validation study was performed in Ontario and may not be generalizable to other regions.
Conclusions:
An algorithm consisting of 1 diagnostic and 1 procedural code can be reliably used to conduct health services research that requires the accurate determination of living kidney donors at the population level.
Keywords: administrative data, health services research, living kidney donation, positive predictive value
Abrégé
Contexte:
Les professionnels de la santé se fient de plus en plus aux données administratives pour évaluer l’issue de l’opération chez les donneurs de rein vivants, étant donné la rareté des complications et les défis posés par la perte des patients au cours du suivi.
Objectif:
Nous souhaitions évaluer la validité des données de néphrectomies sur donneur vivant rapportées dans les bases de données administratives en santé comparativement à la norme de référence qui consiste à consigner les données manuellement dans les dossiers médicaux.
Type d’étude:
Il s’agit d’une étude de cohorte rétrospective.
Cadre de l’étude:
L’étude a été menée dans l’un des cinq principaux centres de transplantation d’Ontario, au Canada.
Participants:
La cohorte était composée de donneurs de rein vivants dont la néphrectomie a eu lieu entre 2003 et 2010.
Mesures:
Sensibilité et valeur prédictive positive (VPP).
Méthodologie:
Dans le cadre d’une étude rétrospective menée à l’aide de bases de données administratives, nous avons examiné la validité des codes de diagnostic et des codes d’intervention dans les cas de néphrectomie sur donneur vivant. Les données de l’organisme ontarien d’approvisionnement en organes, vérifiées manuellement par analyse des dossiers médicaux, ont servi de norme de référence. On a déterminé les paramètres fonctionnels (la sensibilité et la VPP) de plusieurs algorithmes basés sur les codes de diagnostic, les codes d’intervention et les codes de facturation.
Résultats:
Il y a eu 1 199 néphrectomies sur donneur vivant pendant la période couverte par l’étude. Globalement, le meilleur algorithme de repérage des donneurs de rein vivants combinait i) un code de diagnostic attribué à un donneur de rein (ICD-10 Z52.4) et ii) un code d’intervention attribué à l’ablation ou au prélèvement rénal (1PC58, 1PC89, 1PC91). En comparaison avec la norme de référence, cet algorithme présente une sensibilité de 97 % et une VPP de 90 %. Les codes de diagnostic et d’intervention se sont avérés de meilleurs indicateurs que les codes de facturation du médecin (sensibilité de 60 %; VPP de 78 %).
Limites de l’étude:
L’examen des dossiers médicaux des donneurs et l’étude de validation ayant été menés en Ontario, ses conclusions peuvent ne pas être transposables à d’autres régions.
Conclusion:
Un algorithme combinant un code de diagnostic et un code d’intervention s’est avéré fiable pour le dénombrement des donneurs de rein vivants dans la population générale en contexte de recherche en santé.
What was known before
Administrative health care data is increasingly used in many regions to assess outcomes in living kidney donors and to compare risks to other populations, such as the general population, healthy non-donor populations, or other surgical patients. Prior living kidney donors in administrative databases can be identified through linkage with data from transplant centers, regional organ and procurement agencies, national registries, or through the use of administrative diagnostic and procedural codes.
What this adds
The best algorithm for identifying living kidney donors was the presence of 1 diagnostic code for kidney donor (ICD-10 Z52.4) and 1 procedural code for kidney procurement/excision (1PC58, 1PC89, 1PC91). Compared to the reference standard, this algorithm had a sensitivity of 97% and a PPV of 90%.
Introduction
There is growing interest in understanding the short- and long-term outcomes of living kidney donors. Better knowledge of the risks of donor nephrectomy can be used to improve the informed consent process for living kidney donor candidates, maintain the public’s trust in the transplantation system, and increase living donor kidney transplantation rates. Previous studies of outcomes in prior living kidney donors have been limited by single-center data, small sample sizes, short-term follow-up, and high loss to follow-up.1-4 Administrative health care data are increasingly used in many regions to assess outcomes in living kidney donors and to compare risks with other populations, such as the general population, healthy nondonor populations, or other surgical patients.5-18 Prior living kidney donors in administrative databases can be identified through linkage with data from transplant centers, regional organ and procurement agencies, and national registries,5-15,19-22 or through the use of administrative diagnostic and procedural codes.16-18,23 To date, there are no validation studies of these diagnostic and procedural codes for living donor nephrectomy, with the potential for misclassification and erroneous conclusions.24 Using a reference standard of living kidney donors identified from the tissue and organ procurement agency at one province in Canada and verified by manual chart review, we assessed the validity of various algorithms for living kidney donor identification based on data from health care administrative databases.
Methods
Design and Setting
We conducted a retrospective cohort study using linked health care databases in Ontario, Canada. Ontario has ~13 million residents who have universal access to hospital care and physician services.25 This study was approved by the institutional review board at the Sunnybrook Health Sciences Centre, Toronto, Canada. The reporting of this study follows the RECORD guidelines for observational studies (Table S1).26
Data Sources
The Donor Nephrectomy Outcomes Research (DONOR) Network is a multidisciplinary team of nephrologists, surgeons, and epidemiologists with an aim to study short- and long-term outcomes of living kidney donors.5-9,27-30 Many of the outcome studies were made possible through Ontario’s organ and tissue procurement agency, the Trillium Gift of Life Network (TGLN), which captures information on all living kidney donors in the province. To confirm donor status and ensure the accuracy and completeness of the data in this registry, we manually reviewed the perioperative medical charts of all the living kidney donors (>2000 donors) who underwent donor nephrectomy at 1 of 5 major transplantation centers in Ontario from 1992 to 2010. This data source was considered the referent standard and linked to the provincial health care administrative databases at the Institute for Clinical Evaluative Sciences (ICES) using each donor’s encrypted health card number.
Data from TGLN were compared with information in 2 other health care databases within ICES. The Canadian Institute for Heath Information (CIHI) Discharge Abstract Database contains information on diagnostic and procedural information during hospital admissions. The Ontario Health Insurance Plan (OHIP) database contains fee-for-service physician billing claims for both inpatient and outpatient physician services. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Population
We identified all living kidney donors within ICES from January 1, 2003, to March 31, 2010. We started the accrual period in 2003 as the transition from the use of ICD-9 (International Classification of Diseases) to ICD-10 codes occurred in 2002 in Canada. We excluded patients with invalid or missing ICES key number (IKN, identifier used by ICES to link across datasets), date of birth, sex, date of death before the nephrectomy date, or out-of-province residents. For patients identified using CIHI and OHIP codes, we restricted to the first date of any code for each patient. Various algorithms were constructed, a priori, based on previously used algorithms in the literature to identify living kidney donors.10,16,17 For CIHI codes, we tested the validity of 1 diagnostic code, 1 procedural code, and a combination of 1 diagnostic code and 1 procedural code. For OHIP codes, we tested the validity of 1 billing code and 2 billing codes.
Statistics
We assessed the validity of various algorithms using CIHI and OHIP codes compared with the referent standard (TGLN). We determined the probability of identifying living donor nephrectomies in CIHI and OHIP given identification by TGLN (sensitivity), and the probability that the codes in CIHI and OHIP correctly identified living donor nephrectomies (positive predictive value [PPV]). For the concordant nephrectomies that were captured by TGLN and by CIHI and OHIP, we also assessed the accuracy of the recorded nephrectomy dates. For CIHI, this date was taken as the hospital admission date for the donor. Last, for the false positives (patients identified by an algorithm but not registered in TGLN) and false negatives (donors in TGLN that were missed by an algorithm), we reviewed the most common concurrent diagnostic and procedural codes during the index hospitalization to further characterize the patients. Due to the design of this validation study, we did not calculate specificity or negative predictive value. We conducted all analyses with SAS (Statistical Analysis Software) Enterprise Guide Version 7.12.
Results
Validity of the Codes and Algorithms
During the study period, there was a total of 1199 living donor nephrectomies reported by TGLN and confirmed by manual chart review (Figure 1). The codes used to identify living donor nephrectomy from each of the databases are summarized in Table 1. The validity of the CIHI and OHIP algorithms compared with the referent standard, TGLN, is presented in Table 2. Overall, the CIHI algorithms performed better than the OHIP algorithms in identifying living donor nephrectomies. A CIHI algorithm of 1 diagnostic code for kidney donor (ICD-10 Z52.4) and at least 1 procedural code for kidney procurement or excision (1PC58, 1PC89, or 1PC91) was the most accurate algorithm compared with TGLN, with a sensitivity of 97.4% (95% confidence interval [CI], 96.5%-98.3%) and a PPV of 90.1% (95% CI, 88.4%-91.7%). The addition of the procedural codes for kidney excision (1PC89 or 1PC91) improved the sensitivity of the algorithm compared with only including the procedural code for kidney procurement (1PC58) (sensitivity 97.4% vs 92.2%; P < .0001) while the PPV remained similar (PPV 90.1% vs 90.0%; P = .93). Use of the subclassification codes of kidney procurement (1PC58DAXXJ, 1PC58LBXXJ, 1PC58FXXJ, or 1PC58QPXXJ) had similar performance to use of the inclusive code (1PC58). The diagnostic code alone for kidney donor (ICD-10 Z52.4) had a sensitivity of 99.2% (95% CI, 98.7%-99.7%) and a PPV of 84.7% (95% CI, 82.8%-86.6%).
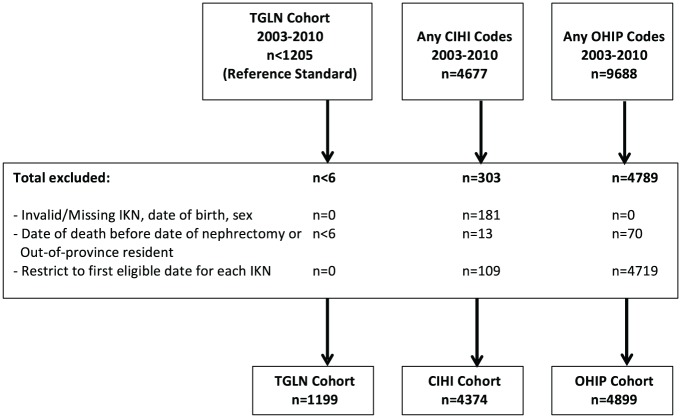
Figure 1.
Cohort creations.
Note. For the CIHI and OHIP cohorts, the presence of any of the study codes, presented in Table 1, was used to identify the cohort. In accordance with ICES privacy policies, cell sizes ≤5 cannot be reported. TGLN = Trillium Gift of Life Network; CIHI = Canadian Institute for Health Information; OHIP = Ontario Health Insurance Plan; IKN = ICES key number; ICES = Institute for Clinical Evaluative Sciences.
Table 1.
Administrative Database Codes Used to Identify Living Donor Nephrectomies.
| Database | Code | Description |
|---|---|---|
| CIHI (Diagnostic) | ICD-10: Z52.4 | Kidney donor |
| CIHI (Procedural) | CCI: 1PC58 | Procurement, kidney |
| CCI: 1PC58DAXXJ | Procurement, kidney using endoscopic (laparoscopic), approach from living donor | |
| CCI: 1PC58LBXXJ | Procurement, kidney open abdominal approach from living donor | |
| CCI: 1PC58PFXXJ | Procurement, kidney open lumbar (flank) approach from living donor | |
| CCI: 1PC58QPXXJ | Procurement, kidney open subcostal transperitoneal approach from living donor | |
| CCI: 1PC89 | Excision total, kidney | |
| CCI: 1PC91 | Excision radical, kidney | |
| OHIP | E753 | Live donor |
| S436 | Donor nephrectomy | |
| S420 | Nephroureterectomy | |
| S416 | Radical nephrectomy |
Note. CIHI = Canadian Institute for Health Information; ICD-10 = International Classification of Diseases, 10th Revision; CCI = Canadian Classification of Health Interventions; OHIP = Ontario Health Insurance Plan.
Table 2.
Accuracy of Living Donor Nephrectomy Algorithms Captured in CIHI and OHIP Compared With TGLN.
| True positive | False positive | False negative | Sensitivity (95% CI) | PPV (95% CI) | |
|---|---|---|---|---|---|
| CIHI Algorithm: 1 diagnostic code | |||||
| Z524 | 1189 | 215 | 10 | 99.2 (98.7-99.7) | 84.7 (82.8-86.6) |
| CIHI Algorithm: 1 procedural code | |||||
| 1PC58 | 1110 | 136 | 89 | 92.6 (91.1-94.1) | 89.1 (87.4-90.8) |
| 1PC58 or 1PC89 or 1PC91 | 1172 | 3094 | 27 | 97.7 (96.9-98.6) | 27.5 (26.1-28.8) |
| CIHI Algorithm: 1 diagnostic code AND 1 procedural code | |||||
| Z524 and 1PC58 | 1106 | 123 | 93 | 92.2 (90.7-93.8) | 90.0 (88.3-91.7) |
| Z524 and (1PC58DAXXJ or 1PC58LBXXJ or 1PC58PFXXJ or 1PC58QPXXJ) | 1100 | 123 | 99 | 91.7 (90.2-93.3) | 89.9 (88.3-91.6) |
| Z524 and (1PC58 or 1PC89 or 1PC91) | 1168 | 129 | 31 | 97.4 (96.5-98.3) | 90.1 (88.4-91.7) |
| OHIP Algorithm: 1 billing code | |||||
| E753 or S436 or S420 or S416 | 988 | 3911 | 211 | 82.4 (80.3-84.6) | 20.2 (19.0-21.3) |
| OHIP Algorithm: 2 billing codes | |||||
| E753 and S436 | 721 | 200 | 478 | 60.1 (57.4-62.9) | 78.3 (75.6-80.9) |
Note. The referent standard was TGLN with verification of donor status performed by manual chart review. During the study period, TGNL reported a total of 1199 living donor nephrectomies in the province. CIHI = Canadian Institute for Health Information; OHIP = Ontario Health Insurance Plan; TGLN = Trillium Gift of Life Network; CI = confidence interval; PPV = positive predictive value.
The algorithm including 1 diagnostic code for kidney donor (ICD-10 Z52.4) and 1 procedural code for kidney procurement (1PC58) had similar validity to the use of the procedural code alone (sensitivity 92.2% vs 92.6%, PPV 90.0% vs 89.1%) suggesting that the diagnostic code did not enhance the validity of the procurement procedural code. On the contrary, the addition of the diagnostic code for kidney donor significantly increased the PPV (90.1% vs 27.5%; P < .0001) for the algorithm including the procedural codes for kidney procurement or excision (1PC58, 1PC89, or 1PC91).
For the donor nephrectomies captured by both TGLN and the databases, the median absolute difference between the recorded nephrectomy dates was 0 days (interquartile range [IQR], 0 to 0) for all of the CIHI and OHIP algorithms.
Characterization of the False Positives and False Negatives
To characterize the false positives and false negatives for the CIHI algorithms, we reviewed the concurrent diagnostic and procedural codes during the index hospitalization (Tables 3 and 4). For the CIHI algorithms using 1 diagnostic code and 1 procedural code, the false-positive cases appear to include true living kidney donors. The most frequently reported concurrent hospitalization codes include the diagnostic code for kidney donor or removal of an organ as well as other possible perioperative complication codes, such as gastroesophageal reflux and accidental laceration. The algorithm with only 1 diagnostic code for kidney donor appears to include deceased donors. For the algorithms using only 1 procedural code for kidney procurement or excision, the false-positive cases appear to comprise deceased donors, kidney transplant recipients, and patients with chronic kidney disease. The algorithm that includes the additional kidney excision codes (1PC89 or 1PC91) appears to also capture patients undergoing nephrectomy for other purposes, such as malignancy.
Table 3.
Most Frequent Diagnostic and Procedural Codes During the Index Hospitalization for the False-Positive Cases.
| Diagnostic code | Description | Procedural code | Description |
|---|---|---|---|
| CIHI Algorithm: 1 diagnostic code (Z524, n = 215) | |||
| Z524 | Kidney donor | 1PC58DAXXJ | Kidney procurement (living donor) |
| Z526 | Liver donor | 1GZ31CAND | Ventilation |
| Z528 | Donor of other organs and tissues | 1PC58PFXXJ | Kidney procurement (living donor) |
| Z527 | Heart donor | 1PC58LBXXJ | Kidney procurement (living donor) |
| G9381 | Other specified disorders of brain | 3AN20WA | CT brain |
| CIHI Algorithm: 1 procedural code (1PC58, n = 136) | |||
| Z524 | Kidney donor | 1PC58DAXXJ | Kidney procurement (living donor) |
| Y836 | Removal of organ | 1PC58LBXXJ | Kidney procurement (living donor) |
| N180 | Chronic kidney disease | 1PC58PFXXJ | Kidney procurement (living donor) |
| I12 | Hypertensive renal disease | 1PC58LBXXK | Kidney procurement (deceased donor) |
| CIHI Algorithm: 1 procedural code (1PC58 or 1PC89 or 1PC91, n = 3094) | |||
| C64 | Malignant neoplasm of kidney | 1PC91DA | Kidney excision, radical |
| I100 | Primary hypertension | 1PC91LB | Kidney excision, radical |
| Y836 | Removal of organ | 1PC89DA | Kidney excision, total |
| N180 | Chronic kidney disease | 1PZ21HQBR | Dialysis |
| I12 | Hypertensive renal disease | 1PC89LB | Kidney excision, total |
| CIHI Algorithm: 1 diagnostic code AND 1 procedural code (all algorithms, n = 123-129) | |||
| Z524 | Kidney donor | 1PC58DAXXJ | Kidney procurement (living donor) |
| Y836 | Removal of organ | 1PC58PFXXJ | Kidney procurement (living donor) |
| K219 | Gastroesophageal reflux disease | 1PC58LBXXJ | Kidney procurement (living donor) |
| T812 | Accidental laceration | 3OT20WA | CT abdomen |
Note. CIHI false-positive cases are those identified by the algorithm but not in the TGLN registry (referent standard). Frequencies are not reported because in accordance with ICES privacy policies, cell sizes ≤5 cannot be reported. CIHI = Canadian Institute for Health Information; CT = computed tomography; ICES = Institute for Clinical Evaluative Sciences; TGLN = Trillium Gift of Life Network.
Table 4.
Most Frequent Diagnostic and Procedural Codes During the Index Hospitalization for the False-Negative Cases.
| Diagnostic code | Description | Procedural code | Description |
|---|---|---|---|
| CIHI Algorithm: 1 diagnostic code (Z524, n = 10) | |||
| Z526 | Liver donor | 1PC58DAXXJ | Kidney procurement (living donor) |
| 1PC85LAXXJ | Transplant, kidney using living donor | ||
| CIHI Algorithm: 1 procedural code (1PC58, n = 89) | |||
| Z524 | Kidney donor | 1PC89DA | Kidney excision, total |
| Y836 | Removal of organ | 1ZZ35HAP2 | Pharmacotherapy, analgesics |
| K913 | Post-operative intestinal obstruction | 1PC87DA | Kidney excision, partial |
| R21 | Rash | 1PC89LB | Kidney excision, total |
| 1PC85LAXXJ | Transplant, kidney using living donor | ||
| 1PC87LA | Kidney excision, partial | ||
| CIHI Algorithm: 1 procedural code (1PC58 or 1PC89 or 1PC91, n = 27) | |||
| Z524 | Kidney donor | 1PC87DA | Kidney excision, partial |
| 1PC85LAXXJ | Transplant, kidney using living donor | ||
| 1PC87LA | Kidney excision, partial | ||
| CIHI Algorithm: 1 diagnostic code AND 1 procedural code (Z524 and 1PC58, n = 93) | |||
| Z524 | Kidney donor | 1PC89DA | Kidney excision, total |
| Y836 | Removal of organ | 1ZZ35HAP2 | Pharmacotherapy, analgesics |
| Z526 | Liver donor | 1PC87DA | Kidney excision, partial |
| K913 | Post-operative intestinal obstruction | 1PC89LB | Kidney excision, total |
| R21 | Rash | 1PC85LAXXJ | Transplant, kidney using living donor |
| 1PC87LA | Kidney excision, partial | ||
| CIHI Algorithm: 1 diagnostic code AND 1 procedural code (Z524 and 1PC58 or 1PC89 or 1PC91, n=31) | |||
| Z524 | Kidney donor | 1PC87DA | Kidney excision, partial |
| Z526 | Liver donor | 1PC85LAXXJ | Transplant, kidney using living donor |
| 1PC87LA | Kidney excision, partial | ||
| 1PC58DAXXJ | Kidney procurement (living donor) | ||
Note. CIHI false-negative cases are those identified in the TGLN registry (referent standard) but not by the algorithm. Frequencies are not reported because in accordance with ICES privacy policies, cell sizes ≤5 cannot be reported. CIHI = Canadian Institute for Health Information; TGLN = Trillium Gift of Life Network; ICES = Institute for Clinical Evaluative Sciences.
We also assessed the concurrent diagnostic and procedural codes during the index hospitalization for the false-negative cases, to determine whether there were other codes that could be used to strengthen the validity of each of the algorithms (Table 4). Many of the donors who did not have a procedural code for kidney procurement (1PC58) had the kidney excision codes instead (1PC89 or 1PC91), resulting in a decrease of the false-negative cases when these additional codes were included (n = 89 vs n = 27). For the algorithms including both the procedural codes for kidney procurement and total/radical excision, the other most frequent associated code for the false-negative cases was for partial kidney excision (1PC87).
Discussion
To our knowledge, this is the first validation study of living donor nephrectomy codes, made possible through the manual perioperative chart review of over 1200 cases during the study period. In this study, the most valid algorithm tested included 1 diagnostic code for kidney donor (ICD-10 Z52.4) and at least 1 procedural code for kidney procurement or excision (1PC58, 1PC89, or 1PC91), yielding a sensitivity of 97.4% and a PPV of 90.1%, compared with the referent standard (TGLN with manual perioperative chart review). This CIHI algorithm outperformed the OHIP algorithms based on physician billing claims. The hospital-based codes from CIHI are abstracted by medical coders who are trained to assign standardized codes on the basis of physician-recorded diagnoses and procedures in a patient’s medical chart.7 In contrast, the information contained in the OHIP database is derived from physician billing claims and an overreporting of cases may have occurred if physicians mistakenly used codes for cases of nondonor nephrectomy, deceased kidney donor procurement, or living donor kidney transplantation.
Data on short- and long-term outcomes of living kidney donors have been challenged by loss to follow-up in single- and multicenter studies, and the limited scope, duration, and completeness of follow-up in regional and national organ registries.3,24,31 In the United States, the United Network for Organ Sharing (UNOS) has required that transplant centers submit information on donor follow-up and outcomes for 2 years post-donation since 2007. Compliance with complete follow-up information at 2 years was as low as 50% for clinical data and 30% for laboratory data in UNOS, although reporting has improved over time,3 especially since implementation of mandated follow-up thresholds.32 Our results suggest that regional and national administrative databases can use diagnostic and procedural codes to identify and follow living kidney donors for postnephrectomy outcomes, even if they already have existing linkage with national organ registries. This methodology may facilitate more research on living kidney donor outcomes by supplementing and expanding national and regional registries when they exist, and providing a novel data source in regions where data may be currently limited to single-center records with small sample sizes, incomplete data, or loss to follow-up.
An understanding of the validity of the living donor nephrectomy codes not only facilitates future research but it also allows for better interpretation of previous studies on donor outcomes.16-18,23 For example, Schold et al. used a similar algorithm of 1 diagnostic code for kidney donor (ICD-9 V59.4) and 1 procedural code for nephroureterectomy (ICD-9 55.51) to identify prior US living kidney donors in the National Inpatient Sample (NIS) database from 1998 to 2010 (n = 69 117). In their sample of donors, the incidence of perioperative complication was 7.9% while the perioperative mortality was reported as 0.17%. While low, the incidence of mortality was higher than previous estimates generated through linkage of national organ and transplant registry data with death records.13,33 This discrepancy led to concerns regarding the inadvertent inclusion of nondonors into the study sample.24 The inclusion of patients who underwent nephrectomy for indications unrelated to donation, such as malignancy, may have biased the results. In our study, the algorithm of 1 diagnostic code for kidney donor (ICD-10 Z52.4) and 1 procedural code for kidney procurement (1PC58) had a sensitivity of 92.2% and a PPV of 90.0%. The addition of procedural codes for kidney excision (1PC89 or 1PC91) improved the sensitivity of the algorithm (97.4% vs 92.2%), although the PPV remained unchanged (90.1% vs 90.0%). Another strategy to improve the validity is to confirm donor status identified through codes by linking with national organ and transplant registries, as has been done in previous studies.10
The main strength of this validation study is the verification of donor status from TGLN through manual perioperative chart review of over 2000 charts, resulting in assurance in the almost 1200 true positive cases in the current study. TGLN receives donor information from the transplant centers, and thus, the false-positive cases identified by the algorithm may be true living kidney donors if there was underreporting to TGLN by the transplant center. By assessing concurrent hospitalization diagnostic and procedural codes for the false-positive and false-negative cases, we were able to assess whether the various algorithms were capturing true living kidney donors, deceased donors, kidney transplant recipients, patients with chronic kidney disease or nephrectomies unrelated to donation, such as for malignancy. We were also able to determine whether there were additional codes that could be used to further strengthen the algorithm. It is likely that the use of the procedural code for partial nephrectomy in the algorithm would only result in further false-positive cases.
There are limitations to this study. The donor chart review and validation study was performed in Ontario and may not be generalizable to other regions. We also performed a validation of the ICD-10 codes alone, rather than the ICD-9 codes, given that they are more currently in use. Previous validation studies have shown minimal differences between ICD-9 and ICD-10 codes for similar diagnoses.34-38
Conclusion
The results of this study suggest that the algorithm of 1 diagnostic code for kidney donor and 1 procedural code for kidney procurement or excision has a high sensitivity and PPV in identifying living kidney donors compared with information from a provincial tissue and organ registry, with verification through manual chart review. This algorithm can be reliably used to conduct health services research that requires the accurate determination of living kidney donors at the population level. This information can be used to monitor living kidney donation activity, evaluate the donor assessment process, and assess postdonation outcomes. Further research on the short- and long-term outcome of living kidney donors from different regions is needed to better understand geographic and demographic variability in postdonation outcomes.
Supplemental Material
Supplemental material, LKD_Validation_-_Appendix_-_20170822 for Validation of Living Donor Nephrectomy Codes by Ngan N. Lam, Krista L. Lentine, Scott Klarenbach, Manish M. Sood, Paul J. Kuwornu, Kyla L. Naylor, Gregory A. Knoll, S. Joseph Kim, Ann Young and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES) Western site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD) and its Clinical Departments, Western University, and the Lawson Health Research Institute (LHRI). The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, or the MOHLTC is intended or should be inferred. The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Western facility, who are supported by a team grant from the Canadian Institutes of Health Research (CIHR). Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of CIHI. The authors acknowledge that the data used in this study were provided by the Trillium Gift of Life Network (Toronto, Ontario), which is funded by the Government of Ontario. The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of TGLN. The authors acknowledge the active investigators of the Donor Nephrectomy Outcomes Research (DONOR) Network.
Footnotes
Abbreviations: CI, confidence interval; CIHI, Canadian Institute for Heath Information; DONOR, Donor Nephrectomy Outcomes Research; ICD, International Classification of Diseases; ICES, Institute for Clinical Evaluative Sciences; IKN, ICES key number; IQR, interquartile range; NIS, National Inpatient Sample; OHIP, Ontario Health Insurance Plan; PPV, positive predictive value; SAS, Statistical Analysis Software; TGLN, Trillium Gift of Life Network; UNOS, United Network for Organ Sharing
Ethics Approval and Consent to Participate: This study was approved by the institutional review board at the Sunnybrook Health Sciences Centre, Toronto, Canada.
Consent for Publication: All authors consent for publication.
Availability of Data and Materials: Additional data is available in the Appendix.
Authors’ Contributions: NNL, PJK, and AXG participated in the research design, data analysis, and performance of the research. NNL drafted the manuscript and all authors contributed to the completion of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NNL was supported by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) New Investigator Award. KLL was supported by grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R01DK102981). MMS is supported by the Jindal Research Chair in the Prevention of Kidney Disease. KLN is supported by the Canadian Institute of Health Research Fellowship and the Canadian National Transplant Research Program Astellas Training Award. AXG received an investigator-initiated grant from Astellas to support CIHR-funded research in living kidney donation. He is also supported by a Dr. Adam Linton Chair in Kidney Analytics and a Clinical Investigator award from the CIHR.
Supplemental Material:Supplementary material is available for this article online.
References
- 1. Boudville N, Prasad GVR, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185-196. [DOI] [PubMed] [Google Scholar]
- 2. Young A, Storsley L, Garg AX, et al. Health outcomes for living kidney donors with isolated medical abnormalities: a systematic review. Am J Transplant. 2008;8(9):1878-1890. [DOI] [PubMed] [Google Scholar]
- 3. Schold JD, Buccini LD, Rodrigue JR, et al. Critical factors associated with missing follow-up data for living kidney donors in the United States. Am J Transplant. 2015;15(9):2394-2403. [DOI] [PubMed] [Google Scholar]
- 4. Kim SH, Hwang HS, Yoon HE, et al. Long-term risk of hypertension and chronic kidney disease in living kidney donors. Transplant Proc. 2012;44(3):632-634. [DOI] [PubMed] [Google Scholar]
- 5. Lam N, Huang A, Feldman LS, et al. Acute dialysis risk in living kidney donors. Nephrol Dial Transplant. 2012;27(8):3291-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lam NN, McArthur E, Kim SJ, et al. Gout after living kidney donation: a matched cohort study. Am J Kidney Dis. 2015;65(6):925-932. [DOI] [PubMed] [Google Scholar]
- 7. Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372(2):124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012;344:e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ordon M, Welk B, McArthur E, et al. Risk of nephrectomy in previous living kidney donors. Transplantation. 2016;100(6):1313-1317. [DOI] [PubMed] [Google Scholar]
- 10. Lentine KL, Lam NN, Axelrod D, et al. Perioperative complications after living kidney donation: a national study. Am J Transplant. 2016;16(6):1848-1857. [DOI] [PubMed] [Google Scholar]
- 11. Lam NN, Garg AX, Segev DL, et al. Gout after living kidney donation: correlations with demographic traits and renal complications. Am J Nephrol. 2015;41(3):231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959-966. [DOI] [PubMed] [Google Scholar]
- 14. Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11(8):1650-1655. [DOI] [PubMed] [Google Scholar]
- 15. Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86(1):162-167. [DOI] [PubMed] [Google Scholar]
- 16. Schold JD, Goldfarb DA, Buccini LD, et al. Comorbidity burden and perioperative complications for living kidney donors in the United States. Clin J Am Soc Nephrol. 2013;8(10):1773-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schold JD, Goldfarb DA, Buccini LD, et al. Hospitalizations following living donor nephrectomy in the United States. Clin J Am Soc Nephrol. 2014;9(2):355-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman AL, Cheung K, Roman SA, Sosa JA. Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg. 2010;145(4):356-362. [DOI] [PubMed] [Google Scholar]
- 19. Lentine KL, Lam NN, Schnitzler MA, et al. Gender differences in use of prescription narcotic medications among living kidney donors. Clin Transplant. 2015;29(10):927-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lentine KL, Schnitzler MA, Xiao H, et al. Depression diagnoses after living kidney donation: linking U.S. Registry data and administrative claims. Transplantation. 2012;94(1):77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lentine KL, Schnitzler MA, Xiao H, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014;97(3):316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363(8):724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel S, Cassuto J, Orloff M, et al. Minimizing morbidity of organ donation: analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation. 2008;85(4):561-565. [DOI] [PubMed] [Google Scholar]
- 24. Lentine KL, Segev DL. Better understanding live donor risk through big data. Clin J Am Soc Nephrol. 2013;8(10):1645-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Statistics Canada. Population and dwelling count highlight tables, 2016 census. Date unknown. http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/pd-pl/Table.cfm?Lang=Eng&T=101&S=50&O=A. Accessed May 30, 2017.
- 26. Benchimol EI, Smeeth L, Guttmann A, et al. Das RECORD-Statement zum Berichten von Beobachtungsstudien, die routinemäßig gesammelte Gesundheitsdaten verwenden. Z Evid Fortbild Qual Gesundhwes. 2016;115-116:33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas SM, Lam NN, Welk BK, et al. Risk of kidney stones with surgical intervention in living kidney donors. Am J Transplant. 2013;13(11):2935-2944. [DOI] [PubMed] [Google Scholar]
- 28. Thomas SM, Lam NN, Huang A, et al. Risk of serious gastrointestinal bleeding in living kidney donors. Clin Transplant. 2014;28(5):530-539. [DOI] [PubMed] [Google Scholar]
- 29. Clemens K, Boudville N, Dew MA, et al. The long-term quality of life of living kidney donors: a multicenter cohort study. Am J Transplant. 2011;11(3):463-469. [DOI] [PubMed] [Google Scholar]
- 30. Garg AX, Pouget J, Young A, et al. Fracture risk in living kidney donors: a matched cohort study. Am J Kidney Dis. 2012;59(6):770-776. [DOI] [PubMed] [Google Scholar]
- 31. Ommen ES, LaPointe Rudow D, Medapalli RK, Schröppel B, Murphy B. When good intentions are not enough: obtaining follow-up data in living kidney donors. Am J Transplant. 2011;11:2575-2581. [DOI] [PubMed] [Google Scholar]
- 32. Henderson ML, Thomas AG, Shaffer A, et al. The national landscape of living kidney donor follow-up in the United States. Am J Transplant. 2017;17(12):3131-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis CL, Cooper M. The state of U.S. living kidney donors. Clin J Am Soc Nephrol. 2010;5(10):1873-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khokhar B, Jette N, Metcalfe A, et al. Systematic review of validated case definitions for diabetes in ICD-9-coded and ICD-10-coded data in adult populations. BMJ Open. 2016;6(8):e009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quach S, Blais C, Quan H. Administrative data have high variation in validity for recording heart failure. Can J Cardiol. 2010;26(8):306-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Coster C, Li B, Quan H. Comparison and validity of procedures coded with ICD-9-CM and ICD-10-CA/CCI. Med Care. 2008;46(6):627-634. [DOI] [PubMed] [Google Scholar]
- 37. Quan H, Li B, Duncan Saunders L, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776-1781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, LKD_Validation_-_Appendix_-_20170822 for Validation of Living Donor Nephrectomy Codes by Ngan N. Lam, Krista L. Lentine, Scott Klarenbach, Manish M. Sood, Paul J. Kuwornu, Kyla L. Naylor, Gregory A. Knoll, S. Joseph Kim, Ann Young and Amit X. Garg in Canadian Journal of Kidney Health and Disease