Abstract
Background:
Benzodiazepines (BZDs) are among the most prescribed sedative hypnotics and among the most misused and abused medications by patients, in parallel with opioids. It is estimated that more than 100 million Benzodiazepine (BZD) prescriptions were written in the United States in 2009. While medically useful, BZDs are potentially dangerous. The co-occurring abuse of opioids and BZD, as well as increases in BZD abuse, tolerance, dependence, and short- and long-term side effects, have prompted a worldwide discussion about the challenging aspects of medically managing the discontinuation of BZDs. Abrupt cessation can cause death. This paper addresses the challenges of medications suggested for the management of BZD discontinuation, their efficacy, the risks of abuse and associated medical complications. The focus of this review is on the challenges of several medications suggested for the management of BZD discontinuation, their efficacy, the risks of abuse, and associated medical complications.
Methods:
An electronic search was performed of Medline, Worldwide Science, Directory of Open Access Journals, Embase, Cochrane Library, Google Scholar, PubMed Central, and PubMed from 1990 to 2017. The review includes double-blind, placebo-controlled studies for the most part, open-label pilot studies, and animal studies, in addition to observational research. We expand the search to review articles, naturalistic studies, and to a lesser extent, letters to the editor/case reports. We exclude abstract and poster presentations, books, and book chapters.
Results:
The efficacy of these medications is not robust. While some of these medicines are relatively safe to use, some of them have a narrow therapeutic index, with severe, life-threatening side effects. Randomized studies have been limited. There is a paucity of comparative research. The review has several limitations. The quality of the documents varies according to whether they are randomized studies, nonrandomized studies, naturalistic studies, pilot studies, letters to the editors, or case reports.
Conclusions:
The use of medications for the discontinuation of BZDs seems appropriate. It is a challenge that requires further investigation through randomized clinical trials to maximize efficacy and to minimize additional risks and side effects.
Keywords: Benzodiazepine discontinuation, benzodiazepine substitution, benzodizaepine dependence, benzodiazepine withdrawal
Benzodiazepines (BZDs) belong to a class of drugs that modulate the neurotransmitter γ-aminobutyric acid (GABA), especially GABA-A, to open the chloride channel (Figure 1). The result can have a sedative effect, a sleep-inducing effect, and an aborting impact on convulsion and ability to relax the muscles. It was in Kraków, a city in the south of Poland, around 1930 that the story of BZDs began. Around 1957, chlordiazepoxide, also known as librium, was the first BZD synthesized. The clinical practice opted to accept BZDs over barbiturates due to the better safety profile and tolerability of the former. The drug reached a market value of approximately $2 billion worldwide in 1991. Data on the nonmedical use of BZDs in the United States have been reported at a prevalence of 2.3% in 2011.1 In one year, 2009, more than 100 million BZD prescriptions were written in the United States.2 The number of BZD prescriptions increased substantially from 1996 to 2013.3 Parallel to the increase in BZD prescriptions (an observation that is current for opioids also), reports of deaths caused by overdoses also increased from 0.58 to 3.07 deaths per 100,000 adults.3
Figure 1.
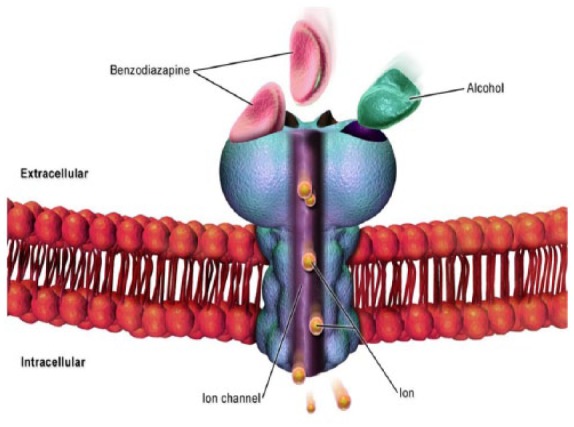
Benzodiazepine binds to γ-aminobutyric acid (GABA) receptor (blue) to open chloride channel (purple). Alcohol (green) cross reacts with the same receptor. The result is the modulation of GABA producing an anxiolytic effect.
Adapted from the work of: BruceBlaus. Cell GABA Receptor. November 12 2015. https://commons.wikimedia.org/wiki/File:Cell_GABA_Receptor.png. This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license. No permission is required.
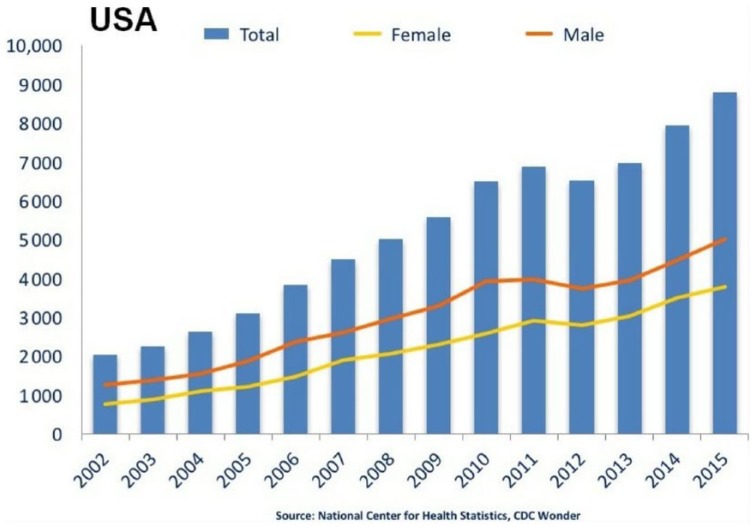
The number of individuals seeking treatment for problems related to BZD abuse is increasing and still on the rise, and after pain relievers (opioids), BZDs have been the drug class most frequently involved in drug-related suicide attempts.4 An estimated 440,000 emergency visits were reported for opiate abuse, and approximately 400,000 emergency visits included BZDs among the Drug Abuse Warning Network (DAWN) estimates in 2010.5 Deaths due to BZDs are not only attributed to the solitary use of the drug, but alcohol is also involved in BZD abuse, leading to emergency visits because of the comorbidity of alcohol and BZD abuse. The association of BZDs with alcohol multiplies the risk of life-threatening overdoses and death by central nervous system depression. The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) analyzed 2010 data from 13 states on deaths related to opioid prescriptions, alcohol, and BZDs, and they found that alcohol was involved in both opiate and BZD drug-related deaths (approximately 22% of deaths were caused by BZDs).6 The number of deaths from BZDs trended upward (from 2002 to 2015) and it is higher for men than for women (Figure 2).
Figure 2.
The number of deaths from benzodiazepines is trending up (from 2002 to 2015). Note that the number is higher for men than for women.
Adapted from the work of the National Institutes of Health, part of the United States Department of Health and Human Services. “National Overdose Deaths—Number of Deaths from Benzodiazepines, with and without opioids.
Source 2002–2015 chart from Overdose Death Rates. By National Institute on Drug Abuse (NIDA).
BZDs have a powerfully calming effect on the brain. BZDs can modulate GABA-A receptors as an agonist. GABA has a fantastic property of being an inhibitory neurotransmitter with the ability to reduce the excitability of neurons.7,8 BZD use can cause physical dependence and tolerance. BZDs can be misused, abused, or diverted. Several short-term trials have endorsed the effectiveness of BZDs for sleep, in addition to their clinical evidence in decreasing anxiety, increasing sedation, inducing muscle relaxation, and exerting antiseizure effects.9 After a period of 1–6 months of use, the abrupt cessation of BZDs can cause life-threatening seizures, delirium, and death.10
BZDs provoke fears of liability in the event of overdose and death; they also carry significant medical complications, such as memory impairment, vehicle accidents, falls, overdoses, and severe withdrawal symptoms, including life-threatening delirium. For these reasons, discontinuation of BZDs after a certain period of use and in cases of nonmedical or medical misuse is often indicated. There are several methods to discontinue BZDs, ranging from pharmacological management to psychotherapies, such as cognitive behavior therapy (CBT).11
Under the umbrella of pharmacological management, several broad categories of medications have been proposed, including anticonvulsants, antidepressants, antihypertensives, endogenous steroids, antiemetics, myorelaxants, anxiolytics, antihistamines, sleeping aids, barbiturates, BZD antagonists, long-acting BZDs, and plant-based derivatives. The efficacy of these medications is not robust, and although some are relatively safe to use, many have a narrow therapeutic index. Some of them can present compounded risks of severe, life-threatening side effects. It seems prudent to wean patients off BZDs from both an ethical and a liability perspective. This review addresses the pharmacological management of BZD discontinuation, expanding on efficacy, risks of addiction, and medical complications.
Methods
This review includes randomized, double-blind, placebo-controlled studies for the most part, as well as open-label pilot studies, animal studies, observational studies, review papers, and to a lesser extent, letters to the editor and case reports (due to lack of weight of evidence). We consulted databases including Worldwide Science (1990–2017), Medline (2000–2017), Directory of Open Access Journals (2010–2017), Embase (2008–2017), Cochrane Library, Google Scholar (2017), PubMed Central, and PubMed (1990–2017). Publications in English (78%), German, Japanese, Korean, French, and Chinese were included in the search. We used keywords such as benzodiazepine, dependence, withdrawal, tapering, substitution, and addiction. Two independent reviewers retrieved approximately 1583 publications. The information collected was placed on a spreadsheet, and then the two reviewers analyzed the relevance of these data to the interest of this paper. The two reviewers then called upon a third reviewer to resolve discrepancies and biases in accepting papers that were selected. A total of 109 additional publications were identified; 545 articles remained after duplicate documents in the same databases were removed. After multiple analyses of 545 documents, we eliminated 250 publications that were in Japanese, Chinese, and Korean, and 8 in French. We then proceeded to remove 73 papers focused on the long-term use of BZDs, 29 on research and development, 30 on health, nuclear power, and course and life sciences. We rejected an additional group of papers on positron emission tomography, Environmental Protection Agency (EPA) pesticide fact sheets, applied chemistry, internal medicine, and equilibrium research. From the remaining 85 publications, we selected 50 after exclusion of abstracts, conferences, book reviews, and books. Subsequently, 37 papers were eligible for inclusion. We also analyzed the size of the study of each drug selected. Because there is a paucity of randomized research, we were not able to perform a meta-analysis.
Results
Propranolol
Propranolol hydrochloride is an antihypertensive medication in the category of what are commonly called β-blocker agents. Aside from propranolol’s medical benefits, the drug can cause heart failure with continuous use. Caution is of paramount importance when propranolol is used in patients with kidney and liver damage. The idea of using propranolol for BZD dependence stipulates that its anxiolytic ability may be its peripheral (autonomic) activity instead of its central activity.12–14 In a randomized, double-blind trial conducted over 17 weeks, Cantopher and colleagues enrolled patients (n = 31) who were determined to be dependent on BZDs. These patients were on a BZD (namely, diazepam) for approximately 6 months and were divided into two groups or two types of withdrawal, which the authors called ‘slow withdrawal’ (SW) or ‘abrupt withdrawal’. Both groups were placed on propranolol. Propranolol was substituted for diazepam in this study for an estimated 10 weeks.15 An essential point in this study is that some of the patients also received counseling during the BZD withdrawal period. The study concluded that patients in the slow withdrawal group, as well as one in the abrupt withdrawal group, became less anxious by the end using the baseline Hamilton Anxiety and Depression Scale.15 The authors recommended the use of counseling with the substitution method. There was no recommendation of propranolol as a method to discontinue BZD. If we must extrapolate the possible benefit of propranolol in anxiety, Steenen and colleagues did not find a noticeable difference in the advantage of propranolol over BZDs for anxiety disorder. Based on this conclusion, one can predict the results of the study by Cantopher and colleagues.16
Clonidine
Clonidine is an antihypertensive medication that is an agonist in the anterior hypothalamus, while at the level of the medulla and in the posterior hypothalamus, clonidine plays the role of antagonist.17 Physicians use clonidine cautiously due to severe rebound hypertension in cases of sudden cessation. Abrupt cessation can cause agitation, headache, and tremor. Clonidine has been used off label to soothe the adrenergic surge of patients in acute anxiety. In a double-blind study, Hoehn-Saric and colleagues reported that clonidine decreased anxiety in patients (n = 23). The antianxiety effects of clonidine can be explained by its postsynaptic effect, neutralizing its presynaptic noradrenergic effects.18 Two letters to the editor suggested the efficacy of clonidine for BZD withdrawal. One was from Keshavan and colleagues, sent to the Lancet in 1985 under the rubric of clonidine in benzodiazepine withdrawal. The group reported successful withdrawal management compared with placebo with clonidine over 3 weeks in a 37-year-old man who had been taking 7.5 mg of lorazepam for many years. The second letter was from Vinogradov and colleagues to the American Journal of Psychiatry in 1986. It is interesting to note that both letters stipulated a similar hypothesis that clonidine’s property as an α2 adrenergic agonist lessened opiate withdrawal symptoms. The latter group reported the results of withdrawal facilitated by clonidine in a 30-year-old woman who was on alprazolam.19 However, these findings were challenged by Goodman and colleagues, who studied three women who were on BZDs for 1 year, and then the group determined whether clonidine was efficiently capable of controlling withdrawal symptoms of BZDs. The study concluded that clonidine markedly reduced blood pressure. However, the measure of plasma MHPG (3-methoxy-4-hydroxyphenethyleneglycol) (brain level of MHPG) increased after BZD withdrawal when BZDs were antagonized (by RO-15-1788) to determine the efficacy of clonidine, which did not suppress the signs and symptoms of BZD withdrawal.20,21
Progesterone
Progesterone is known to be a steroid in the group of sex hormones. Progesterone, in humans, is involved in regulation of the menstrual cycle and protection of the embryo in pregnancy. Progesterone can phosphorylate progesterone receptors to cause the activation of transcription factors and subsequently inhibiting estrogen, and it can induce secretory changes in the endometrium and decrease uterine contractility.22 It has been noted that the use of exogenous progesterone can produce several side effects, such as mood changes and gastrointestinal disturbances. The anxiolytic properties of progesterone occur through the stimulating effects on GABA/BZD receptor chloride. It seems the three 5α metabolites of progesterone are responsible for its antianxiety properties.23 Schweitzer and colleagues studied patients (n = 43) to analyze the efficacy of progesterone during the period of discontinuation of BZD. The patients were divided into placebo (n = 13) and progesterone (n = 30) groups. The authors concluded that progesterone did not reduce withdrawal symptom severity; in addition, progesterone was not able to facilitate discontinuation of BZDs by patients who were dependent on them.24 Despite finding possible anxiolytic properties of progesterone by activating GABA, the study by Schweitzer and colleagues had negative results, even at a dose of 3600 mg/day.
Baclofen
Baclofen is an antispasticity drug that activates GABA-B receptor as an agonist. Baclofen activates GABA to delay the flux of calcium at the neuromuscular junction. This property engenders the reduction of the evoked release of excitatory amino acids and several other transmitters.25 Baclofen has several side effects, and when the drug is used in excess, it can cause confusion, with the risk of severe injuries such as those from falls and head fractures. It can also cause drowsiness, dizziness, and weakness. The idea of recommending baclofen for BZD withdrawal comes from the property of GABA-B agents having what is said to be a correcting effect on aggression and anxiety in animal studies.25 There have been small studies reporting that baclofen eases alcohol withdrawal. Alcohol possesses cross reactivity with BZDs. It is not entirely evidenced that baclofen can, in fact, control BDZ withdrawal during the discontinuation period. Addolorato and colleagues compared baclofen with a placebo over a 30-day period for alcohol abstinence. The author reported that 79% of patients remained abstinent after the study on baclofen at a dose of 10 mg three times per day.26 Based on the results with baclofen for alcohol abstinence, other observations have suggested the efficacy of baclofen for the management of BZD discontinuation. Shukla and colleagues reported five cases of BZD dependence that were switched to baclofen. Nitrazepam was discontinued in a 45-year-old man who was switched to 20 mg per day of baclofen. A 25-year-old man dependent on barbiturates and BZDs was given the same dose. The third patient was a 50-year-old man who was diagnosed with a substance-induced depressive disorder and was dependent on both alcohol and BZDs. He was initially on 20 mg of baclofen, and then the dose was increased to 40 mg per day over a period of a week. The fourth and fifth cases were 39 and 40 years old, respectively. Both were started on baclofen 20 mg/day and increased to 40 mg/day over 1 week.27 The authors reported that all five patients tolerated the switch and remained abstinent from BZDs. The paper concluded by recommending baclofen for the short-term management of BZD withdrawal for dependent patients. The document also indicated that more extensive trials are needed.
Lamotrigine
Lamotrigine is an anticonvulsant, mood stabilizer, anxiolytic, and antidepressant.28 Analysis of the molecule has shown that lamotrigine can inhibit glutamate via inhibition of neuronal hyperexcitability, and it has essential properties as anxiolytic and mood stabilizer. Lamotrigine, also called lamictal, can modify synaptic plasticity using voltage-dependent inhibition of neuronal voltage-activated Na+ channels. The result is a reduction of excessive transmitter release in the brain of what it is called regulating aberrant intracellular and intercellular signaling in the limbic system.29 The drug’s ability to inhibit glutamate makes it an appealing hypothetical agent for BZD withdrawal and discontinuation. There is concern about a severe and life-threatening rash, Stevens-Johnson syndrome, requiring hospitalization. There is a lack of studies of lamotrigine for BZD dependence and withdrawal. Pavlovic wrote an exciting letter to the Journal of Neuropsychiatry Clinical and Neurosciences that was cited by Zalewska-Kaszubska and colleagues in 2015 in Physiology and Behavior. Pavlovic reported the case of a woman dependent on alcohol and BZDs who was initially withdrawn from diazepam and switched to topiramate. Because of complaints of side effects, the patient received lamotrigine at a dose of 200 mg per day. She then became completely abstinent after 16 weeks of treatment on lamotrigine.30 The paper suggested considering lamotrigine as a likely choice for both alcohol and BZD dependence.
Trazodone
Trazodone is a weak antidepressant that has antihistaminic properties at the H1 receptor. Both antagonist effects at the 5-HT2 A and α1 adrenergic receptor in addition to its antihistaminic properties make the drug multifunctional, such as being a hypnotic at low doses and an antidepressant at higher doses by blocking serotonin transporter. Trazodone blockade of the serotonin receptor makes the drug an appealing anxiolytic,31 hence the idea of using trazodone as an alternative for BZD dependence. Trazodone can cause the dramatic side effect of priapism, requiring urgent surgery to salvage the organ. Possibly, this priapism is related to α-adrenergic blockage. Ansseau and colleagues treated patients with BZD dependence (n = 10) with trazodone (>100 mg a day), while the BZDs were tapered slowly and progressively. All the patients had to be hospitalized to conduct the study. Limited withdrawal from BZDs was observed.32 The study concluded that trazodone might be useful as an alternative for patients who are addicted to BZDs.
Valproic acid
Valproic acid (VPA) is an anticonvulsant used in psychiatry as a mood stabilizer for patients with bipolar disorder. VPA is sedative perhaps due to increased neurotransmission at GABA receptors. Several studies have shown that VPA favors the accumulation of GABA in the brain selectively at GABA neurotransmitter terminals. VPA action inhibits voltage-gated sodium channels, thus inhibiting the action potential and propagation of cells that have been excited.33 Another study in male Wistar rats postulated an anxiolytic property of VPA. VPA is comparable to diazepam in terms of antianxiety capability.34,35 VPA is highly sedative and can cause liver and pancreatic damage. Initially, uncontrolled studies by Keck and colleagues and Manseau and colleagues suggested that valproate and trazodone could help in reducing the withdrawal symptoms that occur when BZDs are tapered. Rickels and colleagues conducted a double-blind study to assess whether trazodone and valproate would minimize withdrawal symptoms from BZDs and could also facilitate the process of discontinuation of BZDs. Seventy-eight chronic BZD users were randomized to receive double-blind treatment with trazodone (n = 41), sodium valproate (n = 19), or placebo (n = 18). Both trazodone and valproate were compared with placebo. Although at the beginning of the discussion of the findings of the study, it was noted that neither trazodone nor valproate reduced withdrawal severity compared with placebo, the study then showed that more patients on trazodone and valproate than placebo could remain BZD free for at least 5 weeks. It seems that the speculation that trazodone induces sleep and its sedative properties could assist patients during the tapering period, and the same benefit applies for valproate. There was no clear evidence that either trazodone or valproate could substitute for BZDs, and neither had a significant effect on BZD withdrawal severity.36
Carbamazepine
Carbamazepine (CBZ) is an anticonvulsant with extensive use in psychiatry as a mood stabilizer for patients diagnosed with bipolar mania. CBZ use for bipolar disorder is decreasing in the United States due to its side-effect profile and the need for blood-level monitoring. It seems that CBZ’s mechanism acts on the limbic system and the temporal lobe by a kindling effect related to constant repetition of subtherapeutic electric stimulation.37,38 The pharmacological explanation is complicated, and it goes beyond this review. CBZ blocks sodium channels that are voltage dependent, and the primary outcome is to remove the hyperpolarization state, causing hyperexcitation.39 Such a mechanism might explain the sedative effects of the drug. CBZ, as a CYP450 inducer, can increase the clearance of phenytoin, erythromycin, isoniazid, and propoxyphene, reducing their concentrations to subtherapeutic levels.
CBZ can cause blood dyscrasia. A double-blind treatment study of patients (n = 55) placed on CBZ or placebo aimed to assess whether CBZ could reduce the withdrawal symptoms of patients gradually tapered off BZDs. In fact, patients (n = 40) participated in the survey after dropping out. The Patient Withdrawal Checklist monitored daily withdrawal symptoms. The authors reported that 95% of patients on CBZ remained free of BZDs for 5 weeks.40 In this study, CBZ was recommended as an adjunctive therapy for patients undergoing withdrawal of BZDs. CBZ is the most studied among the medications proposed or recommended for substitution during the BZD discontinuation period. None of the studies could identify the central mechanism or theory of choosing CBZ; it seems to be historical only. A discontinued systematic review stated that CBZ could be useful for BZD discontinuation during taper.41
Gabapentin
Although gabapentin has a structural analog of GABA, it does not possess the exact capability of action on GABA receptors, and it does not block either the metabolism or uptake of GABA. Gabapentin does treat partial onset seizures seemingly due to its involvement or interaction with a receptor linked to the L-system amino acid transporter protein.42 The L-system is composed of neural amino acid transport agents of the sodium channel. A full explanation of how the system works is beyond the scope of this review. A plausible theory explaining how gabapentin is anxiolytic and why it is recommended for BZD withdrawal is that gabapentin can increase GABA (inhibitory effect) concentrations at the extracellular level in the brain.43 Among the attractive aspects of gabapentin are its limited abuse potential and low side-effect profile, and it does not require blood-level monitoring. Gabapentin does not affect the hepatic metabolism of other medications. Mariani and colleagues, in a pilot trial, evaluated gabapentin for patients with BZD abuse or dependence on methadone maintenance. The study had a limitation in statistical power. Participants (n = 19) were enrolled in either a placebo arm or a gabapentin arm.44 The study concluded that no difference was noted in patients on gabapentin compared with placebo. Despite gabapentin being able to increase GABA concentrations, no benefit was found in this study. There are several unpublished papers on gabapentin’s benefits for BZD dependence and withdrawal. On 25 September 2017, the National Institutes of Health updated a clinical trial of gabapentin for BZD dependence. It seems that the preliminary results are positive [ClinicalTrials.gov identifier: NCT01893632].
Pregabalin
Pregabalin is a controlled substance under schedule V as of July 2005. This GABA analog. (S)-3-(aminomethyl)-5-methylhexanoic acid has several properties, including management of seizures, neuropathic pain, fibromyalgia, anxiety, and low back pain. The antiseizure indication is approved in the United States as an adjunct for patients older than 18 years of age with partial seizures. Several double-blind, randomized clinical trials have proven that pregabalin could control partial seizures.45 Pregabalin possesses side effects such as those of all central nervous system depressing drugs. Pregabalin can cause fatal renal failure in overdoses. As a schedule V drug, pregabalin has the potential for abuse and dependence. Pregabalin is a GABA analog; its pharmacological properties do not seem to ascribe to the drug the value of being related to the GABA neurotransmitter or the metabolism of GABA. The binding of pregabalin to α2-δ proteins of neurons (calcium channel α2-δ subunit) might confer its primary targeting. This target appears to decrease synaptic release, subsequently decreasing abnormal network hyperexcitability, which is a mechanism that can explain the prevention of seizures and the anxiolytic properties of the drug studied for the most part in animals.46 In a double-blind, placebo-controlled trial conducted in Italy, France, Spain, Mexico, Guatemala, the Czech Republic, and Costa Rica, Hadley and colleagues evaluated the efficacy of pregabalin for patients (n = 106) in facilitating the tapering of BZDs. These patients were diagnosed with generalized anxiety disorder and were treated for weeks with BZDs. The Hamilton Anxiety Rating Scale (Hamilton) and Physician Withdrawal Checklist measured the probable outcomes. At the time of the study, patient enrollment was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria used to diagnose generalized anxiety disorder. The two-arm research included a 12-week double-blind treatment with pregabalin at a dose of 300–600 mg per day, while the other arm, the placebo group, was tapered at the recommended rate suggested to decrease BZDs of 25% per week gradually. After the initial taper was complete, the patients spent an average of 6 weeks free of BZDs.47 The study concluded that pregabalin fared better than placebo.
Flumazenil
Hunkeler and colleagues described and synthesized flumazenil. Flumazenil (Ro 15-1788) is a 1,4- imidazo BZD that possesses specific antagonism at the level of BZD receptors. Flumazenil can treat near-fatal overdoses of BZDs.48 Flumazenil’s role in BZD dependence is the reversal of the sedative effect, resulting in no impact while one is abusing BZDs. In countries such as Italy, flumazenil is the gold standard for the treatment of patients dependent on high dose BZDs. Flumazenil carries the risk of fatal junctional or ventricular tachycardia and seizures. The drug has been the subject of several studies for the management of BZD withdrawal. A pilot study (n = 11) and a double-blind pilot study (n = 10) reported alleviation of BZD withdrawal with flumazenil. A randomized, controlled clinical trial (n = 50) showed a reduction of withdrawal symptoms with flumazenil versus oxazepam. Additionally, the study concluded that flumazenil demonstrated the ability to return BZD receptors to normal function.49 Another approach is the use of a feasible dose of flumazenil, which is a low dose, for the treatment of BZD dependence. A review of treatment for BZD dependence management noted that flumazenil might be able to control BZD tolerance and withdrawal for patients in long-term therapy. An exciting point reported in this review was that a study performed on rats treated with flumazenil did not seem to cause the rats to become tolerant, and they were able to benefit from the antianxiety effects even without being on BZDs. Some other advantages of flumazenil occur regarding memory and psychomotor performance.50 However, this last study was not without conflicts of interest.
Dothiepin
Dothiepin is a tricyclic antidepressant that medicates both serotonin and noradrenaline by the mechanism of reuptake inhibition. Dothiepin is a sedative due to its property of being an antagonist of histamine H1 receptors. It is used as an anxiolytic-like amitriptyline.51 Dothiepin has several side effects comparable to other tricyclic antidepressants, such anticholinergic effects causing dry mouth and constipation relative to its antihistamine-effect side effects; dizziness and drowsiness have been reported, elevating the risks of falls and fractures. The choice of dothiepin among other tricyclic antidepressants remains debatable. In a controlled trial of dothiepin versus placebo to treat BZD withdrawal, patients (n = 87) with no diagnosis of depression but suffering from BZD dependence were selected for a randomized trial of dothiepin and placebo. One group (n = 41) received dothiepin and another group (n = 46) received placebo. The study favored the placebo group over the dothiepin group. In the placebo group, 41% of patients stopped taking BZDs, while 31% of patients in the dothiepin group stopped BZD use.52 In this study, it was argued that dothiepin might not have any weight in the treatment of BZD withdrawal. However, the study related the failure to a possible issue related to a type II error. The idea of drug withdrawal was not clarified.
Diazepam solo
Diazepam is a long-acting BZD that produces several active metabolites, including temazepam, oxazepam, and desmethyldiazepam. Desmethyldiazepam accumulates in a significant manner in the elderly. Note also that each of these products has its own effect.53 Diazepam produces rapid sedation. It accumulates with time in the body due to a prolonged duration of close to 120 h. One must exercise caution when prescribing diazepam because the buildup of several metabolites can cause erratic changes in homeostasis, such as impairment in cognition, memory loss, slowness of time with severe psychomotor retardation, and slow reflexes. The best rationales for choosing a long-acting BZD are to compensate for the early appearance of withdrawal and for the administration of multiple doses with time. In a sophisticated approach, Zitman and colleagues could successfully taper off 26% of patients (n = 230) from BZDs. In the first phase of the study, BZD-dependent patients were switched to an equivalent dose of diazepam. In the second phase, patients received either placebo or 20 mg of paroxetine. In the last phase, the patients were tapered off diazepam. The study concluded that gradual transfer to diazepam is an effective method to discontinue chronic use of BZDs, and selective serotonin reuptake inhibitors had no weight in the study for the success of diazepam. Further analysis of the study, in the section entitled ‘Discussion section: clinical implications and limitations’, seems to pose an issue because the study could not compare the rates between tapered patients and those who became free of depression.54 The addition of paroxetine was not negligible.
Imipramine and buspirone
Imipramine is an antidepressant in the group of tricyclics; it has a lesser sedative effect, making it attractive to use. Imipramine plays a role in the treatment of dysthymia, depression, and bipolar disorder. The drug has the same side effects as the other tricyclic antidepressants (TCAs). Buspirone is an antianxiety medication with a low side-effect profile. It is used alone or as an adjunct with more effective antianxiety medicines. Initially, buspirone’s mechanism of action was believed to be related only to antagonistic effects at D2 receptors; however, buspirone’s effects displace 8-OH-DPAT from 5-HT1A receptor binding sites. A double-blind, placebo-controlled study of patients (n = 107) with depression and anxiety disorders compared imipramine (180 mg per day) and buspirone (38 mg per day) with placebo for BZD discontinuation. The BZD taper period followed the recommendation of 25% per week. Both sets of results were statistically significant compared with placebo. Imipramine provided a higher success rate than buspirone: more than 82% for imipramine and more than 67% for buspirone.55 However, the study found that this result was modest in terms of BZD discontinuation. Imipramine and buspirone could reduce depression and anxiety symptoms with a successful taper.55 Overall, the study suggested imipramine for BZD discontinuation. The same conclusion did not seem to apply to buspirone.
Melatonin
At the level of the pineal gland, melatonin, a hormone under the influence of the circadian rhythm, is implicated in sleep regulation. Two receptors, MT1 and MT2, receive signals via G-protein-coupled receptors. It appears that the MT2 receptor might be predominantly involved in sleep regulation. MT2 receptor’s activation in the suprachiasmatic nucleus seems to control the release of dopamine in the retina. Infusion of a drug that stimulates the MT2 receptor at the reticular thalamus engenders a burst of firing of the GABAergic neuron. This mechanism can also explain the possible anxiolytic effects of melatonin.56–59 Melatonin causes fewer psychomotor problems, an aspect that can be beneficial for the elderly. Melatonin can cause hypotension, drowsiness, headache, dizziness, nausea, vomiting, and abdominal cramps. Cardinalia and colleagues proposed a change of view on the use of BZDs and presented melatonin as a drug with no detrimental effects such as those of BZDs, including addiction and dependence. A study comparing temazepam with melatonin endorsed melatonin over temazepam in the management of sleep and circadian rhythms.60 A relatively small sample (n = 34) of patients on BZDs were compared double blinded with melatonin (the controlled release form of 2 mg) and placebo. In this trial, the patients were recommended to take 50% of their benzodiazepine medication dose in the first two weeks, 25% during weeks 3 and 4, and then to stop the medication during weeks 5 and 6. The study concluded that melatonin in the controlled release form might be used as a facilitator of BZD discontinuation.61 In a different article, a meta-analysis comparing melatonin with placebo with the objective of BZD discontinuation concluded that melatonin had no substantial benefit for BZD discontinuation.62 Despite its great activity of modulating GABA receptor, the use of melatonin for BZD discontinuation is unsatisfactory.
Lormetazepam
Lormetazepam is a BZD with a high addiction potential, and it is used especially in Italy, where the drug accounts for approximately 50% of BZD use disorder.63 Note that lormetazepam is not approved for sale in the United States or Canada. Lormetazepam has similar liability risks to those of all BZDs. Lormetazepam is a BZD with frequent use in Italy at a rate of 13.3 per 1000 inhabitants per day.64 Petrovic and colleagues substituted lorazepam or placebo for BZDs in geriatric patients with no recorded mental illness. The group determined a good response to either lormetazepam or placebo by the improvement of sleep quality and the control of withdrawal symptoms. The lormetazepam arm showed better improvement in sleep and withdrawal symptoms from BZD discontinuation than the placebo group: approximately 80% for lormetazepam and 50% for placebo.65 The study had some limitations: one was a small sample that was not well defined, and the other was a dropout rate that the author classified as unequal. Another concern is that 1 mg of lormetazepam was predetermined at the beginning of the study, based on success with lormetazepam for sleep improvement. There was no recommendation that lormetazepam could be a substitute for BZD during the discontinuation period.
Cyamemazine
Cyamemazine (CMZ) is not approved for sale in the United States. CMZ is a typical antipsychotic in the class of thiazines, which are phenothiazines on the market basically as insecticides. In the 1940s, it was found that CMZ had no intrinsic property to kill insects, but it had two important properties: an antihistaminic that makes the molecule an antiallergic, in addition to its possessing a strong sedative effect. The molecular property of the drug propelled it into the market as an anxiolytic antipsychotic for patients with schizophrenia and patients with severe major depressive disorder with suicidality. Around 1980, CMZ was the subject of a study for the treatment of anxiety, impulsiveness, and aggression with limited study designs and methods. CMZ, as with other antipsychotics, can cause neuroleptic malignant syndrome and extrapyramidal syndrome, but the extrapyramidal syndrome was found to be less common than with atypical antipsychotics due to its higher affinity for h5-HT (2A), h5-HT (2C), and h5- HT (7) receptors. Note the h5-HT (2C) receptor affinity of CMZ grants it anxiolytic properties in human subjects.66 The utilization of antipsychotics for sleep and sedation is not new. Atypical antipsychotics, such as olanzapine, have increased risks of oversedation, and chlorpromazine, a low-potency typical antipsychotic, can also lead to oversedation. In a prospective, open study performed by Bourin and colleagues, the investigators enrolled ambulatory patients (n = 40) diagnosed with anxious-depressive syndrome.67 The 40 patients enrolled were placed on a mean dose of CMZ of 97.5 mg for a period of 37.7 days (mean daily dose intake: 97.5 mg; range: 45–300 mg daily; mean period: 37.7 days). The maximum number of days was 42. The efficacy of CMZ for anxiety and depression was evaluated with the Hamilton scale. Twenty-four patients (n = 24) had scores less than 16 after 21 days of therapy. A score less than 16 is categorized as moderate depression, but the study concluded that CMZ improved anxiety first and depression later. It was Lemoine’s group that investigated CMZ for the possible management of BZD withdrawal based on the anxiolytic ability of the drug to reduce alcohol withdrawal symptoms. The study enrolled 168 patients (n = 168) randomized to CMZ (25–50 mg every day) or bromazepam (3–6 mg every day) for approximately 4 weeks and an additional 2 weeks on placebo.68 The study concluded that there was no extrapyramidal syndrome observed. In addition, 65.5% of the patients in the bromazepam group were successfully withdrawn from BZDs, making CMZ useful for BZD substitution and for controlling bromazepam withdrawal.
Phenobarbital
Phenobarbital is a barbiturate that lengthens the time of the opening of the chloride channel, which is an essential mechanism in GABA-A receptor response. One of the major differences between barbiturates and BZDs is the ability of barbiturates to act directly on the chloride channel and activate that channel, while BZDs increase the frequency of the opening of the chloride channel,69–71 a difference that might explain why barbiturates are more dangerous than BZDs. Phenobarbital, in comparison with CBZ, VPA, and phenytoin, is associated with more withdrawal symptoms. It is difficult to confirm that phenobarbital causes more adverse effects than the other three drugs.72 As with other barbiturates, phenobarbital can be lethal in overdose. In an observational study of patients (n = 310) over a period of 5 years, BZDs were discontinued while patients were switched to phenobarbital. The parameters of efficacy or no efficacy of phenobarbital included the incidences of falls, seizures, delirium, emergency visits, and readmissions. The study concluded positively for phenobarbital as a tapering protocol for BZD discontinuation. Quoting the survey, ‘phenobarbital may be a good choice’ for BZD withdrawal protocols. The study claimed to be the most extensive case series at the time of the publication. As noted, there were limitations in terms of the effectiveness of phenobarbital over the long term, and there was no comparison group.73
Ondansetron
Ondansetron is an antiemetic drug in the class of serotonin receptor antagonist (5-HT3). Ondansetron has been the subject of attention for the management of BZD withdrawal. A study of the clinical correlations of ondansetron was modeled to determine the effect of ondansetron in animals (n = 6) divided into six groups. One group received normal saline, four groups received ondansetron, and another group (n = 1) received diazepam. The elevated plus maze is a test conducted in the laboratory to screen (rats) for compounds with anxiogenic or anxiolytic properties. The animals were subsequently placed in the elevated plus maze test. Ondansetron resulted in a better anxiolytic profile than in the diazepam group. The study concluded that ondansetron (a 5-HT3 antagonist) presented a new mechanistic approach to controlling anxiety.74 As mentioned by the authors, the study was limited due to the inadequate sample size. Ondansetron was not without side effects. QT prolongation can be an issue, especially for patients born with long QT syndrome. A different result was published in a human model study of patients (n = 108) on alprazolam or lorazepam. After approximately 3 weeks, more than 60% of the patients stated that they stopped BZD use, and after 1 year, more than 67% of them remained stable; however, the study concluded that ondansetron did not have any significant effects on the withdrawal symptoms of BZDs. A similar result was reported for the control of anxiety.75 The translation of ondansetron’s benefit, as evidenced by the elevated maze plus test, has had no replication in human clinical trials. The study suggested the need for newer animal models to study what is called chronic state anxiety.
Valerian
Valeriana officinalis is a perennial plant with sedative and soporific properties, in addition to being an anticonvulsant and analgesic. Studies of valerian’s sedative effects, yet to be elucidated, have focused on the implications of its effects on GABA receptor.76 Valerian has no recommendations for patients taking opiates, BZDs, barbiturates, or alcohol due to an increased risk of respiratory depression. There was a case report on delirium and cardiac complications in a patient withdrawing from the herb.77 Valerian also causes hepatic toxicity. Poyares and colleagues studied patients (n = 19) on treatment for insomnia with BZDs but had no improvement. These patients were subsequently withdrawn from the BZDs and restarted on valerian. Valerian seemed to ameliorate their sleep and alleviate their withdrawal symptoms from BZDs tapered.
Hydroxyzine
Hydroxyzine is an antihistamine with anxiolytic properties that belongs to the diphenylmethane and piperazine class.78 The drug is a culprit in causing nightmares and vivid dreams, in addition to weight gain. Lopez-Peig and colleagues designed a study with the purpose of showing that a BZD withdrawal program was feasible in primary care in Spain with the aid of nursing professionals. An exciting aspect of the study indicated both hydroxyzine and valerian for patients taking BZDs. Some of the patients were on BZDs for 6 months. The Goldberg Scale determines the improvement of symptoms of BZD withdrawal. At the end of the study, more than 80% of the patients stopped taking BZDs, and more than 60% of patients stayed abstinent for more than a year.79 The study did not include a placebo arm. It seems that the study did not evaluate whether valerian or hydroxyzine would benefit BZD discontinuation.
Tiagabine
Tiagabine facilitates the reuptake of GABA. Tiagabine has shown benefit in the treatment of seizures refractory to known anticonvulsants. An open-label clinical trial showed improvement of depression and anxiety with tiagabine.80 A 68-year-old female patient abusing bromazepam for 5 years was switched to tiagabine. Improvement of her anxiety was noted in approximately 4 weeks. Initially, the patient had a Hamilton score of 39, and then it decreased to 22. The study was based on one observation of a change in the Hamilton score. The extrapolation of antianxiety benefit makes it a promising drug for the treatment of BDZ dependence.81 Apparently, controlled clinical trials are lacking.
β-Carboline abecarnil
Abecarnil belongs to the family of β carbolines. The drug is an anxiolytic and anticonvulsant as a partial agonist at the BZD site, but it has acted as a non-BZD in animal models. It is hypothesized that abecarnil is less sedative and less addictive than BZDs, but symptoms such as unstable gait, difficulty of concentration, somnolence, and dizziness have been noted. Two studies in mice recommended abecarnil for BZD withdrawal due to possible low tolerance and dependence on abecarnil. Natolino and colleagues analyzed the results of abecarnil in mice that were withdrawn from diazepam. Abecarnil could protect against the occurrence of convulsions from bicuculline induction, even after the administration of [3H]-flumazenil binding in chronic abecarnil-treated mice.82 Pinna and colleagues withdrew mice from alprazolam (Xanax) and replaced it with abecarnil. Abecarnil facilitated the withdrawal period with no signs of dependence reported.83 The study recommended abecarnil as a new method for the rapid tapering of BZDs.
Challenges
It is recommended the discontinuation of BZD be undertaken over time. Prescribing interventions, substitutions, psychotherapies, and pharmacotherapies can all contribute to this process.84 The use of medications to discontinue patients on BZDs requires experienced and credentialed professionals. The overall prevalence of BZD use is estimated at 0.8% for both men and women. Increasing age is correlated with an increased rate of BZD use.85,86 Some behaviors seem to be predictors of BZD use, dependence or misuse, including doctor shopping and obtaining prescriptions from different pharmacies.87 To prevent complications of BZD withdrawal, such as seizures, confusion, and delirium that can be life threatening, guidelines recommend the conversion of any BZD to an equivalent dose of diazepam. The recommendation for diazepam comes from the pharmacodynamic properties of the drug, which include its long half life in weeks. Recall that all BZDs are similar in their clinical effects, but their pharmacodynamic properties make them different. It is appropriate then to reduce BZDs to one-eighth of the dose over a 2-week period. The BZD in question should gradually be discontinued over 4–6 weeks.88–90 Long-term use of BZDs causes several problems not limited to dependence but also impairment in memory and difficulty in coordinating movements with high risk of falls. A report stated that patients not misusing BZDs do not suddenly escalate the dose prescribed, and BZDs are considered sufficiently safe when used appropriately, even in the case of discontinuation.91 Based on a discontinued Cochrane review, the study pointed out that CBZ was the only drug which demonstrated benefit in controlled trials92; however, flumazenil had also shown good results. There have been controlled trials favoring flumazenil for the substitution method for discontinuation of BZDs. A meta-analysis published in 2008 by Parr and colleagues showed that a pharmacotherapeutic approach for BZD discontinuation seems promising, but there is a lack of evidence to support its use now.93 In their study, Rickels and colleagues adopted a firmer tone by concluding that a pharmacological approach for the management of BZD discontinuation, aiding the tapering method, has proven benefits with drugs such as CBZ, imipramine, valproate, and trazodone.94 CBZ, as an inducer, should be administered with caution. Patients on polypharmacy can have a resurgence of symptoms due to reducing the concentrations of their medications to subtherapeutic levels. CBZ requires monitoring with blood work and it can cause a life-threatening rash in patients with the HLA-B* 1502 allele, aplastic anemia, and agranulocytosis. Clonidine and propanol can cause hypotension. The efficacy of both clonidine and propanol is poor. Flumazenil carries the risk of fatal arrhythmia and seizures. It might require cardiac monitoring. VPA involves control of blood levels; it causes sedation and risks of falls, in addition to hepatitis, pancreatitis, and encephalopathy. The rationale to use diazepam as a sole method for substitution is based on the pharmacokinetics of the drug. It has a long half life; however, diazepam has erratic absorption and a low therapeutic index when it approaches the level of adverse tolerance. Additionally, diazepam will cause cross tolerance with other drugs that utilize GABA receptors. Diazepam’s potential risk is elevated in the elderly with aging kidneys, prolonging the time of the drug in the body by the accumulation of N-demethylated, a metabolite of diazepam, causing lengthy sedation. The same is observed in newborns.95 Drugs such as phenobarbital, a barbiturate, can be fatal with overdose. BZDs were initially marketed to replace barbiturates due to the better safety profiles of BZDs. One can question the rationale of utilizing a barbiturate to discontinue a BZD. Fatal cases of barbiturate overdose have mentioned coma lasting days.96 Additionally, there have been limited data recommending phenobarbital for pharmacological management of BZD dependence. It seems that the risk outweighs the benefit. Both imipramine and buspirone showed fair results in treating depression and anxiety, respectively. Imipramine is a tricyclic antidepressant with an elevated risk of suicide by overdose with fatal arrhythmia. The overall incidence of arrhythmias is low97 and hypotension is more common.98 Lamotrigine carries the risk of a severe, life-threatening rash, Stevens-Johnson syndrome, requiring hospitalization. There is insufficient evidence to support lamotrigine in the management of BZD dependence. It was also reported that lamotrigine overdose was associated with acute pancreatitis.99 CMZ, an antipsychotic, can cause neuroleptic malignant syndrome and extrapyramidal syndrome. There are insufficient data to support CMZ in the management of BZD discontinuation. CMZ is not FDA approved in the United States.
There are also other publications of rapid BZD withdrawal treated successfully with topiramate100 and patients detoxified with oxcarbamazepine who completed withdrawal from BZDs with no reported withdrawal symptoms.101 Using long-acting BZDs as a tapering method was not only attempted with diazepam but also with clonazepam. Patients (n = 37) who were dependent on Xanax were tapered with the substitution of clonazepam safely and efficiently. Note that clonazepam was used in an open-label fashion.102 α-β Laspartate magnesium was not efficacious for BZD cessation.103 Paroxetine and mirtazapine were both helpful for BZD withdrawal.104,105 Many clinicians seem comfortable with and believe that the best method for tapering alprazolam is to switch to clonazepam due to the longer half life and a longer delay in the appearance of withdrawal.
Discussion
Summary of main results
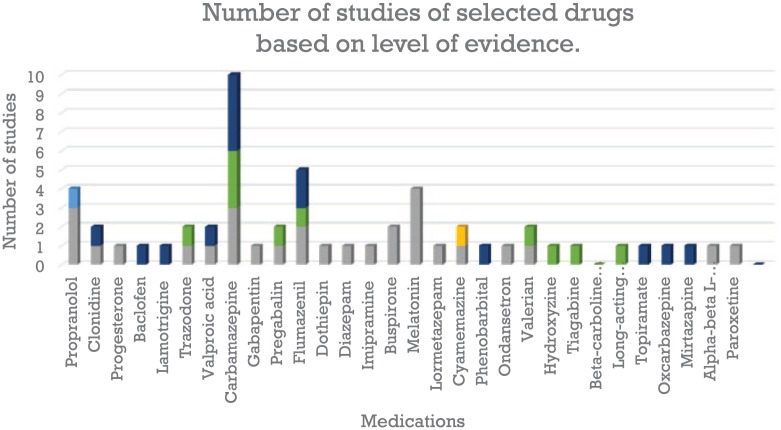
Overall seven (7) medications fared better compared to others from the list of drugs we reviewed for benzodiazepine substitution or discontinuation methods (Table 1). Based on the level of evidence,106 CBZ, flumazenil, propranolol, and melatonin seem to be the most studied. The highest number of studies for CBZ is at level III, but the top for melatonin is at level I; 67% of the drugs fall at least in one randomized controlled trial (Figure 3). Some of the medications recommended for BZD discontinuation are relatively safe to use; some of them have a narrow therapeutic index, with severe, life-threatening side effects. There is no gold standard approach which is a fundamental problem. The most common method is tapering. Tapering involves a hypothetical percentage of gradual reduction of the BZD prescribed, and to avoid discomfort, tapering also combines other medications as substitution. Psychotherapy alone has not been shown to suffice for the discontinuation recommendation. There are research gaps in medications that have been used for substitution, such as the paucity of double-blind, randomized, comparative studies. Some of the drugs suggested for substitution are only based on anecdotal, single case reports, and opinion. The neurobiological basis for substitution is drawn mostly from extrapolation of medications that can alter withdrawal state and discomfort. Some of them can soothe adrenergic responses and a medication like flumazenil can entirely block the effect of BZD. The significant challenges of the pharmacological management of BZD dependence and discontinuation include a lack of analysis of the side effects, risks, and benefits.
Table 1.
Pharmacological management for benzodiazepine discontinuation. Summary of the results section.
| Study | Sample/number of studies | Purpose of the study | Level of evidence106 | Study designs | Key findings |
|---|---|---|---|---|---|
| Propranolol | Level I | ||||
| Cantopher et al. (1990)15 | Patients (n = 31) | Propranolol substituted for diazepam | Randomized controlled trials | No sufficient evidence to support the routine use | |
| Steenen et al. (2016)16 | Studies (n = 8) | Propranolol versus BZDs for anxiety | Meta-analysis | No advantage | |
| Clonidine | Leve III/I | ||||
| Keshavan et al. (1987)107 | Patient (n = 1) | Clonidine for BZD withdrawal | Descriptive study | Successful withdrawal management versus placebo | |
| Vinogradov et al. (1986)19 | Patient (n = 1) | Clonidine for alprazolam withdrawal | Descriptive study | Successful withdrawal management | |
| Goodman et al. (1986)20 | Patients (n = 3) | Clonidine for BZD withdrawal | Double blind, placebo-controlled | Failed | |
| Progesterone | Level I | ||||
| Schweitzer et al. (1995)108 | Patients (n = 43) | Efficacy of progesterone for discontinuation of BZDs | Randomized controlled trials | No difference compared with placebo | |
| Baclofen | Level III | ||||
| Shukla et al. (2014)27 | Patients (n = 5) | For BZD withdrawal and abstinence | Descriptive study | Patients tolerated the switch and remained abstinent | |
| Lamotrigine | Level III | ||||
| Pavlovic (2010)30 | Patient (n = 1) | Lamotrigine for diazepam withdrawal | Descriptive study | Complete abstinence after 16 weeks | |
| Trazodone* | Level I/II-2 | ||||
| Rickels et al. (1999)36,94 (trazodone + VPA) | Patients (n = 78) | Minimize withdrawal symptoms from BZD | Double-blind study | No clear evidence | |
| Ansseau et al. (1992)32 (trazodone alone) | Patients (n = 10) | Treated BZD dependence | Controlled trials | Limited withdrawal from BZD | |
| without randomization | |||||
| VPA* | Level I | ||||
| Rickels et al. (1999)36,94 (trazodone + VPA) | Patients (n = 78) | Minimize withdrawal symptoms from BZD | Double-blind study | No clear evidence | |
| CBZ$ | Level I | ||||
| Schweizer et al. (1991)40 | Patients (n = 40) | Gradual taper off BZD | Randomized controlled trials | 95% of patients on CBZ remained free of BZD | |
| Denis et al. (2006)41 | Eight trials, participants (n = 458) | Pharmacological interventions for BZDD mono dependence | Systematic review | CBZ could be useful | |
| Gabapentin | Level I | ||||
| Mariani et al. (2016)44 | Participants (n = 19) | For patients with BZD abuse/dependence | Pilot trial | No difference between gabapentin and placebo | |
| On methadone maintenance | |||||
| Pregabalin$ | Level I | ||||
| Hadley et al. (2012)47 | Patients (n = 106) | Facilitating the tapering of BZDs | Randomized controlled trials | Pregabalin fared better than placebo | |
| Flumazenil$ | Level I | ||||
| Gerra et al. (1993)109 | Participants (n = 50) | Flumazenil versus oxazepam tapering for BZD withdrawal | Randomized controlled trials | Ability to control BZD withdrawal and tolerance | |
| Dothiepin$ | Level I | ||||
| Tyrer et al. (1996)52 | Patients (n = 87) | Dothiepin versus placebo to treat BZD withdrawal | Randomized controlled trials | May reduce BZD withdrawal | |
| Diazepam$ | Level I | ||||
| Zitman et al. (2001)54 | Patients (n = 230) | Gradual transfer of other BZD to diazepam | Randomized controlled trials | Effective way of discontinuing chronic BZD use | |
| Imipramine*$ | Level I | ||||
| Rickel et al. (2001)55 (imipramine and buspirone) | Patients (n = 107) | BZD discontinuation | Randomized controlled trials | Imipramine had higher success rate than buspirone | |
| Modest success for BZD discontinuation | |||||
| Buspirone* | Level I | ||||
| Rickel et al. (2001)55 (imipramine and buspirone) | Patients (n = 107) | BZD discontinuation | Randomized controlled trials | No advantage | |
| Melatonin | Level I | ||||
| Cardinalia et al. (2016)60 | Patients (n = 34) | BZD discontinuation | Randomized controlled trials | Can be a facilitator of BZD discontinuation | |
| Wright et al. (2015)62 | Six trials. Participants (n = 322) | BZD discontinuation | Meta-analysis | Unsatisfactory for BZD discontinuation | |
| Effect of melatonin on sleep quality | Effect on sleep quality varied | ||||
| Lormetazepam* | Level I | ||||
| Petrovic et al. (2002)110 | Sample was not well defined | Sleep quality and control of withdrawal symptoms from BZD (geriatric patients) | Randomized controlled trials | No recommendation | |
| CMZ$ | Level I/II-1 | ||||
| Bourin et al. (2004)67 | Patients (n = 40) | CMZ for anxiety and depression | Controlled no randomization | CMZ improves anxiety | |
| Lemoine et al. (2006)68 | Patients (n = 168) | Management of BZD withdrawal | Randomized controlled trials | Useful for BZD substitution | |
| CMZ versus bromazepam | Controls bromazepam withdrawal | ||||
| Phenobarbital | Level III | ||||
| Kawasaki et al. (2012)73 | Participants (n = 310) | Phenobarbital as a tapering protocol for BZD discontinuation | Observational study | May be effective | |
| Ondansetron | Level I | ||||
| Romach et al. (1998)75 | Participants (n = 97) | Adjunct medication for BZD discontinuation | Randomized controlled trials | No advantage, high placebo response | |
| Valerian* | Level I/II-3 | ||||
| Poyares et al. (2002)77 | Patients (n = 19) | Withdrawal of BZD, sleep | Uncontrolled trials | Shows improvement | |
| Lopez-Peig et al. (2012)79 (valerian and hydroxyzine) | Participants (n = 51) | Analysis of BZD withdrawal with valerian and hydroxyzine | Pseudoexperimental study | No conclusion of the study | |
| Hydroxyzine* | Level II-3 | ||||
| Lopez-Peig et al. (2012)79 (valerian and hydroxyzine) | Participants (n = 51) | Analysis of BZD withdrawal with valerian and hydroxyzine | Pseudoexperimental study | No conclusion of the study | |
| Tiagabine | Level II-3 | ||||
| Oulis et al. (2009)81 | Participants (n = 1) | Bromazepam switched to tiagabine | Open-label clinical trial | Promising for BZD discontinuation | |
| Aim: improvement of depression and anxiety | |||||
| β-Carboline abecarnil* | None | ||||
| Natolino et al. (1996)82 | Unknown | Abecarmil replaces discontinued diazepam | Experimental study | Possible new method for rapid tapering of BZD | |
| Pinna et al. (1997)83 | Unknown | Abecarmil replaces discontinued alprazolam | Experimental study | Possible new method for rapid tapering of BZD | |
| Long-acting BZDs | Level III | ||||
| Patterson (1990)102 | Patients (n = 37) | Clonazepam substituted alprazolam | Uncontrolled trials | Safe and efficient substitution | |
| Topiramate | Level III | ||||
| Cheseaux et al. (2003)100 | Patient (n = 1) | Rapid BZD withdrawal with topiramate | Descriptive study | Successful withdrawal | |
| Oxcarbazepine | Level II-3 | ||||
| Croissant et al. (2008)101 | Patients (n = 10) | Oxcarbazepine for BZD detoxification | Descriptive study | Successful withdrawal | |
| Mirtazapine | Level III | ||||
| Chandrasekaran (2008)105 | Patient (n = 1) | Mirtazapine for BZD withdrawal | Descriptive study | Alleviate withdrawal symptoms | |
| Paroxetine | Level I | ||||
| Nakao et al. (2006)104 | Participants (n = 97) | Paroxetine for tapering BZD | Randomized controlled trials | SSRI may be beneficial for BDZ withdrawal | |
| A-β L-aspartate magnesium | Level I | ||||
| Hantouche et al. (1998)103 | Participants (n = 144) | α-β L-aspartate magnesium in discontinuation of long-term BZD use | Randomized controlled trials | Promising. Needs more clinical trials |
Level I: evidence obtained from at least one properly designed randomized controlled trial; level II-1: evidence obtained from well designed controlled trials without randomization; level II-2: evidence obtained from well designed cohort studies or case-control studies, preferably from more than one center or research group; level II-3: evidence obtained from multiple time series designs with or without the intervention; dramatic results in uncontrolled trials might also be regarded as this type of evidence; level III: opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees.
For β-carboline abecarnil, the study was done in mice. The sample is unknown. Lormetazepam: the study aimed at withdrawal from BZDs in geriatric inpatients, not specifically for BZD discontinuation. Trazodone: the study by Rickels and colleagues includes both trazodone and VPA. Imipramine and buspirone: the study by Rickels and colleagues includes both drugs. Valerian: the study by Lopez-Peig and colleagues includes both valerian and hydroxyzine.
Seven medications showed positive results. All of them have at least one randomized controlled trial, level I.
BZD, benzodiazepine; CBZ, carbamazepine; CMZ, cyamemazine; SSRI, selective serotonin reuptake inhibitor.
Figure 3.
Number of times a drug appeared at a level of evidence. Carbamazepine, flumazenil, propranolol, and melatonin seem to be the most studied. The highest number of studies for carbamazepine is at level III, but the highest number of studies for melatonin is at level I; 67% of the drugs fall at least in one randomized controlled trial (level I). Grey: level I; yellow: level II-1; light blue: level II-2; green: level II-3; dark blue: level III.
Clinical implications
It should not be dangerously difficult to discontinue patients on long-term BZDs using one of the substitutes suggested with patient consent and proper education. Clinicians have the choice to apply evidence-based guideline for the use of BZD taper and select among seven medications as methods of substitution during the withdrawal period. There is a discrepancy between clinical trials and application of these drugs in practice. It is possible to use them off label judiciously and cautiously. Another aspect is the need to manipulate some of those medications by credentialed professionals. Proper training and credentialing are essential.
Quality of the evidence
Our review has several limitations. The quality of the documents selected for the review varied. It contains not only randomized trials, but review articles, case reviews, and naturalistic studies due to limited randomized studies. There is no comparative study except for CBZ. Although some of the studies have moderate effect size, most of them were not blinded. None of the studies considered the side-effect profile or severe adverse effects of medications suggested for BZD discontinuation. Besides, hypotheses suggesting a drug for BZD substitution are based on extrapolation of the benefit of the drug property in controlling insomnia, adrenergic burst, anxiety, irritability, and cross reaction with other drugs with no evidence of specific pharmacodynamic or pharmacokinetic properties.
Potential biases in the review process
Our methods of extraction are limited to, mostly, publications in English, which can lead to bias. Also, we expand our search beyond randomized controlled studies, which is used to present a generalized and valuable aspect of the topic. This expansion limits our effort to offer a review at a higher level on the scale of the hierarchy of evidence.
Conclusion
Due to impending liabilities, such as overdose, dependence, and life-threatening withdrawal, and the challenges in discontinuing BZDs, many clinicians are overly cautious. Some of them avoid prescribing BZDs to their patients, leading to patient frustration. Patients then remain untreated for symptoms that are genuinely damaging their lives and wellbeing. BZDs carry a high risk of liability; some medical complications, such as memory impairment, respiratory arrest, vehicle accidents, falls, and severe withdrawal symptoms including life-threatening delirium, are frightening. The fear of dependence and tolerance in cases of nonmedical or medical use is a fact. Several medications have been used in pharmacological approaches to alleviate patient suffering during the discontinuation period. Drugs such as buspirone are relatively safe, as is melatonin. Trazodone can relieve anxiety and depression and promote sleep, and hydroxyzine can be obtained over the counter. However, CBZ, depakote, lamotrigine, and phenobarbital have high side-effect profiles and risks of medical complications. They must be administered by specialists who are familiar with them and comfortable using them.
The efficacy of these medications as a method of BZD discontinuation is not robust (Table 1). However, the pharmacological approach seems to be an alternative to abrupt cessation of BZDs, and despite the limitations, it is appropriate to discontinue BZDs after a certain period of use. The discontinuation must be performed in a manner that promotes dignity and reduces suffering. The management of BZD dependence and discontinuation remains a challenge that requires more randomized clinical trials, bearing in mind that the choice of any pharmacological agent must balance side effects, benefits, and risks. A double-blind study with larger effect sizes is necessary. Additionally, new studies must target drugs with fewer side effects and more in-depth studies regarding the specific pharmacodynamic and pharmacokinetic properties of the suggested medications.
Supplemental Material
Supplemental material, TPP-17-09-015_contributor_form for Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation by Dimy Fluyau, Neelambika Revadigar and Brittany E. Manobianco in Therapeutic Advances in Psychopharmacology
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Dimy Fluyau  https://orcid.org/0000-0002-9272-2259
https://orcid.org/0000-0002-9272-2259
Contributor Information
Dimy Fluyau, Emory University School of Medicine, 1648 Pierce Dr NE, Atlanta, GA 30307, USA.
Neelambika Revadigar, Department of Psychiatry, Columbia University, New York, NY, USA.
Brittany E. Manobianco, Emory University School of Medicine, Atlanta, GA, USA
References
- 1. Pal R, Galloway GP. The pharmacology of nonalcohol sedative–hypnotics. In: Herron A, Koehler T. (eds) The ASAM essential of addiction medicine. 2nd ed American Society of Addiction Medicine, 2015; 53–54. [Google Scholar]
- 2. U.S Department of Justice. Drug enforcement administration. Office of diversion control. Benzodiazepines, http://www.deadiversion.usdoj.gov/drugs-concern/benzo-html (2012, accessed May 2017).
- 3. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health 2016; 106: 686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 2015; 49: 493–501. [DOI] [PubMed] [Google Scholar]
- 5. SAMSHA. The DAWN report: highlights of the 2010 Drug Abuse Warning Network finding in drug-related emergency department visits. Rockville, MD: SAMSHA, 2012. [Google Scholar]
- 6. Jones CM, Paulozzi LJ, Mack KA, et al. ; Centers for Disease Control and Prevention. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse–related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep 2014; 63: 881–885. [PMC free article] [PubMed] [Google Scholar]
- 7. Griffin CE, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J 2013; 13: 214–223. [PMC free article] [PubMed] [Google Scholar]
- 8. Sieghart W. Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci 1994; 19: 24–29. [PMC free article] [PubMed] [Google Scholar]
- 9. Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry 2005; 18: 249–255. [DOI] [PubMed] [Google Scholar]
- 10. Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr 2015; 38: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Otto MW, McHugh RK, Simon NM, et al. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: further evaluation. Behav Res Ther 2010; 48: 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kathol RG, Noyes R, Jr, Slymen DJ, et al. Propranolol in chronic anxiety disorders. A controlled study. Arch Gen Psychiatry 1980; 37: 1361–1365. [DOI] [PubMed] [Google Scholar]
- 13. Clark DB, Agras WS. The assessment and treatment of performance anxiety in musicians. Am J Psychiatry 1991; 148: 598–605. [DOI] [PubMed] [Google Scholar]
- 14. Meibach RC, Dunner D, Wilson LG, et al. Comparative efficacy of propranolol, chlordiazepoxide, and placebo in the treatment of anxiety: a double-blind trial. J Clin Psychiatry 1987; 48: 355–358 [PubMed] [Google Scholar]
- 15. Cantopher T, Olivieri S, Cleave N, et al. Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. Br J Psychiatry 1990; 156: 406–411. [DOI] [PubMed] [Google Scholar]
- 16. Steenen SA, van Wijk AJ, van der Heijden GJ. Propranolol for the treatment of anxiety disorders: systematic review and meta-analysis. J Psychopharmacol 2016; 30: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isaac L. Clonidine in the central nervous system: site and mechanism of hypotensive action. J Cardiovasc Pharmacol 1980; 2(Suppl. 1): 5–19. [PubMed] [Google Scholar]
- 18. Hoehn-Saric R, Merchant AF, Keyser ML, et al. Effects of clonidine on anxiety disorders. Arch Gen Psychiatry 1981; 38: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 19. Vinogradov S, Reiss AL, Csernansky JG. Clonidine therapy in withdrawal from high-dose alprazolam treatment. Am J Psychiatry 1986; 143: 1188. [DOI] [PubMed] [Google Scholar]
- 20. Goodman WK, Charney DS, Price LH, et al. Ineffectiveness of clonidine in the treatment of the benzodiazepine withdrawal syndrome: report of three cases. Am J Psychiatry 1986; 143: 900–903. [DOI] [PubMed] [Google Scholar]
- 21. Redmond DE., Jr. Does clonidine alter anxiety in humans? Trends Pharmacol Sci 1982; 3: 477–480. [Google Scholar]
- 22. King TL, Brucker MC. Pharmacology for women’s health. 2nd ed. Sudbury: Jones & Bartlett Publishers, 2010, pp.372–373. [Google Scholar]
- 23. Picazo O, Fernández-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res 1995; 680: 135–141. [DOI] [PubMed] [Google Scholar]
- 24. Schweizer E, Case WG, Garcia-Espana F, et al. Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology (Berl) 1995; 117: 424–429. [DOI] [PubMed] [Google Scholar]
- 25. Davidoff RA. Antispasticity drugs: mechanisms of action. Ann Neurol 1985; 17: 107–116. [DOI] [PubMed] [Google Scholar]
- 26. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomized, double-blind controlled study. Lancet 2007; 370: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 27. Shukla L, Kandasamy A, Kesavan M, et al. Baclofen in the short term maintenance treatment of benzodiazepine dependence. J Neurosci Rural Pract 2014; 5(Suppl. 1): S53–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng F, Hallam K, Lucas N, et al. The role of lamotrigine in the management of bipolar disorder. Neuropsychiatr Dis Treat 2007; 3: 463–474. [PMC free article] [PubMed] [Google Scholar]
- 29. Xie X, Hagan RM. Cellular and molecular actions of lamotrigine: possible mechanisms of efficacy in bipolar disorder. Neuropsychobiology 1998; 38: 119–130. [DOI] [PubMed] [Google Scholar]
- 30. Pavlovic ZM. Long-term treatment and relapse prevention of alcohol and benzodiazepine dependence with lamotrigine. J Neuropsychiatry Clin Neurosci 2010; 22: E25–E26. [DOI] [PubMed] [Google Scholar]
- 31. Stahl S. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr 2009; 14: 536–546. [DOI] [PubMed] [Google Scholar]
- 32. Ansseau M, De Roeck J. Trazodone in benzodiazepine dependence. Eur Neuropsychopharmacol 1992; 2: 382. [Google Scholar]
- 33. Morland C, Nordengen K, Gundersen V. Valproate causes reduction of the excitatory amino acid aspartate in nerve terminals. Neurosci Lett 2012; 527: 100–104. [DOI] [PubMed] [Google Scholar]
- 34. Large CH, Kalinichev M, Lucas A, et al. The relationship between sodium channel inhibition and anticonvulsant activity in a model of generalized seizure in the rat. Epilepsy Res 2009; 85: 96–106. [DOI] [PubMed] [Google Scholar]
- 35. Lal H, Shearman GT, Fielding S, et al. Effect of valproic acid on anxiety-related behaviors in the rat. Brain Res Bull 1980; 5: 575–577.6159056 [Google Scholar]
- 36. Rickels K, Schweizer E, Garcia España F, et al. Trazodone and valproate in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal symptoms and taper outcome. Psychopharmacology (Berl) 1999; 141: 1–5. [DOI] [PubMed] [Google Scholar]
- 37. Birkhimer LJ, Curtis JL, Jann MW. Use of carbamazepine in psychiatric disorders. Clin Pharm 1985; 4: 425–434. [PubMed] [Google Scholar]
- 38. Elliott P. Action of antiepileptic and anesthetic drugs on Na- and Ca-spikes in mammalian non-myelinated axons. Eur J Pharmacol 1990; 175: 155–163. [DOI] [PubMed] [Google Scholar]
- 39. Macdonald RL, Kelly KM. Antiepileptic drug mechanism of action. Epilepsia 1993; 34(Suppl. 5): Sl–SR. [DOI] [PubMed] [Google Scholar]
- 40. Schweizer E, Rickels K, Case WG, et al. Carbamazepine treatment in patients discontinuing long-term benzodiazepine therapy. Effects on withdrawal severity and outcome. Arch Gen Psychiatry 1991; 48: 448–452. [DOI] [PubMed] [Google Scholar]
- 41. Denis C, Fatséas M, Lavie E, et al. Pharmacological interventions for benzodiazepine mono-dependence management in outpatient settings. Cochrane Database Syst Rev 2006; CD005194. [DOI] [PubMed] [Google Scholar]
- 42. Suman-Chauhan N, Webdale N, Hill DR, et al. Characterization of [3H]-gabapentin binding to a novel site in rat brain: homogenate binding studies. Eur J Pharmacol 1993; 244: 293–301. [DOI] [PubMed] [Google Scholar]
- 43. Löscher W, Hocack D, Taylor CP. Gabapentin increases aminooxyacetic acid-induced GABA accumulation in several regions of rat brain. Neurosci Lett 1991; 128: 150–154. [DOI] [PubMed] [Google Scholar]
- 44. Mariani JJ, Malcolm RJ, Mamczur AK, et al. Pilot trial of gabapentin for the treatment of benzodiazepine abuse or dependence in methadone maintenance patients. Am J Drug Alcohol Abuse 2016; 42: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. French JA, Kugler AR, Robins JL, et al. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003; 60: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 46. Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2–δ (alpha2–delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2006; 73: 137–150. [DOI] [PubMed] [Google Scholar]
- 47. Hadley SJ, Mandel FS, Schweizer E. Switching from long-term benzodiazepine therapy to pregabalin in patients with generalized anxiety disorder: a double-blind, placebo-controlled trial. J Psychopharmacol 2012; 26: 461–470. [DOI] [PubMed] [Google Scholar]
- 48. Kasson BJ. Flumazenil: a specific benzodiazepine antagonist. J Am Assoc Nurse Anesth 1992; 60: 464–471. [Google Scholar]
- 49. Gerra G, Zaimovic A, Giusti F, et al. Intravenous flumazenil versus oxazepam tapering in the treatment of benzodiazepine withdrawal: a randomized, placebo-controlled study. Addict Biol 2002; 7: 385–395. [DOI] [PubMed] [Google Scholar]
- 50. Hood SD, Norman A, Hince DA, et al. Benzodiazepine dependence and its treatment with low dose flumazenil. Br J Clin Pharmacol 2014; 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lancaster SG, Gonzalez JP. Dothiepin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs 1989; 38: 123–147. [DOI] [PubMed] [Google Scholar]
- 52. Tyrer P, Ferguson B, Hallström C, et al. A controlled trial of dothiepin and placebo in treating benzodiazepine withdrawal symptoms. Br J Psychiatry 1996; 168: 457–461. [DOI] [PubMed] [Google Scholar]
- 53. Griffin CE, III, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J 2013; 13: 214–223. [PMC free article] [PubMed] [Google Scholar]
- 54. Zitman FG, Couvée JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper-off. Br J Psychiatry 2001; 178: 317–324. [DOI] [PubMed] [Google Scholar]
- 55. Rickels K, DeMartinis N, García-España F, et al. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am J Psychiatry 2001; 157: 1973–1979. [DOI] [PubMed] [Google Scholar]
- 56. Rihel J, Schier AF. Sites of action of sleep and wake drugs: insights from model organisms. Curr Opin Neurobiol 2013; 23: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleepwake cycle. Sleep 2010; 33: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT (1)/MT (2) receptor knockout mice and comparison with MT (1) and MT (2) receptor knockout mice. Behav. Brain Res 2013; 243: 231–238. [DOI] [PubMed] [Google Scholar]
- 59. Ochoa-Sanchez R, Comai S, Lacoste B, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci 2011; 31: 18439–18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cardinali DP, Golombek DA, Rosenstein RE, et al. Assessing the efficacy of melatonin to curtail benzodiazepine/Z drug abuse. Pharmacol Res 2016; 109: 12–23. [DOI] [PubMed] [Google Scholar]
- 61. Garfinkel D, Zisapel N, Wainstein J, et al. Facilitation of benzodiazepine discontinuation by melatonina new clinical approach. Arch Intern Med 1999; 159: 2456–2460. [DOI] [PubMed] [Google Scholar]
- 62. Wright A, Diebold J, Otal J, et al. The effect of melatonin on benzodiazepine discontinuation and sleep quality in adults attempting to discontinue benzodiazepines: a systematic review and meta-analysis. Drugs Aging 2015; 32: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 63. Magro L, Faccini M, Leone R. Neuropathology of drug addictions and substance misuse. 2016; 3: 273–282. [Google Scholar]
- 64. Faccini M, Leone R, Pajusco B, et al. Lormetazepam addiction: data analysis from an Italian medical unit for addiction. Risk Manag Healthc Policy 2012; 5: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Petrovic M, Pevernagie D, Mariman A, et al. Fast withdrawal from benzodiazepines in geriatric inpatients. A randomized double-blind, placebo-controlled trial. Eur J Clin Pharmacol 2002; 57: 759–764. [DOI] [PubMed] [Google Scholar]
- 66. Hameg A, Bayle F, Nuss P, et al. Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes. Biochem Pharmacol 2003; 65: 435–440. [DOI] [PubMed] [Google Scholar]
- 67. Bourin M, Dailly E, Hascöet M. Preclinical and clinical pharmacology of cyamemazine: anxiolytic effects and prevention of alcohol and benzodiazepine withdrawal syndrome. CNS Drug Rev 2004; 10: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lemoine P, Kermadi I, Garcia-Acosta S, et al. Double-blind, comparative study of cyamemazine vs. bromazepam in the benzodiazepine withdrawal syndrome. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 131–137. [DOI] [PubMed] [Google Scholar]
- 69. Mathers DA, Barker JL. Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science 1980; 209: 507–509. [DOI] [PubMed] [Google Scholar]
- 70. Olsen RW, DeLorey TM. GABA receptor physiology and pharmacology. Basic neurochemistry: molecular, cellular and medical aspects. Lippincott-Raven, https://www.ncbi.nlm.nih.gov/books/NBK28090 (1999, accessed May 2017). [Google Scholar]
- 71. Löscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia 2012; 53: 12–25. [DOI] [PubMed] [Google Scholar]
- 72. Zhang LL, Zeng LN, Li YP. Epileptic Disord 2011; 13: 349. [DOI] [PubMed] [Google Scholar]
- 73. Kawasaki SS, Jacapraro JS, Rastegar DA. Rastegar. Safety and effectiveness of a fixed-dose phenobarbital protocol for inpatient benzodiazepine detoxification. J Subst Abuse Treat 2012; 43: 331–334. [DOI] [PubMed] [Google Scholar]
- 74. Kumar AA, Sravani P, Priya V. Anxiolytic profile of 5-HT3 antagonist ondansetron and its clinical correlation. Int J Pharm Bio Sci 2014; 5: 1–5. [Google Scholar]
- 75. Romach MK, Kaplan HL, Busto UE, et al. A controlled trial of ondansetron, a 5-HT3 antagonist, in benzodiazepine discontinuation. J Clin Psychopharmacol 1998; 18: 121–131. [DOI] [PubMed] [Google Scholar]
- 76. Garges HP, Varia I, Doraiswamy PM. Cardiac complications and delirium associated with valerian root withdrawal. JAMA 1998; 280: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 77. Poyares DR, Guilleminault C, Ohayon MM, et al. Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 539–545. [DOI] [PubMed] [Google Scholar]
- 78. Schram WS. Use of hydroxyzine in psychosis. Diseases of the Nervous System 1959; 20: 126–129. [PubMed] [Google Scholar]
- 79. Lopez-Peig C, Mundet X, Casabella B, et al. Analysis of benzodiazepine withdrawal program managed by primary care nurses in Spain. BMC Res Notes 2012; 5: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rosenthal M. Tiagabine for the treatment of generalized anxiety disorder: a randomized, open-label, clinical trial. J Clin Psychiatry 2003; 64: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 81. Oulis P, Masdrakis VG, Karapoulios E, et al. For publication: Tiagabine in the discontinuation of long-term benzodiazepine use. Psychiatry Clin Neurosci 2009; 63: 122 DOI:. [DOI] [PubMed] [Google Scholar]
- 82. Natolino F, Zanotti A, Contarino A, et al. Abecarnil, a beta-carboline derivative, does not exhibit anticonvulsant tolerance or withdrawal effects in mice. Naunyn Schmiedebergs Arch Pharmacol 1996; 354: 612–617. [DOI] [PubMed] [Google Scholar]
- 83. Pinna G, Galici R, Schneider HH, et al. Alprazolam dependence prevented by substituting with the β-carboline abecarnil. Proc Natl Acad Sci USA 1997; 94: 2719–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr 2015; 38: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72: 136–142. [DOI] [PubMed] [Google Scholar]
- 86. Petitjean S, Ladewig D, Meier CR, et al. Benzodiazepine prescribing to the Swiss adult population: results from a national survey of community pharmacies. Int Clin Psychopharmacol 2007; 22: 292–298. [DOI] [PubMed] [Google Scholar]
- 87. James J. Dealing with drug-seeking behaviour. Aust Prescr 2016; 39: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Department of Health (England) and the devolved administrations. Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health (England) the Scottish Government, Welsh Assembly Government and the Northern Ireland Executive, http://www.nta.nhs.uk/uploads/clinical_guidelines_2007.pdf (2007, accessed November 2017). [Google Scholar]
- 89. Diaper AM, Law FD, Melichar JK. Pharmacological strategies for detoxification. Br J Clin Pharmacol 2014; 77: 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Michael S. Treatment of benzodiapenine dependence. N Engl J Med 2017; 376: 1147–1157. [DOI] [PubMed] [Google Scholar]
- 91. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev 1992; 44: 151–347. [PubMed] [Google Scholar]
- 92. Grunze HCR. The effectiveness of anticonvulsants in psychiatric disorders. Dialogues Clin Neurosci 2008; 10: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Parr JM, Kavanagh DJ, Cahill L, et al. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction 2008; 104: 13–24. [DOI] [PubMed] [Google Scholar]
- 94. Rickels K, DeMartinis N, Rynn M, et al. Pharmacologic strategies for discontinuing benzodiazepine treatment. J Clin Psychopharmacol 1999; 19(6 Suppl. 2): S12–S16. [DOI] [PubMed] [Google Scholar]
- 95. Mandelli M, Tognoni G, Garattini S. Clinical pharmacokinetics of diazepam. Clin Pharmacokinet 1978; 3: 72–91. [DOI] [PubMed] [Google Scholar]
- 96. Goldberg MJ, Berlinger WG. Treatment of phenobarbital overdose with activated charcoal. JAMA 1982; 247: 2400–2401. [PubMed] [Google Scholar]
- 97. Thorstrand C. Clinical features in poisonings by tricyclic antidepressants with special reference to the ECG. Acta Med Scand 1976; 199: 337–344. [DOI] [PubMed] [Google Scholar]
- 98. Frommer DA, Kulig KW, Marx JA, et al. Tricyclic antidepressant overdose. JAMA 1987; 257: 521–526. [PubMed] [Google Scholar]
- 99. Benedict Nwogbe, Julia Ferié, Hannah Smith, Gunawardena Indunil, Ketan Dhatariya. Significant lamotrigine overdose associated with acute pancreatitis. J R Soc Med 2009; 102: 118–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cheseaux M, Monnat M, Zullino DF. Topiramate in benzodiazepine withdrawal. Hum Psychopharmacol Clin Exp 2003; 18: 375–377. [DOI] [PubMed] [Google Scholar]
- 101. Croissant B, Grosshans M, Diehl A, et al. Oxcarbazepine in rapid benzodiazepine detoxification. Am J Drug Alcohol Abuse 2008; 34: 534–540. [DOI] [PubMed] [Google Scholar]
- 102. Patterson JF. Withdrawal from alprazolam dependency using clonazepam: clinical observations. J Clin Psychiatry 1990; 51(Suppl): 47–94; discussion 50–53. [PubMed] [Google Scholar]
- 103. Hantouche EG, Guelfi JD, Comet D. Alpha-beta L-aspartate magnesium in treatment of chronic benzodiazepine abuse: controlled and double-blind study versus placebo. Encephale 1998; 24: 469–479. [PubMed] [Google Scholar]
- 104. Nakao M, Takeuchi T, Nomura K, et al. Clinical application of paroxetine for tapering benzodiazepine use in non-major-depressive outpatients visiting an internal medicine clinic. Psychiatry Clin Neurosci 2006; 60: 605–610. [DOI] [PubMed] [Google Scholar]
- 105. Chandrasekaran PK. Employing mirtazapine to aid benzodiazepine withdrawal. Singapore Med J 2008; 49: e166–e167. [PubMed] [Google Scholar]
- 106. U.S. Preventive Services Task Force. Guide to clinical preventive services: report of the U.S. Preventive Services Task Force. 3rd ed. Philadelphia: DIANE Publishing, 1989, p.24. [Google Scholar]
- 107. Keshavan MS. Clonidine in benzodiazepine withdrawal. Am J Psychiatry 1987; 144: 530. [DOI] [PubMed] [Google Scholar]
- 108. Schweitzer E, et al. Progesterone co-administration in patients discontinuing long-term benzodiazepine theraphy: effects on withdrawal severity and taper outcome. Psychopharmacology 1995; 117(4): 424–429. [DOI] [PubMed] [Google Scholar]
- 109. Gerra G, et al. (1993) Effectiveness of flumazenil (RO 15-1788) in the treatment of benzodiazepine withdrawal. Current therapeutic research 1993; 54(5): 580–587. [Google Scholar]
- 110. Petrovic M, et al. Fast withdrawal from benzodiazepines in geriatric inpatients: a randomised double-blind, placebo-controlled trial. European journal of clinical pharmacology 2002; 57(11): 759–764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TPP-17-09-015_contributor_form for Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation by Dimy Fluyau, Neelambika Revadigar and Brittany E. Manobianco in Therapeutic Advances in Psychopharmacology