Abstract
The purpose of the present study was to characterize and compare supragingival and salivary microbiotas during a 10-d period of oral hygiene discontinuation. We tested the hypothesis that the composition of the salivary microbiota will reflect local microbial changes associated with accumulated biofilm formation and maturation. Pooled supragingival plaque (n = 145) and stimulated saliva (n = 145) samples were collected and plaque and gingival indices were recorded from 29 orally healthy individuals at baseline, during oral hygiene discontinuation (days 4, 7, and 10), and 14 d after resumption of oral hygiene. Supragingival and salivary microbiotas were processed by next-generation sequencing (Human Oral Microbe Identification using Next Generation Sequencing) and microbial community profiles were compared. Microbial composition of supragingival plaque samples collected after 4, 7, and 10 d of oral hygiene discontinuation, as well as 14 d after reuptake of oral hygiene, differed significantly from baseline samples, by a 3-fold increase in relative abundance Leptotrichia species and a 2-fold decrease in Streptococcus species (adjusted P < 0.01). In saliva samples, a significant increase in relative abundance of Leptotrichia species (adjusted P < 0.01) was evident at day 7 but completely reversed 14 d after resumption of oral hygiene. While the salivary microbiota was resistant to accumulated local biofilm formation, data from this study showed that compositional changes of supragingival microbiotas were not reversed 14 d after resumption of oral hygiene, despite the restoration of plaque to baseline levels. (ClinicalTrials.gov UCPH_OI_002, NCT02913235).
Knowledge Transfer Statement: Data from this study showed compositional changes of supragingival microbiotas as a consequence of a 10-d period of oral hygiene discontinuation, that was not reversed 14 d after resumption of oral hygiene. Notably, oral hygiene discontinuation was associated with a significant increase in relative abundance of potential cariogenic Leptotrichia species and a decrease in Streptococcus species. Thus, findings from this study highlight the necessity of regular oral hygiene in the maintenance of oral homeostasis.
Keywords: microbiology, bacteria, 16S rRNA, gingivitis, saliva, dental plaque
Introduction
Saliva contains a wide variety of biological substances of human and microbial origin (Miller et al. 2010). While salivary flow rate, physicochemical properties, and concentration of specific antimicrobial substances are pivotal for maintenance of oral homeostasis (Dawes et al. 2015), saliva is also applicable in biomarker research (Giannobile et al. 2011; Yoshizawa et al. 2013). The obvious advantage of using saliva for biomarker research is the ease and efficacy of collecting saliva compared to blood and local microbial sampling.
Saliva is sterile when entering the human oral cavity (Schroder et al. 2017). However, when sampled, saliva contains a diverse microbiota (Zaura et al. 2017). As saliva does not harbor an endogenous microbiota, the salivary microbiota is believed to be a compilation of bacteria shed from oral surfaces (Kilian et al. 2016; Krishnan et al. 2017). In oral health, the core salivary microbiota is closely related to microbiotas found on the tongue and the throat, and it differs from supragingival plaque, especially in terms of higher relative abundance of Firmicutes (Segata et al. 2012). Moreover, salivary carriage of specific oral bacterial species, including Porphyromonas gingivalis and Streptococcus mutans, might be associated with periodontitis (Liljestrand et al. 2014; Belstrøm, Paster, et al. 2016) and dental caries, respectively (ElSalhy et al. 2016; Row et al. 2016).
In oral health, the composition of the salivary microbiota has been shown to be highly individualized (Hall et al. 2017). However at present, perturbation studies addressing the impact of local microbial alterations on the compositional stability of salivary microbiota are lacking. Such information is critical when evaluating the robustness of using salivary carriage of specific bacterial species as markers of periodontitis, dental caries, and other human diseases.
The experimental gingivitis model has been used in multiple studies since it was introduced in 1965 (Loe et al. 1965). In this study, we used the experimental gingivitis model to facilitate undisturbed biofilm formation and maturation during a 10-d period. The purpose of the study was 2-fold: to characterize and compare the impact of oral hygiene discontinuation on supragingival and salivary microbiotas and to learn if any such alteration was reversible after resumption of regular oral hygiene. The hypothesis was that undisturbed growth of supragingival biofilms would cause transient compositional changes of the salivary microbiota but return to baseline after resumption of oral hygiene.
Materials and Methods
Study Population and Baseline Clinical Examination
A total of 31 students from Copenhagen University were enrolled. Inclusion criteria were as follows: age ≥18 y and willingness to discontinue oral hygiene for 10 d. Exclusion criteria were as follows: presence of treatment required for caries, gingivitis, or periodontitis (presence of 1 site with probing pocket depth ≥4 mm + bleeding on probing); hyposalivation (participants were excluded if they were not able to produce at least 1 mL saliva during the collection time of 3 min or if they reported the sensation of hyposalivation); current smokers; presence of systemic diseases and current use of any medication; use of local or systemic antibiotics within the past 3 mo; and professional dental cleaning within the past 3 mo. All clinical examinations were performed by the same investigator (DB). Caries and periodontitis were registered full-mouth clinically and by use of bite-wing radiographs. Twenty-nine participants completed the study (2 participants were excluded because of antibiotic treatment during the study). Background data of the cohort are detailed in the supplemental material (Appendix Table 1). The study was performed in accordance with the STROBE guidelines, approved by the regional ethical committee of the capital region of Denmark (H-16016368), reported to the Danish Data Authority (SUND-2016-58), and registered at ClinicalTrials.gov (NCT02913235).
Study Design
Participants discontinued all oral hygiene on the day of baseline collection and refrained from all oral hygiene until samples had been collected at day 10. Then all participants had a professional dental cleaning, and regular oral hygiene was resumed for 14 d until final sample collection (day 24). To ensure biofilm formation sufficient for sampling, participants were instructed not to perform any oral hygiene in the morning on baseline day and day 24.
Clinical Examination and Sample Collection
A total of 290 samples (supragingival plaque: n = 145, saliva: n = 145) were collected by the same examiner (DB) and before clinical instrumentation was performed (5 time points from 29 subjects). Participants were instructed to avoid eating and drinking at least 2 h before sampling. Samples were collected from 8 a.m. to 6 p.m., and great care was taken to collect samples from each participant at the same time of day to reduce the influence of diurnal variation. At first, a stimulated saliva sample was collected as previously described (Bardow et al. 2014), followed by collection of a supragingival sample. After careful desiccation, supragingival plaque was removed by a periodontal probe from the buccal and lingual surface of each tooth in 2 quadrants (1 in the lower jaw and 1 in the upper jaw based on computerized randomization). Supragingival plaque was immediately pooled and transferred to sterile saline and placed on dry ice. All samples were stored at –80°C until further analysis.
Plaque formation and gingival inflammation were recorded at baseline, day 4, day 7, day 10, and day 24. Plaque formation and gingival inflammations were scored using the simplified plaque index (P-index) and gingival inflammation index (GI-index) based on recordings on 6 teeth (Loe et al. 1965).
Human Oral Microbe Identification Using Next Generation Sequencing
DNA was extracted using protocol Pathogen_Universal_200 (Roche) in accordance with the manufacturer’s guidelines. The laboratory procedures of Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) have previously been presented in detail (Gomes et al. 2015; Belstrøm et al. 2016b). A NanoDrop 8000 spectrophotometer (Thermo Scientific) was used to measure the quantity and quality of DNA. Samples were excluded if DNA <5 ng/µL was recorded. Universal primers (forward: 341F, reverse: 806R) targeting the V3 to V4 region of the 16S ribosomal RNA (rRNA) coding genes were used for polymerase chain reaction (PCR)–based amplification of bacterial DNA. AMPure beads were used for purification of amplicons. Libraries (100 ng) were pooled, gel-purified, and quantified using quantitative PCR (qPCR) before being processed by next-generation sequencing (MiSeq; Illumina), according to a protocol modified from Caporaso et al. (2011). After filtering of bad reads and chimeric sequences, >22.5 million sequences were included for further analysis.
Statistics
A sample size of n = 31 was calculated using longitudinal data on α-diversity in saliva samples (Belstrøm et al. 2016b). All data were checked for normality. Clinical data (P-index, GI-index) were compared using the Freidman test with Dunn’s comparison. For these analyses, a P value <0.05 was considered statistically significant. Relative abundance of bacterial DNA reads was compared between groups of samples at genus and species level using the Kruskal-Wallis and Mann-Whitney tests with Benjamini-Hochberg correction for multiple dependent analyses (Hochberg and Benjamini 1990). For these analyses, an adjusted P value <0.01 was considered significant. Principal component analysis (PCA) was used for data reduction and visualization of comparing microbial profiles at the individual level. MeV version 4_9_0 (Saeed et al. 2006) and GraphPad Prism (GraphPad Software) were used as statistical software.
Results
Clinical Data
Clinical data of plaque accumulation and gingival inflammation are detailed in the Table. Plaque and gingivitis levels increased progressively from baseline to day 10 (P < 0.0001) and reverted to baseline levels at day 24 (P > 0.05).
Table.
Plaque and Bleeding Index at Each Time Point.
| Baseline | Day 4 | Day 7 | Day 10 | Day 24 | |
|---|---|---|---|---|---|
| P-index | 0.5 (0.0–1.0) | 1.3 (0.3–2.0)a | 1.7 (1.2–2.3)a | 2.6 (1.7–3.0)a | 0.5 (0.3–1.8) |
| GI-index | 0.0 (0.0–1.0) | 0.5 (0.0–1.3)a | 1.0 (0.5–1.7)a | 1.5 (1.0–2.0)a | 0.0 (0.0–1.2) |
P-index and GI-index expressed as median (range) at each time point. Baseline levels were compared with each time point using the Freidman test with Dunn’s comparison.
GI-index, gingival inflammation index; P-index, plaque index.
P < 0.0001.
General Information on Sequencing Data
A total of 22,622,996 sequences were generated from 285 microbial samples (plaque: n = 143, saliva: n = 142) with a mean of 76,738 (range: 39,588–144,341) per sample. DNA reads were assigned to a total of 489 different bacterial species with a mean of 170 species (range: 71–247) per sample. The mean number of bacterial species identified was comparable in supragingival plaque (n = 168) and saliva (n = 171) (P = 0.45).
Discontinuation of Oral Hygiene Has Pronounced Influence on Supragingival Community Profiles
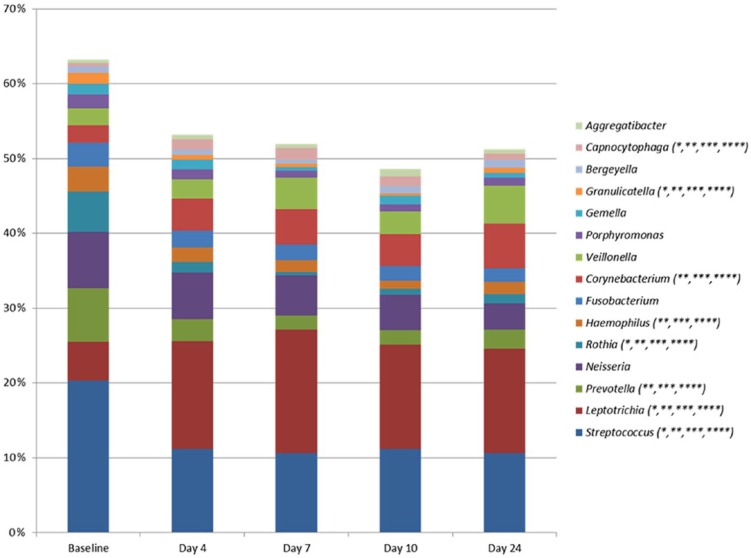
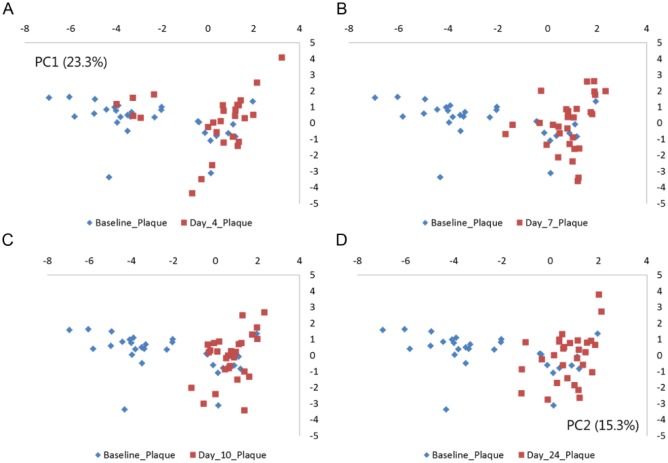
Prominent alterations of supragingival community profiles were evident 4 d after discontinuation of oral hygiene, as shown by an increase in the relative abundance of species of Leptotrichia (day 4: 15.2% vs. baseline: 5.3%) and Capnocytophaga (2.8% vs. 1.0%), in concert with a decrease in species of Streptococcus (baseline: 21.0% vs. day 4: 11.5%) and Rothia (6.0% vs. 1.6%) in samples collected on day 4 (Fig. 1, adjusted P < 0.01). Furthermore, the relative abundance of 75 bacterial species was significantly changed during the 10-d period of oral hygiene discontinuation (adjusted P < 0.01, Appendix Table 2). PCA showed almost complete separation of samples collected at baseline and day 4 (Fig. 2A). These structural changes of microbial community profiles remained stable throughout the 10 d of oral hygiene discontinuation and were clearly not reversed even 14 d after resumption of normal oral hygiene (Figs. 2A–D), despite the restoration of plaque to baseline levels.
Figure 1.
Genus-level abundance of supragingival microbiota. Mean relative abundance of the 15 predominant bacterial genera in a pooled supragingival sample. Relative abundance in baseline samples (n = 29) was compared to the relative abundance in samples collected at day 4 (n = 29), day 7 (n = 29), and day 10 (n = 29) after discontinuation of oral hygiene and 14 d (n = 29) after reuptake of oral hygiene. *Adjusted P < 0.01, baseline vs. day 4. **Adjusted P < 0.01, baseline vs. day 7. ***Adjusted P < 0.01, baseline vs. day 10. ****Adjusted P < 0.01, baseline vs. day 24.
Figure 2.
Principal component analysis of supragingival microbiota. Principal component analysis comparing baseline samples with samples collected at day 4, day 7, day 10, and day 24. The x-axis is expressed as PC1 (26.3%), and y-axis is expressed as PC2 (15.3%). (A) Baseline (blue) vs. day 4 (red). (B) Baseline (blue) vs. day 7 (red). (C) Baseline (blue) vs. day 10. (D) Baseline vs. day 24 (red).
Salivary Microbiota Is Resistant to Local Microbial Alterations
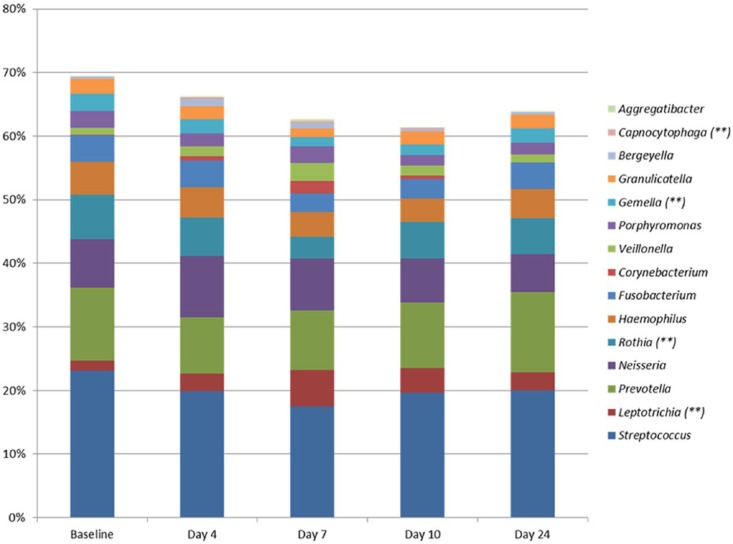
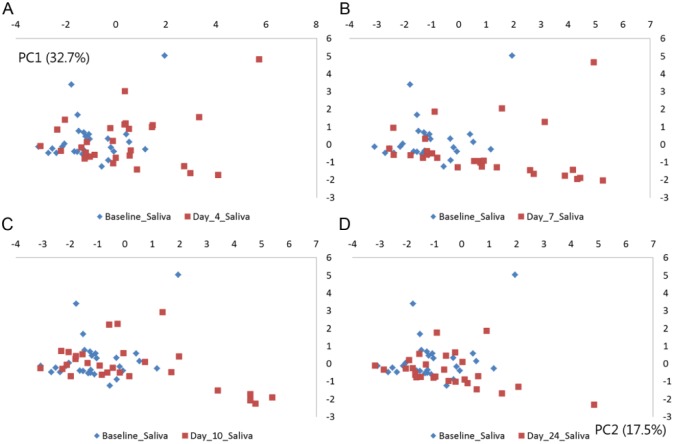
The composition of the salivary microbiota was largely not influenced by accumulated biofilm formation, as demonstrated by limited alterations in relative abundance at genus and species level (Fig. 3, Appendix Table 3). PCA of saliva samples showed random distribution of samples collected at baseline, day 4, day 7, day 10, and day 24, respectively (Fig. 4A–D).
Figure 3.
Genus-level abundance of salivary microbiota. Mean relative abundance of the 15 predominant bacterial genera in saliva samples. Relative abundance in baseline samples (n = 29) was compared to relative abundance in samples collected at day 4 (n = 29), day 7 (n = 29), and day 10 (n = 29) after discontinuation of oral hygiene and 14 d (n = 29) after reuptake of oral hygiene. **Adjusted P < 0.01, baseline vs. day 7.
Figure 4.
Principal component analysis of salivary microbiota. Principal component analysis comparing baseline samples with samples collected at day 4, day 7, day 10, and day 24. The x-axis is expressed as PC1 (32.7%), and y-axis is expressed as PC2 (17.5%). (A) Baseline (blue) vs. day 4 (red). (B) Baseline (blue) vs. day 7 (red). (C) Baseline (blue) vs. day 10. (D) Baseline vs. day 24 (red).
At day 7, a significant increase in α-diversity (2.67 vs. 2.51, P < 0.01), an increase in relative abundance in Leptotrichia species (day 7: 6.1% vs. baseline: 1.7%), and a significant decrease in Rothia species (baseline: 7.4% vs. day 7: 3.7%) (adjusted P < 0.01) were recorded, compared to baseline samples. These alterations were leached already at day 10. No differences were observed between baseline samples and samples collected 14 d after uptake of oral hygiene (Figs. 3, 4).
Discussion
The purpose of this study was to characterize alterations of supragingival microbial community profiles in relation to discontinuation of oral hygiene and to learn if such a perturbation had any impact on the composition of the salivary microbiota. To our knowledge, this is the first study to perform simultaneous characterization of supragingival and salivary microbial community profiles in relation to discontinuation of oral hygiene.
The experimental gingivitis setup has been used since the mid-1960s (Loe et al. 1965) to study the effect of accumulated biofilm formation on oral homeostasis. Traditionally, when using this protocol, oral hygiene is omitted for 21 d and sampling is usually performed at day 7, day 14, and day 21 (Morelli et al. 2014; Schincaglia et al. 2017). The protocol used in this study was slightly modified from the traditional experimental gingivitis model in 2 ways. First of all, the duration of oral hygiene discontinuation was 10 d, with more intense sampling on approximately every third day. Second, another sampling was repeated 14 d after reuptake of oral hygiene. These modifications were made to comply with the primary purpose of the investigation: to characterize the impact and reversibility of perturbation-induced alterations on supragingival and salivary microbial community profiles associated with oral hygiene discontinuation. The clinical data on day 10 demonstrated that all participants had rampant plaque formation and developed moderate to severe gingivitis during the study period (Table). However, we cannot exclude that more pronounced and generalized gingivitis associated with continued discontinuation of oral hygiene would have an impact on the composition of supragingival and salivary microbial communities.
Major compositional changes of supragingival microbial community profiles were evident already at day 4 after oral hygiene discontinuation, as mean relative proportions of Streptococcus and Prevotella species were significantly decreased, and Leptotrichia significantly increased compared to baseline levels (Fig. 1). Clinical data clearly demonstrated a significantly progressive plaque accumulation during the study (Table), but the relative abundance of predominant bacterial genera remained stable from day 4 until day 10 (Fig. 1). Furthermore, no statistical differences were noted between samples from day 4, day 7, and day 10 of oral hygiene discontinuation. These data suggest that discontinuation of oral hygiene quickly facilitated an ecological alteration of the local microbial environment, which favored propagation of Leptotrichia species, which remained the dominant genus until day 10. Leptotrichia species are gram-negative, anaerobic rod-shaped bacteria, which phylogenetically belong to the family of Fusobacteriaceae, which, by use of culturing methods, has been reported as a minor component of dental plaque (Thompson and Pikis 2012). However, the advent of modern molecular methods has revealed potent sugar metabolism in oral species of Leptotrichia (Thompson and Pikis 2012). As participants were instructed not to change their diet during the period of oral hygiene discontinuation, it is likely that undisturbed biofilm formation in combination with regular sugar intake created local ecological conditions in favor of Leptotrichia species.
It is noteworthy that the microbial composition of supragingival plaque collected 14 d after resumption of oral hygiene remained significantly different from baseline samples (Figs. 1, 2). This finding could not be explained by clinical findings of the P-index and GI-index (Table). Therefore, even though the amount of biofilm was reversed by reuptake of regular oral hygiene, compositional changes of the supragingival biofilm were still evident (Figs. 1, 2). Alterations in expression of antimicrobial peptides and gingival tissues and in saliva have been reported in gingivitis (Gursoy et al. 2013; Dommisch et al. 2015). It is therefore possible that this finding highlights equilibrium between the supragingival biofilm and the immune system, which were not reversed after reuptake of oral hygiene. On the other hand, it is also possible that another round of sampling (e.g., 1 mo after reuptake of oral hygiene procedures) would have revealed a complete return of compositional changes to the supragingival microbiota. Thus, further studies are needed to reveal the impact of this finding.
Undisturbed supragingival biofilm formation had only a minor impact on the salivary microbiota (Figs. 3, 4). A 4-fold increase in mean relative abundance of Leptotrichia species was recorded at day 7, while relative abundance of all other predominant genera remained unchanged compared to baseline (Fig. 3). The level of relative abundance of Leptotrichia recorded in saliva in this study from individuals during a period of oral hygiene discontinuation is, however, significantly higher than what we have noted in previous studies on the salivary microbiota using the same molecular method (Belstrøm et al. 2016a, 2016b; Belstrøm, Holmstrup, et al. 2016; Belstrøm, Paster, et al. 2016). Thus, with this overabundance, the Leptotrichia species were likely shed from abundant supragingival biofilm formation, affecting the salivary abundance at day 7 of oral hygiene discontinuation (Fig. 3). It is surprising, however, that the salivary abundance of Leptotrichia species was almost reversed at day 10. One possible explanation of this finding could be a structural alteration of supragingival biofilms from day 7 to day 10, resulting in the positioning of Leptotrichia species in the deeper layers of the maturing biofilm, including less intensive interaction with saliva. This assumption is supported by the clinical data, which showed a major increase in amounts of visible plaque from day 7 to day 10 (Table). However, as we did not perform ultra-structural analysis of supragingival biofilms, this remains speculative. Nevertheless, when comparing saliva sampled at baseline and 14 d after resumption of regular oral hygiene, comparable microbial profiles were identified (Figs. 3, 4). Thus, the composition of the salivary microbiota is apparently resistant to reversible perturbations. Obviously, this is advantageous when considering the use of salivary microbial profiles as biomarkers of periodontitis or dental caries.
Possible limitations to this investigation were the young age (22–29 y) and the predominance of female participants (female: n = 24 vs. male: n = 5), which reflects the population of dental students. However, this limits the external validity of the data presented, as differences in hormone levels between sexes could potentially have an impact on supragingival and salivary microbiotas. On the other hand, based on cross-sectional analysis of a large Danish cohort, we were not able to find any sex- or age-associated salivary microbial characteristics (Belstrøm et al. 2014). Another limitation was that subgingival plaque samples were not collected, and therefore it was not possible to address the influence of oral hygiene discontinuation on the composition of the subgingival microbiota. Thus, further studies characterizing both supragingival and subgingival plaque microbiotas in experimental gingivitis are warranted.
In conclusion, accumulated supragingival biofilm formation and maturation had only a minor impact on the composition of the salivary microbiota, whereas oral hygiene discontinuation was associated with a significant increase in the relative abundance of Leptotrichia species in supragingival plaque, which was not reversed 14 d after reuptake of oral hygiene.
Author Contributions
D. Belstrøm, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; M.L. Sembler-Møller, contributed to data acquisition and analysis, critically revised the manuscript; M.A. Grande, contributed to data acquisition, critically revised the manuscript; N. Kirkby, S.L. Cotton, contributed to data analysis, critically revised the manuscript; B.J. Paster, contributed to conception, design, data analysis and interpretation, critically revised the manuscript; S. Twetman, P. Holmstrup, contributed to conception, design, data acquisition and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This study was supported financially by the Foundation of Mutual Efforts in Dental Care.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Authors’ Note: Access to all data, including DNA sequences, will be granted upon request (dbel@sund.ku.dk).
References
- Bardow A, Lykkeaa J, Qvist V, Ekstrand K, Twetman S, Fiehn NE. 2014. Saliva composition in three selected groups with normal stimulated salivary flow rates, but yet major differences in caries experience and dental erosion. Acta Odontol Scand. 72(6):466–473. [DOI] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. 2016. a. Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. J Oral Microbiol. 8:30112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. 2016. b. Temporal stability of the salivary microbiota in oral health. PLoS One. 11(1):e0147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Fiehn NE, Rosing K, Bardow A, Paster BJ, Lynge Pedersen AM. 2016. Bacterial composition in whole saliva from patients with severe hyposalivation—a case-control study. Oral Dis. 22(4):330–337. [DOI] [PubMed] [Google Scholar]
- Belstrøm D, Holmstrup P, Nielsen CH, Kirkby N, Twetman S, Heitmann BL, Klepac-Ceraj V, Paster BJ, Fiehn NE. 2014. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol. 6:23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Paster BJ, Fiehn NE, Bardow A, Holmstrup P. 2016. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J Oral Microbiol. 8:30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 108(Suppl 1):4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C, Pedersen AM, Villa A, Ekström J, Proctor GB, Vissink A, Aframian D, McGowan R, Aliko A, Narayana N, et al. 2015. The functions of human saliva: a review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol. 60(6):863–874. [DOI] [PubMed] [Google Scholar]
- Dommisch H, Staufenbiel I, Schulze K, Stiesch M, Winkel A, Fimmers R, Dommisch J, Jepsen S, Miosge N, Adam K, et al. 2015. Expression of antimicrobial peptides and interleukin-8 during early stages of inflammation: an experimental gingivitis study. J Periodontal Res. 50(6):836–845. [DOI] [PubMed] [Google Scholar]
- ElSalhy M, Söderling E, Honkala E, Fontana M, Flannagan S, Kokaras A, Paster BJ, Varghese A, Honkala S. 2016. Salivary microbiota and caries occurrence in mutans streptococci-positive school children. Eur J Paediatr Dent. 17(3):188–192. [PubMed] [Google Scholar]
- Giannobile WV, McDevitt JT, Niedbala RS, Malamud D. 2011. Translational and clinical applications of salivary diagnostics. Adv Dent Res 23(4):375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ. 2015. Microbiomes of endodontic-periodontal lesions before and after chemomechanical preparation. J Endod. 41(12):1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy M, Gursoy UK, Sorsa T, Pajukanta R, Kononen E. 2013. High salivary estrogen and risk of developing pregnancy gingivitis. J Periodontol. 84(9):1281–1289. [DOI] [PubMed] [Google Scholar]
- Hall MW, Natasha S, Kester FNG, Lam DK, Goldberg MB, Tenenbaum HC, Neufild JD, Beiko RG, Senadheera DB. 2017. Inter-personal diversity and temporal dynamics of dental, tongue and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes. 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. 1990. More powerful procedures for multiple significance testing. Stat Med. 9(7):811–818. [DOI] [PubMed] [Google Scholar]
- Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. 2016. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 221(10):657–666. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Chen T, Paster BJ. 2017. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 23(3):276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrand JM, Gursoy UK, Hyvarinen K, Sorsa T, Suominen AL, Kononen E, Pussinen PJ. 2014. Combining salivary pathogen and serum antibody levels improves their diagnostic ability in detection of periodontitis. J Periodontol. 85(1):123–131. [DOI] [PubMed] [Google Scholar]
- Loe H, Theilade E, Jensen SB. 1965. Experimental gingivitis in man. J Periodontol. 36:177–187. [DOI] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW, et al. 2010. Current developments in salivary diagnostics. Biomark Med. 4(1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli T, Stella M, Barros SP, Marchesan JT, Moss KL, Kim SJ, Yu N, Aspiras MB, Ward M, Offenbacher S. 2014. Salivary biomarkers in a biofilm overgrowth model. J Periodontol. 85(12):1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row L, Repp MR, Kingsley K. 2016. Screening of a pediatric and adult clinic population for caries pathogen Scardovia wiggsiae. J Clin Pediatr Dent. 40(6):438–444. [DOI] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. 2006. TM4 microarray software suite. Methods Enzymol. 411:134–193. [DOI] [PubMed] [Google Scholar]
- Schincaglia GP, Hong BY, Rosania A, Barasz J, Thompson A, Sobue T, Panagakos F, Burleson JA, Dongari-Bagtzoglou A, Diaz PI. 2017. Clinical, immune, and microbiome traits of gingivitis and peri-implant mucositis. J Dent Res. 96(1):47–55. [DOI] [PubMed] [Google Scholar]
- Schroder SA, Bardow A, Eickhardt-Dalboge S, Johansen HK, Homoe P. 2017. Is parotid saliva sterile on entry to the oral cavity? Acta Otolaryngol. 137(7):762–764. [DOI] [PubMed] [Google Scholar]
- Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. 2012. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 13(6):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Pikis A. 2012. Metabolism of sugars by genetically diverse species of oral Leptotrichia. Mol Oral Microbiol. 27(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT. 2013. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 26(4):781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E, Brandt BW, Prodan A, Teixeira de, Mattos MJ, Imangaliyev S, Kool J, Buijs MJ, Jagers FL, Hennequin-Hoenderdos NL, Slot DE, et al. 2017. On the ecosystemic network of saliva in healthy young adults. ISME J. 11(5):1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.