Abstract
Aims
Carotid intima media thickness (CIMT) predicts cardiovascular (CVD) events, but the predictive value of CIMT change is debated. We assessed the relation between CIMT change and events in individuals at high cardiovascular risk.
Methods and results
From 31 cohorts with two CIMT scans (total n = 89070) on average 3.6 years apart and clinical follow-up, subcohorts were drawn: (A) individuals with at least 3 cardiovascular risk factors without previous CVD events, (B) individuals with carotid plaques without previous CVD events, and (C) individuals with previous CVD events. Cox regression models were fit to estimate the hazard ratio (HR) of the combined endpoint (myocardial infarction, stroke or vascular death) per standard deviation (SD) of CIMT change, adjusted for CVD risk factors. These HRs were pooled across studies.
In groups A, B and C we observed 3483, 2845 and 1165 endpoint events, respectively. Average common CIMT was 0.79mm (SD 0.16mm), and annual common CIMT change was 0.01mm (SD 0.07mm), both in group A. The pooled HR per SD of annual common CIMT change (0.02 to 0.43mm) was 0.99 (95% confidence interval: 0.95–1.02) in group A, 0.98 (0.93–1.04) in group B, and 0.95 (0.89–1.04) in group C. The HR per SD of common CIMT (average of the first and the second CIMT scan, 0.09 to 0.75mm) was 1.15 (1.07–1.23) in group A, 1.13 (1.05–1.22) in group B, and 1.12 (1.05–1.20) in group C.
Conclusions
We confirm that common CIMT is associated with future CVD events in individuals at high risk. CIMT change does not relate to future event risk in high-risk individuals.
Introduction
Carotid intima media thickness (CIMT) has been debated as a screening tool[1,2] and as a surrogate marker of vascular event risk.[3] Recent publications have raised doubts about the clinical usefulness,[4] and of the surrogacy[5,6] of CIMT. In a large study on general population individuals without prevalent cardiovascular disease (CVD), we were unable to show an association between rate of change of CIMT estimated by two measurements assessed some years apart and the subsequent risk of future CVD events, although the association between CIMT, estimated as an average of the two CIMT measures at different time points, and future risk was robust and consistent.[7]
Several hypotheses have been suggested to explain this discrepancy. One credible argument is that the small CIMT change, assessed with reasonable to considerable measurement error in cohort studies, and the low event risk in the asymptomatic general population make it difficult to discern such association. Acting on this hypothesis, we aimed to study individuals at high risk, to explore whether a relation between CIMT change and CVD event risk is present. For the present analyses, we identified studies that included asymptomatic individuals with at least three CVD risk factors, asymptomatic individuals with carotid plaque, and individuals with pre-existing CVD as indicators of high risk. With individual participant data (IPD) meta-analysis we assessed the relation between CIMT, CIMT change, and subsequent vascular event risk in these groups.
Materials and methods
To identify relevant studies for this meta-analysis, we performed a comprehensive literature research. With the search terms “intima media” AND (“myocardial infarction” OR”stroke” OR”death” OR “mortality”) we screened PubMed. In addition, we hand searched reference lists of CIMT review papers. We included publications in all languages, published until 1st October 2015. Using predefined inclusion criteria (Table 1), original articles and research reports were assessed by reading both the abstracts and the full texts. When eligibility for our analysis could not be decided, we sent a short screening questionnaire to the relevant study team. If a study fulfilled all inclusion criteria, the study team was invited to join the collaboration, share their data, and participate in the project. We included cohorts with at least two CIMT scans several years apart, and a subsequent clinical follow-up.
Table 1. Inclusion criteria.
| Population cohorts | Risk cohorts |
|---|---|
| Prospective longitudinal study design | |
| Investigation of a population based sample or a sample similar to the general population | Investigation of one, or including one of the following risk populations: • Individuals with at least 3 CVD risk factors • Individuals with carotid plaque • Individuals with previous MI or stroke |
| Well-defined and disclosed inclusion criteria and recruitment strategy | |
| At least two ultrasound visits where carotid IMT was determined | |
| A clinical follow-up after the second ultrasound visit, recording MI, stroke, death, vascular death or a subset of these. | |
| A minimum of 10 events per endpoint before exclusions | |
The datasets underwent central plausibility checks and transformation into a standard data format with uniform variable names, units, and coding. Ordinal variables were recoded into binary balanced categories. Mean common carotid IMT (mean CCA-IMT) was calculated as the mean from all available mean CIMT measurements in the common carotid arteries, including left and right carotids, near and far wall, and all insonation angles. From the first two ultrasound visits of each study, two CIMT variables were derived: ‘average CIMT’ is the mean of the baseline and the first follow-up scan; and ‘annual CIMT change’ is the difference between the baseline and the first follow-up scan, divided by the time between scans in years. Mean CCA-IMT was used in most analyses, in some sensitivity analyses we used maximal CCA-IMT in the same way. Differences in the ultrasound measurement protocols between studies were tabulated and considered in sensitivity analyses.
We used a combined endpoint for most analyses, defined as the first event of myocardial infarction (MI), stroke (including non-traumatic intracerebral hemorrhage), or vascular death, occurring after the second ultrasound visit. For these component endpoints, the definition used in each study was adopted. When vascular death was not available in a study, total mortality was used instead. For some sensitivity analyses, we also studied the endpoints MI, stroke, and total mortality separately.
From all cohorts except one, IPD were sent to the coordinating center at Frankfurt University, where they were harmonized. The harmonized data were forwarded to the statistics center at Cambridge University for fitting of the Cox models and pooling of their estimates. One cohort (AtheroGene) was unable to forward IPD due to legal restrictions. For this cohort, the plausibility checks and the fitting of the Cox models were done locally, following the programming codes developed by the statistics center, and their estimates were sent to Cambridge for pooling.
Statistical analyses
In order to identify individuals with high CVD risk, we used three subject groups:
-
A)
Individuals with three or more CVD risk factors, including (i) male sex or age ≥ 60 years, (ii) LDL cholesterol>160mg/dl and/or lipid-lowering medication, (iii) HDL cholesterol<40mg/dl, (iv) systolic blood pressure>140mmHg, diastolic blood pressure>90mmHg and/or antihypertensive medication, (v) prediagnosed diabetes or fasting glucose>110mg/dl and/or antidiabetic medication, (vi) current smoking, (vii) triglycerides >200mg/dl and (viii) family history of CVD, without previous MI or stroke. This definition followed the inclusion criteria of the IMPROVE study[8];
-
B)
Individuals with carotid plaques without previous MI or stroke, irrespective of the number of risk factors;
-
C)
Individuals with previous MI or stroke.
From general population cohorts, individuals satisfying these respective criteria were selected. Cohorts in dedicated risk groups and hospital cohorts were included when they matched our criteria, or a relevant proportion of their individuals could be selected by our criteria.
The statistical analysis followed a pre-specified plan. For cohorts A and B, individuals who had a CVD event (MI or stroke) before the second ultrasound visit were excluded. In cohort C, individuals with endpoint events between the two ultrasound visits were excluded. For every study, considering clinical events after the second ultrasound visit, we fitted a Cox regression model for the chosen endpoint (usually combined: MI or stroke or vascular death). The hazard ratio (HR) of annual CIMT change was expressed per (within study) standard deviation (SD) of annual CIMT change. Two levels of adjustment were defined: model 1 included age, sex, and average CIMT, and model 2 included these covariates plus a large set of CVD risk factors (ethnicity, socioeconomic status, body mass index, systolic blood pressure, antihypertensive medication, total cholesterol, lipid-lowering medication, diabetes, smoking status, hemoglobin, creatinine). The log HR estimates were then pooled across all studies using random effects meta-analysis.[9] Heterogeneity between the cohorts was assessed using the I2 statistic.[10] If multiple studies had each less than 20 endpoint events, a Cox regression model was fitted on a merged dataset of these, stratified for the cohort, and the resulting HR was pooled with the HRs of the other cohorts. The effects of study-level variables were assessed by random effects meta-regression. All analyses were based on unimputed data (complete case analysis) since previous work had shown no material differences when using multiple imputation.[7]
The rationale and methods of the PROG-IMT project have been published beforehand.[11] The first author had full access to the data (except the IPD of AtheroGene, as explained above) and takes responsibility for their integrity. All authors have read and agreed to the manuscript as written. The PROG-IMT project and the work leading to this publication have been approved by the Ethics Committee of Frankfurt University Hospital (Geschaeftsnummer 304/13). All contributing studies had approval of their local IRB.
Results
2513 publications were screened and 610 screening questionnaires sent. After the screening process, 60 cohorts were known to be eligible (S1 Fig). Of these, 18 declined collaboration, and 9 accepted but did not provide their dataset in time. We were able to include 23 population cohorts and 10 risk cohorts across the world. One population cohort and one risk cohort had to be excluded subsequently, because after the construction of the groups A-C, no endpoint events were left. The remaining cohorts are shown in Table 2. In group A, 23406 individuals were included, of which 3462 suffered an endpoint event. In group B, 14496 individuals with 2852 endpoint events were analyzed. Group C comprised 3628 individuals who developed 1174 endpoint events. Given our criteria, the subjects selected into group C did not overlap with those in group A or B, but A overlapped with B in 17 cohorts (by 24–79% of group A). In the Cardiovascular Health Study (CHS), individuals of Caucasian ethnicity (cohort 1) had a different follow-up regime than African Americans (cohort 2): they were considered as two separate cohorts (CHS1 and CHS2).
Table 2. Cohorts and subsamples.
| Cohort | Cohort type | Country | Mean age (years) | Mean duration between the first 2 ultrasound visits (years) | Mean clinical follow-up after the second ultrasound visit (years) | Total number of individuals (combined endpoint events) |
Number of individuals (combined endpoint events) included in A (at least 3 RF) | Number of individuals (combined endpoint events) included in B (carotid plaque) | Number of individuals (combined endpoint events) included in C (previous CVD event) |
|---|---|---|---|---|---|---|---|---|---|
| AIR[12] | Population | Sweden | 58.2 | 3.2 | 5.5 | 391 (23) | 129 (9) | 106 (6) | n.a. |
| ARIC[13] | Population | USA | 54.2 | 2.9 | 14.2 | 15040 (2089) | 4486 (933) | 3672 (707) | 408 (176) |
| AtheroGene*[14] | Hospital | Germany | 62.4 | 0.6 | 5.9 | 335 (36) | 181 (14) | n.a. | 154 (22) |
| BHS*[15] | Population | USA | 36.3 | 2.5 | 4.5 | 1392 (13)# | 179 (2) | n.a. | n.a. |
| Bruneck*[16] | Population | Italy | 62.9 | 5.0 | 8.3 | 821 (113) | 372 (58) | n.a. | 61 (23) |
| CAPS[17] | Population | Germany | 51.0 | 3.2 | 5.2 | 6972 (151)+ | 610 (40) | n.a. | 95 (27) |
| CCCC*[18] | Population | Taiwan | 54.9 | 5.0 | 6.9 | 3602 (116)+ | 456 (47) | 250 (32) | 25 (2) |
| CHS1[19] | Population | USA | 72.8 | 2.9 | 8.5 | 5201 (1943) | 1957 (750) | 2633 (963) | 777 (358) |
| CHS2[19] | Population | USA | 73.0 | 6.0 | 5.0 | 687 (206) | 177 (42) | 217 (50) | 58 (16) |
| CMCS[20] | Population | China | 59.9 | 5.4 | 4.9 | 1324 (28) | 369 (8) | 182 (3) | 43 (2) |
| CSN*[21] | Risk population | Italy | 55.0 | 2.5 | 3.6 | 13843 (14) | 1374 (1) | n.a. | n.a. |
| DIWA[22] | Population | Sweden | 64.5 | 5.4 | 2.4 | 644 (53) | 259(9) | n.a. | 26 (4) |
| EAS[23] | Population | UK | 69.0 | 6.6 | 5.3 | 1593 (316) | 513 (29) | 381 (22) | 93 (11) |
| EPICARDIAN[24] | Population | Spain | 67.7 | 3.1 | 5.6 | 446 (53) | 156 (19) | n.a. | 9 (1) |
| EVA[25] | Population | France | 65.1 | 2.0 | 14.0 | 1135 (41)# | 594 (25) | 182 (13) | 81 (6) |
| HOORN[26] | Population | Netherlands | 68.2 | 5.2 | 2.7 | 3103 (458) | 123 (1) | n.a. | 7 (0) |
| IMPROVE[27] | Risk population | Finland, France, Italy, Netherlands, Sweden | 64.2 | 1.2 | 1.8 | 3703(49) | 2471 (41) | n.a. | n.a. |
| INVADE[28] | Population | Germany | 67.7 | 2.2 | 3.9 | 3908 (602)+ | 1183 (135) | 1319 (138) | 408 (97) |
| KIHD[29] | Population | Finland | 52.4 | 4.1 | 13.7 | 1399 (478) | 669 (216) | 239 (96) | 98 (54) |
| Landecho et al.*[30] | Hospital | Spain | 54.5 | 3.6 | 3.2 | 250 (11) | 124 (5) | n.a. | n.a. |
| MDCS plaque substudy*[31] | Risk population | Sweden | 59.5 | 2.1 | 12.2 | 1544 (260) | 654 (157) | n.a. | 31 (12) |
| Niguarda-Monzino*[32] | Hospital | Italy | 56.2 | 3.4 | 4.1 | 1790 (101) | 168 (7) | n.a. | n.a. |
| NOMAS/INVEST[33] | Population | USA | 65.5 | 3.6 | 2.9 | 778 (27) | 378 (15) | 344 (18) | n.a. |
| OSACA-2[34] | Hospital | Japan | 65.0 | 2.8 | 6.0 | 291 (13) | 79 (2) | n.a. | 109 (8) |
| PIVUS*[35] | Population | Sweden | 70.0 | 5.1 | 1.9 | 1017 (114)++ | 386 (17) | 398 (15) | 65 (2) |
| PLIC[36] | Population | Italy | 55.2 | 2.2 | 4.1 | 1782 (25) | 759 (11) | 343 (10) | 88 (4) |
| RIAS[37] | Hospital | Switzerland | 64.4 | 2.7 | 4.8 | 145 (43) | 11 (4) | n.a. | 54 (14) |
| Rotterdam[38] | Population | Netherlands | 70.6 | 6.5 | 5.5 | 7983 (4011)+ | 1192 (317) | 1227 (310) | 383 (160) |
| SAPHIR[39] | Population | Austria | 51.4 | 4.6 | 8.5 | 1800 (70) | 445 (32) | 286 (17) | 39 (3) |
| SHIP[40] | Population | Germany | 49.8 | 5.3 | 5.9 | 4308 (127) | 1262 (71) | 1006 (63) | 130 (18) |
| SPARC*[41] | Hospital | Canada | 70.3 | 1.1 | 2.1 | 349 (23) | 182 (5) | n.a. | n.a. |
| Tromsø[42] | Population | Norway | 59.5 | 6.3 | 8.0 | 4827 (850) | 2091 (461) | 1711 (389) | 540 (176) |
*included in sensitivity analyses only
+combined endpoint MI or stroke or death
#vascular death
++total mortality
AIR = Atherosclerosis and Insulin Resistance Study; ARIC = Atherosclerosis Risk in Communities; BHS = Bogalusa Heart Study; CAPS = Carotid Atherosclerosis Progression Study; CCCC = Chin-Shan Community Cardiovascular Cohort Study; CHS = Cardiovascular Health Study; CMCS = Chines multi-Provincial Cohort Study; CSN = The Campania Salute Network; DIWA = Diabetes and Impaired Glucose Tolerance in Women and Atherosclerosis; EAS = Edinburgh Artery Study; EVA = Étude de Vieillissement Arteriél; IMPROVE = Carotid Intima-Media Thickness and IMT-Progression as Predictors of Vascular Events in a High Risk European Population; INVADE = Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg; KIHD = Kuopio Ischemic Heart Disease Risk Factor Study; MDCS = Malmø Diet and Cancer Study; NOMAS = Northern Manhattan Study; INVEST = Oral Infections and Vascular Disease Epidemiology Study; OSACA = Osaca Follow-up Study for Atherosclerosis; PIVUS = Prospective Investigation of the Vasculature in Uppsala Seniors; PLIC = Progression of Lesions in the Intima of the Carotid; RIAS = Resistive Index in Atherosclerosis; SAPHIR = Salzburg Atherosclerosis Prevention program in subjects at High Individual Risk; SHIP = Study of Health in Pomerania; SPARC = Progression of Carotid Plaque volume predicts cardiovascular events
The distributions of average common CIMT, annual CIMT change, and of crude event rates are summarized by cohort and subgroup in S1 Table. The mean time interval between the first and second ultrasound visit was 3.57 years. Mean average common CIMT ranged from 0.68 to 1.10mm (mean 0.79mm, SD 0.16mm), and mean annual CIMT change from -0.10 to 0.05 mm/year (mean 0.01mm, SD 0.07mm, both group A). The study-specific SD for average common CIMT ranged from 0.09 to 0.75mm, the study-specific SD of annual CIMT change varied between 0.02 and 0.43mm. After the second ultrasound measurement, participants were followed up for endpoind events on average for 7.1 years. The crude event rates varied between 0.2 and 82.9 events per 1000 person years (average 19 events per 1000 person years).
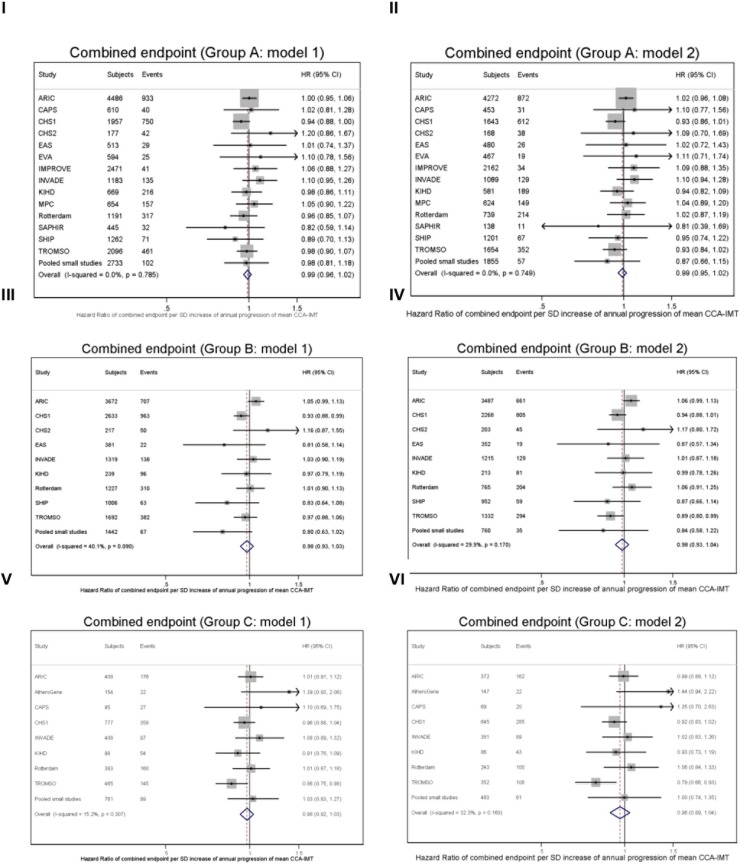
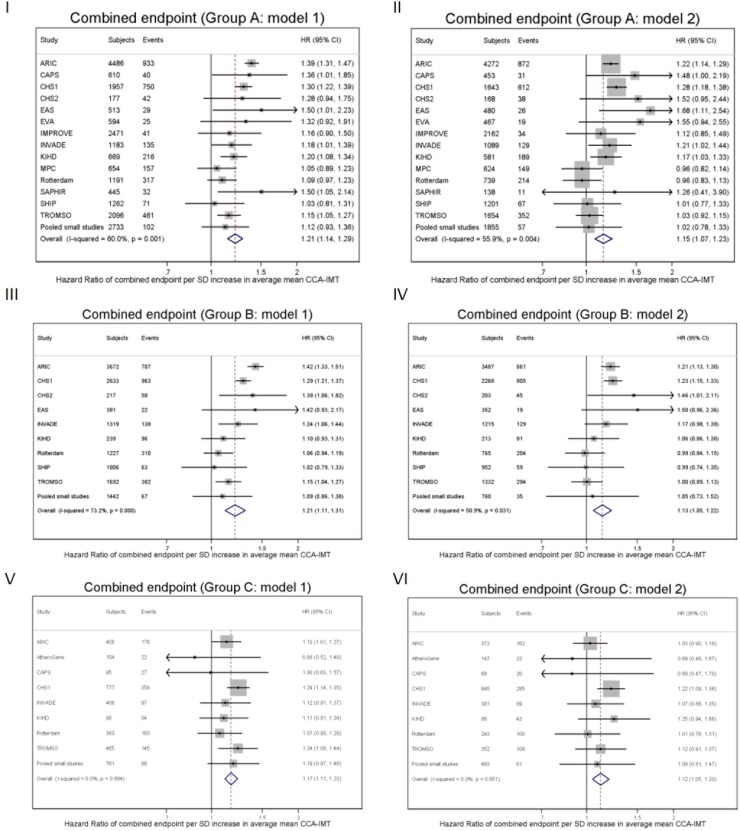
In Fig 1 we show the association between annual common CIMT change and the combined endpoint in all three groups. There was no significant relation in any group, whether adjusted for CVD risk factors or not. Between the cohorts, I2 statistics indicated no substantial heterogeneity. Fig 2 displays the relation between average common CIMT and the combined endpoint. In all three groups, there were significant and consistent positive associations, which attenuated on adjustment for CVD risk factors; the HRs were somewhat heterogeneous between the cohorts (statistically significant in groups A and B). Sensitivity analyses showed very similar results for the separate endpoints MI, stroke, and total mortality; and also for maximal CCA-IMT (shown for group A in S2–S5 Figs). To allow for a non-linear association, we assessed the association between CIMT and risk in Cox regression model including a quadratic term of CIMT change. We found a HR of 0.98 (95% CI 0.95–1.02) per SD of annual mean CCA-IMT progression (I2 = 10.9%, p for heterogeneity = 0.331) and of 1.22 (1.14–1.30) per SD of average mean CCA-IMT (i2 = 60.6%, p for heterogeneity = 0.001) for the combined endpoint.
Fig 1. Forest plots of the HR of the combined endpoint per one SD of annual mean CCA-IMT change (with 95% CIs).
Panel I: Group A (asymptomatic individuals with three or more CVD risk factors), HR adjusted for age, sex and average mean CCA-IMT (model 1). Panel II: Group A (asymptomatic individuals with three or more CVD risk factors), HR adjusted for age, sex, average mean CCA-IMT and other CVD risk factors (model 2). Panel III: Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex and average mean CCA-IMT (model 1). Panel IV: Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex, average mean CCA-IMT and other CVD risk factors (model 2). Panel V: Group C (individuals with previous CVD events), HR adjusted for age, sex and average mean CCA-IMT (model 1). Panel VI: Group C (individuals with previous CVD events), HR adjusted for age, sex, average mean CCA-IMT and other CVD risk factors (model 2).
Fig 2. Forest plots of the HR of the combined endpoint per one SD of average mean CCA-IMT (with 95% CIs).
Panel I: Group A (asymptomatic individuals with three or more CVD risk factors), HR adjusted for age, sex and annual mean CCA-IMT change (model 1). Panel II: Group A (asymptomatic individuals with three or more CVD risk factors), HR adjusted for age, sex, annual mean CCA-IMT change and other CVD risk factors (model 2). Panel III: Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex and annual mean CCA-IMT change (model 1). Panel IV: Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex, annual mean CCA-IMT change and other CVD risk factors (model 2). Panel V: Group C (individuals with previous CVD events), HR adjusted for age, sex and annual mean CCA-IMT change (model 1). Panel VI: Group C (individuals with previous CVD events), HR adjusted for age, sex, annual mean CCA-IMT change and other CVD risk factors (model 2).
In three cohorts (ARIC, INVADE, KIHD), CIMT measurements were available from four visits. In these cohorts, we estimated the correlation between the annual common CIMT change from visit 1 to visit 2, with the annual CIMT change from visit 3 to visit 4. This correlation was -0.021 in ARIC (p = 0.60), -0.065 in INVADE (p = 0.11), and -0.082 in KIHD (p = 0.11).
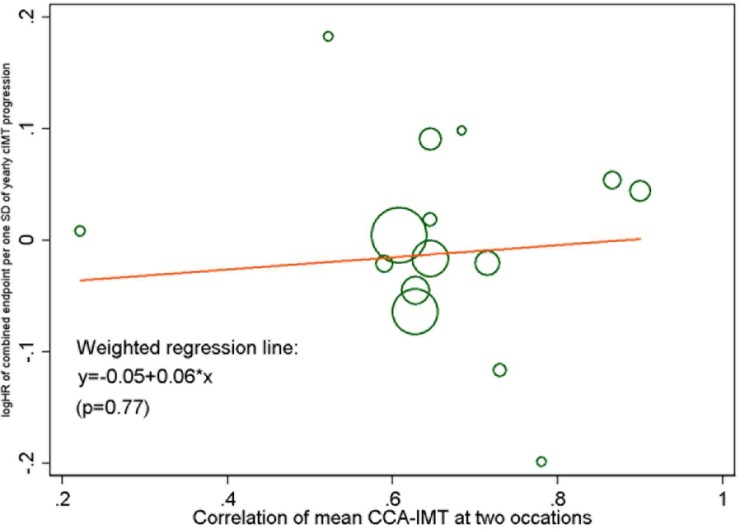
We studied the influence of the accuracy of CIMT measurement on the association between annual common CIMT change and risk in meta-regression analyses. There was no significant relation between the year of the study start and the HR for the combined endpoint per SD of annual common CIMT change (S6 Fig). Fig 3 shows the meta-regression of the correlation between the two CIMT measurements (as an indicator of measurement precision) and the HR, again with no significant relation. To assess the influence of the ultrasound protocol, we repeated the meta-analysis for individuals with prevalent carotid plaques (group B) and grouped the cohorts into those where CIMT measurement included carotid plaques and those where plaques were avoided (S7 Fig). The pooled HR for the combined endpoint did not differ between these two groups.
Fig 3. Meta-regression plot for the HR (combined endpoint) per SD of annual mean CCA-IMT change (model 1), by the correlation of baseline and follow-up common CIMT.
The size of each circle represents the precision of the log HR.
Discussion
Within a global collaborative project (see www.prog-imt.org), we managed to amass a large proportion of the worldwide available data in high-risk individuals (52% of all eligible cohorts), in order to assess the association between common CIMT change, and vascular event risk. Even in the selected high-risk individuals studied here, we were unable to demonstrate any association. In contrast, the known association between CIMT and vascular event risk was reproduced in a very consistent way.
There may be both methodological reasons and biological explanations for this discrepancy. One key methodological finding is that, even in high-risk populations, annual CIMT change was not a stable property of individuals, and therefore not a reproducible biomarker. When we compared–in three cohorts with the necessary data–CIMT change from visit 1 to visit 2 with CIMT change from visit 3 to visit 4 (all several years apart), we found no correlation.
But what is behind this lack of reproducibility? As can be seen in S1 Table, the range of common CIMT change, compared to CIMT, is very wide both within and between cohorts, indicating that measurement error is a major issue. For example in group A, average common CIMT is 3 to 8fold higher than its study-specific standard deviation, whereas annual common CIMT change is always smaller than its SD. It is plausible that the small systematic changes of CIMT within a few years are dwarfed by measurement error and random fluctuations.
A key problem of measuring CIMT change is to pinpoint the exact same measurement site in the carotid artery, years after the first measurement, and often done by a different technician. Despite multiple provisions in the ultrasound protocols, this seems to be an unresolved problem. As many of the studies shown here–and in particular the largest of them–were planned and started decades ago, we may hope that the newest studies and trials perform better. At least among the available cohorts, neither the year of study start, nor the accuracy of CIMT measurement had any significant effect on the CIMT-risk association we studied.
A plausible biological reason for these null findings is the complexity of the atherosclerotic process. CIMT reflects not only atherosclerosis, but also an adaptive component of the muscular wall, sometimes referred to as ‘remodelling’ [43–48]. In addition, in patients with high event risk, focal plaques may superimpose CIMT. Although overall, CIMT and plaques are progressing in parallel, there are individuals with low CIMT and impressive plaques (focal type), and vice versa (diffuse type of atherosclerosis).[49] Risk factors can act differently on CIMT and plaques,[50–52] and the association between plaque and CVD event risk may be closer than between CIMT and risk.[53]
In sensitivity analyses we studied cohorts where plaques were excluded from the CIMT measurement separately, but found no significant differences. However, it may not always be possible to avoid focal lesions when they are very distinct, and in the ultrasound measurement, the differentiation between diffuse (CIMT) and focal (plaque) atherosclerotic lesions is not clear-cut. So perhaps an isolated investigation of CIMT is too limited. Unfortunately, given the complex spatial structure of plaques, it is much more difficult to study plaque and plaque change, compared to CIMT. The standardization process for plaque measurement is years behind CIMT, where there is at least an international consensus.[54] Moreover, the amount of data that is available to analyze plaque change with standardized measurements is considerably lower than for CIMT change.
Linked with the previous argument, individuals with multiple risk factors, with carotid plaques, and stroke or MI patients, are often subjected to intensive risk factor management, life style modifications, and polypharmacy. Although we attempted to adjust for antihypertensive and lipid lowering medication, complex interactions between risk factors, nutrition, exercise, drugs and CIMT may obscure the association between CIMT and risk.
It is very important to distinguish between the ‘surrogacy’ at an individual level, as assessed here, and surrogacy at a group level, which is important for the interpretation of clinical trials about CIMT change. In this paper we addressed whether individuals whose CIMT progresses have higher subsequent event risk. For the interpretation of clinical trials with the endpoint CIMT change, we need to know whether a group of individuals treated with a drug whose CIMT progressed on average less than another group treated with another drug (or placebo), exhibits a lower event risk in the same period. This latter question has not been answered satisfactorily yet, as the current findings are contradictory.[5,6] The criteria of surrogacy in clinical trials, that is whether the effects of interventions on CIMT parallel the effects on risk, will be addressed in stage 3 of the PROG-IMT project.[11]
Limitations
It may be argued that many of the individuals included here were already studied in our previous work on general population cohorts.[7] Three arguments counteract this point: First, we selected only individuals at high cardiovascular risk out of these population cohorts. This could well have improved the ratio between the hypothesized association, and measurement error. Second, we added a number of population based studies [18, 20, 22, 24, 26, 39], risk cohorts [21, 27, 31] and hospital cohorts [14, 30, 32, 34, 37, 41] since the above cited publication. These new cohorts (15 of 31) comprise 33% of the sample size, and 10% of the endpoint events. Third, group C included only individuals that were explicitly excluded from the analyses of our previous work.
Conclusions
Although common CIMT is associated with future CVD event risk, this is not apparently true for common CIMT change over time. Reasons may include the complexity of atherosclerotic process, and technical limits of current CIMT measurement.
Do these null findings mean that CIMT (change) is not scientifically useful? Our results confirm that CIMT is still a very useful biomarker, with close associations with both risk factors and future endpoints. The change of CIMT, however, should be interpreted with care.
Supporting information
*mean CCA-IMT not available, maximal CCA-IMT used instead.
&combined endpoint not available, total mortality used instead.
(DOCX)
+plaques purposely included.
#internal landmarks in computer aided navigation aid.
++2D images extracted from 3D dataset.
n.s. = not specified.
(DOCX)
(PDF)
(PDF)
(DOCX)
Left panel: HR for MI per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for MI per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for stroke per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for stroke per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for total mortality per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for total mortality per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for the combined endpoint per one SD of annual maximal CCA-IMT change, adjusted for age, sex and average maximal CCA-IMT (model 1).
Right panel: HR for the combined endpoint per one SD of average maximal CCA-IMT, adjusted for age, sex and annual maximal CCA-IMT change (model 1).
(DOCX)
The size of each circle represents the precision of the log HR.
Left panel: Model 1 (HR adjusted for age, sex, and average mean CCA-IMT): weighted regression line y = 7.07+0.004*x (p = 0.34).
Right panel: Model 2 (HR adjusted for age, sex, average mean CCA-IMT and other CVD risk factors): weighted regression line y = -9.43+0.005*x (p = 0.32).
(DOCX)
Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex and average mean CCA-IMT (model 1).
(DOCX)
Acknowledgments
We used a restricted access dataset of the Atherosclerosis Risk In Communities (ARIC) Study. The ARIC Study was supported by National Heart, Lung and Blood Institute (Bethesda, MD, USA) in collaboration with the ARIC study investigators. This Article does not necessarily convey the opinions or views of the ARIC Study or the National Heart, Lung and Blood Institute. The Bruneck study was supported by an excellence initiative (Competence Centers for Excellent Technologies—COMET) of the Austrian Research Promotion Agency FFG: “Research Center of Excellence in Vascular Ageing–Tyrol, VASCage” (K-Project No. 843536) funded by the BMVIT, BMWFW, Wirtschaftsagentur Wien and Standortagentur Tirol, the Pustertaler Verein zur Praevention von Herz- und Hirngefaesserkrankungen, Gesundheitsbezirk Bruneck, and the Assessorat fuer Gesundheit (Province of Bolzano, Italy). The Carotid Atherosclerosis Progression Study was supported by the Stiftung Deutsche Schlaganfall-Hilfe. The Cardiovascular Health Study research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 to N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke (Bethesda, MD, USA). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (Bethesda, MD, USA). A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Etude sur le vieillissement artériel was organised with an agreement between INSERM and Merck, Sharp, and Dohme-Chibret. The Northern Manhattan Study/The Oral Infections and Vascular Disease Epidemiology Study is funded by the National Institute of Neurological Disorders and Stroke grant R37 NS 029993 and the Oral Infections, Carotid Atherosclerosis, and Stroke study by the National Institute of Dental and Craniofacial Research (Bethesda, MD, USA) grant R01 DE 13094. The Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg study was supported by AOK Bayern. The Rotterdam Study was supported by the Netherlands Foundation for Scientific Research, ZonMw, Vici 918-76-619. The Study of Health in Pomerania is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (BMBF 01ZZ9603 and 01ZZ0103), the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. The PROG-IMT project was funded by the Deutsche Forschungsgemeinschaft (DFG Lo 1569/2-1). The IMPROVE Study was supported by by the European Commission (Contract number: QLG1- CT- 2002–00896), Ministero della Salute Ricerca Corrente, Italy, LUA/ALF Gothenburg (agreement concerning research and education of doctors), the Swedish Heart-Lung Foundation, the Swedish Research Council (projects 8691 and 0593), the Foundation for Strategic Research, the Stockholm County Council (project 562183), the Foundation for Strategic Research, the Academy of Finland (Grant #110413) and the British Heart Foundation (RG2008/014). Simon Thompson is supported by the British heart Foundation (CH/12/2/29428).
Data Availability
Data are not owned by the PROG-IMT project, but provided under limitations to accomplish the project aims. Data are subject to limitations specific for each study, but distribution of data to third parties are generally prohibited.
Funding Statement
The PROG-IMT project, which includes this publication, has been funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, www.dfg.de) under the grants DFG Lo 1569/2-1 and DFG Lo 1569/2-3, received by MWL. The DFG had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Simon Thompson is supported by the British heart Foundation (CH/12/2/29428). Some of the contributing studies were funded by different parties, as listed in the acknowledgement section. Here, too, the funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Falk E, Shah PK The SHAPE guideline: ahead of its time or just in time. Curr Atheroscler Rep 2011;13: 345–352. doi: 10.1007/s11883-011-0195-y [DOI] [PubMed] [Google Scholar]
- 2.Beller GA The Texas Heart Attack Prevention Bill mandating coverage for CAD screening tests. J Nucl Cardiol 2009;16: 681–682. doi: 10.1007/s12350-009-9139-0 [DOI] [PubMed] [Google Scholar]
- 3.Bots ML Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006;22: 2181–2190. doi: 10.1185/030079906X148472 [DOI] [PubMed] [Google Scholar]
- 4.Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308: 796–803. doi: 10.1001/jama.2012.9630 [DOI] [PubMed] [Google Scholar]
- 5.Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J. Am. Coll. Cardiol. 2010;56: 2006–2020. doi: 10.1016/j.jacc.2010.05.059 [DOI] [PubMed] [Google Scholar]
- 6.Goldberger ZD, Valle JA, Dandekar VK, Chan PS, Ko DT and Nallamothu BK. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction? A critical review and meta-regression analysis. Am. Heart J. 2010;160: 701–714. doi: 10.1016/j.ahj.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 7.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Toumainen TP et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379: 2053–2062. doi: 10.1016/S0140-6736(12)60441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldassarre D, Nyyssönen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J. 2010;31:614–22. doi: 10.1093/eurheartj/ehp496 [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thompson SG, Deeks JJ, Altman Measuring inconsistency in meta-analyses. BMJ 2003;327: 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz MW, Bickel H, Bots ML, Breteler MMB, Catapano AL, Desvarieux M et al. Individual progression of carotid intima media thickness as a surrogate for vascular risk (PROG-IMT): Rationale and design of a meta-analysis project. Am. Heart J. 2010;159: 730–736.e2. doi: 10.1016/j.ahj.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallenfeldt K, Bokemark L, Wikstrand J, Hulthe J, Fagerberg B. Apolipoprotein B/apolipoprotein A-I in relation to the metabolic syndrome and change in carotid artery intima-media thickness during 3 years in middle-aged men. Stroke 2004; 35: 2248–52. doi: 10.1161/01.STR.0000140629.65145.3c [DOI] [PubMed] [Google Scholar]
- 13.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997; 146: 483–494. [DOI] [PubMed] [Google Scholar]
- 14.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G et al. Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke 2000;31:2127–33. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 2003; 290: 2271–76. doi: 10.1001/jama.290.17.2271 [DOI] [PubMed] [Google Scholar]
- 16.Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation 2001; 103: 1064–70. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006; 37: 87–92. doi: 10.1161/01.STR.0000196964.24024.ea [DOI] [PubMed] [Google Scholar]
- 18.Su TC, Jeng JS, Chien KL, Sung FC, Hsu HC, Lee YT. Hypertension status is the major determinant of carotid atherosclerosis: a community-based study in Taiwan. Stroke 2001;32:2265–71. [PubMed] [Google Scholar]
- 19.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14–22. doi: 10.1056/NEJM199901073400103 [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Hong Y, D'Agostino RB Sr, Wu Z, Wang W, Sun J et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA 2004;291:2591–9. doi: 10.1001/jama.291.21.2591 [DOI] [PubMed] [Google Scholar]
- 21.Lembo G, De Luca N, Battagli C, Iovino G, Aretini A, Musicco M et al. A common variant of endothelial nitric oxide synthase (Glu298Asp) is an independent risk factor for carotid atherosclerosis. Stroke 2001;32:735–40. [DOI] [PubMed] [Google Scholar]
- 22.Behre CJ, Brohall G, Hulthe J, Wikstrand J, Fagerberg B. Are serum adiponectin concentrations in a population sample of 64-year-old Caucasian women with varying glucose tolerance associated with ultrasound-assessed atherosclerosis? J Intern Med 2006;260:238–44. doi: 10.1111/j.1365-2796.2006.01683.x [DOI] [PubMed] [Google Scholar]
- 23.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25: 1172–81. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel R, Alonso M, Reviriego B, Muñiz J, Vega S, López I et al. Ten-year fatal and non-fatal myocardial infarction incidence in elderly populations in Spain: the EPICARDIAN cohort study. BMC Public Health 2009;9:360 doi: 10.1186/1471-2458-9-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol 1996; 16: 310–16. [DOI] [PubMed] [Google Scholar]
- 26.Spijkerman AM, Henry RM, Dekker JM, Nijpels G, Kostense PJ, Kors JA et al. Prevalence of macrovascular disease amongst type 2 diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the Hoorn Screening Study. J Intern Med 2004;256:429–36. doi: 10.1111/j.1365-2796.2004.01395.x [DOI] [PubMed] [Google Scholar]
- 27.Baldassarre D, Nyyssönen K, Rauramaa R, de Faire U, Hamsten A, Smit AJ et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J 2010;31:614–22. doi: 10.1093/eurheartj/ehp496 [DOI] [PubMed] [Google Scholar]
- 28.Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation 2000; 102: 1536–41. [DOI] [PubMed] [Google Scholar]
- 29.Lynch J, Kaplan GA, Salonen R, Salonen JT. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol 1997; 17: 513–19. [DOI] [PubMed] [Google Scholar]
- 30.Landecho MF, Colina I, Huerta A, Fortuño A, Zalba G, Beloqui O. [Connection between the early phases of kidney disease and the metabolic syndrome]. [Article in Spanish] Rev Esp Cardiol 2011;64:373–8. doi: 10.1016/j.recesp.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 31.Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 2005;257:430–7. doi: 10.1111/j.1365-2796.2005.01485.x [DOI] [PubMed] [Google Scholar]
- 32.Baldassarre D, Amato M, Bondioli A, Sirtori CR, Tremoli E. Carotid artery intima-media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke 2000;31:2426–30. [DOI] [PubMed] [Google Scholar]
- 33.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology 2008; 70: 1200–07. doi: 10.1212/01.wnl.0000303969.63165.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa K, Hougaku H, Yamagami H, Hashimoto H, Itoh T, Shimizu Y et al. Carotid intima-media thickness and risk of cardiovascular events in high-risk patients. Results of the Osaka Follow-Up Study for Carotid Atherosclerosis 2 (OSACA2 Study). Cerebrovasc Dis 2007;24:35–42. doi: 10.1159/000103114 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Macias KA, Lind L, Naessen T. Thicker carotid intima layer and thinner media layer in subjects with cardiovascular diseases. An investigation using noninvasive high-frequency ultrasound. Atherosclerosis 2006; 189: 393–400. doi: 10.1016/j.atherosclerosis.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 36.Norata GD, Garlaschelli K, Ongari M, Raselli S, Grigore L, Catapano AL. Eff ects of fractalkine receptor variants on common carotid artery intima-media thickness. Stroke 2006; 37: 1558–61. doi: 10.1161/01.STR.0000221803.16897.22 [DOI] [PubMed] [Google Scholar]
- 37.Staub D, Meyerhans A, Bundi B, Schmid HP, Frauchiger B. Prediction of cardiovascular morbidity and mortality: comparison of the internal carotid artery resistive index with the common carotid artery intima-media thickness. Stroke 2006;37:800–5. doi: 10.1161/01.STR.0000202589.47401.c6 [DOI] [PubMed] [Google Scholar]
- 38.Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JC. Carotid intima-media thickness at diff erent sites: relation to incident myocardial infarction; the Rotterdam Study. Eur Heart J 2002; 23: 934–40. doi: 10.1053/euhj.2001.2965 [DOI] [PubMed] [Google Scholar]
- 39.Sourij H, Schmoelzer I, Dittrich P, Paulweber B, Iglseder B, Wascher TC. Insulin resistance as a risk factor for carotid atherosclerosis: a comparison of the Homeostasis Model Assessment and the short insulin tolerance test. Stroke 2008;39:1349–51. doi: 10.1161/STROKEAHA.107.502799 [DOI] [PubMed] [Google Scholar]
- 40.von Sarnowski B, Lüdemann J, Völzke H, Dörr M, Kessler C, Schminke U. Common carotid intima-media thickness and Framingham risk score predict incident carotid atherosclerotic plaque formation: longitudinal results from the study of health in Pomerania. Stroke 2010; 41: 2375–77. doi: 10.1161/STROKEAHA.110.593244 [DOI] [PubMed] [Google Scholar]
- 41.Wannarong T, Parraga G, Buchanan D, Fenster A, House AA, Hackam DG et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013;44:1859–65. doi: 10.1161/STROKEAHA.113.001461 [DOI] [PubMed] [Google Scholar]
- 42.Stensland-Bugge E, Bønaa KH, Joakimsen O, Njølstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromsø study. Stroke 2000; 31: 574–81. [DOI] [PubMed] [Google Scholar]
- 43.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988; 112: 1018–31. [PubMed] [Google Scholar]
- 44.Crouse JR, Goldbourt U, Evans G, Pinsky J, Sharrett AR, Sorlie P et al. Arterial enlargement in the Atherosclerosis Risk In Communities (ARIC) cohort. In vivo quantification of carotid arterial enlargement. Stroke. 1994; 25: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 45.Bonithon-Kopp C, Touboul PJ, Berr C, Magne C, Ducimetière P. Factors of carotid arterial enlargement in a population aged 59 to 71 years. The EVA study. Stroke. 1996; 27: 654–660. [DOI] [PubMed] [Google Scholar]
- 46.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997; 28: 2442–7. [DOI] [PubMed] [Google Scholar]
- 47.Sitzer M, Puac D, Buehler A, Steckel DA, von Kegler S, Markus HS et al. Internal carotid artery angle of origin: a novel risk factor for early carotid atherosclerosis. Stroke 2003; 34: 950–5. doi: 10.1161/01.STR.0000060895.38298.C4 [DOI] [PubMed] [Google Scholar]
- 48.Lloyd KD, Barinas-Mitchell E, Kuller LH, Mackey RH, Wong EA, Sutton-Tyrrell K. Common carotid artery diameter and cardiovascular risk factors in overweight or obese postmenopausal women. Int J Vasc Med. 2012; 2012: 169323 doi: 10.1155/2012/169323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiechl S, Willeit J The natural course of atherosclerosis. Part I: incidence and progression. Arterioscler. Thromb. Vasc. Biol. 1999;19: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 50.Fagerberg B, Wikstrand J, Berglund G, Samuelsson O, Agewall S Mortality rates in treated hypertensive men with additional risk factors are high but can be reduced: a randomized intervention study. Am. J. Hypertens. 1998;11: 14–22. [DOI] [PubMed] [Google Scholar]
- 51.Agewall S, Fagerberg B, Berglund G, Schmidt C, Wendelhag I and Wikstrand J. Multiple risk intervention trial in high risk hypertensive men: comparison of ultrasound intima-media thickness and clinical outcome during 6 years of follow-up. J. Intern. Med. 2001;249: 305–314. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt C, Fagerberg B, Wikstrand J, Hulthe J Multiple risk factor intervention reduces cardiovascular risk in hypertensive patients with echolucent plaques in the carotid artery. J. Intern. Med. 2003;253: 430–438. [DOI] [PubMed] [Google Scholar]
- 53.Johnsen SH, Mathiesen EB Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep 2009;11: 21–27. [DOI] [PubMed] [Google Scholar]
- 54.Touboul P, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012;34: 290–296 doi: 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*mean CCA-IMT not available, maximal CCA-IMT used instead.
&combined endpoint not available, total mortality used instead.
(DOCX)
+plaques purposely included.
#internal landmarks in computer aided navigation aid.
++2D images extracted from 3D dataset.
n.s. = not specified.
(DOCX)
(PDF)
(PDF)
(DOCX)
Left panel: HR for MI per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for MI per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for stroke per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for stroke per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for total mortality per one SD of annual mean CCA-IMT change, adjusted for age, sex and average mean CCA-IMT (model 1).
Right panel: HR for total mortality per one SD of average mean CCA-IMT, adjusted for age, sex and annual mean CCA-IMT change (model 1).
(DOCX)
Left panel: HR for the combined endpoint per one SD of annual maximal CCA-IMT change, adjusted for age, sex and average maximal CCA-IMT (model 1).
Right panel: HR for the combined endpoint per one SD of average maximal CCA-IMT, adjusted for age, sex and annual maximal CCA-IMT change (model 1).
(DOCX)
The size of each circle represents the precision of the log HR.
Left panel: Model 1 (HR adjusted for age, sex, and average mean CCA-IMT): weighted regression line y = 7.07+0.004*x (p = 0.34).
Right panel: Model 2 (HR adjusted for age, sex, average mean CCA-IMT and other CVD risk factors): weighted regression line y = -9.43+0.005*x (p = 0.32).
(DOCX)
Group B (asymptomatic individuals with carotid plaques), HR adjusted for age, sex and average mean CCA-IMT (model 1).
(DOCX)
Data Availability Statement
Data are not owned by the PROG-IMT project, but provided under limitations to accomplish the project aims. Data are subject to limitations specific for each study, but distribution of data to third parties are generally prohibited.