Cold temperature blocks used to synchronize protein trafficking inhibit GBF1 function, leading to a decrease in ARF1-GTP levels and mislocalization of the ARF1 effector golgin-160. Several other, but not all, Golgi proteins including ARL1 also mislocalize. ARF1 activity and golgin-160 localization require more than 30 min to recover from these blocks.
Abstract
ADP-ribosylation factor (ARF) proteins are key regulators of the secretory pathway. ARF1, through interacting with its effectors, regulates protein trafficking by facilitating numerous events at the Golgi. One unique ARF1 effector is golgin-160, which promotes the trafficking of only a specific subset of cargo proteins through the Golgi. While studying this role of golgin-160, we discovered that commonly used cold temperature blocks utilized to synchronize cargo trafficking (20 and 16°C) caused golgin-160 dispersal from Golgi membranes. Here, we show that the loss of golgin-160 localization correlates with a decrease in the levels of activated ARF1, and that golgin-160 dispersal can be prevented by expression of a GTP-locked ARF1 mutant. Overexpression of the ARF1 activator Golgi brefeldin A–resistant guanine nucleotide exchange factor 1 (GBF1) did not prevent golgin-160 dispersal, suggesting that GBF1 may be nonfunctional at lower temperatures. We further discovered that several other Golgi resident proteins had altered localization at lower temperatures, including proteins recruited by ARF-like GTPase 1 (ARL1), a small GTPase that also became dispersed in the cold. Although cold temperature blocks are useful for synchronizing cargo trafficking through the Golgi, our data indicate that caution must be taken when interpreting results from these assays.
INTRODUCTION
In humans, the ARF family of proteins consists of five small GTPases, four of which are critical for the function of the secretory pathway through their interactions with effector proteins (reviewed in D’Souza-Schorey and Chavrier, 2006; Donaldson and Jackson, 2011). The ARFs play semiredundant but essential roles in cargo transit throughout the secretory system by acting as molecular switches, a function dependent on their GTPase cycles (Volpicelli-Daley et al., 2005). The cis-Golgi role of ARF1 has been well studied. After activation by its guanine nucleotide exchange factor (GEF), GBF1, the Golgi membrane-bound ARF1-GTP recruits coat protein I (COPI) coat complexes to interact with cargo proteins (reviewed in Jackson, 2014). The ARF GTPase-activating protein (ARFGAP) -stimulated hydrolysis of GTP by ARF1 is required for the recruitment of cargo into COPI-coated vesicles and the subsequent removal of those coats (Teal et al., 1994; Nickel et al., 1998; Pepperkok et al., 2000; Lee et al., 2005).
ARF1-GTP can recruit several other effector proteins, one of which is golgin-160 (also called GOLGA3 and GCP-170). Found only in vertebrates, golgin-160 has been reported to promote Golgi structure as well as facilitate the proper targeting and efficient trafficking of specific cargo proteins (Hicks et al., 2006; Yadav et al., 2009). Many golgins promote bulk cargo movement through the Golgi, by acting as tethers or maintaining Golgi structure (Munro, 2011). Golgin-160’s trafficking role is unique because it promotes the trafficking of only a small, specific subset of cargoes. We previously found that loss of golgin-160 causes glucose transporter type 4 (GLUT4) to traffic directly to the plasma membrane, bypassing its proper localization to insulin-sensitive vesicles (Williams et al., 2006). We also reported that golgin-160 directly binds to the beta-1 adrenergic receptor (β1AR), and that depletion of golgin-160 decreases β1AR steady-state surface levels as well as the rate of arrival of the receptor at the plasma membrane (Hicks et al., 2006; Gilbert et al., 2014). Intriguingly, the cis-localized golgin-160 affects the rate of β1AR trafficking at a post-trans-Golgi step (Hicks et al., 2006). These findings led us to become interested in how the localization of golgin-160 impacts its function in protein trafficking.
Synchronization of cargo exit from a specific compartment is a commonly used tool to study protein trafficking at distinct stages of the secretory pathway. Several new synchronization techniques have been developed in the last several years; however, they require protein modifications that can have unintended effects on trafficking or localization (reviewed in Boncompain and Perez, 2013; Roboti et al., 2013; Feng and Arnold, 2016). One of the oldest and most broadly useful techniques is the use of cold temperature blocks, which allows for synchronization of untagged, endogenous cargo proteins. First reported in 1983, it was shown that incubating cells at 20°C leads to a block in cargo trafficking at the trans-Golgi network (TGN), whereas an incubation at 15–16°C leads to retention of cargo proteins in the early Golgi or ER-Golgi intermediate compartment (ERGIC; Matlin and Simons, 1983; Saraste and Kuismanen, 1984; Saraste and Svensson, 1991). Although some data, like those showing the inability of brefeldin A (BFA) to collapse the Golgi into the ER at these temperatures (Lippincott-Schwartz et al., 1990), suggest that these blocks may be due to inhibiting membrane dynamics, the exact mechanism behind these temperature blocks has never been elucidated.
In this study, we attempted to use cold temperature blocks to study golgin-160’s role in cargo trafficking at distinct compartments of the secretory pathway. However, we found that these temperatures disrupt golgin-160 localization at the Golgi. This led us to analyze the effects of cold temperature shifts on ARF1 (the protein responsible for recruitment of golgin-160 to Golgi membranes) and other Golgi-localized proteins.
RESULTS
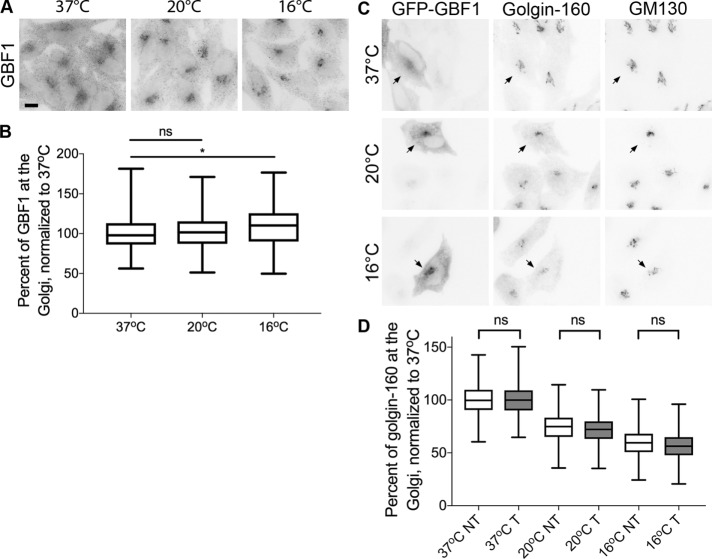
Golgin-160 disperses from Golgi membranes in cells subjected to 20 or 16°C trafficking blocks
We and other groups have reported that golgin-160 facilitates the trafficking of specific cargo proteins (Bundis et al., 2006; Hicks et al., 2006; Williams et al., 2006; Gilbert et al., 2014). To dissect golgin-160’s role in this process, we attempted to employ two commonly used temperature blocks to stop cargo trafficking at different subcompartments of the Golgi complex. However, when we assessed the localization of golgin-160 by indirect immunofluorescence microscopy in HeLa cells after incubation for 3 h at 20 or 16°C, we found that golgin-160 signal was lost from the Golgi region (Figure 1A). To quantify this effect, we defined the Golgi region using the fluorescence of another cis-Golgi–localized peripheral membrane protein, GM130, which did not show a dramatic change in localization (Figure 1A; quantification in Supplemental Figure 1A). We then measured the golgin-160 fluorescence intensity within both the Golgi region and the whole cell. The percent of the signal within the Golgi region was calculated and the values were normalized to those in cells kept at 37°C. There was a progressive loss of golgin-160 localization at the lower temperatures, with a 40% decrease in golgin-160 at the Golgi at 20°C, and a 48% decrease at 16°C (Figure 1B).
FIGURE 1:
Two temperature blocks lead to dispersal of golgin-160 from Golgi membranes. (A) Representative images of HeLa cells incubated at 37, 20, or 16°C for 3 h are shown. Cells were labeled with rabbit anti–golgin-160 and mouse anti-GM130, followed by Alexa Fluor 488 anti-rabbit IgG and Alexa Fluor 546 anti-mouse IgG. Scale bar, 10 μm. (B) Quantification of golgin-160 dispersal. The GM130 signal was used to outline the Golgi region in each cell, and the amount of golgin-160 fluorescent signal was calculated within the Golgi and divided by the total golgin-160 fluorescence in the cell to obtain the percent of golgin-160 at the Golgi. Each fluorescence intensity value was normalized to the average value at 37°C. More than 160 cells from three separate experiments were quantified for each temperature. p < 0.001 for all comparisons.
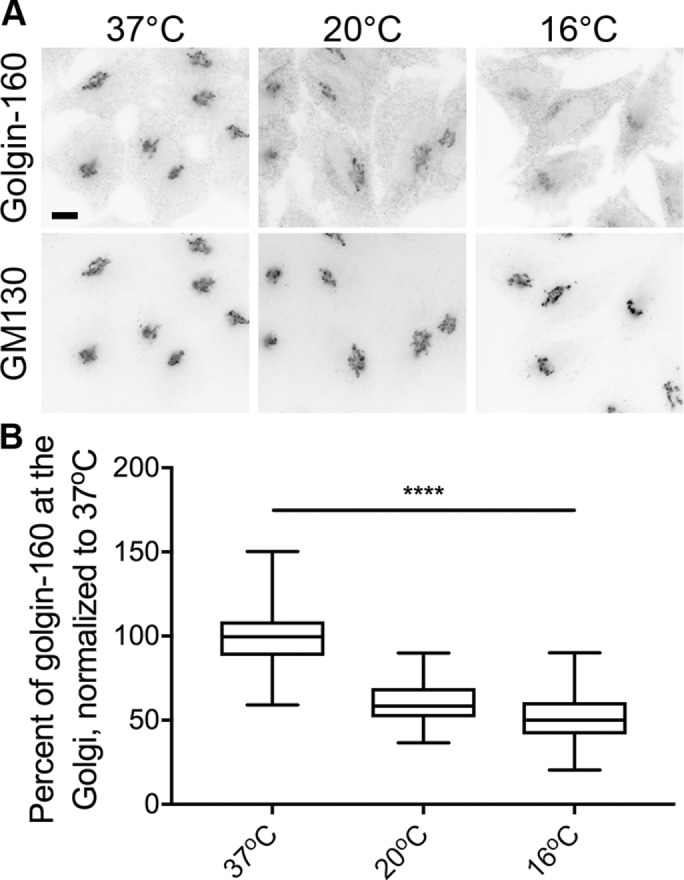
It was possible that the loss of Golgi-localized golgin-160 could be due to protein degradation. However, a Western blot of lysates from HeLa cells incubated at 37, 20, or 16°C showed no difference in golgin-160 levels (Figure 2). Thus, it appears that cold temperatures cause the release of golgin-160 from Golgi membranes into a cytosolic pool.
FIGURE 2:
Loss of golgin-160 localization at cold temperatures is not due to protein degradation. (A) Lysates of HeLa cells incubated at 37, 20, or 16°C for 3 h were analyzed by Western blot with rabbit anti–golgin-160 or mouse anti-actin, followed by 680RD donkey anti-rabbit IgG or 680RD donkey anti-mouse IgG. (B) Golgin-160 signal was first normalized to actin and then to the 37°C control. ns, not significant (p = 0.23), n = 5.
Manolea et al. (2010) discovered that exogenously expressed GFP-tagged ARF3 loses its Golgi localization upon shifting cells to 20°C. This relocation to the cytoplasm was nearly complete within 20 min, and ARF3 Golgi localization could be restored within 20 min of shifting cells back to 37°C (Manolea et al., 2010). Thus, we examined the kinetics of golgin-160 dispersal, and whether the effect was reversible. Golgin-160 dispersal was not as rapid as that reported for ARF3: after 30 min at 16°C, Golgi-localized golgin-160 decreased by only 32%, compared with 48% after 3 h (Figure 3). Golgin-160 localization also recovered more slowly than that of ARF3: when HeLa cells incubated for 3 h at 16°C were shifted back to 37°C for 30 min, golgin-160 still had a 12% decrease in Golgi localization compared with cells kept at 37°C for the entire time course (Figure 3B).
FIGURE 3:
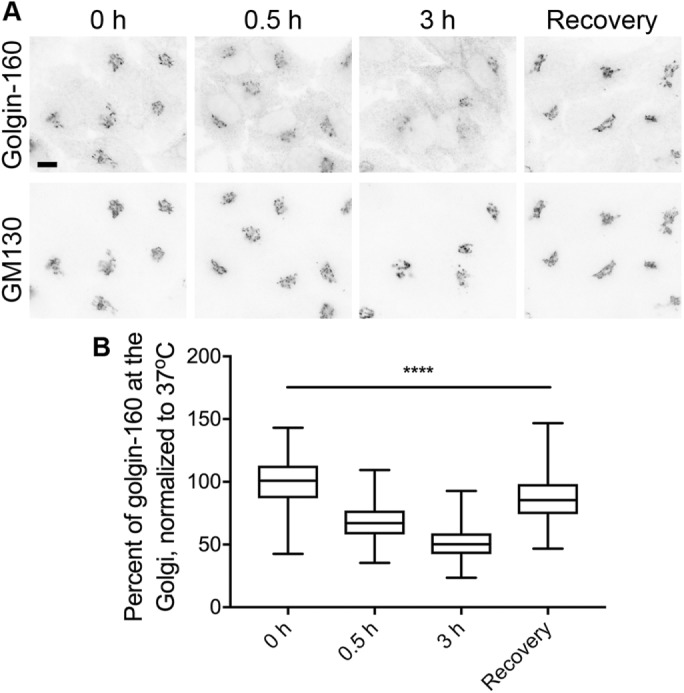
Golgin-160 disperses from and recovers to the Golgi slowly during temperature shifts. (A) HeLa cells were shifted from 37°C to 16°C for 0, 0.5, or 3 h. For the recovery condition, cells were incubated 3 h at 16°C and then returned to 37°C for 0.5 h. Cells were labeled with rabbit anti–golgin-160 and mouse anti-GM130, followed by Alexa Fluor 488 anti-rabbit IgG and Alexa Fluor 546 anti-mouse IgG. Scale bar, 10 μm. (B) Quantification of the percent of golgin-160 signal in the Golgi region was calculated as in Figure 1. More than 170 cells from three separate experiments were analyzed for each temperature condition. p < 0.001 for all conditions.
ARF1 activation status is altered at cold temperatures
Golgin-160 localization at the Golgi is dependent on the activation status of the small GTPase ARF1 (Yadav et al., 2012). We predicted that the temperature blocks might cause golgin-160 dispersal by affecting ARF1 activation. Thus, we determined the relative amount of GTP-bound ARF1 in HeLa cells incubated at 37, 20, or 16°C using an effector Golgi-associated, gamma adaptin ear-containing, ARF-binding protein 3 (GGA3)–based pull-down kit (see Materials and Methods). We observed a 30% decrease in the amount of GTP-bound ARF1 in cells incubated for 3 h at 20°C, and a 60% decrease at 16°C, when compared with cells incubated at 37°C (Figure 4, A and B). After a 3 h incubation at 16°C, a 30 min recovery at 37°C partially restored levels of activated ARF1, with only an 11% decrease in ARF1-GTP levels compared with control cells maintained at 37°C. We observed a wide range of biological variability in this assay, but the progressive loss of GTP-bound ARF1 at cold temperatures and the ability to restore ARF1-GTP levels after a recovery period correlated with the progressive decrease and restoration of Golgi-localized golgin-160 (Figures 1B and 3B).
FIGURE 4:
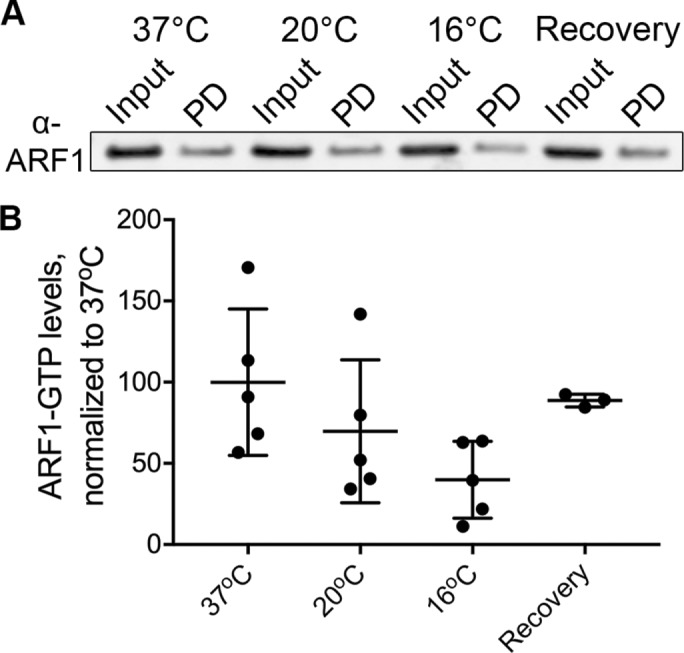
ARF1-GTP levels decrease during cold temperature shifts and recover upon return to 37°C. (A) Representative Western blot of active (GTP-bound) ARF1. The input and pull-down (PD) samples were run side by side. HeLa cells were incubated at 37, 20, or 16°C for 3 h before being lysed. For the recovery sample, cells were incubated at 16°C for 3 h before being shifted back to 37°C for 0.5 h. Active ARF1 was removed from lysates using agarose beads conjugated with the ARF1-GTP–binding domain of GGA3. (B) The amount of active ARF1 was analyzed by Western blot and was normalized first to input and then to the 37°C control. n = 5 for 37, 20, and 16°C. Three of these replicates also contained the recovery time point. p = 0.08 comparing 37° and 16°C; p > 0.2 for all other comparisons.
Localization of ARF1 and ARF1 effector proteins is differentially altered by cold temperature shifts
Manolea et al. (2010) also examined the localization of GFP-tagged ARF1 at 20°C and found that ARF1, unlike ARF3, was insensitive to this temperature shift and did not dissociate from the Golgi. We confirmed that GFP-tagged ARF1 does not disperse from Golgi membranes at 20°C, and also observed no change in GFP-ARF1 localization at 16°C, despite the decrease in cellular ARF1-GTP levels (Figure 5A). We examined the localization of several additional ARF1-effector proteins to further investigate the effects of the cold on ARF1 activation, predicting that they would show the same dispersal as golgin-160. Instead, three trans-Golgi cargo adaptor proteins had three unique cold phenotypes: GGA2 did not change localization at cold temperatures, AP-1 increased its Golgi localization at cold temperatures, and GGA3 only had increased localization at 20°C (Figure 5B). The reason for these different localization patterns is currently unclear.
FIGURE 5:
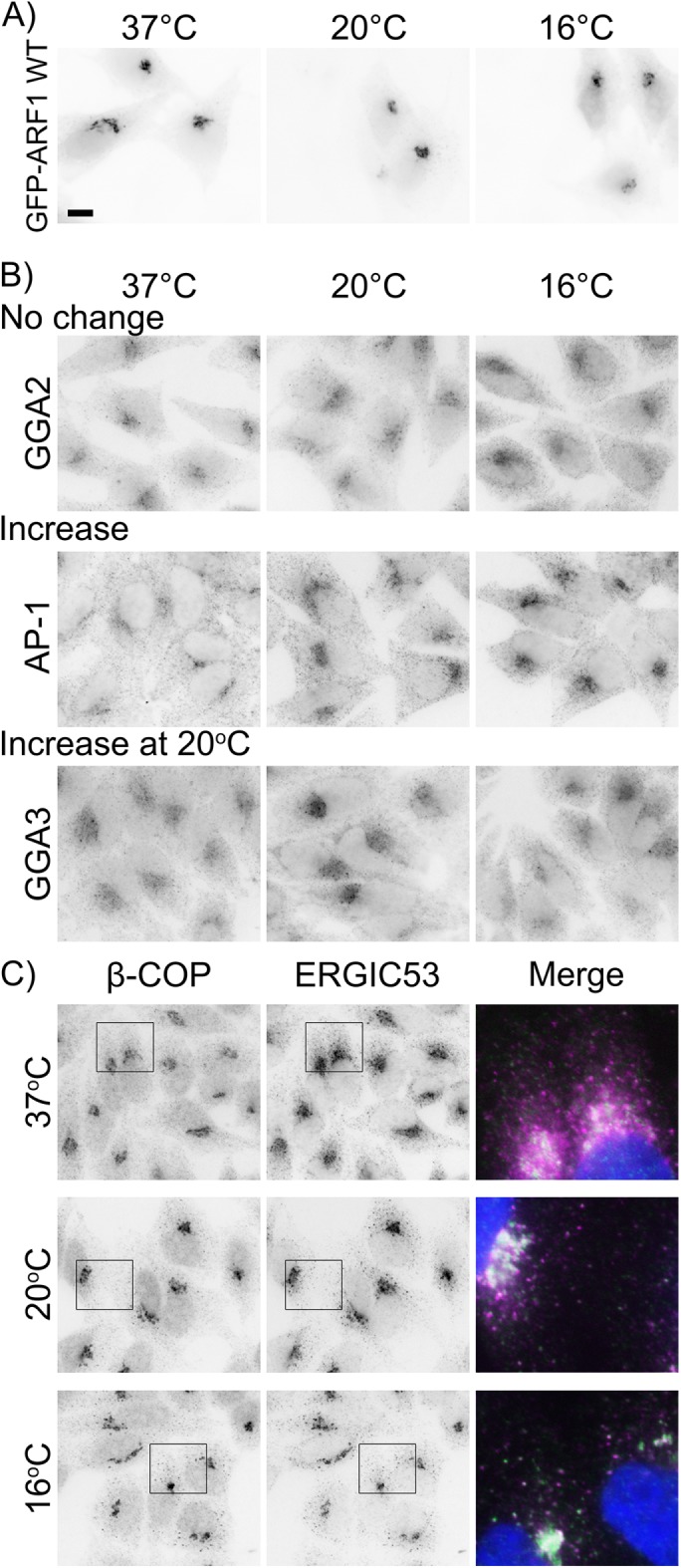
ARF1 and ARF1 effector proteins have differential localization at cold temperatures. (A) HeLa cells expressing GFP-ARF1 WT for 16–17 h were shifted to 20 or 16°C for 3 h and the GFP signal was visualized after fixation. (B) HeLa cells were incubated as described in A and endogenous trans-Golgi ARF1 effectors were visualized by staining with mouse anti-GGA2, -AP-1, or -GGA3 followed by Alexa Fluor 546 anti-mouse IgG. (C) HeLa cells were incubated as in A and were stained with rabbit anti-β-COP and mouse anti-ERGIC53 followed by Alexa Fluor 546 anti-mouse and Alexa Fluor 488 anti-rabbit IgG. Boxed regions are enlarged in the merged image: green, β-COP; magenta, ERGIC53; blue, nuclei. Scale bar, 10 μm.
β-COP is a subunit of the COPI coat complex (Donaldson et al., 1992), which is stabilized on Golgi membranes by ARF1-GTP (Presley et al., 2002). Although binding rates decreased with decreasing temperature, both ARF1 and β-COP can bind to Golgi membranes at temperatures as low as 4°C (Presley et al., 2002). At 15°C, it was previously shown that β-COP and the ERGIC protein ERGIC53 accumulate in extra-Golgi puncta (Klumperman et al., 1998). We also observed the formation of extra-Golgi β-COP puncta that localized with or near to ERGIC53 puncta, both at 20 and 16°C, and additionally observed an increase in the Golgi localization of both these proteins (Figure 5C).
Activated ARF1 can prevent cold-induced dispersal of golgin-160
Although activated ARF1-GTP is canonically the predominant form of ARF1 thought to be stably associated with Golgi membranes, the dominant inactive ARF1 T31N mutant (which mimics the inactive ARF1-GDP) is also Golgi localized, possibly through stabilized interaction with GBF1 (Niu et al., 2005; Szul et al., 2005). The Golgi localization of ARF1-GFP observed by fluorescence microscopy at cold temperatures, coupled with the decrease in cellular ARF1-GTP levels, suggest that the Golgi-localized ARF1 could be stabilized on Golgi membranes in its GDP-bound form. This predicts that the decrease in Golgi localization of golgin-160 should be prevented by providing activated ARF1 to the cells. To test this, we expressed the ARF1 Q71L mutant, which mimics the active ARF1-GTP–bound protein (Teal et al., 1994). ARF1 Q71L prevented golgin-160 dispersal at both 20 and 16°C (Figure 6A). Although nontransfected cells on the same coverslip had a decrease in golgin-160 Golgi localization by 28 and 44%, ARF1 Q71L–transfected cells had reductions of only 11 and 26%, respectively (Figure 6B). In contrast, GM130 localization is ARF1 independent, and expression of ARF1 Q71L did not alter GM130 localization at cold temperatures (Supplemental Figure 1C). Thus, it appears that the cold-induced dissociation of golgin-160 from Golgi membranes is due to reduced levels of ARF1 activation.
FIGURE 6:
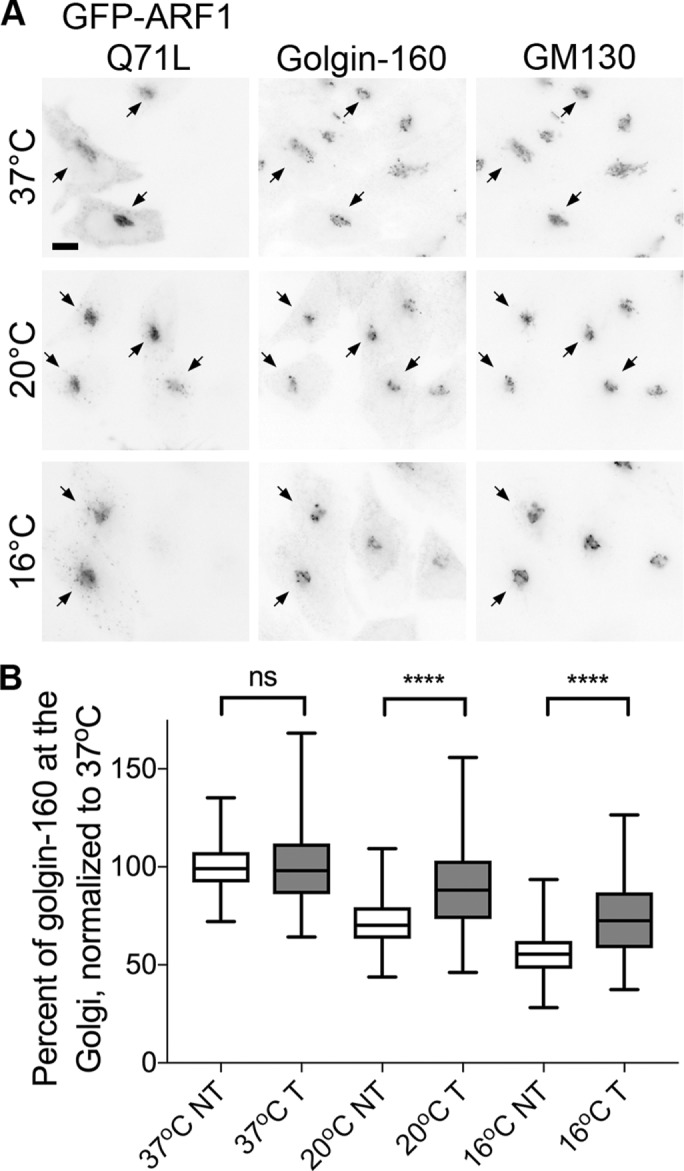
The active ARF1 Q71L mutant can prevent golgin-160 dispersal from the Golgi at cold temperatures. (A) HeLa cells expressing GFP-ARF1 Q71L for 16–17 h were shifted to 20 or 16°C for 3 h. GFP fluorescence was used to identify transfected cells and golgin-160 and GM130 localization was determined by indirect immunofluorescence with rabbit anti–golgin-160 and mouse anti-GM130, followed by Alexa Fluor 546 anti-mouse IgG and Cy5 anti-rabbit IgG. Scale bar, 10 μm. Arrows indicate transfected cells. (B) The percent of golgin-160 at the Golgi was calculated in both transfected (T) and nontransfected (NT) cells for each experiment as described in Figure 1. For each population of cells (T and NT), values were normalized to 37°C. The differences between temperatures were significant for both T and NT populations (p < 0.001 for all comparisons). Expression of ARF1 Q71L was protective, with a significant difference between ARF1 Q71L transfected and nontransfected cells at both 20 and 16°C (p < 0.001). More than 135 cells from four separate experiments were analyzed for each temperature and transfection condition.
Cold temperatures affect the function of the ARF1 activator GBF1
GBF1 depletion experiments have indicated that GBF1 is required for ARF1 activation to facilitate golgin-160 recruitment (Yadav et al., 2012). Thus, we examined the distribution of endogenous GBF1 in cells incubated at reduced temperatures. As shown in Figure 7A and quantified in 7B, GBF1 displayed a slight increase in Golgi localization at 20 and 16°C, by 2 and 8%, respectively. The increased level of Golgi-associated GBF1 is consistent with the Golgi-localized ARF1 being in an inactive form, as GBF1 is stabilized on membranes in the presence of the ARF1-GDP and is released from membranes when ARF1 becomes activated by binding GTP (Szul et al., 2005). The increased localization of GBF1 and inactive form of ARF1 at the Golgi during cold treatments suggests that the catalytic activity of GBF1 might be inhibited at lower temperatures.
FIGURE 7:
GBF1 has increased Golgi localization at cold temperatures and GBF1 overexpression does not prevent cold-induced dispersal of golgin-160. (A) HeLa cells were shifted to 20 or 16°C for 3 h, followed by staining with mouse anti-GBF1 and Alexa Fluor 546 anti-mouse IgG. Scale bar, 10 μm. (B) Percent of GBF1 at the Golgi was quantified similarly to golgin-160 localization in Figure 1. More than 137 cells from three separate experiments were analyzed for each temperature condition. *, p = 0.043. (C) HeLa cells expressing GFP-GBF1 A795E for 16–17 h were shifted to 20 or 16°C for 3 h. Microscopy and analysis were performed as in Figure 5. Arrows indicate transfected cells. (D) The differences in golgin-160 Golgi localization between temperatures were significant (p < 0.001 for all comparisons); however, there were no significant differences between the GBF1 transfected and NT populations at each temperature. More than 135 cells from four separate experiments were analyzed for each temperature and transfection condition.
To further probe this point, we expressed GFP-tagged GBF1 and assessed its ability to support golgin-160 localization in the cold. We did not observe any protective effect of overexpressing GBF1 on golgin-160 dispersal at lower temperatures (Figure 7C). When normalized to cells incubated at 37°C, the Golgi localization of golgin-160 decreased by ∼27 and ∼41% at 20 and 16°C, respectively, in both GBF1 transfected and nontransfected cells (Figure 7D). This suggests that GBF1, while localized to the Golgi at cold temperatures, is not functioning to promote conversion of ARF1-GDP to its GTP-bound form.
ARL1-dependent proteins also have disrupted localization at cold temperatures
Although ARF1 recruits many essential proteins to the Golgi, we also examined a panel of ARF1-independent proteins for cold-induced changes in cellular localization. Our localization data from all ARF-dependent and -independent proteins examined at cold temperatures are summarized in Table 1. A number of ARF1-independent transmembrane proteins did not have altered localization in the cold (Supplemental Figure 2). The lack of phenotype in transmembrane proteins is not surprising given the overall lack of change in Golgi morphology observed at cold temperatures.
TABLE 1:
Numerous Golgi-localized proteins show disrupted localization patterns during cold shifts.
| Protein name | Localization | ARF/ARL dependent? | Change at lower temperatures? |
|---|---|---|---|
| ERGIC53 | ERGIC | No | No change |
| Golgin-160 | CGN | ARF1 | Decrease |
| β-COP | CGN | ARF1 | Increase; puncta |
| GBF1 | CGN and TGN | No | Small increase |
| p115 | CGN | No | No change |
| GM130 | CGN | No | Small decrease |
| GRASP65 | CGN | No | No change |
| Giantin | Medial | No | No change |
| ManII | Medial | No | No change |
| AP-1 | TGN | ARF1/3 | Increase |
| GGA2 | TGN | ARF1/3 | No change |
| GGA3 | TGN | ARF1/3 | Increase at 20oC |
| BIG1 | TGN | ARF4/5, ARL1 | No change |
| BIG2 | TGN | ARF4/5, ARL1 | Decrease at 16oC |
| Golgin-97 | TGN | ARL1 | Puncta |
| GCC1 | TGN | ARL1 (?) | Variable decrease |
| GCC2 | TGN | ARL1 (?) | No change |
| p230 | TGN | ARL1 | Increase; puncta |
| TGN46 | TGN | No | No change |
Multiple Golgi-localized proteins were analyzed by indirect immunofluorescence microscopy after incubation at 37, 20, or 16°C for 3 h. Sub-Golgi localization and ARF or ARL dependence do not correlate with cold shift phenotypes. TGN, trans-Golgi network; CGN, cis-Golgi network; ERGIC, ER-Golgi intermediate compartment.
Six of the proteins we examined are recruited to the Golgi by the ARF-like small GTPase ARL1. This includes the four GRIP (golgin-97, RanBP2α, Imh1p, and p230/golgin-245) domain-containing proteins (p230/golgin-245, golgin-97, GCC1/GCC88, and GCC2/GCC185) and the two BIG family GEFs (BIG1 and BIG2). These ARL1-dependent proteins showed a variety of phenotypes at cold temperatures, including no change, appearance in extra-Golgi puncta, and decreased Golgi localization at cold temperatures (Figure 8A). We then investigated the localization of ARL1 itself at cold temperatures by exogenously expressing GFP-tagged ARL1. We found that, unlike ARF1, ARL1 became increasingly cytosolic at 20 and 16°C (Figure 8B). Although we do not currently know the molecular mechanism responsible for the differential response of these proteins to cold temperature shifts, our data suggest that caution should be taken when interpreting results obtained using these methods.
FIGURE 8:
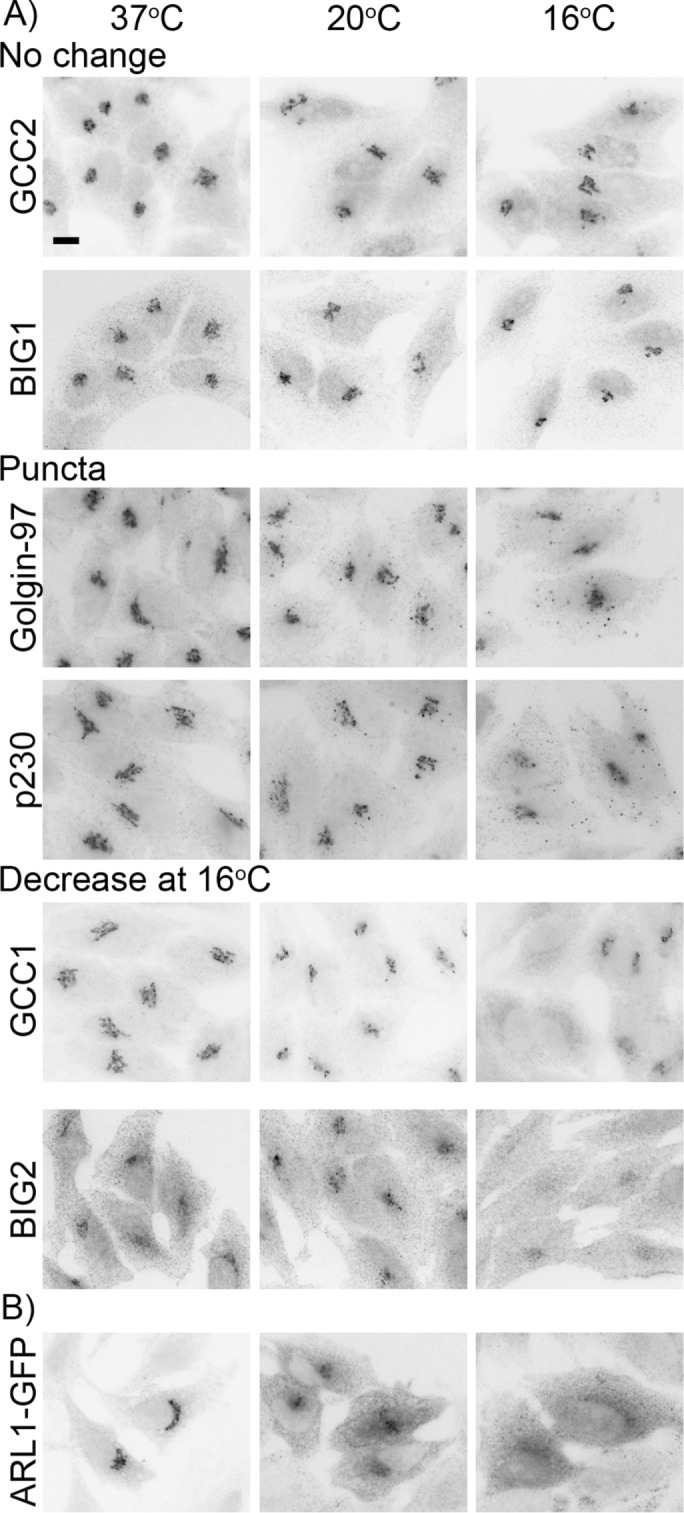
ARL1 effectors have differential localization and ARL1-GFP disperses from Golgi membranes at cold temperatures. (A) HeLa cells were shifted to 16 or 20°C for 3 h before the localization patterns of endogenous ARL1 effectors were examined. Cells were stained with rabbit anti-BIG1, -BIG2, -GCC1, or -GCC2 or mouse anti–golgin-97 followed by Alexa Fluor 546 anti-mouse or Alexa Fluor 488 anti-rabbit IgG. Scale bar, 10 μm. (B) HeLa cells expressing ARL1-GFP for 16–17 h were shifted as in A. Cells were stained with rabbit anti-GFP followed by Alexa Fluor 546 anti-rabbit IgG.
DISCUSSION
We previously reported that golgin-160 is a cis-Golgi concentrated golgin that facilitates the post-Golgi trafficking of several specific cargo proteins (Hicks et al., 2006; Williams et al., 2006; Gilbert et al., 2014). In our attempts to further characterize the function of golgin-160, we used two common cold temperature blocks to synchronize protein trafficking. We report here that these temperature blocks lead to the disruption of not only golgin-160’s localization (Figure 1), but the localization and/or function of multiple Golgi resident proteins, including the small GTPases ARF1 and ARL1 and several of their effectors (Figures 4–8 and Table 1). Although the effects on golgin-160 localization are reversible upon return to 37°C, the recovery is not immediate (Figure 3), indicating that the full complement of Golgi-localized proteins that impact cargo trafficking may not be available when cargo proteins are initially released from the cold temperature block.
ARF1 has many effector proteins, including golgin-160, which facilitate ARF1’s critical roles in promoting anterograde and retrograde trafficking at the Golgi. At the cis-Golgi, the balance between the active ARF1-GTP and the inactive ARF1-GDP is maintained both by its GEF, GBF1, and multiple GAPs. Disruption of this balance has a negative impact on trafficking through the secretory pathway: overexpression of the activated Q71L mutant and the triple depletion of the semiredundant ARFGAP1, 2, and 3 both lead to blocks in trafficking through the Golgi (Zhang et al., 1994; Claude et al., 1999; Saitoh et al., 2009). Both wild-type (WT) ARF1 and ARF1 Q71L bind to the N-terminus of golgin-160 in vitro (Yadav et al., 2012), which contains its Golgi targeting information (Hicks and Machamer, 2002). Here, we demonstrated that the degree and timing of golgin-160 dispersal from Golgi membranes directly correlates with the ARF1-GTP levels in cells (Figures 1, 3, and 4). These data, in addition to the discovery that ARL1 becomes cytoplasmic in the cold (Figure 8) and the previously reported finding that ARF3 localization is sensitive to a 20°C shift (Manolea et al., 2010), suggest that trafficking blocks induced by cold temperature shifts could be caused by a reduction in the activation of multiple Golgi-localized small GTPases.
A reduction in ARF1 activity at lower temperatures is supported by an effector-based pull-down assay (Figure 4) and the rescue of golgin-160’s dispersal phenotype in cells expressing the constitutively active ARF1 Q71L (Figure 6). However, this conclusion is complicated by the fact that ARF1-GFP is recruited to Golgi membranes at temperatures as low as 4°C (Presley et al., 2002) and by our observation that ARF1-GFP is maintained at the Golgi in the cold (Figure 5). To the first point, although ARF1-GFP was shown by fluorescence recovery after photobleaching (FRAP) to recover to the Golgi at cold temperatures, the rate of recovery decreased as the temperature decreased (Presley et al., 2002). Our data suggest that this reduction in rate could be due to decreased, although not abolished, levels of ARF1-GTP at lower temperatures. To the second point, it has typically been thought that only GTP-bound ARF GTPases have high membrane affinity. However, it was previously reported that that Golgi localization and cold temperature sensitivity are separable for both ARF1 and ARF3 (Manolea et al., 2010). Manolea et al. (2010) determined that the Golgi subcompartment localization of these two ARFs was dictated by their C-termini. In contrast, a difference in two residues in their N-terminal amphipathic helix caused ARF3 localization to be sensitive to cold temperature and ARF1 to be insensitive (Manolea et al., 2010). An earlier study on ARF1 indicated that these residues stabilized ARF1 on Golgi membranes (Antonny et al., 1997). Thus, it is possible that ARF1 localization is separable from its nucleotide binding status at cold temperatures, possibly due to stabilized interactions between the ARF1 N-terminus and Golgi membranes.
That the golgin-160 dispersal phenotype can be rescued by expression of ARF1 Q71L but not overexpression of the ARF1 GEF GBF1 (Figures 6 and 7) indicates that GEF activity is reduced at lower temperatures. The inactive ARF1-GDP is bound by GBF1 on Golgi membranes, and both inhibition of GDP release from or GTP incorporation into ARF1 leads to stabilized ARF1-GBF1 interactions on Golgi membranes (Szul et al., 2005). This could further explain why ARF1 is maintained at the Golgi at cold temperatures, when our data suggest that ARF1 activity has been reduced in those conditions. Although the mammalian GEF for ARL1 is unknown (Mahajan et al., 2013), ARF3 can be activated at the trans-Golgi by BIG1 and BIG2 (Manolea et al., 2010). However, the BIGs themselves are recruited by small GTPases. ARL1, ARF4, and ARF5 have been implicated in recruiting the BIGs to Golgi membranes (Christis and Munro, 2012; Lowery et al., 2013), and the effects of cold on ARF4 and ARF5 have yet to be investigated. Despite similar GTPase requirements, only BIG2 became dispersed at colder temperatures (Figure 8). As ARF3 is dispersed at 20°C (Manolea et al., 2010), this suggests that BIG2 may be more important for ARF3 recruitment at reduced temperatures. It also suggests that BIG1 localization could be regulated by additional unknown factors.
An interesting finding that requires further study is the variable changes in ARF1 and ARL1 effector protein localization at cold temperatures (Figures 5 and 8 and Table 1), in particular the finding that multiple, but not all, coat proteins examined here had increased Golgi localization under these conditions. One of these is the trans-Golgi clathrin coat adaptor protein and ARF effector, AP-1. AP-1 is thought to be recruited to the Golgi by ARF1 and ARF3 (which has a semiredundant role in vesicle coating and trafficking at the trans-Golgi with ARF1; Shin et al., 2004; Dong et al., 2010). As ARF3 is most likely dissociated from the Golgi at these temperatures (Manolea et al., 2010) and we have suggested here that ARF1 has decreased activity (Figure 4), we would predict that AP-1 would have decreased Golgi localization in the cold. It has been shown, however, that AP-1 binding to a cargo sorting signal enhances subsequent cargo binding and stabilizes AP-1 binding to ARF1-GTP (Lee et al., 2008). The high concentration of cargo proteins induced by trapping cargo at the trans-Golgi, in addition to altered Golgi membrane dynamics at these temperatures, may allow for stable association of cargo adaptors like AP-1 on Golgi membranes even when levels of ARF1-GTP are reduced. This cargo-induced or -stabilized recruitment of coat proteins is not unique to AP-1. The ARF1-effectors and cargo adaptor proteins GGA2 and GGA3 also have enhanced recruitment to the trans-Golgi in the presence of overexpressed cargo, even in the presence of BFA when ARF1 should be inactive (Hirst et al., 2007). Interestingly, GGA2 localization does not change in the cold, whereas GGA3 has increased Golgi localization only at 20°C (Table 1 and Supplemental Figure 2). The GGA3 localization phenotype could be due to cargo being trapped at the trans-Golgi at 20°C, compared with at the cis-Golgi at 16°C, although why it is different from GGA2 has yet to be explored. Although increased Golgi localization of β-COP is a phenotype previously associated with an increase in activated ARF1 (Teal et al., 1994; Saitoh et al., 2009), it has been reported that the γ-COP subunit of the COPI coat can bind directly to GBF1 (Deng et al., 2009), which we observed here to have increased Golgi localization in the cold (Figure 7). This suggests that GBF1 could be stabilizing the COPI complex on Golgi membranes. Further, the formation of β-COP and ERGIC53 containing extra-Golgi puncta, which was previously described at 15°C (Klumperman et al., 1998) and observed here (Figure 5C), was also observed in cells depleted of both ARF1 and ARF3 (Volpicelli-Daley et al., 2005). This phenotype was also detected upon triple depletion of ARFGAPs 1, 2, and 3 (Saitoh et al., 2009), which inhibits removal of COPI coats (Pepperkok et al., 2000). Whether these puncta result from unnatural vesicle formation or inability of retrograde vesicles to uncoat and fuse with the ERGIC requires further investigation.
What is particularly interesting is that proteins that contain similar localization signals have distinct localization patterns at cold temperatures. In particular, ARL1 can bind to the GRIP domain and recruit GRIP domain–containing proteins (Lu and Hong, 2003; Panic et al., 2003), although its ability to recruit GCC1 and GCC2 has been questioned (Derby et al., 2004). We have shown that although ARL1 becomes dispersed from the Golgi at cold temperatures (Figure 8B), the four GRIP domain–containing proteins had three distinct phenotypes: p230 and golgin-97 had increased Golgi localization and are observed in extra-Golgi puncta, GCC2 had decreased localization, and GCC1 was unaffected (Figure 8A). This may reflect various degrees of dependence on ARL1 or a role of unknown cofactors for their localization.
Although the localizations of ARF- and ARL-independent proteins examined here were unaltered by cold treatment (Table 1 and Supplemental Figure 2), other proteins that fall under this category may have different phenotypes. Previous work examining Golgi structure at 15°C demonstrated that glycosylation enzymes and several SNARE and Rab proteins involved in intra-Golgi transport rapidly localized to Golgi-derived tubules (Martínez-Alonso et al., 2005, 2007a, b). These data support the idea of a complex system of protein and membrane interactions being disturbed at lower temperatures.
Cold temperature trafficking blocks are undeniably useful due to their ability to stop all cargo proteins in specific subcompartments of the Golgi complex, which is why they are still in use 3 decades after their initial discovery (Park et al., 2011; Lavieu et al., 2013, 2014; Jensen et al., 2014; Farr et al., 2015). However, the effect of these blocks on Golgi function itself has not been studied in depth. Although we cannot state that a decrease in GTPase or GEF function is the sole mechanism by which these trafficking blocks work, altering the activity of ARF1 has been associated with complete cargo trafficking blocks (Zhang et al., 1994; Saitoh et al., 2009). The effects of the cold temperature blocks on golgin-160 localization are reversible; however, although cargo trafficking immediately restarts at permissive temperatures, it takes more than 30 min for golgin-160 to completely recover its Golgi localization. Therefore, the trafficking roles of mislocalized proteins like golgin-160 may not be fully reconstituted immediately upon return to 37°C.
Although more specialized in scope, several new techniques to synchronize cargo trafficking have been developed in the past few years, including the RUSH, IM-LARIAT, and CUTE systems (Boncompain et al., 2012; Abraham et al., 2016; Nguyen et al., 2016). Additionally, improvements in microscopy and photoactivatable constructs allow for visual pulse chases to be performed in cells. Genetic editing techniques are also allowing for the introduction of tagged proteins that are expressed at endogenous levels. These techniques may provide better methods of synchronization of cargo proteins in the future.
MATERIALS AND METHODS
Cell culture
HeLa cells (American Type Culture Collection CCL-2) were maintained in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA) and 0.1 mg/ml Normocin (InvivoGen, San Diego, CA) at 37°C with 5% CO2. The cells were authenticated by STR profiling in the Johns Hopkins Genetics Resources Facility and are tested for Mycoplasma contamination every 6 mo.
Expression constructs and transient transfection
The plasmid encoding GFP-ARF1 WT was obtained from Addgene (#39554; Cambridge, MA). The Q71L mutation was introduced via Quikchange mutagenesis and confirmed by sequencing (Stratagene, La Jolla, CA), using the following mutagenic primers: 5′-GACGTGGGTGGCCTGGACAAGATCCGG-3′ and its complement, 5′-CCGGATCTTGTCCAGGCCACCCACGTC-3′. The Venus-GBF1 A795E construct was previously described (Lanke et al., 2009). The A795E mutation provides resistance to BFA but otherwise this construct behaves similarly to WT (Lanke et al., 2009). The GBF1 A795E coding region was subcloned into the pEGFP N1 backbone (Clontech, Mountain View, CA). The plasmid encoding ARL1-GFP was a gift from Richard Kahn through Addgene (plasmid #67395).
Transfection was performed using the X-tremeGENE 9 DNA transfection reagent (Roche, Indianapolis, IN) in accordance with the manufacturer’s protocol. For all transfection experiments, 35-mm dishes of HeLa cells were transfected with 0.2 μg of plasmids encoding GFP-ARF1 WT, GFP-ARF1 Q71L, or ARL1-GFP, or 0.5 μg of the plasmid encoding GFP-GBF1 A795E. Experiments were performed 16-17 h posttransfection.
Antibodies
Rabbit anti–golgin-160 N-terminal head domain was previously described and validated (Chandran and Machamer, 2008). Rabbit anti–golgin-160 C-terminus was also previously described and validated (Hicks and Machamer, 2002). Mouse anti-GBF1, -GM130, -GGA2, -GGA3, -p115, and -p230 were obtained from BD Transduction Laboratories (San Jose, CA). Rabbit anti-β-COP was provided by J. Lippincott-Schwartz (National Institutes of Health, Bethesda, MD). Mouse anti-actin (A3853), -ERGIC-53, and -AP-1 (gamma adaptin) were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit anti-GCC1 and -GCC2 were obtained from Atlas Antibodies (Bromma, Sweden). Mouse anti–golgin-97 and rabbit anti-GFP were obtained from Molecular Probes/Thermo Fisher Scientific (Rockford, IL). Rabbit anti-giantin was obtained from Covance/BioLegend (San Diego, CA). Mouse anti-ARF1 was obtained from Cell Biolabs (San Diego, CA). Rabbit anti-BIG1 and -BIG2 were previously described (Lowery et al., 2013). Mouse anti-GORASP1 (GRASP65) was obtained from Novus Biologicals (Littleton, CO). Rabbit anti-mannosidase II (ManII) was from Marilyn Farquhar (University of California, San Diego). Sheep anti-TGN46 was from Serotec/Bio-Rad (Hercules, CA). Alexa Fluor 568 anti-mouse immunoglobulin G (IgG) and Alexa Fluor 488 anti-rabbit IgG were from Life Technologies (Grand Island, NY). Cy5-conjugated AffiniPure Donkey anti-rabbit and Texas Red anti-sheep IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). IRDye 680RD goat anti-mouse IgG and 680RD donkey anti-rabbit IgG were obtained from LI-COR (Lincoln, NE).
Temperature shifts
For all temperature shifts, cells were placed in normal growth medium containing 20 mM HEPES (Cellgro, Manassas, VA) that had been prechilled to 20 or 16°C. Dishes were parafilmed closed and placed in water baths set to 20 or 16°C for 3 h (or the indicated time). For recovery at 37°C, after cells were first incubated at 16°C for 3 h, the medium was exchanged for media lacking HEPES that had been prewarmed at 37°C and cells were returned to a 37°C incubator with 5% CO2.
Indirect immunofluorescence microscopy and quantification
Cells were plated on 15- or 35-mm dishes with coverslips for 24 h before transfection with GBF1 or ARF1 constructs, or 48 h for analysis of endogenous proteins, before temperature shifts were performed. At the indicated times post–temperature shift, cells were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. For most antibodies, cells were permeabilized for 10 min with 0.02% saponin (CalBiochem/Sigma) in PBS (Sigma) containing 10 mM glycine (Sigma; Sap/PBS/gly buffer). Coverslips were incubated 15 min in primary antibody diluted in Sap/PBS/gly buffer with 1% bovine serum albumin (BSA). Cells were washed two times with Sap/PBS/gly buffer before being incubated in secondary antibody diluted as above. Cells were washed again before being incubated for 3 min in 0.1 μg/ml Hoescht 33258 diluted in PBS. Coverslips were then mounted in glycerol with 0.1 M N-propyl gallate (Sigma).
Several antibodies (BIG1, BIG2, β-COP, ERGIC53) required permeabilization in 0.5% Triton X-100 (Sigma) in PBS/10 mM glycine for 3 min. For these antibodies, coverslips were washed in PBS/glycine buffer without saponin, and antibodies were diluted in PBS/glycine with 1% BSA.
Cells stained with the TGN46 antibody were fixed in paraformaldehyde as described above and permeabilized in PBS containing 1% gelatin from cold water fish skin (Sigma) and 0.1% saponin. Coverslips were washed in PBS and antibodies were diluted in PBS containing 1% fish skin gelatin.
Coverslips were imaged on an Axioskop microscope (Zeiss, Thornwood, NY) equipped for epifluorescence using an ORCA-03G charge-coupled device camera (Hamamatsu, Japan) using iVision software (BioVision Technologies, Exton, PA). All images are shown inverted for better visualization of cytoplasmic localization. For quantification of golgin-160 localization, regions of interest (ROI) were created around the Golgi region using the GM130 image as well as around the whole cell, and the integrated pixel density of the golgin-160 and GM130 signal in each ROI was determined using FIJI (National Institutes of Health, Bethesda, MD). Quantification of GBF1 signal was performed in the same manner, using giantin fluorescence to define the Golgi region. For each experiment, control images where fluorescent light was blocked from reaching the camera (no photon controls) were also taken to account for variations in camera light detection. Adjusted integrated pixel densities for each ROI were calculated by subtracting the pixel density measured in the control images from the corresponding experimental image pixel densities. To calculate the percent of golgin-160 at the Golgi, the adjusted integrated pixel density at the Golgi was divided by that of the whole cell. This percent was then normalized to 37°C by dividing each individual percent by the average percent at 37°C. For endogenous protein quantification, three independent experiments were performed and between 180 and 300 cells were quantified per condition. For transfected cell experiments, four independent experiments were performed and between 130 and 190 cells were quantified per condition. For all box and whiskers plots, the center bar represents the mean, the box extends from the 25th to the 75th percentile, and the whiskers extend to the minimum and maximum values.
Golgin-160 expression levels
HeLa cells were seeded on 35-mm dishes and allowed to grow for 48 h before being shifted to 20 or 16°C as described above. After 3 h, cells were washed with PBS twice and then lysed on ice for 10 min in lysis buffer (1% NP-40, 0.4% DOC, 50 mM Tris, pH 8.0, 62.5 mM EDTA, pH 8.0) containing protease inhibitors. The lysate was separated by SDS–PAGE (10% acrylamide) and golgin-160 and actin were detected by immunoblotting after transfer to Immobilon-FL PVDF (EMD Millipore, Billerica, MA), by near-infrared fluorescent imaging on the Odyssey CLx Imaging System (LI-COR, Lincoln, NE). The amount of protein in each lane was measured using Quantity One volume analysis tools (Bio-Rad, Hercules, CA). To calculate expression levels of golgin-160 in each condition, the golgin-160 values were first normalized to actin and then to 37°C. The graph represents the mean value of five independent replicates, and the error bars represent SD.
Active ARF1-GTP pull down
The amount of active ARF1-GTP in HeLa cells at each temperature condition was determined using the ARF1 Activation Assay kit (Cell Biolabs, San Diego, CA). In brief, three 6-cm dishes of HeLa cells were incubated at each condition, 37, 20, or 16°C, for 3 h as described above. Three recovery dishes were incubated at 16°C for 3 h before being returned to 37°C for 0.5 h. The cells were then lysed on ice using the kit lysis buffer with protease inhibitors for 10 min. After clarification of the lysate, 10% input was removed and the remaining lysate was added to 40 μl of GGA3 protein binding domain (PBD) agarose bead slurry, which specifically binds to GTP-bound ARF1. The lysates and beads were rotated for 1 h at 4°C before the beads were washed three times in lysis buffer. The beads were resuspended in 40 μl SDS–PAGE loading sample buffer (0.1 M Tris, pH 6.8, 0.04% SDS, 30% glycerol, 0.1% bromophenol blue) with 4% BME and boiled for 3 min at 95°C. The input and 50% of the pull down for each condition were then separated on a 4–12% NuPAGE Bis/Tris gel (Thermo Fisher Scientific), transferred to PVDF, and analyzed for ARF1 via Western blotting. Blots were imaged on the Odyssey CLx Imaging System and the amount of protein in each lane was measured using Image Studio (LI-COR, Lincoln, NE). n = 5 for the 37, 20, and 16°C conditions; three of these replicates also included the recovery condition. The center line on the scatter plot represents the mean and the error bars represent the SD.
Statistical analysis
Graphs and statistical analyses were obtained using GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA). For all quantified experiments, one-way analysis of variance followed by Tukey’s multiple comparisons test were performed. For the microscopy data, outliers were identified using Prism’s Robust Regression and Outlier removal (ROUT) tool with Q set to 1%. Between 0 and 1 outliers (out of 130–300 measurements) per condition were identified per experiment and removed.
Supplementary Material
Acknowledgments
We thank Jason Westerbeck and Jeanne Sisk for helpful discussions and comments on the manuscript. The work was supported by the Biochemistry, Cellular and Molecular Biology graduate program (National Institutes of Health T32 GM007445) and MCB13-510 from the National Science Foundation to E.S.
Abbreviations used:
- AP
adaptor protein
- ARF1
ADP-ribosylation factor 1
- ARL1
ARF-like GTPase 1
- β1AR
beta-1 adrenergic receptor
- BIG
brefeldin A–inhibited guanine nucleotide exchange factor
- CGN
cis-Golgi network
- COPI
coat protein I
- ERGIC
ER-Golgi intermediate compartment
- GAP
GTPase-activating protein
- GBF1
Golgi brefeldin A–resistant guanine nucleotide exchange factor 1
- GEF
guanine nucleotide exchange factor
- GRIP
golgin-97, RanBP2α, Imh1p, and p230/golgin-245
- NT
nontransfected
- T
transfected
- TGN
trans-Golgi network
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-11-0622) on February 19, 2018.
REFERENCES
- Abraham O, Gotliv K, Parnis A, Boncompain G, Perez F, Cassel D. (2016). Control of protein trafficking by reversible masking of transport signals. Mol Biol Cell , 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. (1997). N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry , 4675–4684. [DOI] [PubMed] [Google Scholar]
- Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F. (2012). Synchronization of secretory protein traffic in populations of cells. Nat Methods , 493–498. [DOI] [PubMed] [Google Scholar]
- Boncompain G, Perez F. (2013). Fluorescence-based analysis of trafficking in mammalian cells. Methods Cell Biol , 179–194. [DOI] [PubMed] [Google Scholar]
- Bundis F, Neagoe I, Schwappach B, Steinmeye K. (2006). Involvement of golgin-160 in cell surface transport of renal ROMK channel: co-expression of golgin-160 increases ROMK currents. Cell Physiol Biochem Biochem , 1–12. [DOI] [PubMed] [Google Scholar]
- Chandran S, Machamer CE. (2008). Acute perturbations in Golgi organization impact de novo sphingomyelin synthesis. Traffic , 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christis C, Munro S. (2012). The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2. J Cell Biol , 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude A, Zhao B, Kuziemsky CE, Dahan S, Berger SJ, Yan J, Armold AD, Sullivan EM, Melançon P. (1999). Exchange factor that displays specificity for ADP-ribosylation factor 5. J Cell Biol , 71–84. [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. (2009). A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep , 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby MC, van Vliet C, Brown D, Luke MR, Lu L, Hong W, Stow JL, Gleeson PA. (2004). Mammalian GRIP domain proteins differ in their membrane binding properties and are recruited to distinct domains of the TGN. J Cell Sci , 5865–5874. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. (1992). ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA , 6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. (2011). ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol , 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhang X, Zhou F, Dou H, Duvernay MT, Zhang P, Wu G. (2010). ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther , 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. (2006). ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol , 347–358. [DOI] [PubMed] [Google Scholar]
- Farr GA, Hull M, Stoops EH, Bateson R, Caplan MJ. (2015). Dual pulse-chase microscopy reveals early divergence in the biosynthetic trafficking of the Na,K-ATPase and E-cadherin. Mol Biol Cell , 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Arnold DB. (2016). Techniques for studying protein trafficking and molecular motors in neurons. Cytoskeleton , 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CE, Zuckerman DM, Currier PL, Machamer CE. (2014). Three basic residues of intracellular loop 3 of the beta-1 adrenergic receptor are required for golgin-160-dependent trafficking. Int J Mol Sci , 2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SW, Horn TA, McCaffery JM, Zuckerman DM, Machamer CE. (2006). Golgin-160 promotes cell surface expression of the beta-1 adrenergic receptor. Traffic , 1666–1677. [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE. (2002). The NH2-terminal domain of Golgin-160 contains both Golgi and nuclear targeting information. J Biol Chem , 35833–35839. [DOI] [PubMed] [Google Scholar]
- Hirst J, Seaman MNJ, Buschow SI, Robinson MS. (2007). The role of cargo proteins in GGA recruitment. Traffic , 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LP. (2014). Structure and mechanism of COPI vesicle biogenesis. Curr Opin Cell Biol , 67–73. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Watanabe S, Rasmussen HB, Schmitt N, Olesen S-P, Frost NA, Blanpied TA, Misonou H. (2014). Specific sorting and post-Golgi trafficking of dendritic potassium channels in living neurons. J Biol Chem , 10566–10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J, Schweizer A, Clausen H, Tang BL, Hong W, Oorschot V, Hauri HP. (1998). The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J Cell Sci (Pt 2), 3411–3425. [DOI] [PubMed] [Google Scholar]
- Lanke KHW, van der Schaar HM, Belov GA, Feng Q, Duijsings D, Jackson CL, Ehrenfeld E, van Kuppeveld FJM. (2009). GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication. J Virol , 11940–11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Dunlop MH, Lerich A, Zheng H, Bottanelli F, Rothman JE. (2014). The Golgi ribbon structure facilitates anterograde transport of large cargoes. Mol Biol Cell , 3028–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Zheng H, Rothman JE. (2013). Stapled Golgi cisternae remain in place as cargo passes through the stack. Elife , e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Doray B, Govero J, Kornfeld S. (2008). Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1–GTP. J Cell Biol , 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. (2005). ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol , 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell , 821–836. [DOI] [PubMed] [Google Scholar]
- Lowery J, Szul T, Styers M, Holloway Z, Oorschot V, Klumperman J, Sztul E. (2013). The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN). J Biol Chem , 11532–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hong W. (2003). Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol Biol Cell , 3767–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan D, Boh BK, Zhou Y, Chen L, Cornvik TC, Hong W, Lu L. (2013). Mammalian Mon2/Ysl2 regulates endosome-to-Golgi trafficking but possesses no guanine nucleotide exchange activity toward Arl1 GTPase. Sci Rep , 3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolea F, Chun J, Chen D, Clarke I, Summerfeldt N, Dacks JB, Melançon P. (2010). Arf3 is activated uniquely at the trans-Golgi network by brefeldin A-inhibited guanine nucleotide exchange factors. Mol Biol Cell , 1836–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Alonso E, Ballesta J, Martínez-Menárguez JA. (2007a). Low-temperature-induced Golgi tubules are transient membranes enriched in molecules regulating intra-Golgi transport. Traffic , 359–368. [DOI] [PubMed] [Google Scholar]
- Martínez-Alonso E, Egea G, Ballesta J, Martínez-Menárguez JA. (2005). Structure and dynamics of the Golgi complex at 15°C: low temperature induces the formation of Golgi-derived tubules. Traffic , 32–44. [DOI] [PubMed] [Google Scholar]
- Martínez-Alonso E, Tomás M, Ballesta J, Martínez-Menárguez JA. (2007b). Low temperature (15°C) induces COPII dissociation from membranes and slow exit from the endoplasmic reticulum in HeLa cells. Histochem Cell Biol , 379–384. [DOI] [PubMed] [Google Scholar]
- Matlin KS, Simons K. (1983). Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell , 233–243. [DOI] [PubMed] [Google Scholar]
- Munro S. (2011). The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol , 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MK, Kim CY, Kim JM, Park BO, Lee S, Park H, Heo WDO. (2016). Optogenetic oligomerization of Rab GTPases regulates intracellular membrane trafficking. Nat Chem Biol , 431–436. [DOI] [PubMed] [Google Scholar]
- Nickel W, Malsam J, Gorgas K, Ravazzola M, Jenne N, Helms JB, Wieland FT. (1998). Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPγS in vitro. J Cell Sci (Pt 2), 3081–3090. [DOI] [PubMed] [Google Scholar]
- Niu T-K, Pfeifer AC, Lippincott-Schwartz J, Jackson CL. (2005). Dynamics of GBF1, a brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol Biol Cell , 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic B, Perisic O, Veprintsev DB, Williams RL, Munro S. (2003). Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell , 863–874. [DOI] [PubMed] [Google Scholar]
- Park JJ, Gondre-Lewis MC, Eiden LE, Loh YP. (2011). A distinct trans-Golgi network subcompartment for sorting of synaptic and granule proteins in neurons and neuroendocrine cells. J Cell Sci , 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok R, Whitney JA, Gomez M, Kreis TE. (2000). COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J Cell Sci (Pt 1), 135–144. [DOI] [PubMed] [Google Scholar]
- Presley JF, Ward TH, Pfeifer AC, Siggia ED, Phair RD, Lippincott-Schwartz J. (2002). Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature , 187–193. [DOI] [PubMed] [Google Scholar]
- Roboti P, Witkos TM, Lowe M. (2013). Biochemical analysis of secretory trafficking in mammalian cells. Methods Cell Biol , 85–103. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Shin HW, Yamada A, Waguri S, Nakayama K. (2009). Three homologous ArfGAPs participate in coat protein I-mediated transport. J Biol Chem , 13948–13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. (1984). Pre- and post-golgi vacuoles operate in the transport of semliki forest virus membrane glycoproteins to the cell surface. Cell , 535–549. [DOI] [PubMed] [Google Scholar]
- Saraste J, Svensson K. (1991). Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci (Pt 3), 415–430. [DOI] [PubMed] [Google Scholar]
- Shin H-W, Morinaga N, Noda M, Nakayama K. (2004). BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell , 5283–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szul T, Garcia-Mata R, Brandon E, Shestopal S, Alvarez C, Sztul E. (2005). Dissection of membrane dynamics of the ARF-guanine nucleotide exchange factor GBF1. Traffic , 374–385. [DOI] [PubMed] [Google Scholar]
- Teal SB, Hsu VW, Peters PJ, Klausner RD, Donaldson JG. (1994). An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J Biol Chem , 3135–3138. [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. (2005). Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol Biol Cell , 4495–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Hicks SW, Machamer CE, Pessin JE. (2006). Golgin-160 is required for the Golgi membrane sorting of the insulin-responsive glucose transporter GLUT4 in adipocytes. Mol Biol Cell , 5346–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Puri S, Linstedt AD. (2009). A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell , 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Puthenveedu MA, Linstedt AD. (2012). Golgin160 recruits the dynein motor to position the Golgi apparatus. Dev Cell , 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Rosenwald AG, Willingham MC, Skuntz S, Clark J, Kahn RA. (1994). Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J Cell Biol , 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.