Abstract
Background
Euglena gracilis, a photosynthetic protist, produces protein, unsaturated fatty acids, wax esters, and a unique β-1,3-glucan called paramylon, along with other valuable compounds. The cell composition of E. gracilis was investigated in this study to understand how light and organic carbon (photo-, mixo- and heterotrophic conditions) affected growth and cell composition (especially lipids). Comparisons were primarily carried out in cultures grown at 23 °C, but the effect of growth at higher temperatures (27 or 30 °C) was also considered.
Cell growth
Specific growth rates were slightly lower when E. gracilis was grown on glucose in either heterotrophic or mixotrophic conditions than when grown photoautotrophically, although the duration of exponential growth was longer. Temperature determined the rate of exponential growth in all cultures, but not the linear growth rate during light-limited growth in phototrophic conditions. Temperature had less effect on cell composition.
Cell composition
Although E. gracilis was not expected to store large amounts of paramylon when grown phototrophically, we observed that phototrophic cells could contain up to 50% paramylon. These cells contained up to 33% protein and less than 20% lipophilic compounds, as expected. The biomass contained about 8% fatty acids (measured as fatty acid methyl esters), most of which were unsaturated. The fatty acid content of cells grown in mixotrophic conditions was similar to that observed in phototrophic cells, but was lower in cells grown heterotrophically. Heterotrophic cells contained less unsaturated fatty acids than phototrophic or mixotrophic cells. α-Linolenic acid was present at 5 to 18 mg g-1 dry biomass in cells grown in the presence of light, but at < 0.5 mg g-1 biomass in cells grown in the dark. Eicosapentaenoic and docosahexaenoic acids were detected at 1 to 5 mg g-1 biomass. Light was also important for the production of vitamin E and phytol.
Introduction
Microalgae, as a source of nutrients, bioactive compounds, biofuels and chemicals, have gained attention from both academia and industry [1–8]. The unicellular, photosynthetic protist Euglena gracilis, often considered with eukaryotic algae, contains compounds of commercial interest. E. gracilis accumulates paramylon (a type of β-1,3-glucan that is unique to euglenoids, particularly Euglena species) for energy and carbon storage, especially when grown on organic carbon (i.e. in mixo- or heterotrophic conditions) [4,9]. Similar to other beta glucans, paramylon is considered to have anti-tumor [10] and anti-HIV [11] activity. Paramylon can accumulate to contribute more than 80% of the total cell dry biomass in cells grown in the dark, but accumulated to only 23% of the biomass in phototrophically grown cells [4]. In anaerobic conditions, wax esters become the main storage compounds in E. gracilis [12]. These esters are composed of medium chain fatty acids and fatty alcohol, which can be converted into biofuels [13,14].
The fatty acid profile of E. gracilis features a high content (e.g. > 50% of total lipid [15]) of polyunsaturated fatty acids (PUFAs), including the highly desired α-linolenic (ALA), arachidonic (ARA), eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids for human and animal nutrition [15,16]. α-Tocopherol, the most bioactive form of vitamin E is also present in E. gracilis biomass in a relatively high amount [17]. As a photosynthetic organism, E. gracilis also contains phytol, a C20 diterpenoid associated with chlorophyll. Phytol has been used to produce synthetic vitamin E, but it and its derivatives have other pharmacological properties of interest to the health industry [18]. Thus, the lipid composition of E. gracilis makes it suitable for nutritional applications. In addition, the unique cell wall (pellicle) of E. gracilis is mostly composed of protein [19], making the nutritious E. gracilis biomass more digestible than other microalgae, which are protected by non-digestible polysaccharides [20]. Around one quarter of the cell biomass may be protein, regardless of whether the cells are grown in the light or dark [12].
E. gracilis grows in habitats, which vary widely in temperature and pH [3]. It can metabolise many organic carbon sources even in the presence of light [17], and has consequently been studied in autotrophic, and heterotrophic conditions. Photoautotrophic cultivations of E. gracilis can produce more than 3 g L-1 biomass (e.g. [4]). The specific growth rate of E. gracilis in autotrophic cultures is around 1.1 d-1 in favourable growth conditions [21,22]. Ogbonna et al. [23] reported growth rates between 0.9 and 1.1 g L-1 d-1 during the linear growth phase of E. gracilis in phototrophic conditions. Higher biomass concentrations and growth rates were achieved in heterotrophic and mixotrophic (photoheterotrophic) conditions [23]. As with other microalgae, in which light is reported to affect the production of carotenoids, polysaccharides and bioactive molecules [24, 25, 26, 27], the presence or absence of light affects cell composition in E. gracilis, so that mixotrophic growth continues to be of interest in E. gracilis studies. Some of the cell components are only formed in light, and the formation of some other components (e.g. PUFAs) is enhanced by light [22,28]. Recent studies have shown how the provision of light or organic carbon affect production of lipid-soluble cellular components such as α-tocopherol [4] and unsaturated fatty acids [28].
E. gracilis is considered to be a promising candidate for development of a photosynthetic biorefinery [3,29] and companies, like Euglena CO. [30], Ltd and Algaeon Ltd, are now producing E. gracilis biomass and pure β-1,3-glucan for the food and feed industries.
Here we assess growth of E. gracilis in photo-, mixo- and heterotrophic conditions, and analyse the E. gracilis cell composition of these cultures at 23 °C as a low temperature, in comparison with 27 or 30 °C as high temperatures. A detailed analysis of the fatty acid composition of E. gracilis lipids produced in photo-, mixo- and heterotrophic conditions was carried out for cultures grown at 23 °C, as well as for lipids produced at 30 °C in phototrophic conditions. Protein and paramylon content was determined only in phototrophic cultivations (23 and 30 °C), since previous studies have focused on their production in hetero- and mixotrophic conditions [4, 12]. Wax esters were not individually characterised, since all cultures were grown aerobically.
Material and methods
Strain, medium, and inocula preparation
Axenic Euglena gracilis (NIES-48) was purchased from the National Institute of Environmental Studies of Japan. Cells were maintained in chemically defined medium at room temperature (~22 °C) in non-agitated flasks with low light intensity illumination and sub-cultured every three weeks.
Chemically defined medium was adapted from Ogbonna, Tanaka et al. [17] for macronutrients and Bischoff and Bold [31] for trace elements. For photoautotrophic cultivations, the medium contained (per litre): 0.3 g (NH4)2SO4, 0.08 g (NH4)2HPO4, 0.16 g K2HPO4, 0.2 g MgSO4∙7H2O, 0.06 g CaCl2∙2H2O, 0.5 mg vitamin B1, 0.002 mg vitamin B12, 3.36 mg FeSO4∙7H2O, 2.88 mg H3BO3, 4 g Na2EDTA∙2H2O, 0.314 mg CuSO4∙5H2O, 1.765 mg ZnSO4∙7H2O, 0.236 mg MnCl2∙2H2O, 0.238 mg Na2MoO4∙2H2O and 0.108 mg CoCl2∙6H2O. For heterotrophic and mixotrophic cultivations, the same medium was modified to provide five-fold more vitamins and trace elements, and 2.5-fold more magnesium to ensure that growth would not be limited by these compounds with the higher biomass concentrations achieved in hetero- and mixotrophic conditions. Specifically, the heterotrophic and mixotrophic medium contained (per litre): 0.3 g (NH4)2SO4, 0.1 g (NH4)2HPO4, 0.2 g K2HPO4, 0.5 g MgSO4∙7H2O, 0.06 g CaCl2∙2H2O, 16.6 ± 0.7 g glucose, 2.5 mg vitamin B1, 0.01 mg vitamin B12, 16.8 mg FeSO4∙7H2O, 14.4 mg H3BO3, 20 g Na2EDTA∙2H2O, 1.57 mg CuSO4∙5H2O, 8.82 mg ZnSO4∙7H2O, 1.18 mg MnCl2∙2H2O, 1.19 mg Na2MoO4∙2H2O and 0.54 mg CoCl2∙6H2O.
Inocula for bioreactor cultivations were grown in 50 mL photoautotrophic growth medium in 250 mL Erlenmeyer flasks on a shaking platform (Infors AG Switzerland) at 90 rpm, 24 °C. One or two fluorescent tubes (8 W) were placed on the edge of the shaker to provide light intensities of 100 to 150 μmol photons m-2s-1 on culture surfaces. Light intensity was measured with a quantum sensor connected to a radiometer (Li-Cor. Inc. USA). Inocula were cultivated until the optical density at 780 nm (OD780) was 0.3.
Stirred tank (photobioreactor) cultivations
Sartorius BioStat B 2.5 L glass vessel bioreactors, with a water jacket, (Sartorius AG, Germany) were set up as described by Wang et al. [32]. Two-litre working volume cultures were maintained at pH 6.0 (with 1 M H3PO4 or 1 M NaOH for titration), 200 rpm agitation, and 0.1 volume of air per volume culture per minute (vvm) aeration with CO2 enriched air (1.8% CO2, 20.9% O2 and 77.7% N2). Cultures were maintained at pH 6.0 to prevent precipitation of salts in the medium, since this was within the optimal pH range (between pH 3 and 6) for E. gracilis [21]. Cultivations were carried out at 23 °C (low temperature), 27 °C or 30 °C (high temperatures, Table 1). The light intensity of the cultures was adjusted by using either two or three externally mounted fluorescent lamps (11 W, 20 cm, with reflective back cover, Lival Oy, Finland), vertically mounted at equal distances around the reactor vessel, each providing light intensities of approx. 400 μE m-2 s-1 at the inner surface of the reactor directly facing the lamps. Light intensity is thus described as either 2 or 3 x 400 μE m-2 s-1, to reflect the illumination provided to the surface of the cultures. The same bioreactors were wrapped in foil to eliminate stray light for heterotrophic cultures (at 23 and 27 °C).
Table 1. Cultivation conditions and analyses carried out from each cultivation.
| Cultivation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Conditions | Temperature (°C) | 23 | 23 | 23 | 23 | 27 | 27 | 30 | 30 |
| Light (x 400 μE m-2 s-1) | 2 | 2 | 0 | 3 | 2 | 0 | 3 | 3 | |
| Glucose added | - | + | + | - | + | + | - | - | |
| Analyses | Specific growth rate | Fig 1 | Fig 2 | Fig 2 | Fig 1 | Fig 2 | Fig 2 | Fig 1 | Fig 1 |
| Linear growth rate | Fig 1 | Fig 2 | Fig 2 | Fig 1 | Fig 2 | Fig 2 | Fig 1 | Fig 1 | |
| Lipids (total) | + | - | - | + | - | - | + | - | |
| Lipids (composition) | - | + | + | + | - | - | (+) | + | |
| Protein and paramylon | - | - | - | + | - | - | (+) | + | |
| Figure legend | ○ | ⊕ | ● | □ | ◇ | ◆ | Δ | ∇ |
+ indicates that glucose was added to the cultivation or that the specified analysis was carried out. (+) indicates the analysis was carried out only at the end of the cultivation.—indicates that glucose was not added (photoautotrophic culture) or that the indicated analysis was not carried out. The figure in which data on specific and linear growth rate determination is indicated.
Cultivations 7 and 8 provided independent replicates, although not all analyses were carried out for each replicate.
The composition of the CO2 enriched gas stream and the exhaust gas (percent O2, CO2 and N2) from the cultivations was analysed by a photoacoustic IR gas analyser (Innova-1313/LumaSense, United States), with air as reference or a Prima Pro Process mass spectrometer (Thermo Scientific, Winsford, UK) calibrated with 3% CO2 in Ar, 5% CO2 with 0.99% Ar and 15% O2 in N2, 20% O2 plus 20% Ar in N2, and 0.04% ethanol in N2. The CO2 enriched gas stream composition was measured before inoculating each cultivation.
Analyses
Cell biomass
Cell biomass was measured as OD780 and dry weight. OD780 was used to monitor increase in biomass at low cell densities and was measured in triplicate against deionised water using an infrared spectrophotometer (Hitachi U-2000, Japan). Since phototrophic algal cultures only grew exponentially at low biomass concentrations, OD780 was used to calculate specific growth rates in these cultures. For dry weight determination, at least 3 mL of culture was centrifuged (3000 rpm 10 min), and the pellet was washed twice with deionised water. During the washing step, the cell biomass was transferred to a 2 mL pre-dried (105 °C) and weighed microcentrifuge tube. The tube containing the cell biomass was dried by lyophilisation (Christ® lyophiliser, Sartorius AG, Germany) and re-weighed. Dry weight was determined for a minimum of 2 replicates (for low biomass concentrations which required large sample volumes), but usually for 4 and occasionally 6 replicates, depending on the volume required to obtain an accurate measurement. The dry algal biomass was used for lipid, protein and paramylon content analyses. Supernatant was retained for ammonium and glucose analysis.
Ammonium analysis
Ammonium was measured using an ion selective ammonium electrode (C-CIT AG, Switzerland) with a detection limit of 0.3 mmol L-1.
Total lipid extraction and analysis
Total lipid content was determined gravimetrically after chloroform-methanol (2:1) extraction adapted from Folch et al. [33], as described by Wang et al. [32].
Fatty acid analysis by gas chromatography
Freeze-dried samples (2–25 mg) were homogenised with a Retsch Mixer Mill homogenizer (Retsch GmbH, Haan, Germany) for 2 x 30 s (20 Hz, 2 steel grinding balls ø 3 mm) and extracted twice with 1 ml chloroform:methanol (2:1). The chloroform-methanol extracts, containing the lipids, were combined to obtain the total lipids and 30.45 μg C17:0 triacylglycerol (TG, 17:0/17:0/17:0) and 15.0 μg C17:0 free fatty acid (FFA) were added to a 300 μl aliquot before removal of the solvent under nitrogen flow. Lipids were re-dissolved in 1 ml petroleum ether (bp 40–60 °C). Transesterification was performed by adding 500 μl of 0.5 N sodium methoxide in methanol and two boiling stones to the solubilised lipids, then boiling at 45 °C for 5 min. Fifteen percent (w/v) NaHSO4 (1 ml) was added to acidify the samples, after which methyl esters and free fatty acids were extracted with petroleum ether. The separated petroleum ether layer was evaporated and the residue re-dissolved in 150 μl hexane. Fatty acid methyl esters were analysed on an Agilent 7890A GC equipped with an Agilent FFAP silica capillary column (25 m × 0.2 mm × 0.3 μm). Hydrogen was used as carrier gas with a split ratio of 20:1. The oven temperature was increased from 70 °C (2 min) to 235 °C at a rate of 10 °C/min, with a total run time of 30 min.
After quantifying fatty acid methyl esters (FAME) by GC, the same samples were trimethylsilylated to determine FFAs and other polar compounds by GC-MS (Agilent 7890A GC combined with 5975C MSD). The solvents were evaporated and samples re-dissolved in 50 μl dichloromethane and silylated with 25 μl N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) for 20 min at 80 °C. The samples were analysed on an Agilent DB-5 silica capillary column (30 m x 0.25 mm x 0.25 μm). The split ratio was 20:1 and the oven temperature programme from 70 °C (1 min) to 240 °C at a rate of 10 °C/min, the total run time was 30 min. The samples (2 μl) were injected by a Gerstel MPS injection system and the data were collected in EI mode (70 eV) at a mass range of m/z 50–600.
Extraction and measurement of protein and paramylon
Protein extraction and quantification was based on the methods developed by Slocombe et al. [34]. Approximately 5 mg freeze-dried E. gracilis biomass was weighed and re-suspended in 250 μl 10% (w/v) trichloroacetic acid (TCA). The suspension was ultrasonicated for 15 min (Fritsch ultrasonic cleaner, FRITSCH GmbH, Germany) and then incubated in a 65 °C water bath for 30 min. The samples were vortexed several times during the incubation to ensure complete cell disruption. After cooling to room temperature, samples were centrifuged at 15,000 g, 4 °C, for 20 min. The pellets were resuspended in 0.5 ml alkaline solution containing 20 g L-1 Na2CO3 and 4 g L-1 NaOH to dissolve the proteins. The suspension was centrifuged to remove the non-soluble particles, including paramylon, from the protein extract. Protein was quantified using the DC protein assay kit (Bio-RAD), based on the Lowry method [35].
Paramylon extraction was adapted from Barsanti et al. [9]. Protein free pellets from the extracted E. gracilis cells were washed with 3 ml ethanol, resuspended in 3 ml sodium dodecyl sulfate (SDS) solution (2 g L-1) and incubated at 37 °C for 24 h. Solids were collected by centrifugation and the supernatant discarded. SDS washing was repeated without incubation. The paramylon pellets were washed twice with 3 ml distilled water, then dissolved in 2 ml NaOH (1 M). The concentration of paramylon was determined using the phenol-sulfuric acid method described by Albalasmeh et al. [36].
Results
Autotrophic growth of E. gracilis
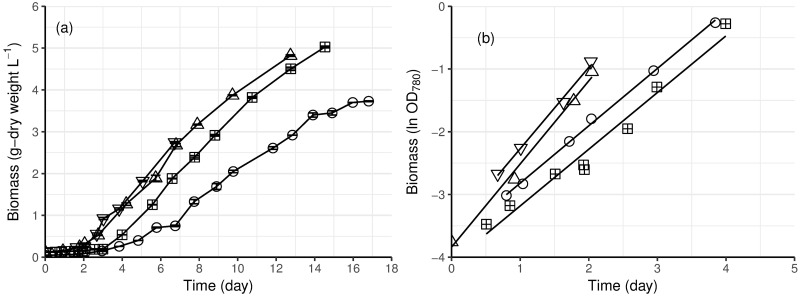
E. gracilis initially grew exponentially in photoautotrophic batch cultures (Fig 1b), with no apparent lag phase. The exponential phase lasted for approximately 2 to 4 days (depending on the temperature), until there was about 0.5 g l-1 biomass, after which the cultures became light limited and increase in biomass was linear (Fig 1a). The cultures were stopped before stationary phase. At 23 °C, the specific growth rate of E. gracilis during the exponential phase was 0.90 d-1, regardless of the light intensity. At 30 °C with 3 x 400 μE m-2 s-1 light it was 40% higher (1.30 d-1, Table 1 and Fig 1b) than at 23 °C.
Fig 1. Growth of photoautotrophic cultures of E. gracilis at 23 (circle) or 30 °C (upward and downward triangles) with two (square) or three (circle and triangles) light sources of 400 μE m-2 s-1 intensity (2 x 400 or 3 x 400 μE m-2 s-1).
(a), Growth curves measured by cell dry weight. Error bars (which were generally smaller than the symbol) represent the standard error of the mean (n = 2–6). (b), Natural logarithms (ln) of cell density measured by OD780, and the least square regression lines.
The growth rate of E. gracilis in the linear phase (0.50 g L-1 d-1, Table 2, Fig 1a), i.e. after the 2–4 days exponential growth, was similar at 23 or 30 °C, for cultures at 3 x 400 μE m-2 s-1 light.
Table 2. Specific growth rates (μ) and volumetric growth rates (r-lin) of photoautotrophic cultures of E. gracilis at 23 and 30 °C with 2 x 400 or 3 x 400 μmol m-2 s-1 light.
| Light (μmol m-2 s-1) | 2 x 400 | 3 x 400 | 3 x 400 | 3 x 400 |
| T (°C) | 23 | 23 | 30 | 30 |
| μ (d-1) | 0.91±0.05 | 0.90±0.16 | 1.31±0.35 | 1.30±0.28 |
| r-lin (g L-1d-1) | 0.35±0.04 | 0.50±0.04 | 0.49±0.06 | 0.52±0.09 |
The data are presented with ± 95% confidence interval
Comparison of the linear growth rates with more (3 x 400 μE m-2 s-1) or less (2 x 400 μE m-2 s-1) light was made at 23 °C and demonstrated that the linear growth rate (0.35 g L-1d-1) was lower with less light provided than with 3 x 400 μE m-2 s-1 (Table 2). Providing more light also allowed more biomass to be produced: after 13 or 14 days, 5.0 g L-1 biomass had been produced in cultures receiving 3 x 400 μE m-2 s-1 light; while the culture with 2 x 400 μE m-2 s-1 light generated 3.7 g L-1 biomass in 17 days.
Heterotrophic and mixotrophic growth of E. gracilis
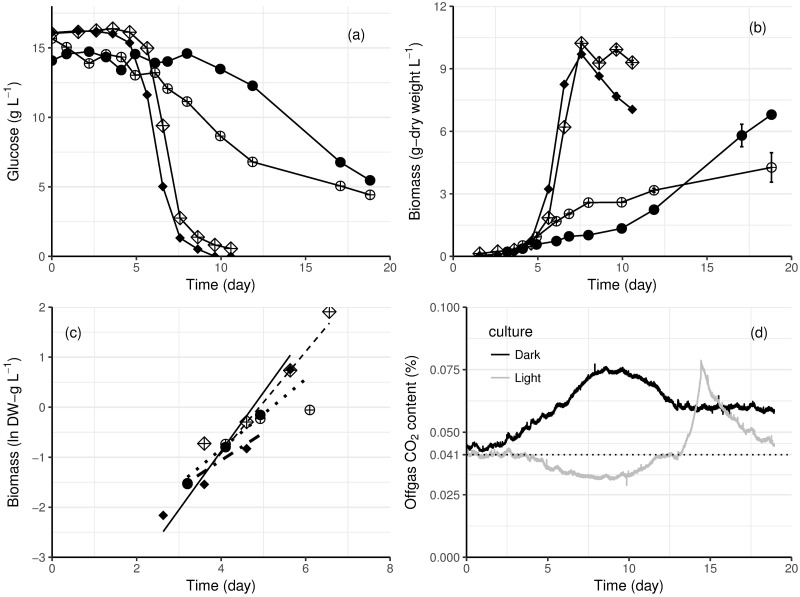
E. gracilis cells in both heterotrophic and mixotrophic conditions only started to consume glucose after an adaptation phase (up to 8 days for the mixotrophic culture at 23 °C, Fig 2a), reflecting that the inocula had been grown photoautotrophically in medium without glucose. Glucose consumption was observed 1–2 days after biomass began to increase in the mixotrophic cultures (Fig 2). No net CO2 production occurred during initial growth in light, whereas CO2 production was correlated to growth throughout the cultivation in the dark (cf. Fig 2b and 2d). The specific growth rate of heterotrophic cultures was higher than that of the mixotrophic cultures (Table 3). However, the final cell densities of mixotrophic cultures at both temperatures were greater than in heterotrophic cultures (Fig 2b).
Fig 2. Growth and glucose consumption of E. gracilis in heterotrophic and mixotrophic conditions.
(a) Glucose consumption and (b) biomass production of heterotrophic (solid symbols) and mixotrophic (symbols with cross) cultures of E. gracilis. Cultures were grown at 27 °C (diamonds) and 23 °C (circles). Error bars represent ± standard error of the mean (n = 4 for cultures at 23 °C, n = 2 for cultures at 27 °C). (c) The exponential growth phase is shown by plotting the biomass dry weight on a logarithmic scale. The least square regression lines are shown by the dashed lines. (d), Carbon dioxide content in the off gas in heterotrophic (black) and mixotrophic (grey) cultures of E. gracilis at 23 °C. The dotted line indicates the CO2 content of air, as measured with the MS.
Table 3. Specific growth rates (μ) during exponential phase of mixotrophic (primarily phototrophic growth) and heterotrophic cultures of E. gracilis at 23 and 27 °C.
| μ (d-1) | Mixotrophic | Heterotrophic |
|---|---|---|
| 23 °C | 0.55±0.14 | 0.70±0.34 |
| 27 °C | 1.02±0.41 | 1.17±0.36 |
The data are presented with ± 95% confident interval.
The specific growth rate of E. gracilis was higher at 27 °C than at 23°C, in both heterotrophic and mixotrophic conditions (Table 3 and Fig 2c). The higher temperature also shortened the adaptation phase for mixotrophic culture to consume glucose (Fig 2a).
E. gracilis lipid content and profile
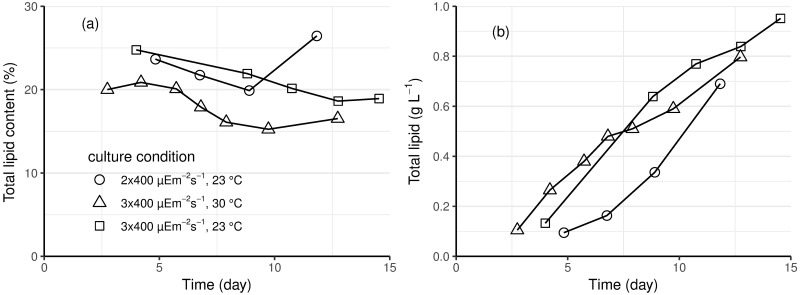
E. gracilis total lipid content was relatively constant during both exponential and linear growth phases in photoautotrophic cultures (Fig 3a). At 23 °C, the E. gracilis cells contained more lipids (on average 22%) than at 30 °C (on average 18%). However, since the total lipid produced in a culture is a function of the lipid content of the biomass and the biomass concentration, the total concentration of lipids of the photoautotrophic cultures receiving the same light intensity (3 x 400 μE m-2s-1) were similar, but less lipid was produced when less light was provided (Fig 3b).
Fig 3. Total lipid (a) content, as percentage of dry weight, and (b) concentration of E. gracilis biomass generated in autotrophic conditions.
Cultures grown at 23 °C (circle and square) with 2 x 400 (circle) or 3 x 400 μmol m-2 s-1 (square) light or at 30 °C (triangle) with 3 x 400 μmol m-2 s-1 light.
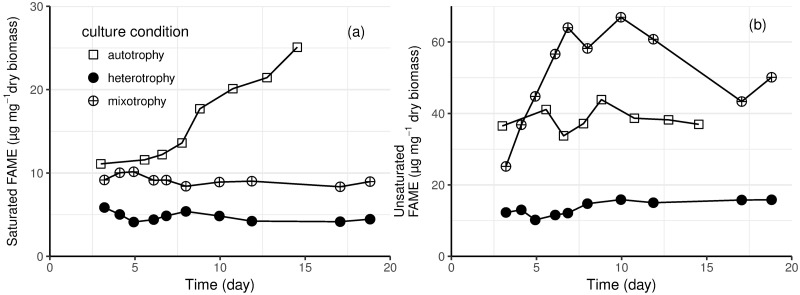
Total lipids measured gravimetrically in photoautotrophic cultures contained only 30% fatty acids that could be transesterified to fatty acid methyl esters (FAMEs) and less than 5% free fatty acids; the remaining ca. 65% probably contained lipophilic compounds such as wax esters, pigments (including chlorophyll and carotenoids), phytol, and unidentified compounds. Fig 4 shows the saturated and unsaturated FAME content of E. gracilis biomass in photoautotrophic, heterotrophic and mixotrophic batch cultivations at 23 °C. In all conditions, E. gracilis biomass contained more unsaturated than saturated fatty acids. E. gracilis biomass from heterotrophic cultures contained the lowest amount of fatty acids (both saturated and unsaturated), and their content remained constant throughout the cultivation. The maximum unsaturated fatty acid content (67 μg mg-1) was obtained in mixotrophic culture at the end of the exponential phase (ca. 7 days after inoculation; Fig 4b). The photoautotrophic culture (23 °C) accumulated 25 μg mg-1 saturated fatty acids by the end of the cultivation, which was higher than in mixotrophic and heterotrophic cultures (Fig 4a).
Fig 4. The (a) saturated and (b) unsaturated FAME content of E. gracilis grown in photoautotrophic (square), heterotrophic (solid circle) and mixotrophic (circle with cross) batch cultivations at 23 °C.
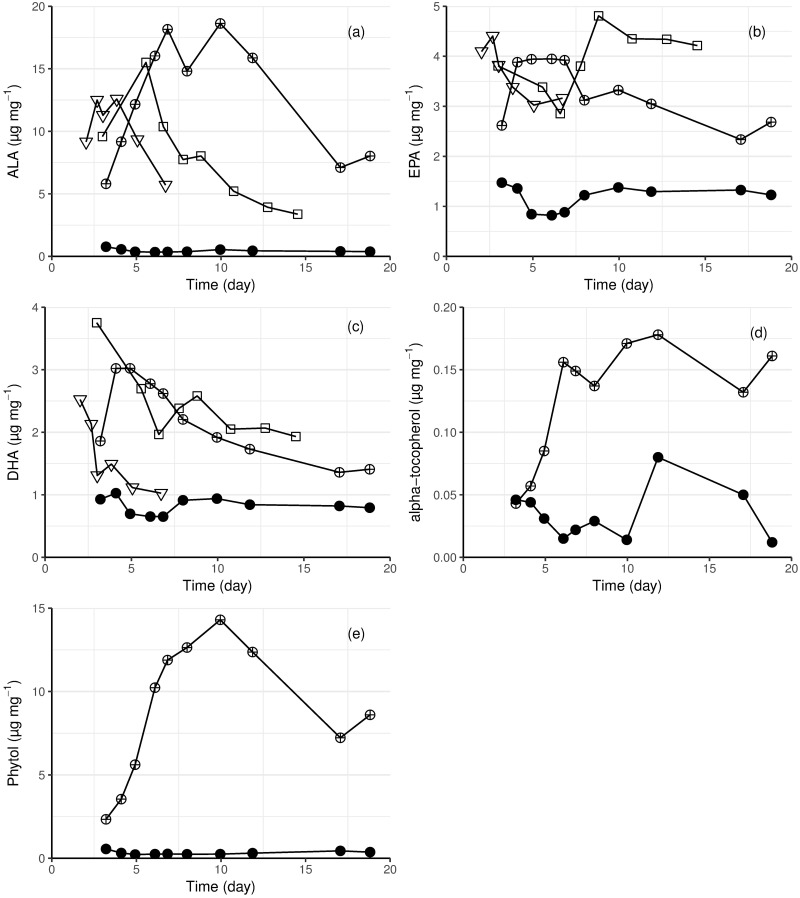
In light grown E. gracilis, the most abundant PUFA was ALA (see supporting information files S1 and S2 Figs for comparison with other fatty acids). Its maximum concentration (18 mg g-1) was obtained from the mixotrophic culture at 23 °C. ALA content was low (< 0.5 mg g-1) throughout the heterotrophic culture (Fig 5a). Long chain PUFAs, such as EPA and DHA, were present in E. gracilis biomass from all cultures. The maximum concentrations of EPA and DHA were 4.8 and 3.8 mg g-1, respectively, in cells from the photoautotrophic culture (Fig 5b and 5c). The EPA content was similar among light grown cultures (autotrophic and mixotrophic), but it was one third lower in the heterotrophic culture (Fig 5b). The heterotrophically grown biomass also contained the lowest amount of DHA. Both light grown cultures at 23 °C contained more DHA than the culture at 30 °C (autotrophic). In all light grown cultures the DHA content decreased during cultivation (Fig 5c).
Fig 5. The content of (a) ALA, (b) EPA, (c) DHA, (d) α-tocopherol and (e) phytol in E. gracilis biomass from autotrophic (23 °C square, 30 °C triangle), heterotrophic (23 °C solid circle) and mixotrophic (23 °C circle with cross) cultivations.
Wax esters were detected in both mixotrophically (maximum 2.6 mg g-1 cell dry weight) and heterotrophically (maximum 3.2 mg g-1 cell dry weight) grown cells. The majority were esters of C14 and C16 fatty acids and fatty alcohols (14:0–14:0, 14:0–16:0 and 16:0–16:0). In mixotrophic cultures the α-tocopherol content increased during exponential growth (from 0.04 to 0.18 mg g-1), whereas in the heterotrophic culture, it decreased to 0.01 mg g dry biomass-1 (Fig 5d). Phytol, associated with photosynthesis, was present in concentrations about 80-fold higher than that of α-tocopherol in mixotrophically grown E. gracilis cells (up to 14 mg g-1 biomass), but not in the heterotrophically grown ones (< 0.5 mg g-1 biomass). The phytol content was highest (1.4% of the cell dry weight) at the end of the exponential growth phase. Its content gradually decreased during the linear growth phase (Fig 5e). The content of other fatty acids, measured as FAME and ranging in length from C12:0 to C22:6, in the cells is shown in supporting information S1 and S2 Figs.
Paramylon and protein in photoautotrophically grown E. gracilis
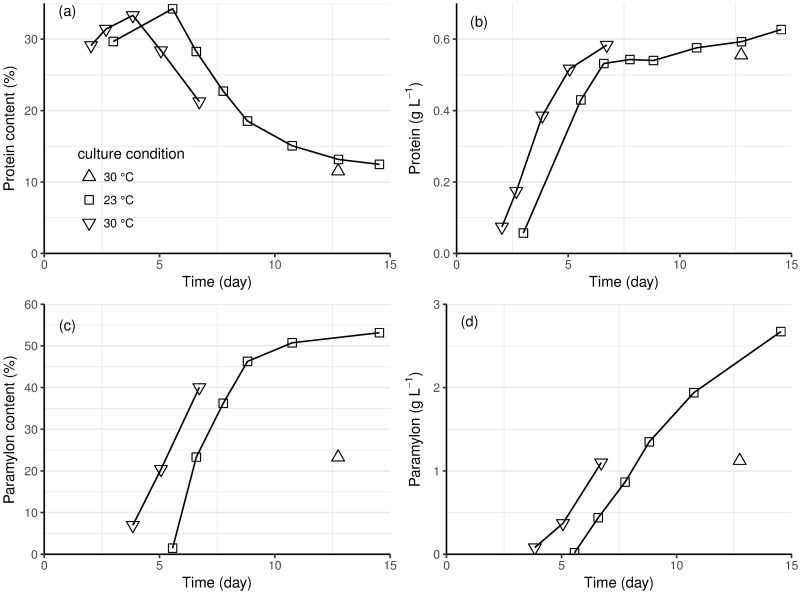
E. gracilis cells grown in autotrophic conditions contained 12–34% protein. The cell protein content (as a proportion of cell dry weight) was highest during the exponential growth phase (observed maximum protein content was 34.2% at day-6 of the culture at 23 °C, Fig 6a). Both protein content and cell biomass increased during the exponential phase, so total protein concentration in the culture increased rapidly (Fig 6b). The total protein concentration of the culture remained constant during the linear growth phase, as the relative proportion of protein in the cells decreased, and the biomass concentration increased. Protein content was similar in the cultures at 23 and 30 °C (Fig 6).
Fig 6. The content (as percent, a and c) and concentration (b and d) of protein (a and b) and paramylon (c and d) in photoautotrophically grown E. gracilis.
Cultures grown at 23 °C (square) and at 30 °C (up and down triangles) with 3 x 400 μmol m-2 s-1 light. One culture at 30 °C provided samples during the initial 6 days of growth, whereas the second culture included only an end-point sample, the data from which is included here for comparison with the longer cultivation at 23 °C.
The paramylon content of the cells was 1.5% (23 °C) or 7.0% (30 °C) at the end of the exponential growth phase, and increased during the linear growth phase. After 9 days, 46.3% of the total cell dry biomass was paramylon, increasing to 53.2% at the end of the cultivation in the culture at 23 °C (Fig 6c). The total amount of paramylon in the cultures increased throughout the cultivation, as also observed at 30 °C (Fig 6d).
Discussion
The specific growth rate of photoautotrophic E. gracilis culture was 45% higher at 30 °C than at 23 °C (Table 2), as observed in previous studies [22,37]. The optimal growth temperature for E. gracilis is between 27 and 30 °C [22]. However, we observed that temperature only affected the specific growth rate (i.e. the exponential growth phase), in which all substrates, including light, were sufficient, and not the light-limited volumetric growth rate (Table 2). For photoautotrophic microalgal cultures, sustaining exponential growth is dependent on sufficient light penetration, and is thus limited by cell density and reactor geometry. In the stirred tank photobioreactor used here, provided with 3 x 400 μE m-2s-1 light, exponential growth of E. gracilis stopped when the biomass reached 0.5 g L-1 (Fig 1). Lower overall light intensity did not affect the specific growth rate during the exponential phase, but the critical biomass level was lower (< 0.3 g L-1) when less light (2 x 400 μE m-2s-1) was provided than with 3 x 400 μE m-2 s-1 (Fig 1a, Table 2). A similar critical biomass level was observed by Li et al. in Scenedesmus sp. cultures [38]. Above the critical biomass concentration, the culture is light limited because of self-shading, but linear increase in biomass occurs [39]. In the linear growth phase, the specific growth rate gradually decreases, as the self-shading increases, and the volumetric rate of biomass production becomes the parameter by which growth is evaluated. That the volumetric biomass production rate was affected by light provision, but not temperature (Table 2), confirmed that light availability was the limiting factor for E. gracilis growth during this phase. Various reactor designs for improving light transfer in photobioreactors have been tested, which include internal illumination, enhanced mixing and shortening the light path [40], but a culture inevitably reaches a critical biomass concentration, at which light limitation results in linear growth. Thus the temperature of operation (within the range permissive of growth) is unlikely to be critical for E. gracilis biomass production at commercial scales, which would be light limited most of the time. This would allow various geographical regions with both warm and cool climates to be used for E. gracilis cultivation [30].
When grown heterotrophically and mixotrophically, exponential growth of E. gracilis was no longer limited by light. As in photoautotrophic cultures, the specific growth rate was affected by temperature, but temperature also affected the time required for adaptation to glucose utilization in mixotrophic cultures. Low temperature (23 °C) delayed growth on glucose (for 8 days). Light is also known to inhibit glucose transport in E. gracilis [28,41], although the mechanism of the inhibition remains unclear and the inhibition only applies to glucose, not other organic carbon sources. In E. gracilis, photoautotrophic and heterotrophic metabolic activities are reported to occur simultaneously only at very low light intensity [23]. Nicolas et al. observed no glucose consumption for 6 to 7 days when E. gracilis was grown in nitrogen-limited conditions with only 600 lux (~8 μmol m2 s-1) light and longer delays in its consumption in the presence of higher light intensity [41]. In the current study, we provided 3 x 400 μmol m2 s-1 and observed a delay of 8 days before glucose consumption started at 23 °C, reduced to only 4 days at 27 °C (Fig 2). Although the specific growth rate in mixotrophic cultures was slightly lower than in heterotrophic cultures (Table 3), the final biomass concentration was higher in the mixotrophic conditions, as observed by Yamane et al. [42] and Zeng et al. [43]. Providing light to the mixotrophic culture at 27 °C was sufficient to prevent cell death and lysis after glucose had been consumed.
Despite the inhibition of glucose utilisation by light, E. gracilis has been grown in mixotrophic [44] as well as heterotrophic (e.g. to produce 39.6 g L-1 biomass [17]) conditions to generate high biomass concentrations. Although providing light incurs cost, mixotrophy remains of interest since specific products, such as unsaturated fatty acids, are only produced or are produced in larger amounts when cultures are exposed to light [42,43,45].
In general, E. gracilis accumulates much less lipid than oleogenic microalgal species like Chlorella (30–57% lipid) [32] and Nannochloropsis (26–42% lipid) [46], but is known to contain a large proportion of unsaturated lipids [47]. Although lipid content as high as 37% has been reported from photoautotrophic conditions [48], the lipid content is typically only 8 to 18% of the cell biomass [43,44]. Neutral lipids are not the main storage compounds of E. gracilis, so much of the fatty acids extracted from E. gracilis are derived from the phospholipids of the cell and organelle membranes [44,49]. Only 11–18% of the lipids in E. gracilis are triacylglycerols [49]. Considerable amounts of lipophilic compounds other than fatty acids are included in gravimetric measurements of lipid content [48,50]. In the present study, about 20% of the dry biomass of cells grown in photoautotrophic conditions was extracted as total lipid (gravimetric measurement), less than half of which was detected as fatty acids.
Most of the unsaturated fatty acids In E. gracilis are found in chloroplast membranes [48] and the unsaturated fatty acid content of the cells is affected by the activity and amount of chloroplasts, which, in turn, are dependent on light availability. Up to 80% of the lipids in light grown cells may be unsaturated, while the proportion in dark grown cells was 32% [43,51]. In the current study, we observed 78 to 88% unsaturated fatty acids in cells grown in light and around 72% in cells grown without light. The content of both saturated and unsaturated fatty acids was essentially constant in the dark grown cells, but the content of unsaturated fatty acids initially increased in mixotrophic conditions (Fig 4). A subsequent decrease in unsaturated fatty acids content corresponded to the start of glucose utilization. The mixotrophically grown E. gracilis contained up to five times more unsaturated fatty acids than the cells grown in the dark (Fig 4b). The same trend has also been observed by Schwarzhans et al. [28] and Zeng et al. [43].
ALA was the major PUFA in light grown E. gracilis; it was nearly absent in dark grown cells (Fig 5), as previously observed [28,43,48]. Two long-chain omega-3 fatty acids important for human physiology, EPA and DHA, were also present in higher amounts in light grown cultures than in heterotrophic cultures (Fig 5); i.e. the synthesis of omega-3 fatty acids, like other unsaturated fatty acids in E. gracilis, was stimulated by light. Growth at 23 °C, rather than 27 °C also had a positive effect on DHA production. Although E. gracilis did not show higher productivity of any single PUFA in the conditions tested here, compared to other PUFA producing microalgal species, E. gracilis contains all basic omega-3 and omega-6 fatty acids, making its biomass suitable for high quality feed [52].
In the current study, α-tocopherol content was also light dependent, but the concentration was lower than observed by Grimm et al. in either heterotrophic or mixotrophic conditions [4] and more comparable to that observed by Kusmic et al. [53]. α-Tocopherol production is associated with photosynthetic organisms [54], but can be produced in mitochondria as well as in chloroplasts [53]. None-the-less, production of α-tocopherol, even in E. gracilis lacking chloroplasts is stimulated by light [53].
Although E. gracilis is expected to synthesise wax esters in anaerobic conditions by converting storage paramylon to wax esters [55], we observed that wax esters were constantly present at low concentrations in aerobic heterotrophic and mixotrophic cultures of E. gracilis.
Kunne and de Groot found that protein synthesis in E. gracilis was dependent on both temperature and light, with high light and low-temperature favouring protein synthesis [56]. However, we observed similar protein production at 30 and 23 °C, with the protein content being dependent on the stage of the culture, not the temperature. Protein content was highest at the end of the exponential phase, i.e. before light limitation occurred. During the light-limited, linear growth phase, E. gracilis protein content decreased (Fig 6). The total protein content of the population increased very slowly during this phase, suggesting that little new protein was synthesized. Carell et al. also observed that cells in older cultures contained about half the maximum protein content per cell [57].
Unlike protein, most paramylon accumulated during the linear growth phase in photoautotrophic E. gracilis cultures (Fig 6). Up to ~50% of the cell biomass, 2.7 g L-1, was paramylon at 23 °C, which was comparable to that reported for heterotrophic paramylon production (35 to 85% of cell biomass) [28,12]. This was much higher than previously reported for a photosynthetic culture of E. gracilis (23% of cell biomass) [4]. Accumulation of paramylon in heterotrophic and mixotrophic cultures has been associated with exponential growth [9], whereas we observed accumulation of paramylon in phototrophic conditions only after the cells and shifted into a slower, linear increase in biomass (cf. Figs 1 and 5). It may be that Grimm et al. [4] did not observe high accumulation of paramylon in their photoautotrophic cultures because they stopped sampling too early or because of limitations of growth in the linear phase in shaken flasks, compared to growth in a photobioreactor. As with protein synthesis, paramylon accumulation was not dependent on temperature, but rather on the stage of the culture. Our result indicated that E. gracilis cells can be harvested to provide either protein or paramylon, but that the cultivation process should be optimised for the specific target product, to maximise the yield and productivity.
Conclusions
E. gracilis grew well in photoautotrophic, mixotrophic and heterotrophic conditions at 23 °C, with mixo- and heterotrophic conditions primarily providing a benefit in producing high biomass concentrations. Providing light, whether in photoautotrophic or mixotrophic cultures, however, strongly influenced the cell composition. Total unsaturated fatty acids, omega-3 fatty acids, and α-tocopherol content in the cells was higher in the presence of light than in its absence. However, saturated fatty acids were still highest in cells grown in phototrophic conditions.
Increasing the temperature provided only limited benefit to phototrophic cultures, since the temperature only affected the relatively short exponential growth phase. In phototrophic cultures, temperature also had little impact on the production of protein, paramylon or total lipid, although the production of some specific fatty acids, such as DHA were lower at high than at low temperature. In contrast, cultivation temperature was important when the cells grew on organic carbon, since the exponential phase was sustained until the organic carbon was consumed.
The above-mentioned E. gracilis cell components are all either essential nutrients or compounds that promote human and animal health. Thanks to their ability to produce this unique range of compounds, dried E. gracilis whole cells and E. gracilis extracts are already on the market (sold by companies like Euglena Ltd [30]. Algaeon Inc. and Algal Scientific Corporation) and paramylon derived β-1,3-glucan is also marketed as a health supplement. More specialised E. gracilis products are expected to follow [30]. The results presented by this research will facilitate process development focusing on individual products from E. gracilis. For example, we found that high concentrations of paramylon can be produced in phototrophic conditions, although previously it had been reported this was only possible in mixo- and heterotrophic conditions [4]. In addition to natural products, potential new target products from E. gracilis are being identified by transcriptomics [29], and the production of such can be realised by metabolic engineering of E. gracilis [58].
Supporting information
(TIF)
(TIF)
Acknowledgments
We thank Jaana Rikkinen for her work of strain maintenance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by TEKES, the Finnish Funding Agency for Technology and Innovation (https://www.tekes.fi/en/tekes/), through the ALGIND (40149/11) project, and by the European Commission (grant no. 311932, project acronym: SeaBiotech, http://spider.science.strath.ac.uk/seabiotech/), and as a personal grant to YW by the Ella and Georg Ehrnrooth Foundation (http://www.ellageorg.fi/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. VTT (with funds from TEKES and the EU FP7) provided support in the form of salaries for authors [YW, TS-L, HR, MGW], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Danquah MK, Harun R, Singh M, Forde GM. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew Sustain Energy Rev. 2010;14: 1037–1047. doi: 10.1016/j.rser.2009.11.004 [Google Scholar]
- 2.Jones CS, Mayfieldt SP. Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol. 2012;23: 346–351. doi: 10.1016/j.copbio.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 3.Krajčovič J, Matej Vesteg, Schwartzbach SD. Euglenoid flagellates: a multifaceted biotechnology platform. J Biotechnol. 2015;202: 135–145. [DOI] [PubMed] [Google Scholar]
- 4.Grimm P, Risse JM, Cholewa D, Müller JM, Beshay U, Friehs K, et al. Applicability of Euglena gracilis for biorefineries demonstrated by the production of α-tocopherol and paramylon followed by anaerobic digestion. J Biotechnol. 2015;215: 72–79. doi: 10.1016/j.jbiotec.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Hamed I. The evolution and versatility of microalgal biotechnology: A review (2016). Compr Rev Food Sci Food Saf. 2016;15: 1104–1123. doi: 10.1111/1541-4337.12227 [DOI] [PubMed] [Google Scholar]
- 6.Romano G, Costantini M, Sansone C, Lauritano C, Ruocco N, Ianora A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar Environ Res. 2017;128: 58–69. doi: 10.1016/j.marenvres.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Su P, Zhang W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar Drugs 2015;13: 4231–4254. doi: 10.3390/md13074231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valverde F, Romero-Campero FJ, León R, Guerrero MG, Serranol A. New challenges in microalgae biotechnology. Eur J Protistol. 2016;55(Pt A): 95–101. doi: 10.1016/j.ejop.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Barsanti L, Vismara R, Passarelli V, Gualtieri P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J Appl Phycol. 2001;13: 59–65. doi: 10.1023/A:1008105416065 [Google Scholar]
- 10.Watanabe T, Shimada R, Matsuyama A, Yuasa M, Sawamura H, Yoshida E, et al. Antitumor activity of the beta-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. FOOD Funct. 2013;4: 1685–1690. doi: 10.1039/c3fo60256g [DOI] [PubMed] [Google Scholar]
- 11.Koizumi N, Mori S, Miyatake K, Nakano Y, Nakashima H, Murakami T, et al. Aanti-HIV (Human-Immunodeficiency-Virus) activity of sulfated paramylon. Antiviral Res. 1993;21: 1–14. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda F, Hayashi M, Kondo A. Comparative profiling analysis of central metabolites in Euglena gracilis under various cultivation conditions. Biosci Biotechnol Biochem. 2011;75: 2253–2256. doi: 10.1271/bbb.110482 [DOI] [PubMed] [Google Scholar]
- 13.Furuhashi T, Ogawa T, Nakai R, Nakazawa M, Okazawa A, Padermschoke A, et al. Wax ester and lipophilic compound profiling of Euglena gracilis by gas chromatography-mass spectrometry: toward understanding of wax ester fermentation under hypoxia. Sect Title Ferment Bioind Chem. 2014; 11: 175–183. doi: 10.1007/s11306-014-0687-1 [Google Scholar]
- 14.Kawabata A, Miyatake K, Kitaoka S. Effect of temperature on the contents of the two energy-reserve substances, paramylon and wax esters, in Euglena gracilis. J Protozool. 1982;29: 421–423. doi: 10.1111/j.1550-7408.1982.tb05425.x [Google Scholar]
- 15.Korn ED. The fatty acids of Euglena gracilis. J Lipid Res. 1964;5: 352–362. [PubMed] [Google Scholar]
- 16.Certik M, Shimizu S. Biosynthesis and regulation of microbial polyunsaturated fatty acid production. J Biosci Bioeng. 1999;87: 1–14. [DOI] [PubMed] [Google Scholar]
- 17.Ogbonna JC, Tanaka H, Tomiyamal S. Heterotrophic cultivation of Euglena gracilis Z for efficient production of α-tocopherol. J Appl Phycol. 1998;10: 67–74. doi: 10.1023/A:1008011201437 [Google Scholar]
- 18.Islam MdT, Barros de Alencar MVO, da Conceição Machado K, da Conceição Machado K, de Carvalho Melo-Cavalcante AA, de Sousa DP, de Freitas RM. Phytol in a pharma-medico-stance. Chem Biol Interact. 2015;240: 60–73. doi: 10.1016/j.cbi.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Nakano Y, Urade Y, Urade R, Kitaoka S. Isolation, purification, and characterization of the pellicle of Euglena gracilis. J Biochem. 1987;102: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 20.Vismara R, Barsanti L, Lupetti P, Passarelli V, Mercati D, Dallai R, et al. Ultrastructure of the pellicle of Euglena gracilis. Tissue Cell. 2000;32: 451–456. [DOI] [PubMed] [Google Scholar]
- 21.Olaveson MM, Nalewajko C. Effects of acidity on the growth of two Euglena species. Hydrobiologia. 2000;433: 39–56. doi: 10.1023/A:1004006401516 [Google Scholar]
- 22.Kitaya Y, Azuma H, Kiyota M. Effects of temperature, CO2/O2 concentrations and light intensity on cellular multiplication of microalgae, Euglena gracilis. Adv Space Res. 2005;35: 1584–1588. [DOI] [PubMed] [Google Scholar]
- 23.Ogbonna E, Ichige H, Tanaka J. Interactions between photoautotrophic and heterotrophic metabolism in photoheterotrophic cultures of Euglena gracilis. Appl Microbiol Biotechnol. 2002;58: 532–538. doi: 10.1007/s00253-001-0901-8 [DOI] [PubMed] [Google Scholar]
- 24.Ingebrigtsen RA, Hansen E, Andersen JH, Eilertsen HC. Light and temperature effects on bioactivity in diatoms. J Appl Phycol. 2016;28: 939–950. doi: 10.1007/s10811-015-0631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Kong Z, Chen S, Ran Z, Ye M, Xu J, Zhou C, Liao K, Cao J, Yan X. The comparative study for physiological and biochemical mechanisms of Thalassiosira pseudonana and Chaetoceros calcitrans in response to different light intensities. Algal Res. 2017;27: 87–98. doi: 10.1016/j.algal.2017.08.026 [Google Scholar]
- 26.Gong M, Bassi A. Investigation of Chlorella vulgaris UTEX 265 cultivation under light and low temperature stressed conditions for lutein production in flasks and the coiled tree photo-bioreactor (CTPBR). Appl Biochem Biotechnol. 2017;183: 652–671. doi: 10.1007/s12010-017-2537-x [DOI] [PubMed] [Google Scholar]
- 27.Kumar D, Kvíderová J, Kaštánek P, Lukavsky J. The green alga Dictyosphaerium chlorelloides biomass and polysaccharides production determined using cultivation in crossed gradients of temperature and light. Engin Life Sci. 2017;17: 1030–1038. doi: 10.1002/elsc.201700014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzhans J-P, Cholewa D, Grimm P, Beshay U, Risse J-M, Friehs K, et al. Dependency of the fatty acid composition of Euglena gracilis on growth phase and culture conditions. J Appl Phycol. 2014;27: 1389–1399. doi: 10.1007/s10811-014-0458-4 [Google Scholar]
- 29.O’Neill EC, Trick M, Hill L, Rejzek M, Dusi RG, Hamilton CJ, et al. The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol BioSyst. 2015;11: 2808 doi: 10.1039/c5mb00319a [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K. Large-Scale Cultivation of Euglena. Advances in experimental medicine and biology. 2017;979: 285–293. doi: 10.1007/978-3-319-54910-1_14 [DOI] [PubMed] [Google Scholar]
- 31.Bischoff HW, Bold HC. Some soil algae from enchanted rock and related algae species. Phycol Stud. 1963;44: 1–95. [Google Scholar]
- 32.Wang Y, Rischer H, Eriksen NT, Wiebe MG. Mixotrophic continuous flow cultivation of Chlorella protothecoides for lipids. Bioresour Technol. 2013;144: 608–614. doi: 10.1016/j.biortech.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226: 497–509. [PubMed] [Google Scholar]
- 34.Slocombe SP, Ross M, Thomas N, McNeill S, Stanley MS. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour Technol. 2013;129: 51–57. doi: 10.1016/j.biortech.2012.10.163 [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193: 265–275. [PubMed] [Google Scholar]
- 36.Albalasmeh AA, Berhe AA, Ghezzehei TA. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym. 2013;97: 253–261. doi: 10.1016/j.carbpol.2013.04.072 [DOI] [PubMed] [Google Scholar]
- 37.Kawabata A, Kaneyama M. The effect of growth temperature on wax ester content and composition of Euglena gracilis. J Gen Microbiol. 1989;135: 1461–1467. doi: 10.1099/00221287-135-6-1461 [Google Scholar]
- 38.Li X, Hu H, Zhang Y. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol. 2011;102: 3098–3102. doi: 10.1016/j.biortech.2010.10.055 [DOI] [PubMed] [Google Scholar]
- 39.Ogbonna JC, Yada H, Tanaka H. Light supply coefficient: A new engineering parameter for photobioreactor design. J Ferment Bioeng. 1995;80: 369–376. doi: 10.1016/0922-338X(95)94206-7 [Google Scholar]
- 40.Ogbonna JC, Tanaka H. Light requirement and photosynthetic cell cultivation—Development of processes for efficient light utilization in photobioreactors. J Appl Phycol. 2000;12: 207–218. doi: 10.1023/A:1008194627239 [Google Scholar]
- 41.Nicolas P, Freyssinet G, Nigon V. Effect of light on glucose utilization by Euglena gracilis. Plant Physiol. 1980;65: 631–634. doi: 10.1104/pp.65.4.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamane Y, Utsunomiya T, Watanabe M, Sasaki K. Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol Lett. 2001;23: 1223–1228. doi: 10.1023/A:1010573218863 [Google Scholar]
- 43.Zeng M, Hao W, Zou Y, Shi M, Jiang Y, Xiao P, et al. Fatty acid and metabolomic profiling approaches differentiate heterotrophic and mixotrophic culture conditions in a microalgal food supplement “Euglena”. BMC Biotechnol. 2016;16: 49 doi: 10.1186/s12896-016-0279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezić T, Filipović J, Šantek B. Photo-mixotrophic cultivation of algae Euglena gracilis for lipid production. Agric Conspec Sci. 2013;78: 65–69. Available: http://hrcak.srce.hr/99323?lang=en [Google Scholar]
- 45.Takeyama H, Kanamaru A, Yoshino Y, Kakuta H, Kawamura Y, Matsunaga T. Production of antioxidant vitamins, beta-carotene, vitamin C, and vitamin E, by two-step culture of Euglena gracilis Z. Biotechnol Bioeng. 1997;53: 185–190. doi: 10.1002/(SICI)1097-0290(19970120)53:2<185::AID-BIT8>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 46.Millán-Oropeza A, Fernández-Linares L. Biomass and lipid production from Nannochloropsis oculata growth in raceway ponds operated in sequential batch mode under greenhouse conditions. Environ Sci Pollut Res. 2017;24: 25618–25626. doi: 10.1007/s11356-016-7013-6 [DOI] [PubMed] [Google Scholar]
- 47.Jeong U, Choi J, Kang C, Choi B, Kang S. Effects of culture methods on the growth rates and fatty acid profiles of Euglena gracilis. Korean J Fish Aquat Sci. 2016;49: 38–44. [Google Scholar]
- 48.Constantopoulos G, Bloch K. Effect of Light Intensity on the Lipid Composition of Euglena gracilis. 1967;242: 3538–3542. [Google Scholar]
- 49.Miyatake K, Minamigawa M, Nakano Y, Kitaoka S. Effects of culture conditions on polyunsaturated fatty acids composition in Euglena gracilis. Nippon Eiyo Shokuryo Gakkaishi. 1985;38: 117–122. [Google Scholar]
- 50.Hayashi M, Toda K, Kitaoka S. Enriching Euglena with Unsaturated Fatty Acids. Biosci Biotechnol Biochem. 1993;57: 352–353. doi: 10.1271/bbb.57.352 [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg A. A comparison of lipid patterns in photosynthesizing and nonphotosynthesizing cells of Euglena Gracilis. Biochemistry. 1963;2: 1148–1154. doi: 10.1021/bi00905a042 [DOI] [PubMed] [Google Scholar]
- 52.Ward OP, Singh A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005;40: 3627–3652. doi: 10.1016/j.procbio.2005.02.020 [Google Scholar]
- 53.Kusmic C, Barsacchi R, Barsanti L, Gualtieri P, Passarelli V. Euglena gracilis as source of the antioxidant vitamin E. Effects of culture conditions in the wild strain and in the natural mutant WZSL. J Appl Phycol. 1999;10: 555–559. doi: 10.1023/A:1008022305865 [Google Scholar]
- 54.Tani Y, Tsumura H. Screening for tocopherol-producing microorganisms and α-tocopherol production by Euglena gracilis Z. Agric. Biol Chem. 1989;53: 305–312. [Google Scholar]
- 55.Inui H, Ishikawa T, Tamoi M. Wax ester fermentation and its application for biofuel production. Advances in experimental medicine and biology. 2017;979: 269–283. doi: 10.1007/978-3-319-54910-1_13 [DOI] [PubMed] [Google Scholar]
- 56.Kunne A, deGroot EJ. Protein synthesis in Euglena gracilis is light- and temperature-dependent, oscillating in a circadian, temperature-compensated manner. Bot ACTA. 1996;109: 57–63. [Google Scholar]
- 57.Carell EF, Johnston PL, Christopher AR. Vitamin B(12) and the macromolecular composition of Euglena. J Cell Biol. 1970;47: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogawa T, Tamoi M, Kimura A, Mine A, Sakuyama H, Yoshida E, et al. Enhancement of photosynthetic capacity in Euglena gracilis by expression of cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase leads to increases in biomass and wax ester production. Biotechnol Biofuels. 2015;8: 80 doi: 10.1186/s13068-015-0264-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.