Abstract
Background
Non-human primate (NHP) diabetic models using chemical ablation of β-cells with STZ have been achieved by several research groups. Chemotherapeutic STZ could lead to serious adverse events including nephrotoxicity, hepatotoxicity, and mortality.
Methods
We implemented a comprehensive therapeutic strategy that included the tether system, permanent indwelling catheter implants, an aggressive hydration protocol, management for pain with IV nubain and anxiety with IV midazolam, moment-by-moment monitoring of glucose levels post-STZ administration, and continuous intravenous insulin therapy.
Results
A triphasic response in blood glucose after STZ administration was fully characterized. A dangerous hypoglycemic phase was also detected in all baboons. Other significant findings were hyperglycemia associated with low levels of plasma leptin, insulin and C-peptide concentrations, hyperglucagonemia, and elevated non-esterified fatty acids (NEFA) concentrations.
Conclusions
We successfully induced frank diabetes by IV administering a single dose of pharmaceutical-grade STZ safely and without adverse events in conscious tethered baboons.
Keywords: triphasic blood glucose response, hydration protocol, hypoleptinemia, hyperglucagonemia, hypoglycemia, fulminant type 1 diabetes
Introduction
Both major forms of diabetes mellitus (DM) involve Beta-cell (β-cell) destruction and dysfunction. Type 1 DM (T1DM) involves severe insulin deficiency caused by autoimmune destruction of islet β-cells. In long-standing T1DM, up to 99% of islet mass is lost [45]. Type 2 diabetes (T2DM) develops when insulin secretory capacity no longer compensates for peripheral insulin resistance. In long-standing T2DM, approximately 65% of islet mass is destroyed by apoptosis, which appears to be mediated primarily by lipotoxicity and/or glucotoxicity [4]. Accordingly, new treatment strategies have focused on replenishing the deficiency of β-cell mass common to both major forms of diabetes by islet transplantation or β-cell regeneration [8].
Islet transplantation has been investigated as a treatment for T1DM in selected patients with inadequate glucose control despite insulin therapy. Although the outcome of the procedure has improved dramatically over the past decade, it remains an invasive procedure with a substantial risk of morbidity [51, 62]. A major cause for the high incidence of failure in human clinical allotransplantations is the poor availability of transplanted pancreatic tissue. To improve the clinical outcome of human islet allotransplantation, the realization of well-designed preclinical studies in animal models remains crucial.
Animal models for T1DM range from animals with spontaneously developing autoimmune diabetes to chemical ablation of the pancreatic β-cells. Some of the most commonly used models of T1DM are chemically-induced, spontaneous autoimmune, genetically-induced and virally-induced models [36]. Islet regeneration has been successful in animal models, primarily targeting liver using viral vectors [19, 37]. Since islet mass is known to increase during obesity and pregnancy, the concept of stimulating pancreatic islet regeneration in vivo is both rational and physiologic [34, 66].
Rodent models of T1DM have been extensively studied. However, these rodent homologs are limited as translational models by their small size and brief life span and that biological outcomes in rodents may not faithfully reflect the clinical manifestations of such findings in humans. Many of these limitations can be overcome by identifying large animals with genetic diseases, such as diabetes, corresponding to their human disease counterparts [9]. Over the years, non-human primates (NHP) have been used in studies regarding important historical and current diseases such as human immunodeficiency virus, tuberculosis, hepatitis, polio, and malaria [5]. Advantages of using NHP are their size and long lifespan compared to mice and rats. Their larger size permits a wider range of procedures to be performed, and the animals live long enough to be able to monitor and track long term effects of conditions and treatments [26].
Baboons are ideal NHP models for studying the biological and genetic aspects of human disease. There is a considerable resemblance when comparing baboon anatomy and physiology to that of humans. The biological processes related to growth, development, maturation, reproduction, senescence, the reproductive system, bone structure, dental growth, cardiac function, and metabolic disturbances in baboons are significantly similar to humans [15]. The Major Histocompatibility Complex (MHC) bears a striking resemblance to humans. The similarity of the MHC between NHP and humans compared to that between rodents and humans has allowed for critical development in standard medical practices including vaccination protocol development, blood transfusion protocol development, and organ compatibility and transplantation [15].
Nevertheless, in large animal models such as the baboon, spontaneous diabetes is relatively rare; therefore, induced NHP models of T1DM are required. Different strategies have been used to induce hyperglycemia in baboons, mainly by pancreatectomy or streptozotocin (STZ). Pancreatectomy has been used to induce diabetes in pigs [47], dogs [20], and primates [27]. This method requires invasive intra-abdominal surgery in which the exocrine pancreas function is removed, leading to pancreatic exocrine deficiency in the animal and the need for enzyme supplementation [17]. This approach also requires a skilled surgeon because the tight adherence of the pancreas to the intestines makes pancreatectomy in baboons a complicated intervention [70].
Multiple NHP diabetic models using chemical ablation of β-cells with STZ have been achieved by several research groups [67]. STZ is widely used to induce experimental diabetes in animals. The mechanism of its action in β-cells of the pancreas has been intensively investigated. The cytotoxic action of this diabetogenic agent is mediated by reactive oxygen species. STZ enters the β-cell via a glucose transporter (GLUT2) and causes alkylation of DNA. DNA damage induces activation of poly ADP-ribosylation, a process that is more important for the diabetogenicity of STZ than DNA damage itself. Poly ADP-ribosylation leads to depletion of cellular NAD+ and ATP. Enhanced ATP dephosphorylation after STZ treatment supplies a substrate for xanthine oxidase resulting in the formation of superoxide radicals. Consequently, hydrogen peroxide and hydroxyl radicals are also generated. Furthermore, STZ liberates toxic amounts of nitric oxide that inhibit aconitase activity and participates in DNA damage. As a result of the STZ action, β-cells undergo the destruction by necrosis [41].
Clinically relevant adverse effects have been reported with the administration of STZ in healthy juvenile and adult NHP [22]. As STZ is an alkylating cytotoxic agent, it could lead to serious adverse events including nephrotoxicity, hepatotoxicity, and mortality resulting from organ failure or severe metabolic perturbations [55]. Except for a recent report [21], there is not sufficient data concerning the appropriate management and treatment of adverse effects for STZ use in NHP in the literature [39].
Here we report our findings with the use of pharmaceutical-grade STZ with the intention of instituting a safer strategy to induce hyperglycemia in conscious tethered baboons. Therefore, the primary aim of this document is the establishment of a model of induced frank diabetes in baboons through β-cell compromise with STZ under a tether system and indwelling catheter implants.
Materials and Methods
Humane Care Guidelines
Animals were strictly handled in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health, the Animal Welfare Act by the United States Department of Agriculture, and the Weatherall report by the Medical Research Council. The protocol was approved by the Institutional Animal Care and Use Committees of the Texas Biomedical Research Institute and Baylor Research Institute (Assurance Number: 10–4233). The study approval ID was IACUC 1251PC.
Animal selection and acclimation
Four healthy adult male baboons (ID No. 18175, 26104, 20105, 26457), age >6 but <16 y.o., weighing 26 – 34 kg were selected to go into the study. The baboons studied are selected from a population at the Southwest National Primate Research Center (SNPRC) located at the Texas Biomedical Research Institute (Texas Biomed) San Antonio, TX [13, 15]. These animals develop spontaneous and diet-induced obesity and its co-morbidities [13]. The DNA sequence of genes such as leptin and adiponectin show a 95% to 99% correspondence of sequence identity at the DNA, RNA, and predicted protein level with humans [63]. Our lab has also documented impaired insulin signaling at the whole body and molecular level and overt diabetes in spontaneously obese baboons [3, 10].
Animals were not included in the study if they had lost more than 3kg of weight in the last three months. However, animals were included if they had not been weighed recently, but they had good health according to veterinarian’s clinical decision. They were monitored regarding their food consumption as well their behavioral traits during the acclimation period. Animal acclimation to technician presence was carefully undertaken prior to the procedure start date. Most of these criteria were subjective and assessed by individuals working closely with the animals. As such, the study veterinarian in conjunction with the lead technical staff was best qualified to make recommendations regarding behavior. An additional animal remained on sham-tether, accompanying the selected baboon in the bay. The accompanying animal was kept on sham-tether through the successful catheter implant of the study animal. In the event something went wrong, a second animal was immediately available to stay on schedule. Once the implanted catheters into the study animal were successful, the sham-tether from the accompanying animal was removed. Eligibility and exclusion criteria followed recommendations previously described to evaluate clinical suitability for STZ administration [21].
The quantity of food offered to each baboon daily was based on the estimated metabolizable energy (ME) requirements for adult captive baboons. Specifically, the animals were fed with standard chow (Monkey Diet 15%, Constant Nutrition Purina 5LE0™) to sustain constant body weight (BW) : 48 kcal * BW (kg) [48]. The 5LE0 food ration was weighed before distribution. All bagged pellets were delivered to the technicians. At the end of the day, any part of the ration remaining in the food hopper was estimated as a percentage according to the best of the veterinary technician’s knowledge. Their diet was enriched with fresh fruits, vegetables, grains and nuts. They were offered water ad libitum. Standard chow (5LE0) was offered in daily rations divided in two equal quantities.
The tether system and indwelling catheter implants
All animals in the study underwent surgical tether implantation. Catheter implants in the femoral artery and vein for tether were prepared as follows: heparin (low-molecular weight)-coated polyurethane catheters (Solomon Scientific, Plymouth, PA, USA) were placed in the femoral artery and vein. An incision was made over the femoral artery and vein just below the inguinal lymph node, and the femoral artery/vein was exposed by blunt dissection. Through a small incision in the vessel (artery/vein), a catheter was introduced. The catheter was advanced, secured and tested for flow. A tension loop was formed and secured under the skin. Both catheters were routed subcutaneously to the midscapular region of the back where they exited the skin into a molded plastic box (backpack) attached to the tether jacket [12].
Intensive hydration protocol, management for discomfort and anxiety, and analgesia for pain relief
After fasting overnight, all baboons were prehydrated with saline solution (NaCl) 0.9% intravenous (IV) at a rate of 30 ml/kg of body weight/day for 24 hours before STZ administration to decrease the likelihood of kidney toxicity [52]. The rate of fluid administration remained the same for 48 hours more after STZ administration. However, fluid administration rate and/or dextrose 5% or higher was adjusted and/or exchanged with the saline solution, according to each animal’s clinical needs as dictated by blood glucose and insulin therapy efficacy.
We administered the synthetic opioid agonist-antagonist analgesic nalbuphine hydrochloride (Nubain) (Endo Pharmaceuticals Inc. Chadds Ford, Pennsylvania, USA) at a dosage of 0.15 mg × kg IV every six hours starting 24 hours before the STZ administration, remaining with the same regime for 48 hours after STZ administration. We also administered midazolam (LGM Pharma, Nashville, TN, USA). This is a short-acting drug in the benzodiazepine class. Midazolam has a fast recovery time and is the most commonly used benzodiazepine as a premedication for sedation. It is the most popular benzodiazepine in the intensive care unit (ICU) because of its short elimination half-life, combined with its water solubility and its suitability for continuous infusion [7]. We administered midazolam IV at an initial priming dose of 0.025 – 0.05 mg/kg using the tether catheter system prior to STZ administration. A continuous IV infusion of midazolam was administered after the priming dose for 24 hours with a dosage range of 0.02 mg/kg/hr to 0.1 mg/kg/hr. This dosage was expected to provide an anxiolytic effect, not a sedative effect. We administered both drugs for comfort and a means of relieving organic stress and pain due to the sudden exposure to the cytotoxic drug.
Induction of the diabetic phenotype with pharmaceutical-grade steptozotocin (STZ) dose administration
A single IV injection of pharmaceutical-grade STZ (Zanosar) at a flat-fixed dosing of 150 mg/kg was administered. STZ (Zanosar) is indicated in the treatment of metastatic islet cell carcinoma of the pancreas in humans. Renal toxicity is dose-related and cumulative. Other side effects are nausea and vomiting. In addition, liver dysfunction, diarrhea, and hematological changes have been observed in some human patients. Each vial of Pharmaceutical-grade STZ (Zanosar; Sicor Pharmaceuticals, Irvine, CA, USA) contains 1g of the active ingredient streptozocin and 220 mg citric acid anhydrous. STZ (Zanosar) is available as a sterile, pale yellow, freeze-dried preparation for intravenous administration. The pH has been adjusted with sodium hydroxide.
To prepare STZ, Zanosar was reconstituted with 9.5 mL of cold 0.9% NaCl for IV administration. To avoid decomposition after reconstitution, immediately after preparation STZ was administered as an IV bolus. When reconstituted as directed, the pH of the solution is between 3.5 and 4.5. STZ (Zanosar) is very soluble in water or physiological saline and must be protected from light. Caution in the handling and preparation of the powder and solution should be exercised, and the use of gloves is recommended. If the sterile powder or a solution prepared from STZ (Zanosar) contacts the skin or mucosa, the affected area must be immediately washed with soap and water [6, 29].
Close monitoring of glucose levels post-STZ administration
The most dangerous and significant adverse effect immediately after STZ administration is severe hypoglycemia [42]. Therefore, repeated measurements of glucose levels are mandatory. Blood samples were drawn as frequently as needed within the first 24 hours, taking advantage of the indwelling arterial catheter. However, 15 minute draws were the standard interval during such 24 hours for an ideal clinical follow up. 0.1mL of arterial blood was drawn to measure glucose on-site with a conventional glucometer. The blood draw interval during the following 48 hours was every 30 – 60 minutes, but such intervals were dictated by the measured circulating glucose levels.
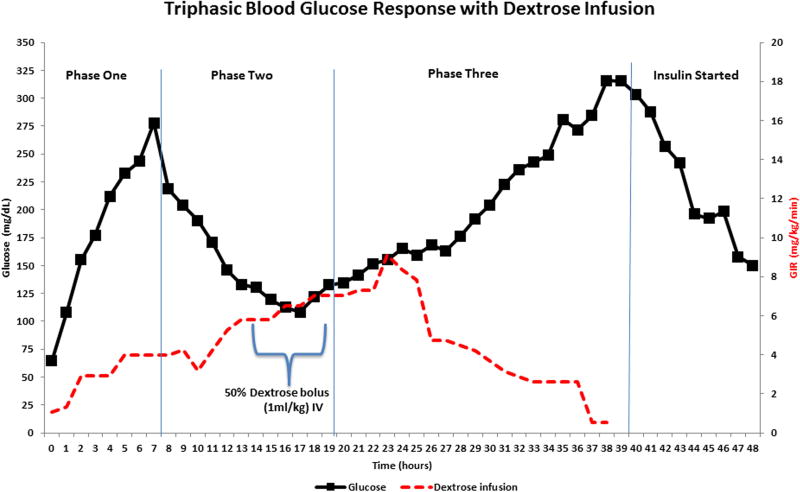
Dextrose 50% (1 ml × kg IV bolus) was administered if blood glucose levels showed a curve of glucose decreasing to levels below 75 mg/dl at any time point after the STZ administration. At the same time, a bag with 10% to 30% dextrose in 1000 ml of saline was continuously administered at a rate of approximately 30–40 ml/hr during the first 48–72 hours. The length of administration for this 10%-30% dextrose bag was dictated by the levels of glucose in the following hours post-STZ administration. We eventually withdrew the continuous glucose administration and exchanged it for saline NaCl 0.9% when frank hyperglycemia was confirmed. Depending upon glucose values, continuous IV insulin was initiated (Fig. 3). The glucose infusion rate (GIR) was calculated according to the established formula:
Figure 3.
Continuous intravenous insulin therapy
We administered a continuous IV insulin infusion (Humulin-R U-100, human, USP, rDNA, Elli Lily Co., Indianapolis, IN) using a Baxter infusion pump (Model AS50, Baxter Health Care Corporation, Deerfield, IL), utilizing a dosage of 0.1- 0.5 U/kg/h. The dosage was adjusted according to the response of the animal and to the glucose levels both in fed and fasting conditions. The goal was to maintain daily circulating glucose levels between 100 and 200 mg/dl. The directions for insulin syringe preparation are as follows: draw 19 ml of 0.9% non heparinized Sodium Chloride into a 20 cc BD syringe. Add 1 ml of whole blood and 20 units insulin (Humulin-R) (0.2 cc) to the syringe. Invert the syringe to mix. Place the syringe on the Baxter pump to begin infusing immediately. Set pump speed to ml/hr equivalent of U/kg/h. It is important to follow the order when mixing the insulin syringes: saline first, then blood, and finally insulin. The reason is that the blood prevents the insulin from binding with the plastic of the syringe, so it is critical that the blood be added to the saline prior to the insulin in the syringe.
Analysis of metabolites and hormones to confirm the induction of the diabetic state
After 36–42 hours, the glucose infusion was stopped, and diabetes was confirmed by the persistence of fasting hyperglycemia. One week before and one week following STZ administration, an intravenous glucose tolerance test (IVGTT) was performed, with glucose, insulin, C-peptide and glucagon concentrations measured for 60 min. Midazolam was administered as an anxiolytic agent during the one hour conscious IVGTT as described above. A bolus of 50% dextrose (0.5mg/kg) was given IV over 60 seconds. Arterial blood samples were collected at 2, 4, 6, 8, 10, 15, 30, 45, and 60 minutes post-dextrose administration. The midazolam was stopped promptly upon collection of the final blood sample.
A fasting plasma glucose level of >200 mg/dl in combination with C-peptide levels of less than 0.4 ng/ml, accompanied with the absence of a stimulated C-peptide response upon an IVGTT (0.3 ng/ml) was considered to be indicative of diabetes in accordance with established criteria [14]. All animals became diabetic between 36 and 42 hours after STZ administration. An excess of circulating levels of glucagon (hyperglucagonemia) was significantly present after STZ administration, both fasting and during the IVGTT. Decreased levels of leptin and increased levels of non-esterified fatty acids (NEFA) were also found after STZ administration.
Serum concentrations of glucose were determined using an ACE® chemistry analyzer (Alfa Wasserman Diagnostic Technologies, LLC; West Caldwell, NJ). Serum insulin, glucagon, leptin and C-peptide were determined using a DPC Immulite 1000 Analyzer ® (Diagnostics Products Corporation; Los Angeles, CA). The Immulite is an automated chemiluminescent peptide and steroid hormone analyzer. For measurements of plasma free fatty acid (FFA) concentrations, enzymatic kits were used (WAKO, Richmond, VA).
Total parenteral nutrition (TPN)
TPN is generally indicated as an anticipation of undernutrition. We established a clinical criteria to consider immediate IV administration of TPN if any of our STZ-treated baboons would eat 20% or less (~100 mg or less) of normal chow 5LE0 in a 48 hour period. The critical period was 72 hours after STZ IV injection. We designed and manufactured our routine TPN treatment to be able to provide fluids (saline 0.9% 30ml/kg/d), energy calories (30–60 Kcal/kg/day, depending on energy expenditure), carbohydrates calculated as 60% from the total amount of Kcal/kg/day (50% dextrose providing 0.5gr/ml), amino acids 1.0 to 2.0 g/kg/day, depending on the degree of catabolism (trophamine 500 ml, 10% specialty amino acids injection), fatty acids calculated as 40% of essential fatty acids administered from the total amount of Kcal/kg/day (intralipid 20% IV fat emulsion in 500 ml), 40 ml of calcium gluconate (calcium gluconate injection, USP, 10%, 23.25 mEq/50ml), 10 ml of potassium chloride (2meq/ml), 2 ml of trace elements (zinc, copper, manganese, chromium, selenium as multitrace 5 concentrate USP), 5 ml of multi-vitamin for infusion (M.V.I Pediatric), and 20 mg of famotidine injection. Calcium gluconate, potassium chloride, the trace elements, the M.V.I, and famotidine were administered in a 24-hour period as a mixture to the bag of TPN. This treatment represents the standard of care in humans.
Euthanasia and histopathology examination
When euthanasia was scheduled, the animal was sedated with ketamine hydrochloride (10 mg/kg). Then, an overdose of sodium pentobarbital (>100mg/kg) was administered IV to effect. This is the preferred form of euthanasia in non-human primates. The procedure was performed by a veterinarian assisted by a senior veterinary technician. This procedure was in accord with guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. After euthanasia, samples of pancreas were collected, refrigerated/frozen and fixed for additional analyses, including histopathology and immunohistochemistry.
Samples of the tissues were cut into pieces, fixed in 10% buffered formalin and 4% paraformaldehyde, and stained with hematoxylin and eosin. Pancreatic cryostat sections 7–10 µm in thickness were fixed in 4% paraformaldehyde for 15 minutes at 4°C and quenched for five minutes with 10mM glycine in phosphate buffered saline (PBS). Sections were then rinsed in PBS three times and permeabilized with 0.5% Triton X-100 in PBS for 10 minutes. Sections were blocked with 10% vol/vol goat serum at 37°C for one hour and washed three times with PBS. Primary antibodies (anti-insulin, anti-glucagon) were added and incubated at 4°C overnight. After washing with PBS three times for five minutes each time, secondary antibody was added and incubated for one hour at 37°C. Sections were rinsed three times with PBS for five minutes each time, DAPI (1:5000 dilutions with PBS) for five minutes, washed two times with PBS, and then mounted.
Early detection of adverse effects
At the time of catheter placement, blood was collected to complete a comprehensive laboratory workup and establish baseline values for complete blood count (CBC) with differential, routine chemistry panel, and HbA1C. The blood chemistries and metabolic factors measured are albumin, alkaline phosphatase, ALT, AST, BUN, serum chloride, creatinine, potassium, serum sodium, total protein, HbA1c, glucose, insulin, C-peptide, NEFA, leptin and glucagon. The complete laboratory workup was repeated after STZ administration for early detection, assessment and close monitoring of adverse effects to kidney and liver.
Data analysis
Data analysis was performed with Statview software (SAS, Cary, NC, USA). The results are expressed as the mean ± one standard deviation. Differences were analyzed by repeated measures ANOVA with Fisher’s post hoc test and considered significant at p<0.05.
Results
Circulating values of fasting insulin-glucose axis phenotypes before and after the administration of pharmaceutical-grade (STZ)
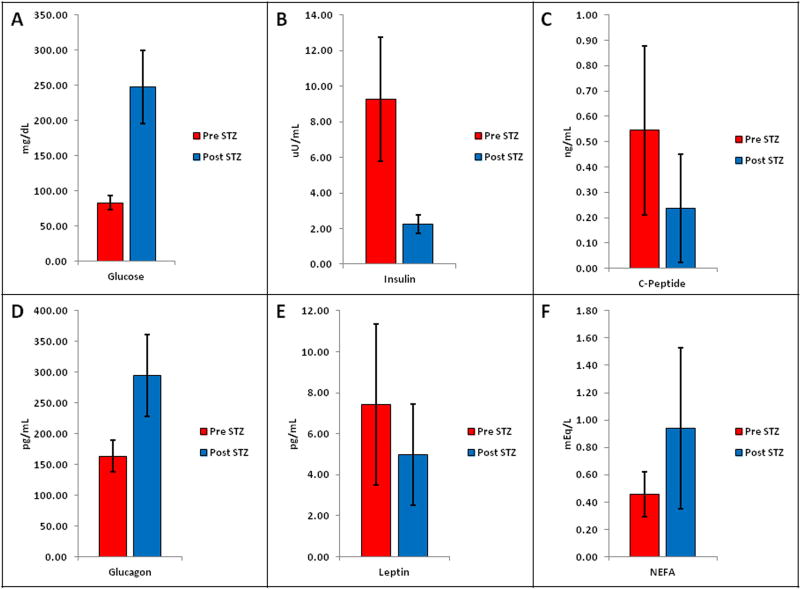
Pre-STZ blood glucose concentrations reported a mean value of 83.2 ± 10.3 mg/dl, exhibiting mean elevated post-STZ glucose levels of 247.25 ± 66.5 mg/dl; all these biochemical changes occurred within eight days after STZ administration (Fig. 1A). Mean insulin levels decreased from 9.3 ± 3.4 uU/ml before STZ injection to 2.3 ± 0.5 uU/ml after STZ injection (Fig. 1B). Mean C-peptide levels decreased from 0.545 ± 0.33 ng/ml before STZ injection to 0.237 ± 0.21 ng/ml after STZ injection (Fig. 1C). Circulating glucagon concentrations ranged from 134.78 pg/ml to 197.81 pg/ml before administration of STZ (Mean values 163.60 ± 26.18 pg/ml), and from 222.06 pg/ml to 363.23 pg/ml after STZ administration (Mean values 294.08 ± 66.5 pg/ml) (Fig. 1D). Pre-STZ leptin mean values were 7.44 ± 3.9 pg/ml. Post-STZ leptin mean values were 4.9 ± 2.4 pg/ml (Fig. 1E). Mean NEFA levels increased from 0.45 ± 0.16 mEq/L before STZ injection to 0.94 ± 0.5 mEq/L after STZ injection (Fig. 1F). This data shows that diabetes was clearly induced in otherwise normal baboons using pharmaceutical-grade STZ. All animals (N=4) developed fasting post-STZ frank hyperglycemia associated with fasting levels of low plasma insulin, C-peptide and leptin concentrations, and high plasma glucagon and NEFA.
Figure 1.
Circulating levels of glucose, insulin, C-peptide and glucagon during the intravenous glucose tolerance test (IVGTT) before and after STZ administration
Glucose
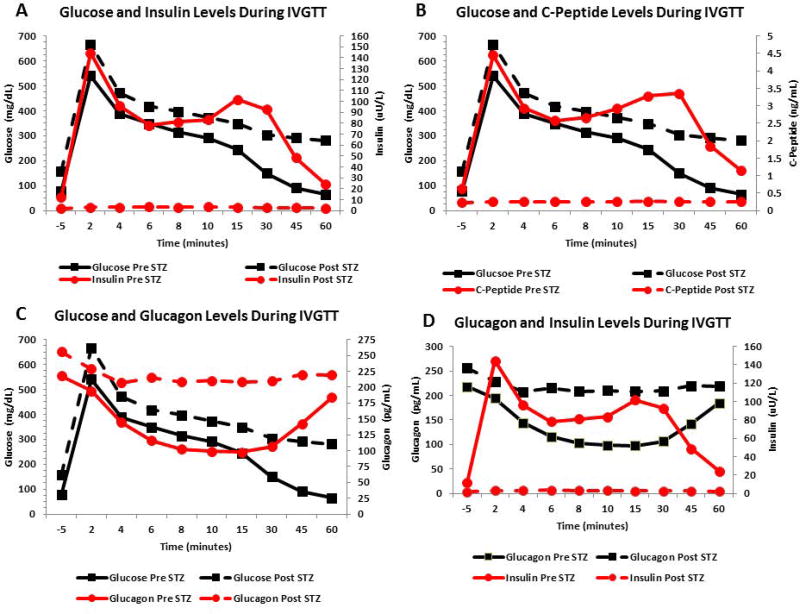
IVGTTs (60 minutes) were performed in all our research animals before and after STZ administration. Baseline pre-STZ blood glucose concentrations peaked within the first two to six minutes to mean values of 415.2 ± 84.6 mg/dl immediately after a 50% dextrose (0.5mg/kg) IV bolus. Mean glucose values at 8 to15 minutes were 283.6 ± 35.3 mg/dl. Mean glucose values at 30 to 45 minutes were 120.2 ± 47.7 mg/dl. Mean glucose levels returned to normal by 60 minutes. (64.5 ± 23.1 mg/dl). In contrast, IVGTTs performed after STZ administration showed post-STZ blood glucose concentrations peaked within the first two to six minutes to mean values of 504.55 ± 127.33 mg/dl. Mean glucose values at 8 to15 minutes were 372.6 ± 67.1 mg/dl. Mean glucose values at 30 to 45 minutes were 297.3 ± 48.4 mg/dl. Mean blood glucose values remained frankly elevated (282 ± 47.8 mg/dl) above values observed at 60 minutes before STZ administration (Fig. 2 A–C).
Figure 2.
Insulin
Pre-STZ mean insulin levels during the first two to six minutes were 102.8 ± 53.4 uU/ml. Mean insulin values at 8 to15 minutes were 88.7 ± 38.8 uU/ml. Mean insulin values at 30 to 45 minutes were 70.5 ± 47.3 uU/ml. Mean insulin levels were 24.2 ± 19.8 uU/ml by 60 minutes. In contrast, post-STZ mean insulin levels were steadily low during the 60 minutes. IVGTT (3.0 ± 1.9 uU/ml) (Fig. 2A).
C-peptide
Pre-STZ mean C-peptide levels during the first two to six minutes were 3.21 ± 1.7 ng/ml. Mean C-peptide values at 8 to 15 minutes were 2.9 ± 1.6 ng/ml. Mean C-peptide values at 30 to 45 minutes were 2.6 ± 1.7 ng/ml. Mean C-peptide levels were 1.14 ± 0.6 ng/ml by 60 minutes. In contrast, post-STZ mean C-peptide levels were steadily low during the 60 minutes. IVGTT (0.26 ± 0.2 ng/ml) (Fig. 2B).
Glucagon
Compared to mean fasting values, IVGTT pre-STZ mean glucagon levels during the first two to six minutes decreased to 147.4 ± 56.9 pg/ml. Mean glucagon values at 8 to15 minutes. also decreased to 99.6 ± 20.2 pg/ml, and continued to be decreased at 30 to 45 minutes. (124.4 ± 50.8 pg/ml). Mean glucagon levels were 184.09 ± 81.9 pg/ml by 60 minutes. In contrast, IVGTTs performed after STZ administration showed increased blood glucagon concentrations within the first two to six minutes (mean values of 215.77 ± 88.5 pg/ml). The glucagon levels remained steadily elevated at 8 to 15 minutes (mean values of 209.06 ± 84.1 pg/ml), and at 30 to 45 minutes (mean values of 214.50 ± 94.3 pg/ml). Mean glucagon levels were 219.18 ± 85.4 pg/ml by 60 minutes post-STZ (Fig. 2C and D).
Triphasic Blood Glucose Response
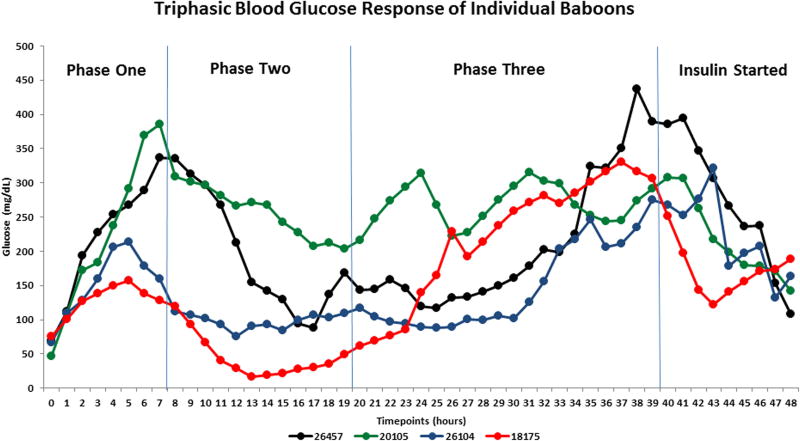
The advantage of the tether system and indwelling surgical catheter implants in all our treated baboons enabled us to plan an accurate strategy to frequently draw blood samples to carefully monitor the metabolic environment before and after pharmaceutical-grade STZ administration. All baboons participating in our research protocol that were treated with pharmaceutical-grade STZ (Zanosar) developed a triphasic blood glucose response to STZ administration (Fig. 3). During the initial phase there was a marked glucose increase reaching a mean of 280 mg/dl approximately six to eight hours after STZ IV administration. This peak was followed by a steady glucose decrease to a mean of 100 mg/dl between 16 and 18 hours after STZ IV injection. This drop was accompanied by a second and sustained mean glucose increase of 325 mg/dl approximately 38 to 40 hours after STZ IV administration, establishing a diagnosis of a frank hyperglycemic state. Continuous dextrose administration started with a mean rate of infusion of 1 mg/kg/min. The rate increased by the end of phase one (7.5 hours after STZ IV injection) to 3 mg/kg/min. The infusion rate was steadily increased from 5 to 8.5 mg/kg/min between 12 and 23 hours after STZ injection to maintain glucose levels above 75 mg/dl. Intermittent 50% dextrose boluses, if clinically needed (1ml/kg IV), were administered between 14 and 18 hours to avoid hypoglycemia. As the glucose levels increased as expected during phase three (20 to 40 hours after STZ injection), the clinical need to infuse dextrose became unnecessary. A continuous IV insulin infusion (0.1–0.5 U/kg/h) was then indicated after 38 to 40 hours of IV STZ administration (Fig. 3). A triphasic blood glucose response of individual baboons during and after successful induction of hyperglycemia after the administration of STZ was observed in each case (Fig 4).
Figure 4.
Morphology of pancreatic islets using triple immunofluorescent staining
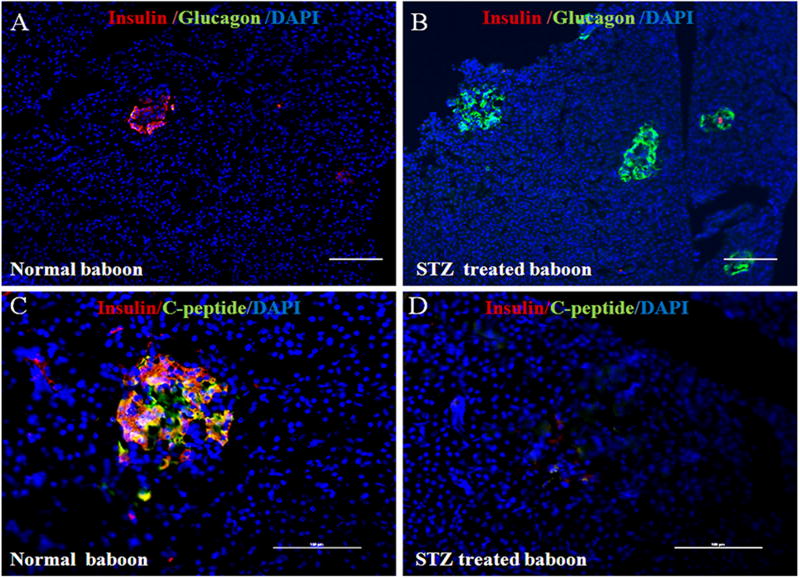
Panels A and B of Figure 5 show staining with anti-insulin (red), anti-glucagon (green), and DAPI (blue) for the cell nucleus. Panels C and D show triple staining for insulin (red), C-peptide (green), and DAPI (blue). One otherwise healthy baboon (ID No. 11692) scheduled for euthanasia that matched the age and weight criteria for the study population (N=4) was opportunistically identified to harvest pancreatic tissue at the time of necropsy and be considered as the normal control for pancreatic staining. In this normal baboon, the β-cells dominate islet structure (Fig. 5A). Interestingly, after administering pharmaceutical-grade STZ to baboon ID No. 18175, all β-cells were initially destroyed, but islet-like structures containing large clusters of alpha cells are abundantly seen (Fig. 5B). The confocal images of triple staining for insulin and C-peptide shows normal β-cell islet architecture for baboon No. 11692 with the presence of insulin staining (Fig. 5C). In contrast, absence of insulin and C-peptide staining is seen for baboon No. 18175 after STZ administration (5D).
Figure 5.
Adverse effects after STZ administration
Kidney and liver toxicity are complications in STZ-injected animals. As shown in Tables 1 and 2, these complications were absent (non-significant) in our treated baboons.
Table 1.
| Phenotypes | Pre-STZ | Post-STZ | P Value |
|---|---|---|---|
| Mean ± SD | |||
| WBC (thous/mm3) | 7.77 ± 2.41 | 11.54 ± 3.48 | 0.114 |
| Hematocrit (%) | 40.56 ± 3.41 | 44.56 ± 17.14 | 0.611 |
| Creatinine (mg/dL) | 1.16 ± 0.13 | 1.62 ± 0.81 | 0.259 |
| BUN (mg/dL) | 9.79 ± 2.25 | 11.80 ± 4.14 | 0.434 |
| ALT/SGPT (U/L) | 36.12 ± 4.88 | 60.00 ± 24.44 | 0.116 |
| AST/SGOT (U/L) | 32.56 ± 4.50 | 43.40 ± 23.19 | 0.408 |
| ALK PHOS (U/L) | 128.97 ± 30.64 | 265.40 ± 180.27 | 0.159 |
| Carbon Dioxide (mEq/L) | 31.08 ± 1.76 | 26.70 ± 7.08 | 0.247 |
| Anion Gap (mEq/L) | 10.19 ± 2.54 | 10.08 ± 1.09 | 0.940 |
p<0.05
n=4 baboons
Table 2.
| Kidney Tests | Electrolytes | ||||||
|---|---|---|---|---|---|---|---|
| Normal Values | Creatinine (mg/dL) 0.8–1.5 |
BUN (mg/dL) 8–22 |
Sodium (mEq/L) 135–148 |
Potassium (mEq/L) 3.0–4.1 |
Chloride (mEq/L) 98–111 |
CarbonDioxide (mEq/L) 24–34 |
Anion Gap 7.5–16.8 |
| Pre STZ | |||||||
| 26457 | 1.1 | 11 | 143 | 4.1 | 102 | 33 | 11.6 |
| 20105 | 1.4 | 8 | 142 | 3.7 | 106 | 31 | 8.9 |
| 26104 | 1.2 | 12 | 143 | 4.0 | 105 | 32 | 10.1 |
| 18175 | 1.1 | 11 | 146 | 4.0 | 107 | 32 | 11.0 |
| PostSTZ | |||||||
| 26457 | 2.8 | 17 | 143 | 3.7 | 116 | 19 | 11.5 |
| 20105 | 2.0 | 21 | 146 | 3.3 | 122 | 17 | 10.3 |
| 26104 | 1.3 | 12 | 140 | 3.6 | 103 | 29 | 11.6 |
| 18175 | 1.1 | 10 | 141 | 3.7 | 106 | 29 | 9.4 |
Discussion
In this study, our results show that we were able to successfully induce frank hyperglycemia in four conscious tethered-baboons with minimal morbidity by IV administering a single dose of pharmaceutical-grade STZ (Zanosar). Mean values of biochemistry tests and laboratory results in individual STZ-injected animals after STZ administration showed minimal (non-significant) changes in kidney (creatinine, BUN), liver profiles (ALT, AST), and electrolytes (Table 1 and 2). We need to begin by clarifying that this was a short-term study in which the safety and efficacy effects of the alkylating cytotoxic agent STZ were analyzed one week after this drug was administered to our study animals. Our four baboons were part of a research project proposing a novel approach in which non-viral gene therapy was delivered to pancreatic islets using ultrasound-targeted microbubble destruction (UTMD) [43]. Our results from the gene therapy protocol have already been published [11]. Here, we will discuss the methods and results and analyze the strengths and limitations of this study in utilizing a tether system and indwelling catheter implants to induce a diabetic phenotype after STZ administration.
All animals were carefully chosen based on maintenance of food intake, general attitude towards study technical staff (submissive, aggressive, etc.), general attitude towards study veterinarian, and comfort level with presence of technical staff in bay. Response to sham-tethering (attitude, time at the front of cage, etc.), tether jacket fit, and normal feeding habits were carefully monitored and documented. Our protocol followed expert behavioral training techniques already established [23].
A major advantage of the tether and indwelling catheter system is that the presence of IV catheter implants allows for a continuous flow of fluids. IV hydration is a key factor in preventing not only the most common and devastating adverse events secondary to the alkylating cytotoxicity of STZ to liver and kidney, but, most importantly, in preventing dangerous hypoglycemia due to very large amounts of insulin released from the destruction of β-cells during STZ administration [58].
IV hydration protocols to prevent the toxic profile of IV STZ administration in NHP have been poorly reported in the literature. One comprehensive paper reported that 10 cynomolgus monkeys with permanent, surgically-implanted, indwelling IV lines were prehydrated with 10ml/kg/h of 0.9% NaCl IV for 8 to 10 hours before STZ administration, increasing the rate of fluid infusion to 15 ml/kg/h for two more hours after STZ injection as a preventive measure to reduce kidney toxicity. This study claimed to be the first to use pharmaceutical-grade STZ (Zanosar) 150 mg/kg due to perhaps greater purity and less variability than traditional-grade STZ routinely used to induce β-cell destruction. The authors concluded that compared to traditional-grade IV STZ administration, their combined IV fluid therapy and pharmaceutical-grade STZ (Zanosar) were key factors in achieving diabetes induction safely and without adverse effects. They did not report any symptoms of severe life-threatening adverse events such as pulmonary edema, rapid onset of advanced nephrotoxicity, or metabolic acidosis. They also reported a triphasic blood glucose response, detecting significantly low blood glucose levels during the second phase of the triphasic response, corresponding to a massive insulin release from dying β-cells [52, 58].
Our comprehensive therapeutic strategy included an intensive IV hydration protocol 24 hours before and 48 hours after STZ injection. STZ is a chemotherapeutic cytotoxic drug associated with devastating side effects, namely liver toxicity, pulmonary edema, organ failure and, most prominently, nephrotoxicity and life-threatening metabolic ketoacidosis [54]. It has been documented that the kidney is the first target post-STZ administration [53]. STZ is rapidly cleared by the kidneys within 48 hours after an acute administration. The drug is primarily excreted in urine [35]; therefore, using intensive IV fluid therapy to induce high urinary flow rates protects the kidney from injury during treatment with this chemotherapeutic agent [49]. Our intensive IV hydration therapy is based on recommended protocols used in humans with the objective of preventing renal toxicity induced by IV STZ injection. Current hydration protocols recommend 2.5 to 3.5 liters of fluid per meter2 per 24 hours, beginning 12 hours before the administration of chemotherapeutic agents associated with kidney toxicity and continuing for 24 to 48 hours. The main goal is to measure fluid intake vs. output to avoid a fluid negative balance and prevent loss of renal function by judicious intensive IV hydration therapy [69].
Recent recommendations [22, 23] to avoid adverse events associated with the use of chemotherapeutic STZ to induce diabetes in NHP have been published. The authors based their recommendations on long-term experience with a cohort of 78 cynomolgus and rhesus macaques. They established elegant up-to-date, state-of-the-art, pretreatement regimens, laboratory evaluations post-STZ administration, flow-charts to assess triggering factors for STZ adverse events, and validated critical clinical tools to detect, prevent and manage the most serious side effects after STZ administration. Their protocol was not based on the presence of permanent surgical indwelling catheter implants and preventive intensive IV hydration. Instead, their methodology was based on training NHP to cooperate with hand feeding and drinking, shifting and limb presentation. They reported that 8% of STZ-treated animals from that cohort manifested severe life-threatening adverse effects [22]. The outstanding measures they established did not fully eliminate the expected major life-threatening complications of kidney toxicity and metabolic acidosis. Therefore, the choice of utilizing permanent surgical indwelling catheter implants and an aggressive preventive and immediate post-STZ IV hydration protocol like the one presented in this paper should strongly be considered when administering IV STZ to NHP, given the recommended preventive intensive IV fluid therapy protocols in humans before the administration of chemotherapeutic agents associated with kidney toxicity [49, 69].
The surgical tether catheter implants offer the crucial advantage of a continuous measurement of circulating blood glucose levels before and after IV STZ injection. We were able to fully characterize the triphasic response in blood glucose after STZ administration. Such triphasic response post-STZ injection [33] has been described in NHP and mice [59, 68]. As shown in Figure 3, during phase one (7–8 hours after IV STZ administration) blood glucose rises. Pancreatic β-cell damage is initiated by STZ, leading to transient hyperglycemia due to acute inflammation, inability of the β-cells to oxidize glucose and sudden and continuous breakdown of liver and muscle glycogen. Phase two (between 9–20 hours post-STZ IV injection) is a critical physiological stage with adverse effects which may be severe enough to lead to death. There is further destruction and disruption of β-cells by STZ resulting in a massive release of insulin, pronounced muscle insulin-mediated glucose uptake, and a steady fall in circulating blood glucose levels, leading to dangerous hypoglycemia. Subsequently, phase three is observed in our protocol between 20 and 40 hours and onwards after STZ injection, and is characterized by the permanent rise in glucose levels, a persistent and irreversible hyperglycemic state and a total degranulation with structural alterations in pancreatic β-cells [25, 40].
The dangerous hypoglycemic phase after IV STZ administration has been discussed in depth only rarely in peer-reviewed papers reporting induction of diabetes in NHP with this chemotherapeutic drug [22, 23, 38, 39, 52]. It has been documented that metabolic acidosis occurred mainly during the second phase when blood glucose fell to low levels after IV STZ injection [52]. In the past, high-dose STZ administered to adult rhesus macaques has resulted in “unacceptable mortality rates attributed to anuria and hypoglycemic crisis” [60]. Hypoglycemia is defined as blood glucose <70 mg/dl [16, 57], whereas severe hypoglycemia is defined as blood glucose < 40 mg/dl [56]. During hypoglycemia, lack of glucose supply to neurons can lead to confusion, brain damage [1], seizures, and death [2, 64]. Six to ten percent of deaths in young people with T1DM are directly attributable to hypoglycemia [18]. It has been hypothesized that abrupt cardiac arrhythmias contribute to severe hypoglycemia-induced sudden death. Sudden deaths caused by insulin-induced severe hypoglycemia are mediated by lethal cardiac arrhythmias triggered by brain neuroglycopenia and a marked sympathoadrenal response [50]. Moreover, glucose counter regulation is a function of prior hypoglycemic episodes which sets up a vicious cycle of one hypoglycemic episode increasing the likelihood of subsequent hypoglycemia and progressive decay in the counterregulatory response. In a normal physiological environment, hypoglycemia immediately elicits a counter regulatory response [44]. However, recurrent hypoglycemia blunts the activities of counter regulatory responses. It has been described that healthy individuals who have two hypoglycemic episodes in a day show inhibited glucose counter regulatory response. The defective counter regulatory response is also affected by the reduced autonomic response which further decreases hepatic glucose production [28].
On our protocol, baboon ID No. 18175 experienced an unexpected case of severe hypoglycemia during the second phase after IV STZ administration lasting nine hours (between 10 and 19 hours post-STZ injection) (Figure 4, red solid line). The presence of the permanent indwelling IV catheter implants and the tether system allowed us to measure moment-by-moment circulating arterial glucose levels and permitted us to administer intermittent 50% dextrose bolus (1ml/kg IV) if clinically needed, while continuously infusing dextrose 10%-30% to keep the glucose levels within normal limits.
It is clear that dangerous hypoglycemia is the hallmark of the second phase after STZ injection. This is probably because β-cell destruction is so rapid that insulin in the destroyed β-cells may enter the blood stream within a short period of time. A similar phenomenon is frequently observed just before the onset of fulminant T1DM [32]. In this deadly pathology, pancreatic β-cells are completely destroyed in the same way as is evident in rodents and NHP when IV STZ is injected into their bloodstreams to provide models of T1DM [65]. In fulminant T1DM the process of β-cell destruction and progression of hyperglycemia and ketoacidosis are extremely rapid. The duration of the disease from a normal β-cell mass and normoglycemia to almost total destruction of β-cells and ketoacidosis rarely exceeds one week. Without appropriate therapy, the death of the patient is inevitable [31]. It was previously reported that the rapid onset of β-cell destruction and ensuing diabetes induced by IV STZ injection mimics that of fulminant T1DM [30].
The results of our study clearly document an acute onset of diabetes and β-cell destruction with similar clinical and pathological findings to fulminant T1DM. Our advantage is that we know STZ administration will cause the pathological scenario. In fulminant T1DM the etiopathogenic factors causing the deadly disease are unknown. Therefore, if we already know what to expect after STZ IV administration, all preventive and intensive clinical procedures recommended in this paper should be implemented.
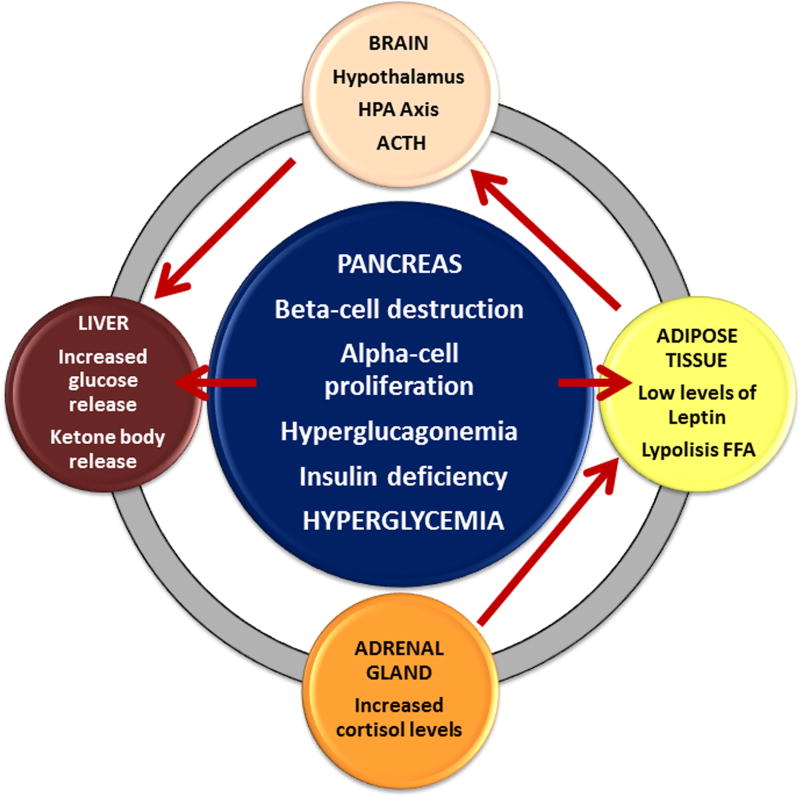
Recent evidence has shown that glucagon, under the control of insulin at paracrine levels, is a key regulator of glucose metabolism, and that hyperglucagonemia (glucagon excess) and unregulated alpha cell dysfunction in T1DM, rather than insulin deficiency, are profoundly involved in the pathophysiology of T1DM. In normal physiologic conditions, glucose-responsive β-cells normally regulate juxtaposed alpha-cells. In the T1DM state, without intraislet insulin, unregulated alpha-cells hypersecrete glucagon, which directly causes hyperglycemia [24]. A series of studies in rodent models of T1DM have confirmed that increased plasma glucagon concentration is also an important mediator of metabolic dysregulation. Glucagon stimulates hepatic glucose production by activating the enzymatic pathways involved in both glycogenolysis and gluconeogenesis. In these rodents, β-cell destruction by STZ administration leads to hypoinsulinemia and hyperglycemia as expected. Interestingly, plasma leptin concentrations are decreased and plasma glucagon concentrations are increased in these animals. The combined effects of hypoinsulinemia, hypoleptinemia, and hyperglucagonemia lead to increased release of NEFA from adipose tissue [46] (Fig. 6).
Figure 6.
A significant finding in all tethered baboons treated with IV STZ was that they developed hyperglucagonemia. In figure 2D, the black solid line shows an IVGTT curve with expected mean glucagon levels returning to normal after a 60 minute glucose challenge, also showing a mean highly abnormal glucagon curve after Zanosar STZ (black dashed line). These findings are consistent with recently published data indicating that the absence of leptin and hyperglucagonemia contributes to the development of T1DM [46]. We found the same biochemistry pattern in our baboons treated with IV STZ. They developed frank hyperglycemia associated with low levels of plasma insulin, C-peptide and leptin concentrations, and high plasma glucagon and NEFA concentrations (Fig. 1A–F). Triple immunofluorescent staining of pancreatic tissue analyzed before and after STZ injection corroborated these findings (Fig. 5). To our knowledge, the present study is the first to perform a comprehensive analysis of the key hormones and metabolites influencing the physiologic control of the insulin-glucose axis and the alpha and β-cell biology by dynamically measuring fasting glucose, insulin, C-peptide, glucagon, leptin and NEFA circulating levels pre and post-STZ administration in conscious tethered baboons.
Our results and findings from recently published, new, cutting-edge research indicating that the absence of leptin and hyperglucagonemia triggers T1DM [46], may have some implications for the diagnostic criteria of the induction of the diabetic phenotype [14] after STZ administration. It seems that the insulino-glucocentric diagnostic view exclusively centered on measurements of glucose, insulin, and C-peptide should be reviewed to consider the diagnosis of the STZ-induced diabetic phenotype with a broader view including a leptino-glucagonocentric makeover according to the new pathophysiology of T1DM [46, 61].
In summary, our comprehensive therapeutic strategy (tether system and permanent indwelling catheter implants during the length of the study, aggressive hydration protocol, management for discomfort and anxiety, analgesia for pain relief, moment-by-moment monitoring of glucose levels post-STZ administration, continuous intravenous insulin therapy, total parenteral nutrition if needed, and early detection of adverse effects with a comprehensive laboratory workup to measure blood chemistries and metabolic factors) was successful to completely induce diabetes with IV pharmaceutical-grade STZ safely and without adverse events in conscious tethered baboons. We were able to characterize a triphasic blood glucose response after IV STZ injection indicating a complete and successful induction of the diabetic phenotype.
References
- 1.Abdelmalik PA, Shannon P, Yiu A, Liang P, AdamchikY Y, Weissapapir M, Samoilova M, Burnham WM, Carlen PL. Hypoglycemic seizures during transient hypoglycemia exacerbate hippocampal dysfunction. Neurobiol Dis. 2007;26:646–660. doi: 10.1016/j.nbd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Auer RN, Olsson Y, Siesjö BK. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984;33:1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- 3.Bastarrachea RA, Veron SM, Vaidyanathan V, Garcia-Forey M, Voruganti VS, Higgins PB, Parks EJ. Protocol for the measurement of fatty acid and glycerol turnover in vivo in baboons. J Lipid Res. 2011;52:1272–1280. doi: 10.1194/jlr.D012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Wier GC. New sources of pancreatic B-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 5.Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- 6.Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001;81(3):527–42. doi: 10.1016/s0039-6109(05)70141-9. [DOI] [PubMed] [Google Scholar]
- 7.Brown TB, Lovato LM, Parker D. Procedural sedation in the acute care setting. Am Fam Physician. 2005;71(1):85–90. [PubMed] [Google Scholar]
- 8.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of B-cells in normal physiology, in disease, and for therapy. Nat Clin Pract Endocrinol & Metab. 2007;3:758–68. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 9.Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Gen. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 10.Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkmol A, Tantiwong P, Voruganti VS, Musi N, Comuzzie AG, DeFronzo RA, Folli F. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Bastarrachea RA, Roberts BJ, Voruganti VS, Frost PA, Nava-Gonzalez EJ, Arriaga-Cazares HE, Chen J, Huang P, DeFronzo RA, Comuzzie AG, Grayburn PA. Successful beta cells islet regeneration in streptozotocin-induced diabetic baboons using ultrasound-targeted microbubble gene therapy with cyclinD2/CDK4/GLP1. Cell Cycle. 2014;13(7):1145–51. doi: 10.4161/cc.27997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho AMJ, Carey KD. A social tethering system for nonhuman primates used in laboratory research. Lab Anim Sci. 1990;40:388–394. [PubMed] [Google Scholar]
- 13.Comuzzie AG, Cole SA, Martin L, Carey KE, MAhaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DK, Casu A. Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes – chapter 4: pre-clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation. 2009;16:229–38. doi: 10.1111/j.1399-3089.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 15.Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, Nathanielsz PW, Nicolella DP, Shade RE, Voruganti S, VandeBerg JL. Baboons as a model to study genetics and epigenetics of human disease. ILAR. 2013;54:106–121. doi: 10.1093/ilar/ilt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Endocrine Society: Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–28. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 17.Ericzon BG, Wijnen RM, Kubota K, vd Bogaard A, Kootstra G. Diabetes induction and pancreatic transplantation in the cynomolgus monkey: methodological considerations. Transpl Int. 1991;4:103–9. doi: 10.1007/BF00336407. [DOI] [PubMed] [Google Scholar]
- 18.Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C, McKinney PA. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care. 2008;31:922–926. doi: 10.2337/dc07-2029. [DOI] [PubMed] [Google Scholar]
- 19.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–72. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 20.Fisher SJ, Shi ZQ, Lickley HL, Efendic S, Vranic M, Giacca A. Low-dose IGF-I has no selective advantage over insulin in regulating glucose metabolism in hyperglycemic depancreatized dogs. J Endocrinol. 2001;168:49–58. doi: 10.1677/joe.0.1680049. [DOI] [PubMed] [Google Scholar]
- 21.Graham ML, Mutch LA, Kittredge JA, Rieke EF, Robinson NA, Zolondek EK, Faig AW, Dufour TA, Munson JW, Schuurman HJ. Management of adverse side-effects after chemotherapy in macaques as exemplified by streptozotocin: case studies and recommendations. Lab Anim. 2012;46(3):178–192. doi: 10.1258/la.2012.011077. [DOI] [PubMed] [Google Scholar]
- 22.Graham ML, Mutch LA, Rieke EF, Kittredge JA, DuFour TA, Munson JW, Zolondek EK, Hering BJ, Schuurman HJ. Refining the high-dose streptozotocin-induced diabetic nonhuman primate model: an evaluation of risk factors and outcomes. Exp Biol Med. 2012;236:1218–1230. doi: 10.1258/ebm.2011.011064. [DOI] [PubMed] [Google Scholar]
- 23.Graham ML, Rieke EF, Mutch LA, Zolondek EK, Faig AW, Dufour TA, Munson JW, Kittredge JA, Schuurman HJ. Successful implementation of cooperative handling eliminates the need for restraint in a complex nonhuman primate disease model. J Med Primatol. 2012;41(2):89–106. doi: 10.1111/j.1600-0684.2011.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RA, Dixit VP. Dose dependent alteration in lipid and carbohydrate metabolites in streptozotocin induced diabetic rats. Endocrinology. 1982;80(3):332–340. [PubMed] [Google Scholar]
- 26.Hau J, Schapiro SJ. Non-human primates in biomedical research. Scand J Lab Anim Sci. 2006;33(1):9–12. [Google Scholar]
- 27.He S, Chen Y, Wei L, Jin X, Zeng L, Ren Y, Zhang J, Wang L, Li H, Lu Y, Cheng J. Treatment and risk factor analysis of hypoglycemia in diabetic rhesus monkeys. Exp Biol Med. 2011;236:212–218. doi: 10.1258/ebm.2010.010208. [DOI] [PubMed] [Google Scholar]
- 28.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 29.Herbai G, Lundin A. Treatment of malignant metastatic pancreatic insulinoma with streptozotocin: review of 21 cases described in detail in the literature and report of complete remission of a new case. Acta Medica Scandinavica. 1976;200:447–452. [PubMed] [Google Scholar]
- 30.Imagawa A, Hanafusa T. Pathogenesis of fulminant type 1 diabetes. Rev Diabet Stud. 2006;3(4):169–77. doi: 10.1900/RDS.2006.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, Kawasaki E, Kawabata Y, Kobayashi T, Shimada A, Shimizu I, Takahashi K, Nagata M, Makino H, Maruyama T. Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM study group. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 33.Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 34.Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 35.Karunanayake EH, Hearse DJ, Mellow G. The synthesis of [14C] Streptozotocin and its distribution and excretion in the rat. Biochem J. 1974;142:673–683. doi: 10.1042/bj1420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King, Aileen JF. The use of animal models in diabetes research. Br J Pharmacol. 2012;166.3:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 38.Koulmanda M, Qipo A, Chebrolu S, O’Neil J, Auchincloss H, Smith RN. The effect of low versus high dose of streptozotocin in cynomolgus monkeys (Macaca fascilularis) Am J Transplant. 2003;3(3):267–272. doi: 10.1034/j.1600-6143.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 39.Koulmanda M. Islet transplant model in nonhuman primates: use of streptozotocin. Transpl Rev. 2006;20(3):126–130. [Google Scholar]
- 40.Lee JH, Yang SH, Oh JM, Lee MG. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol. 2010;62:1–23. doi: 10.1211/jpp.62.01.0001. [DOI] [PubMed] [Google Scholar]
- 41.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 42.Maheandiran M, Mylvaganam S, Wu C, El-Hayek Y, Sugumar S, Hazrati L, del Campo M, Giacca A, Zhang L, Carlen PL. Severe hypoglycemia in a juvenile diabetic rat model: presence and severity of seizures are associated with mortality. PLoS One. 2013;8(12):e83168. doi: 10.1371/journal.pone.0083168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv. 2008;5:1121–1138. doi: 10.1517/17425247.5.10.1121. [DOI] [PubMed] [Google Scholar]
- 44.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59(10):2333–2339. doi: 10.2337/db10-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier JJ. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51:703–713. doi: 10.1007/s00125-008-0936-9. [DOI] [PubMed] [Google Scholar]
- 46.Mittendorfer B, Klein S. Absence of leptin triggers type 1 diabetes. Nat Med. 2014;20(7):705–706. doi: 10.1038/nm.3629. [DOI] [PubMed] [Google Scholar]
- 47.Morel P, Kaufmann DB, Matas AJ, Tzardis P, Field MJ, Lloveras JK, Sutherland DE. Total pancreatectomy in the pig for islet transplantation. Technical alternatives. Transplantation. 1991;52:11–15. doi: 10.1097/00007890-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 48.National Research Council: Chapter 11. Nutrient Requirements of Nonhuman Primates (2nd revised ed.) Washington, DC: National Academies Press; 2003. pp. 191–194. [Google Scholar]
- 49.Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol. 2010;30:570–581. doi: 10.1016/j.semnephrol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes. 2013;62(10):3570–3581. doi: 10.2337/db13-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59:1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ezzelarab M, Busch J, hara H, Trucco M, Cooper DK. Induction of diabetes in cynomolgus monkeys with high-dose streptozotocin: adverse effects and early responses. Pancreas. 2006;33:287–92. doi: 10.1097/01.mpa.0000235307.04110.a2. [DOI] [PubMed] [Google Scholar]
- 53.Safirstein RL. Renal diseases induced by antineoplastic agents. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. 8. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. pp. 1069–1073. [Google Scholar]
- 54.Schacht RG, Feiner HD, Gallo GR, Lieberman A, Baldwin DS. Nephrotoxicity of nitrosoureas. Cancer. 1981;48:1328–1334. doi: 10.1002/1097-0142(19810915)48:6<1328::aid-cncr2820480613>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 55.Schein PS, O’Connell MJ, Blom J, Hubbard S, Magrath IT, Bergevin P, Wiernik PH, Ziegler JL, DeVita VT. Clinical antitumor activity and toxicity of streptozotocin (NSC-85998) Cancer. 1974;34:993–1000. doi: 10.1002/1097-0142(197410)34:4<993::aid-cncr2820340404>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz AV, Vittinghoff E, Sellmeyer DE, Feingold KR, de Rekeneire N, Strotmeyer ES, Shorr RI, Vinik AI, Odden MC, Park SW, Faulkner KA, Harris TB. Health, Aging, and Body Composition Study: Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853–861. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 58.Takimoto G, Jones C, Lands W, Bauman A, Jeffrey J, Jonasson O. Biochemical changes in rhesus monkey during the first days after streptozotocin administration are indicative of selective beta cell destruction. Metabolism. 1988;37(4):364–370. doi: 10.1016/0026-0495(88)90137-0. [DOI] [PubMed] [Google Scholar]
- 59.Tal MG, Hirshberg B, Neeman Z, Bunnell D, Soleimanpour S, Bacher J, Patterson N, Chang R, Harlan DM. Induction of diabetes in nonhuman primates by means of temporary arterial embolization and selective arterial injection of streptozotocin. Radiology. 2004;230(1):163–168. doi: 10.1148/radiol.2301021413. [DOI] [PubMed] [Google Scholar]
- 60.Theriault BR, Thistlethwaite JR, Jr, Levisetti MG, Wardrip CL, Szot G, Bruce DS, Rilo H, Li X, Gray GS, Bluestone JA, Padrid PA. Induction, maintenance, and reversal of streptozotocin-induced insulin-dependent diabetes mellitus in the juvenile cynomolgus monkey (Macaca fascilularis) Transplantation. 1999;68(3):331–337. doi: 10.1097/00007890-199908150-00003. [DOI] [PubMed] [Google Scholar]
- 61.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud. 2011;8(1):51–67. doi: 10.1900/RDS.2011.8.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.VandeBerg JL. Introduction: The baboon in biomedical research. New York: Springer; 2009. pp. xvii–xxiii. 2009. [Google Scholar]
- 64.Velísek L, Velísková J, Chudomel O, Poon KL, Robeson K, Marshall B, Sharma A, Moshe SL. Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures. J Neurosci. 2008;28:9349–9362. doi: 10.1523/JNEUROSCI.3195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waguri M, Yamamoto K, Miyagawa JI, Tochino Y, Yamamori K, Kajimoto Y, Nakajima H, Watada H, Yoshiuchi I, Itoh N, Imagawa A, Namba M, Kuwajima M, Yamasaki Y, Hanafusa T, Matsuzawa Y. Demonstration of two different processes of beta-cell regeneration in a new diabetic mouse model induced by selective perfusion of alloxan. Diabetes. 1997;46:1281–1290. doi: 10.2337/diab.46.8.1281. [DOI] [PubMed] [Google Scholar]
- 66.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 67.Wei L, Lu Y, He S, Jin X, Zeng L, Zhang S, Chen Y, Tian B, Mai G, Yang G, Zhang J, Wang L, Li H, Markmann JF, Cheng J, Deng S. Induction of diabetes with signs of autoimmunity in primates by the injection of multiple-low-dose streptozotocin. Biochem Biophys Res Commun. 2011;412:373–378. doi: 10.1016/j.bbrc.2011.07.105. [DOI] [PubMed] [Google Scholar]
- 68.West E, Simon OR, Morrison EY. Streptozotocin alters pancreatic beta-cell responsiveness to glucose within six hours of injection into rats. West Indian Med J. 1996;45(2):60–62. [PubMed] [Google Scholar]
- 69.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 70.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 16997;226:248–257. doi: 10.1097/00000658-199709000-00004. Discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]