Abstract
Background
Recurrent gastrointestinal bleeding is one of the most significant adverse events in patients with left ventricular assist devices (LVAD).
Methods
We enrolled LVAD patients who had received an intramuscular injection of 20 mg of octreotide LR every 4 weeks as secondary prevention for recurrent gastrointestinal bleeding despite conventional medical therapies and repeated transfusions. The frequency of gastrointestinal bleeding and other associated clinical outcomes before and during octreotide therapy were compared.
Results
Thirty LVAD patients (66.4 ± 8.8 years old and 16 male [53%]) received octreotide therapy for 498.8 ± 356.0 days without any octreotide-associated adverse events. The frequency of gastrointestinal bleeding was decreased significantly during octreotide therapy (from 3.4 ± 3.1 to 0.7 ± 1.3 events/year, p <0.001), accompanied by significant reductions in red blood cell and flesh frozen plasma transfusions, days in hospital, and need for endoscopic procedures (p <0.05 for all).
Conclusions
Octreotide therapy reduced the frequency of recurrent gastrointestinal bleeding and may be considered for secondary prevention.
Keywords: LVAD, pulsatility, somatostatin, HeartMate
Graphical abstract
INTRODUCTION
Left ventricular assist device (LVAD) therapy has improved long-term survival rates in patients with stage D heart failure,1, 2 but gastrointestinal (GI) bleeding remains a significant adverse effect and a major cause of readmissions following LVAD implantation.3, 4 GI bleeding occurs more frequently with continuous-flow devices than the previous pulsatile devices.5–7 The site of bleeding varies among each patient and occurs similarly from both upper and lower GI sources.8
Several factors are believed to contribute to GI bleeding. Originally, acquired von Willebrand disease, characterized by low levels of high molecular weight von Willebrand factor7, was thought to be induced by continuous-flow LVADs and linked to the high GI bleeding rate. However, our understanding today has shifted to the concept that GI arteriovenous malformations (AVMs)3 render patients susceptible to bleeding, particularly in the context of required continuous antiplatelet and anticoagulant therapy.9 We demonstrated recently that thrombin-induced angiopoietin-2 expression in LVAD patients leads to increased angiogenesis in vitro which may contribute to AVM formation in vivo.10
Conservative therapies for GI bleeding include temporary discontinuation of anticoagulant and antiplatelet therapy, intravenous administration of proton pump inhibitors, invasive endoscopic cauterization3 and, only in extreme cases, administration of vitamin K and/or flesh frozen plasma (FFP). These latter strategies should be used cautiously to prevent acute pump thrombosis. GI bleeding refractory to the aforementioned therapies is often encountered in the real-world setting.4
In non-LVAD patients, octreotide, a somatostatin analog, has been used to manage recurrent GI bleeding particularly due to vascular malformations.11 Favorable effects of octreotide have been reported in a small number of case reports and in a phase I study in LVAD patients.12–16 However, these previous studies were underpowered to statistically assess the efficacy of octreotide. In this study, we describe the clinical effects of octreotide therapy in a moderate-sized cohort of continuous-flow LVAD patients with refractory GI bleeding.
METHODS
Patient selection
Patients with continuous-flow LVADs who were hospitalized for treatment of GI bleeding refractory to conventional therapies were enrolled in the octreotide clinic. GI bleeding was defined per INTERMACS as any clinically suspected or documented bleeding from the GI tract as indicated by a new drop in hemoglobin and/or the appearance of melena, hematochezia, hematemesis, or guaiac-positive stool. Validity of the definition of GI bleeding was confirmed by two independent researchers for all events.
All patients received standard guideline-directed medical therapy for LVADs including antiplatelet therapy, proton pump inhibitor, and warfarin with dosing to achieve an international normalized ratio (INR) between 2.0 and 3.0 for HVAD LVAD and between 2.0–2.5 for HeartMate II LVAD, unless recurrent GI bleeding occurred.17 Patients were followed until death, transplant or LVAD explant for any other reason.
Management of GI bleeding
When patients were admitted to the hospital due to GI bleeding, anticoagulants and antiplatelet agents were discontinued. Transfusions of red blood cells (RBC) were performed to a minimum target hemoglobin level of 7.0 g/dL or 8.0 g/dL per the physician’s discretion. Vitamin K and/or FFP were considered when continued bleeding was present in the context of an elevated INR. All patients received proton pump inhibitor therapy by intravenous infusion. Invasive upper and/or lower endoscopy was performed in most cases, along with capsule endoscopy, push eneteroscopy and mesenteric angiography when needed.
After active GI bleeding resolved, anticoagulation was reintroduced using the previous INR target, which is device-dependent. Antiplatelet therapy was typically held or reintroduced at a reduced dose. With repeat bleeding, the INR target could be reduced or warfarin could be discontinued at the discretion of each patient’s physician. Octreotide therapy was considered when GI bleeding was refractory to these modifications. Patients received an intramuscular injection of 20 mg of octreotide LR every four weeks for secondary prevention of GI bleeding after insurance approval. Medical costs were covered by insurance and all octreotide therapy was administered in the outpatient clinic. During octreotide therapy, antiplatelet and anticoagulation therapies were managed similar to the pre-octreotide period, with individual variation based on patient risk.
Variables evaluated
All variables including baseline characteristics were obtained from the electronic medical record. The main endpoint of this study was the number of GI bleeding events encountered during the study period. Also, we recorded the type and number of infused blood products and the number of GI procedures during the overall observational period, including both hospitalizations and the ambulatory setting.
The percent of days on LVAD support with any aspirin administration and the percent of days with INR >2.0 were calculated during the pre- and post-octreotide periods in each patient. The aspirin dose, LVAD speed, and hemoglobin levels were averaged during the pre- and post-octreotide periods in each patient.
Statistical analyses
Statistical analysis was performed using SPSS Statistics 22 (SPSS Inc, Chicago, IL, USA). Two-tailed p-value under 0.05 was considered as statistical significant. Data were expressed as mean ± SD unless otherwise indicated. Continuous parameters obtained before and during octreotide therapy were compared by using the Wilcoxon signed-rank test. Categorical parameters obtrained before and during octreotide therapy were compared by using McNemar’s test. Comparisons of background characteristics stratified by the occurrence of GI bleeding were performed by using an unpaired t-test or Mann–Whitney U test for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Prognostic impact of octreotide was assessed by Kaplan-Meier analysis and compared by Mantel-Cox test.
RESULTS
Baseline Characteristics
Thirty LVAD patients (20 HeartMate II, 9 HVAD, and 1 HeartAssist 5) were enrolled (Table 1). Patients were 66.4 ± 8.8 years old and 16 (53%) were male. The majority of patients were implanted as destination therapy (83%) and 14 (47%) had an ischemic etiology of their cardiomyopathy. Patients were followed for 1243.8 ± 787.6 days following LVAD implantation. The duration between the time of LVAD implantation and octreotide initiation was 745.0 ± 728.9 (42–2613) days. Patients continued octreotide therapy for 498.8 ± 356.0 days without any complications associated with its use. There was no discontinuation of octreotide during the study period.
Table 1.
Background characteristics
| N = 30 | |
|---|---|
| Age, years | 66.4 ± 8.8 |
| Race (Caucasian) | 17 (57%) |
| Body Mass Index | 30.9 ± 8.0 |
| Gender (Male) | 16 (53%) |
| Destination Therapy | 25 (83%) |
| Ischemic Etiology | 14 (47%) |
| Devices | |
| HeartMate II | 20 (67%) |
| HVAD | 9 (30%) |
| HeartAssist 5 | 1 (3%) |
| Hypertension | 20 (67%) |
| Diabetes Mellitus | 14 (47%) |
| Atrial Fibrillation | 11 (37%) |
| History of Ventricular Tachyarrhythmia | 7 (23%) |
Changes in medications and LVAD speed between the pre- and post-octreotide therapy periods are summarized in Table 2. The percent of days with INR >2.0 was significantly increased after octreotide initiation (p = 0.003). Mean aspirin dose and percent of days with any aspirin administration were comparable. Mean LVAD speeds remained unchanged.
Table 2.
Changes in patient management parameters before and after octreotide initiation
| Pre-octreotide period | Post-octreotide period | P value | |
|---|---|---|---|
| %days with INR >2.0, % | 22.7 ± 22.9 | 45.4 ± 37.0 | 0.006* |
| Mean aspirin dose, mg/day | 72.7 ± 111.7 | 57.6 ± 54.7 | 0.77 |
| %days with any aspirin administration, % | 37.5 ± 42.2 | 48.7 ± 39.2 | 0.34 |
| Mean LVAD speed, rpm | |||
| HeartMate II (N = 20) | 9171.5 ± 426.7 | 9127.0 ± 374.2 | 0.68 |
| HVAD (N = 9) | 2588.9 ± 130.5 | 2654.4 ± 164.3 | 0.43 |
| HeartAssist 5 (N = 1) | 8698.4 ± 98.4 | 9089.5 ± 99.7 | - |
p <0.05 by Wilcoxon signed-rank test
GI bleeding events
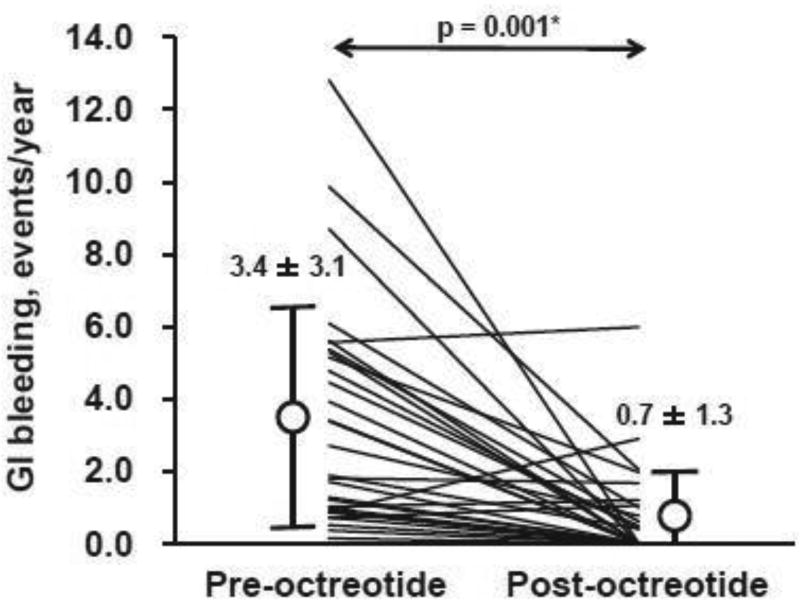
Prior to octreotide initiation, patients had an average of 3.0 ± 2.4 GI bleeding events, for a total of 91 GI bleeding events (3.4 ± 3.1 events/year; median 2.3 event/year). Mean time from device implantation to the first event was 419.9 ± 544.8 days (median 163; IQR, 44–694).
Following octreotide initiation, only 13 patients (43%) experienced GI bleeding events (for a total of 23 events). Time from octreotide initiation to the first GI bleeding event was 257.9 ± 305.9 days (median 114; IQR 31–400 days). Seventeen patients (57%) were free from GI bleeding for an average follow-up time of 466.6 ± 367.5 days (median 334 days; IQR, 169–757 days).
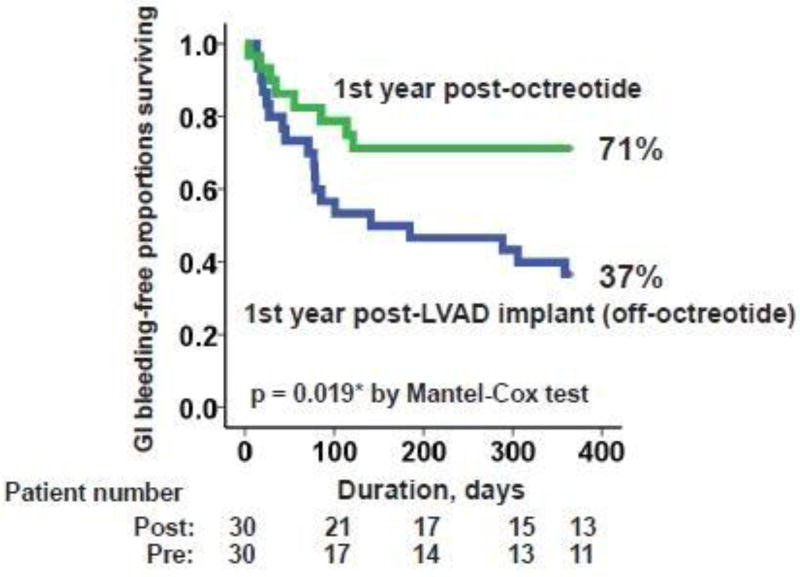
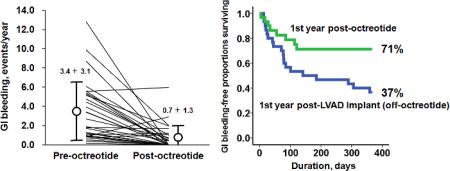
The frequency of GI bleeding decreased significantly during octreotide therapy to 0.7 ± 1.3 events/year (p <0.001; Figure 1), leading to a higher 1-year event-free rate during octreotide therapy compared to the pre-octreotide period (Figure 2; 71% vs. 37%, p = 0.019).
Figure 1.
Comparison of frequency of GI bleeding between pre- and post-octreotide therapy.
GI, gastrointestinal. *p <0.05 by Wilcoxon signed-rank test.
Figure 2.
Comparison of GI bleeding-free proportions surviving between post-LVAD implantation off-octreotide period and post-octreotide therapy period.
*p <0.05 by Mantel-Cox test.
Among 25 patients who continued octreotide therapy for over 6 months, 18 patients (72%) were free from GI bleeding during 6-month octreotide therapy. There was no significant difference in background characteristics between those with and those without GI bleeding during octreotide therapy, including age, co-morbidities, frequency of prior GI bleeding, and concomitant therapy at the time of octreotide initiation (Supplementary Table 1).
Clinical outcomes associating with GI bleeding
There was a significant reduction in blood product utilization following octreotide initiation (Table 3; p <0.05 for all). There was also a significant reduction in the use of GI procedures following octreotide initiation (p <0.05). The mean hemoglobin level increased significantly in the post-octreotide period compared with the pre-octreotide period (9.82 ± 0.83 vs. 9.24 ± 0.84 g/dL, p = 0.006).
Table 3.
Changes in therapeutic parameters associating with GI bleeding
| Pre-octreotide period | Post-octreotide period | P value | |
|---|---|---|---|
| Mean hemoglobin level, g/dL | 9.24 ± 0.84 | 9.82 ± 0.83 | 0.003* |
| Per year amount | |||
| RBC, unit/year | 20.2 ± 24.6 | 7.1 ± 10.3 | 0.002* |
| FFP, unit/year | 13.1 ± 19.1 | 3.6 ± 5.0 | 0.002* |
| LOS, day/year | 35.4 ± 47.3 | 8.7 ± 28.3 | <0.001* |
| GI procedure, time/year | 2.9 ± 3.1 | 0.4 ± 0.7 | <0.001* |
| Per event amount | |||
| RBC, unit/event | 2.5 ± 2.7 | 1.0 ± 1.7 | 0.004* |
| FFP, unit/event | 1.3 ± 2.1 | 0.1 ± 0.4 | 0.001* |
| LOS, day/event | 9.1 ± 6.3 | 4.5 ± 7.9 | 0.015* |
| GI procedure, time/event | 0.8 ± 0.2 | 0.3 ± 0.5 | <0.001* |
RBC, red blood cell; FFP, fresh frozen plasma; LOS, length of stay.
p <0.05 by Wilcoxon signed-rank test
There were 63 endoscopic procedures before octreotide therapy, and 18 endoscopic procedures following octreotide initiation. The endoscopic results before and after octreotide therapy are summarized in Table 4. AVMs were identified during endoscopy 44% of the time prior to octreotide therapy, and 28% of the time following octreotide therapy. The bleeding source was less frequently identified following octreotide therapy (p = 0.03), and fewer invasive therapies were performed during endoscopy following octreotide therapy (p = 0.047).
Table 4.
Results of endoscopic procedures
| Pre-octreotide (N = 63) | Post-octreotide (N = 18) | P value |
|
|---|---|---|---|
| Lesions identified as source of GI bleeding | |||
| Arteriovenous malformation | 28 (44%) | 5 (28%) | 0.18 |
| Erosion, gastritis or colitis | 8 (13%) | 1 (6%) | 0.48 |
| Dieulafoy lesion | 2 (3%) | 0 (0%) | 0.42 |
| Diverticulum | 1 (2%) | 0 (0%) | 0.75 |
| No active site identified | 24 (38%) | 12 (67%) | 0.028* |
| Localization of GI bleeding | |||
| Esophagus | 2 (3%) | 0 (0%) | 0.42 |
| Stomach/duodenum | 17 (27%) | 5 (28%) | 0.94 |
| Small intestine | 12 (19%) | 0 (0%) | 0.042* |
| Large intestine | 8 (13%) | 1 (6%) | 0.52 |
| Unknown origin | 24 (38%) | 12 (67%) | 0.028* |
| Performed therapy | |||
| Clipping | 5 (8%) | 0 (0%) | 0.24 |
| Argon plasma coagulation | 19 (30%) | 3 (17%) | 0.28 |
| Infrared radiation embolization | 2 (3%) | 0 (0%) | 0.42 |
| No invasive therapies | 37 (59%) | 15 (83%) | 0.044* |
p <0.05 by McNemar’s test
The overall survival rate following octreotide therapy was 78% at 1 year and 73% at 2 years. Eleven patients (35%) died during the study period. The leading cause of death was heart failure (seven patients) followed by device thrombosis (three patients) and one patient due to sepsis.
DISCUSSION
In this prospective study, we investigated the impact of octreotide therapy for the secondary prevention of GI bleeding in patients with continuous-flow LVADs. Our main findings are: 1) Tthere was a significant reduction in the frequency of GI bleeding during octreotide therapy without any associated complications despite unchanged antiplatelet therapy and increase in the levels of anticoagulation therapy, 2) There was a significant reduction in blood product utilization during octreotide therapy, and an increase in the hemoglobin level following octreotide initiation, 3) There was a significant reduction in GI procedures as well as invasive therapies during octreotide therapy.
Management of recurrent GI bleeding
Although several agents have been purported to reduce the frequency of GI bleeding in patients with recurrent GI bleeding episodes, there are no definitive studies to guide their use. Medical therapies that may act directly or indirectly to inhibit angiogenesis and generation of immature vascular networks in the GI tract, including hormone therapy and thalidomide, have been investigated.18, 19
Some investigators have suggested that a reduction of LVAD speed to promote aortic valve opening and enhance arterial pulsatility may be effective to reduce GI bleeding.17 However, a recent study of a partial ventricular support device, in which pulse pressure was fully preserved, reported a relatively high rate of non-surgical GI bleeding and epistaxis.20 This observation raises questions about the association of diminished pulsatility with the generation of AVMs and increased bleeding risk.
Long-term reduction of anticoagulation and antiplatelet therapy has also been suggested as a means to prevent recurrent bleeding. However, results of the US-TRACE study showed that despite discontinuation of anticoagulation therapy, antiplatelet therapy or both, patients continued to bleed.21 We observed a similar finding in this study prior to octreotide initiation and it has been shown that reduced anticoagulation is associated with an increased risk of thromboembolic events.9 Thus, to date, no strategy has been proven to reduce the rate of GI bleeding in LVAD patients.
Efficacy and safety of the octreotide therapy
In the present study, octreotide therapy reduced the frequency of GI bleeding. The total number of units of RBCs and FFP infused, and number of days in the hospital decreased for the overall cohort during octreotide therapy. Furthermore, the mean hemoglobin level increased after octreotide therapy, probably related to the reduction in the frequency and magnitude of GI bleeding. The magnitude of each GI bleeding event, as assessed by blood product usage during each event, also decreased during octreotide therapy. Furthermore, the necessity for GI procedures as well as invasive therapies also decreased during octreotide therapy. The percent of days with INR >2.0 was increased following octreotide initiation, which can be explained by a reduction in the frequency of events necessitating warfarin discontinuation. Accordingly, prevention of recurrent GI bleeding by octreotide therapy has the potential to improve patient quality of life and improve cost-effectiveness of LVAD therapy.
The mechanism by which octreotide reduces GI bleeding in LVAD patients has not been investigated. Contributing mechanisms are hypothesized to include decreases of portal vein pressure due to splanchnic vasodilatation, enhancement of platelet aggregation, inhibition of GI angiogenesis as well as suppression of digestive enzymes.11
We did not observe any octreotide-associated adverse events such as bradycardia or digestive symptoms. A prior phase I study also reported no adverse events including GI bleeding following 16-weeks of octreotide therapy among 8 LVAD patients. This study had no comparison group and octreotide was administered for primary prevention of GI bleeding. Therefore, the risk for GI bleeding among patients in that study may not be as high as our patients.15 In our cohort, three patients experienced pump thrombosis during octreotide therapy, possibly related to inability to maintain therapeutic anticoagulation levels due to recurrent GI bleeding.
Future octreotide therapy for GI bleeding in LVAD patients
Age, history of GI bleeding, and implantation as destination therapy are strong risk factors for GI bleeding during LVAD therapy.6, 13 We did not find an association of age, frequency of GI bleeding, or LVAD support duration with refractoriness to octreotide therapy, which was assessed by freedom from an event during 6-months of octreotide therapy. Hemodynamic, echocardiographic, or endoscopic parameters may be helpful to more fully uncover the global effects of octreotide therapy.22, 23 Measurement of GI bleeding-associated factors such as vascular endothelial growth factor and portal vein pressure before and during octreotide therapy may also be useful for optimal patient selection.15
Interestingly, all six patients with HVAD were free from GI bleeding during octreotide therapy. The relationship between device types and GI bleeding remains controversial,24 but this should be analyzed in larger-scale studies, particularly including the most recently introduced HeartMate 3.2
Other therapies such as danazol and thalidomide may have a potential hemostatic role in LVAD patients.18, 19 They lack sufficient evidence for routine clinical use, but can be administered orally and may be cheaper than octreotide. However, the adverse event profile for both agents is significant. Strict patient selection and careful monitoring are required for thalidomide therapy due to pancytopenia and neuropathy. Patients may not tolerate long-term danazol therapy because of its androgenic effects. The relative efficacy, safety, and cost-effectiveness of octreotide and these other agents should be studied.
Finally, the doses and dosing schedules of octreotide we used were based on previous case reports.14, 15 However, optimization of dosing specifically for LVAD patients is warranted. These factors, in addition to varied indications for the use of octreotide among institutions, may underlie discordant reports with some case reports showing positive effects and others showing no effect on bleeding.12–16
Study Limitations
This is a prospective study with a moderate cohort size from a single center. Furthermore, the study was nonrandomized, and pre-treatment data from the same patients were used to assess the prospective effects of octreotide. A randomized control study would be best method to evaluate the use of octreotide in this patient population, but may not be practical given the severe consequences of refractory GI bleeding on patient quality-of-life.
The timing of initiation of octreotide therapy (both in terms of time from LVAD implant and in terms of number of prior GI bleeds) varied widely among patients. These time biases may have affected the outcomes. Most of the enrolled patients were treated with HeartMate II or HVAD. Our results may not simply be adopted in patients with other devices such as HeartMate 3.2
Conclusions
Octreotide therapy may be useful to prevent recurrent GI bleeding refractory to conventional therapies in patients with continuous-flow LVADs.
Supplementary Material
Acknowledgments
Disclosures statement
Teruhiko Imamura receives Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science. Nir Uriel receives consultant fee and grant support from Abbott and Medtronic; Valluvan Jeevanandam receives consultant fee from Abbott. Daniel Burkhoff receives consultant fee from Medtronic, Corvia Medical, Sensible Medical, Impulse Dynamics, Cardiac Implants, and educational grant support from Abiomed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med. 2017;376(5):440–50. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 3.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 4.Forest SJ, Bello R, Friedmann P, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg. 2013;95(4):1276–81. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137(1):208–15. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk Factors and Outcomes of Gastrointestinal Bleeding in Left Ventricular Assist Device Recipients. Am J Cardiol. 2016;117(2):240–4. doi: 10.1016/j.amjcard.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30(8):849–53. doi: 10.1016/j.healun.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Maltais S, Kilic A, Nathan S, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant. 2017;36(1):1–12. doi: 10.1016/j.healun.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Tabit CE, Chen P, Kim GH, et al. Elevated Angiopoietin-2 Level in Patients With Continuous-Flow Left Ventricular Assist Devices Leads to Altered Angiogenesis and Is Associated With Higher Nonsurgical Bleeding. Circulation. 2016;134(2):141–52. doi: 10.1161/CIRCULATIONAHA.115.019692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szilagyi A, Ghali MP. Pharmacological therapy of vascular malformations of the gastrointestinal tract. Can J Gastroenterol. 2006;20(3):171–8. doi: 10.1155/2006/859435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes HM, Dembo LG, Larbalestier R, O'Driscoll G. Management options to treat gastrointestinal bleeding in patients supported on rotary left ventricular assist devices: a single-center experience. Artif Organs. 2010;34(9):703–6. doi: 10.1111/j.1525-1594.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal A, Pant R, Kumar S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93(5):1534–40. doi: 10.1016/j.athoracsur.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Loyaga-Rendon RY, Hashim T, Tallaj JA, et al. Octreotide in the management of recurrent gastrointestinal bleed in patients supported by continuous flow left ventricular assist devices. ASAIO J. 2015;61(1):107–9. doi: 10.1097/MAT.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra R, Shah KB, Chawla R, et al. Tolerability and biological effects of long acting octreotide in patients with continuous flow left ventricular assist devices. ASAIO J. 2016 doi: 10.1097/MAT.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 16.Rennyson SL, Shah KB, Tang DG, et al. Octreotide for left ventricular assist device-related gastrointestinal hemorrhage: can we stop the bleeding? ASAIO J. 2013;59(4):450–1. doi: 10.1097/MAT.0b013e318295232d. [DOI] [PubMed] [Google Scholar]
- 17.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Schettle SD, Pruthi RK, Pereira NL. Continuous-flow left ventricular assist devices and gastrointestinal bleeding: potential role of danazol. J Heart Lung Transplant. 2014;33(5):549–50. doi: 10.1016/j.healun.2014.01.922. [DOI] [PubMed] [Google Scholar]
- 19.Draper K, Kale P, Martin B, Cordero K, Ha R, Banerjee D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant. 2015;34(1):132–4. doi: 10.1016/j.healun.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Meyns B, Rega F, Ector J, et al. Partial left ventricular support implanted through minimal access surgery as a bridge to cardiac transplant. J Thorac Cardiovasc Surg. 2009;137(1):243–5. doi: 10.1016/j.jtcvs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: A one-year analysis of the US-TRACE Study. J Heart Lung Transplant. 2015;34(12):1542–8. doi: 10.1016/j.healun.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Sparrow CT, Nassif ME, Raymer DS, Novak E, LaRue SJ, Schilling JD. Pre-Operative Right Ventricular Dysfunction Is Associated With Gastrointestinal Bleeding in Patients Supported With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail. 2015;3(12):956–64. doi: 10.1016/j.jchf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Jabbar HR, Abbas A, Ahmed M, et al. The Incidence, Predictors and Outcomes of Gastrointestinal Bleeding in Patients with Left Ventricular Assist Device (LVAD) Dig Dis Sci. 2015;60(12):3697–706. doi: 10.1007/s10620-015-3743-4. [DOI] [PubMed] [Google Scholar]
- 24.Lalonde SD, Alba AC, Rigobon A, et al. Clinical differences between continuous flow ventricular assist devices: a comparison between HeartMate II and HeartWare HVAD. J Card Surg. 2013;28(5):604–10. doi: 10.1111/jocs.12158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.