The liver is a critical hub for numerous physiological processes. These include macronutrient metabolism, blood volume regulation, immune system support, endocrine control of growth signaling pathways, lipid and cholesterol homeostasis, and the breakdown of xenobiotic compounds, including many current drugs. Processing, partitioning, and metabolism of macronutrients provide the energy needed to drive the aforementioned processes and are therefore among the liver's most critical functions. Moreover, the liver's capacities to store glucose in the form of glycogen, with feeding, and assemble glucose via the gluconeogenic pathway, in response to fasting, are critical. The liver oxidizes lipids, but can also package excess lipid for secretion to and storage in other tissues, such as adipose. Finally, the liver is a major handler of protein and amino acid metabolism as it is responsible for the majority of proteins secreted in the blood (whether based on mass or range of unique proteins), the processing of amino acids for energy, and disposal of nitrogenous waste from protein degradation in the form of urea metabolism. Over the course of evolution this array of hepatic functions has been consolidated in a single organ, the liver, which is conserved in all vertebrates. Developmentally, this organ arises as a result of a complex differentiation program that is initiated by exogenous signal gradients, cellular localization cues, and an intricate hierarchy of transcription factors. These processes that are fully developed in the mature liver are imperative for life. Liver failure from any number of sources (e.g. viral infection, overnutrition, or oncologic burden) is a global health problem. The goal of this primer is to concisely summarize hepatic functions with respect to macronutrient metabolism. Introducing concepts critical to liver development, organization, and physiology sets the stage for these functions and serves to orient the reader. It is important to emphasize that insight into hepatic pathologies and potential therapeutic avenues to treat these conditions requires an understanding of the development and physiology of specialized hepatic functions.
Liver cellular anatomy
The liver is composed of several cell types of different embryological origin including hepatocytes, biliary epithelial cells (cholangiocytes), stellate cells, Kupffer cells, and liver sinusoidal endothelial cells. Each of these cell types possesses unique functions that cooperatively regulate hepatic function at multiple levels. Hepatocytes are the primary epithelial cell population of the liver. They make up the majority of the liver volume and perform many of the functions ascribed to the liver. Cholangiocytes are the second most abundant epithelial population of the liver and have a more traditional epithelial function as the cells lining the lumen of the bile ducts. Stellate cells represent a dynamic cell population that can exist in a quiescent or activated state. In the quiescent state stellate cells store vitamin A in lipid droplets; however, other functions in this quiescent state remain unclear. Damage to the liver leads to activation of stellate cells. Upon activation, stellate cells proliferate and progressively lose vitamin A stores. Stellate cells are also responsible for deposition and organization of collagen in the injured liver. This process contributes to scarring of the liver, which can progress to cirrhosis, a critical pathology contributing to end stage liver disease. Kupffer cells are the resident macrophage population of the liver. These cells recognize the many pathogenic stimuli introduced through the portal circulation and can attain pro- or anti-inflammatory roles in liver wound healing depending on a number of contributing factors. Finally, liver sinusoidal endothelial cells are a specialized endothelial population with unique characteristics. These cells form fenestrated sieve plates at the sinusoidal lumen. This structure creates pores ranging in size from 50–180 nm in humans or 50–280 nm in mice and rats. This organization is critical for exchange of proteins and particles within these size limits between plasma and the cell types of the liver, while maintaining certain barrier functions.
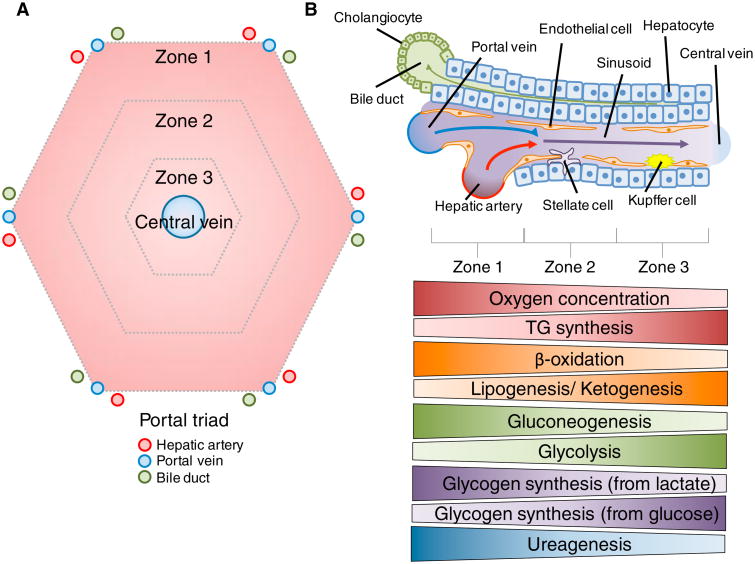
The cells of the liver are organized around the functional structural unit of the liver — the lobule (Figure 1A). This consists of chords of hepatocytes organized in a typically hexagonal shape around the central vein (Figure 1A). At the vertices of this hexagon are the portal triads consisting of closely grouped branches of the hepatic artery, portal vein, and bile ducts (Figure 1A). Circulatory units within the hepatocyte chords differ from a typical capillary bed in that the endothelial cells of the liver do not form tight junctions (Figure 1B). This creates a sinusoidal network that minimizes barriers between hepatocytes and the blood traversing the sinusoid. Oxygen-rich blood from the hepatic artery mixes with nutrient-rich blood from the portal circulation in the sinusoid before flowing over the cells of the lobule and draining into the central vein (Figure 1A, B). This organization causes the blood composition exiting the lobule to have different characteristics than the blood entering the lobule. As blood progresses across the lobule, cells utilize oxygen and process nutrients, while generating metabolites and waste products. Blood becomes deoxygenated and metabolic byproducts are secreted from cells along the length of the sinusoid. This creates gradients of oxygen, nutrients, and waste presented to cells of the liver based on their lobular location. These and other gradients formed across the sinusoids of the lobule result in a partitioning of functions based on localization, such as increased oxidative metabolism in areas with higher blood oxygen content. This partitioning of functions has been termed ‘metabolic zonation’ and typically breaks the lobule into three distinct ‘zones’. Each zone possesses hepatocytes with differential metabolic gene expression and functionality. These metabolic zones are typically depicted as discrete regions (Figure 1A), but hepatic zonation actually exists on a flexible spectrum. For example, hepatocytes from Zone 2 can assume the functional attributes of Zone 1 hepatocytes in the face of damage or loss of function. This may occur in response to various liver-damaging pathologies such as viral hepatitis. A hallmark of the liver is that its diverse cell populations couple with its anatomical organization to maintain hepatic functionality.
Figure 1. Organization of the liver.
(A) Geometric representation of a hepatic lobule. Appearing roughly hexagonal in shape, the vertices represent the portal triad area. Each triad contains branches of the hepatic artery, portal vein, and bile duct. Oxygenated blood from the hepatic artery mixes with nutrient-rich blood from the portal circulation drained from the gut. Upon mixing, this blood equilibrates and fl ows across the lobule through a sinusoidal network before draining in to branches of the central vein. This organization leads to formation of a number of gradients including oxygen, hormones, nutrients, and waste products. This gradient formation and the consequential organization of relevant metabolic processes has been dubbed ‘metabolic zonation’. These zones are depicted as roughly equal, but can shift in size and location based on a number of factors (e.g. hepatocellular damage or altered blood flow). (B) A schematic representation of a sinusoid within the liver and the corresponding zonation of several metabolic processes across the sinusoid. A number of cell types exist within the sinusoid including hepatocytes, biliary epithelial cells (cholangiocytes), endothelial cells, Kupffer cells, and stellate cells. As previously mentioned blood flows through the sinusoid leading to a number of gradients along the length of this vessel. Liver endothelial cells do not form tight junctions, but instead have sieve plate networks between them. This creates a minimal barrier between the circulating blood and hepatocytes. Hepatocytes perform a majority of the hepatic metabolic functions. The gradients depicted below the scheme pertain to both essential molecules (oxygen) and metabolic processes along the sinusoid. These processes are critical to both liver and whole body metabolic homeostasis. Therefore, it is important to note the flexibility of these gradients as they are often modified during times of variable nutrient availability, such as the fasting or fed states.
Initiation of liver development
As the function and organization of the liver are critical to so many processes it is important to understand how these aspects of the liver arise developmentally. Described here is the general organization of hepatic development that occurs in many animals including zebra fish, mice, rats, and humans. The duration and identity of signals involved in each of these developmental aspects may vary between species. The goal of this section is to give a general overview of hepatic development in common model organisms and humans. For simplicity, specific proteins and transcription factors referenced have been derived from studies in mice and rats except where specifically noted.
The definitive endoderm, ectoderm, and mesoderm make up the three major cell layers established during embryonic gastrulation. Cells from the definitive endoderm proceed to form the epithelium of the respiratory and digestive tracts as well as associated organs such as liver and pancreas. The primary metabolic cell population of the liver, hepatocytes, and the bile duct lining epithelial cells, cholangiocytes, arise from the posterior foregut region within the definitive endoderm. Definitive endoderm specification and segregation require a complex array of extracellular growth factor signals in a proper temporal order. Some of the earliest signals for initiation of hepatic bud outgrowth from the posterior foregut endoderm include fibroblast growth factor (FGF), and bone morphogenic proteins (BMPs), which are derived from the overlying mesodermally derived cardiac mesoderm and septum transversum. Other signals include transforming growth factor β (TGF-β), Wnt, and NOTCH. These signals are supported by expression and activity of transcription factors in the FoxA and GATA families within the endodermally derived epithelium. Specific members of these families, notably FoxA1 and GATA4, act as pioneer factors, interacting with their DNA-binding motifs within compact chromatin to modify nucleosome localization. This alteration of chromatin conformation creates an environment of transcriptional competence for these and other downstream transcription factors. The sum of these modifications results in a ‘footprint’ of transcriptional access, leading to the establishment and maintenance of a gene expression program, critical for differentiation and mature function.
Cell patterning and maturation during liver development
Cells of the hepatic bud give rise to bipotential progenitor cells known as hepatoblasts, which further differentiate into the liver parenchymal cells: hepatocytes and cholangiocytes. Importantly, prior to the formation of the bone marrow, the developing liver bud serves as the center of fetal hematopoiesis. Signals from hematopoietic cells, such as oncostatin M, can also govern hepatoblast proliferation and E-cadherin-mediated cell junction formation in hepatoblasts. While there are several other contributors to hepatoblast differentiation, the gradient of TGF-β secreted from the portal vein mesenchyme is integral to cholangiocyte and hepatocyte differentiation. This contributes to the hepatoblast fates that are dependent on portal vein proximity. Mechanistically, higher TGF-β signaling in portal vein proximal hepatoblasts drives cholangiocyte fate by decreasing expression of CCAAT/ enhancer binding protein (C/EBP) α and promoting expression of Hnf6 (aka Oc1) and Hnf1β. This transcription factor profile promotes cholangiocyte-specific gene transcription through HNF6 and HNF1β, while suppressing hepatocyte specific genes by decreasing C/EBPα levels. Hepatoblasts located further from the portal vein develop into hepatocytes, forming chords across the developing hepatic lobules. These cells receive lower levels of TGF-β, which leads to a higher level of C/EBPα. In turn, C/EBPα inhibits the expression of TGF-β receptor II, creating a positive feedback loop of TGF-β signal inhibition. C/EBPα also regulates expression of HNF1α and HNF4α, which in turn act as feed forward co-activators of a number of hepatocyte-specific genes. Finalization of hepatocyte differentiation is linked to oncostatin M, glucocorticoids, hepatocyte growth factor (HGF), Wnt, and yes-associated protein signaling (YAP). Interestingly, Wnt/β-catenin signaling has been implicated in establishment of metabolic zonation. In fact, a balance of stimulation and suppression of genes by HNF4α is influenced by the β-catenin activated transcription factor LEF1 to establish zonal specific expression of various enzymes (e.g. glutamine synthetase).
Perinatal metabolic programming
In the final term of gestation (week 34–37 in humans; day 18–21 in rats and mice), the liver prepares for a metabolic switch from an organ of glucose consumption to one in which glucose is both stored and produced. This is evidenced by a decrease in glycolytic enzyme expression coupled with increases in enzymes critical to gluconeogenic and glycogenic processes. The rate of enzyme activities such as the gluconeogenic enzymes phosphenolpyruvate carboxykinase and glucose-6-phosphatase, and the glycogenic enzyme glycogen synthase, are dependent on the metabolic zones of the hepatic lobule.
The switch from placental to maternal-independent endogenous and exogenous nutrient provision leads to a drop in blood glucose, which is accompanied by a stark rise in the hepatic sinusoidal glucagon to insulin ratio (Figure 2A). These hormones have opposing actions with regards to several hepatic metabolic processes and are the primary physiologic regulators of glucose homeostasis in the adult organism. In the fed state, when insulin is high and glucagon is low, the liver is a site of glucose uptake and anabolic processes are accelerated. Insulin inhibits gluconeogenesis while promoting glycogen and lipid synthesis in hepatocytes, amongst other metabolic effects. As an organism transitions away from placental nutrition, glucagon levels rise and insulin levels fall. Glucagon promotes glycogenosis, gluconeogenesis, fat oxidation and metabolism of other macronutrients by hepatocytes. These effects of the increased glucagon to insulin ratio are required for maintenance of blood glucose concentrations (Figure 2A). The switch in perinatal metabolic programming of the liver can be stimulated by hormone-mediated increases in cyclic adenosine monophosphate (cAMP) and is relatively insensitive to insulin. These processes are also regulated by glucocorticoid signaling, which appears necessary for functional differentiation of glycogen storage and gluconeogenesis. During the perinatal period, a number of transcription factors are induced, which regulate expression of key enzymes in these processes. Among these are FoxO1, glucocorticoid receptor, cAMP response element-binding protein (CREB), peroxisome proliferator activated receptor (PPAR) γ coactivator 1α, HNF6, and HNF4α. Transcription factors such as HNF6, HNF4α, and the glucocorticoid receptor intertwine differentiation and metabolic control in hepatocytes, indicating a potentially important connection in the two processes.
Figure 2. The liver in the fasted and fed state.
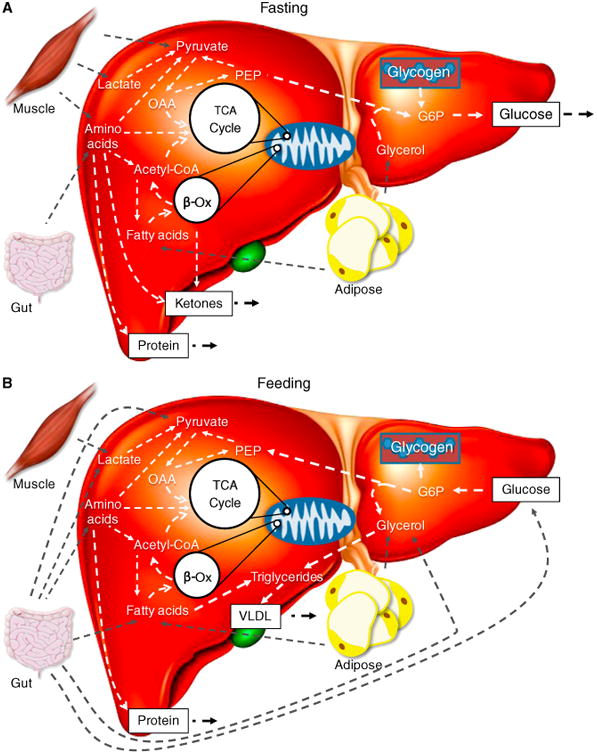
(A) In the fasting state the liver is in a net hepatic glucose output mode due to the low insulin to glucagon ratio. Glucose is derived from both glycogen and gluconeogenesis. Gluconeogenic substrates are provided in the form of amino acids (gut and muscle), lactate (muscle), pyruvate (muscle), and glycerol (adipose tissue). Fatty acids from adipose tissue lipolysis are also directed to several pathways, such as beta-oxidation and the TCA cycle. These processes support gluconeogenesis through production of ATP and reducing equivalents. Ketone bodies may also be produced from lipid oxidation and act as an additional energy shuttle between the liver and other organs. Amino acids can also enter the TCA cycle as anaplerotic substrates and be utilized for synthesis of proteins. Nitrogen released as a result of deamination during amino acid metabolism are disposed of during ureagenesis. Urea is released from the liver and excreted by the kidneys. (B) During feeding, water soluble nutrients enter the portal venous circulation from the intestine. At the liver, the insulin to glucagon ratio is elevated leading to net hepatic glucose uptake. Glucose may undergo glycolysis, as a means of ATP production, or may be stored as glycogen. Amino acids may be oxidized for energy production or utilized as anaplerotic substrates for the TCA cycle. Once again, these amino acids, as in the fasted state, may be used for synthesis of local or secreted proteins. Ingested fats are assembled to form triglycerides from fatty acids and glycerol. These triglycerides are packaged into chylomicrons, which then enter the lymphatic system. Chylomicrons drain from the lymphatics to the circulation and, upon reaching the liver, are unloaded of remaining fatty acids and glycerol. Fatty acids can be used for restoration of energy state, repletion of TCA cycle intermediates, or re-esterified to triglycerides. Triglycerides can be loaded on to very low density lipoproteins, which shuttle lipid to other tissues including muscle and adipose depots.
Glucose metabolism
As discussed at the outset, the ability of the liver to store, synthesize, metabolize, and release glucose is necessary for the postnatal metabolic transition and is maintained throughout the life of an organism. Upon feeding, the liver shifts from a mode of net output to net uptake (Figure 2B). This requires a decline in glucagon and an increase in insulin and results in decreased liver glucose output from glycogen stores and gluconeogenesis (Figure 2B). Glycolysis and glycogen deposition increase in hepatocytes, leading to net hepatic glucose uptake (Figure 2B). The glycogenic response restores glycogen reserves. As an organism transitions from an absorptive state to a fasting state insulin decreases and glucagon increases (Figure 2A). This shifts the liver from glucose storage to net glucose output, which involves glycogen breakdown and gluconeogenesis (Figure 2A). Glucose output is dynamic and responsive to the energy needs throughout the body (e.g. brain, skeletal muscle, and immune system).
One of the growing pathologic concerns in developed countries is the hepatic response to over-nutrition. A key component of this pathologic response is insulin resistance at the liver, which is closely associated with type II diabetes, nonalcoholic fatty liver disease, and cardiovascular disease. Given the immense public health implications of these disease states it is important to understand the functional consequences of this condition. Hepatic insulin resistance is characterized by an impaired ability of insulin to decrease net glucose output from the liver. This contributes to increased blood glucose. While the inhibitory effect of insulin on hepatic glucose output is lost, the stimulatory effect of insulin on lipogenesis is maintained. This dissociation of insulin's effects on carbohydrate and lipid metabolism creates a ‘selective insulin resistance’ and is thought to contribute to a number of pathological conditions (e.g. metabolic syndrome, non-alcoholic fatty liver disease, and cardiovascular disease). There are a number of underlying factors implicated in the development of hepatic insulin resistance. These involve altered coupling of insulin receptor to intracellular signaling proteins, protein levels, kinase activities, nuclear localization of transcription factors, and a number of other molecular mechanisms. Additionally, insulin resistance is associated with an increased flux of gluconeogenic substrates and fatty acids to the liver during over-nutrition. This creates a paradigm where increased substrate fluxes are coupled with a deficit in appropriate molecular control resulting in many of the pathologic consequences of insulin resistance.
Lipid and cholesterol metabolism
The liver is critical for digestive absorption and performs uptake, synthesis, packaging, and secretion of lipids and lipoproteins. The liver's biliary synthesis and secretion system enables efficient absorption of lipid from digestion. Chylomicrons are assembled from lipoproteins and digested lipids in the gut before progressing through the lymphatic system to the circulation. Fatty acids are then extracted from chylomicron remnants by lipoprotein lipase at the liver. These fatty acids are then transported into hepatocytes via a number of transport proteins (e.g. fatty acid transport proteins 2, 4 and 5 and CD36). The liver is able to utilize fatty acids as an internal energy source through oxidative pathways, but can also provide energy to other organs from the ketogenic products (acetoacetate and beta hydroxybutyrate) (Figure 2A,B). This ability to provide ketones as an energetic substrate is necessary for organisms undergoing extreme fasting or consuming extremely low levels of dietary carbohydrates. The release of ketones from the liver prevents excess formation of tricarboxylic acid cycle intermediates and could thereby protect oxidative status. During times of feeding, the liver is also important for providing lipid substrates for the body. The liver can assemble fatty acids and glycerol into triglycerides, which are packaged with very low density lipoprotein particles for secretion from hepatocytes into the bloodstream (Figure 2B). These can then reach other organs such as the adipose tissue for storage or the skeletal muscle for use as an energy source. This lipid handling ability of the liver is also critical for absorption of a number of lipid-soluble vitamins. Without proper hepatic lipid uptake and secretion, vitamin deficiencies at the whole body or organ-specific level can occur. In addition to its function with regards to classical fat molecules, the liver is also critical for cholesterol homeostasis within the body. Cholesterol can be absorbed from the intestine or synthesized de novo in the liver. Cholesterol is a required molecule for assembly of cellular membranes as well as maintenance of membrane fluidity. While a lack of cholesterol can be damaging, an excess is also detrimental to health. Excess cholesterol from the diet and de novo synthesis can result in inappropriate cell membrane dynamics, and may also stimulate pathologic processes contributing to atherosclerosis or cardiovascular disease. Statins are a drug class that inhibits HMG-CoA reductase, the rate limiting enzyme in cholesterol production. This results in a cholesterol-lowering effect that is necessary for statin-mediated improvement of outcomes for cardiovascular events and atherosclerosis. Statins also possess pleiotropic actions which are thought to be significant at higher statin dosages. Therefore, cholesterol-lowering is considered the primary benefit of statins to disease processes with pleiotropic statin effects becoming relevant at higher doses.
Protein and amino acid metabolism
Synthesis and breakdown of proteins are critical to all cellular and organ-level functions. However, these metabolic processes within the liver have broader implications. As a protein synthetic organ, the liver is responsible for 85–90% of circulating protein volume. Albumin is the most abundant of these secreted proteins, contributing 55% of the total plasma protein on average. This protein is essential for maintenance of blood volume and possesses carrier functions in transporting a number of critical molecules such as lipids and hormones. The liver also secretes acute-phase proteins, growth factors, and numerous other peptides that are involved in systemic regulation. Additionally, the liver has a high capacity to break down proteins and metabolize the amino acids that comprise them (Figure 2A,B). Amino acid metabolism can provide energy for the hepatocyte, but requires disposal of nitrogenous wastes (Figure 2A,B). The liver urea cycle is one mechanism for this, disposing of otherwise damaging reactive nitrogenous molecules. The carbon skeleton of specifi c amino acids may also be incorporated into the tricarboxylic acid cycle to serve as gluconeogenic substrates (Figure 2A,B). This allows conversion of amino acids from tissues such as skeletal muscle and intestine to glucose. This conversion of gluconeogenic amino acids to glucose is pertinent to glucose homeostasis and the provision of energy to glucose-dependent organs in times of extended fasting.
Concluding remarks
The liver is a dynamic, heterogeneous organ that is under highly regulated physiological control. Development and control of these functions is established through appropriate timing, localization, and intensity of signals. Despite progress in our understanding of hepatic development, metabolism, and repair, hepatic pathologies continue to have significant global morbidity and mortality burdens. This drives the need to understand these diseases and how to best treat them. This can be accomplished through therapeutic and public health initiatives reinforced by knowledge of hepatic physiology and emerging disease treatment paradigms.
Further Reading
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Gordillo M, Evans T, Gouon-Evans V. Orchestrating liver development. Development. 2015;142:2094–2108. doi: 10.1242/dev.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppuso PA, Sanders JA. Regulation of liver development: implications for liver biology across the lifespan. J Mol Endocrinol. 2016;56:115–125. doi: 10.1530/JME-15-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Trefts E, Williams AS, Wasserman DH. Progress in Molecular Biology and Translational Science. 1st. Vol. 135. Amsterdam: Elsevier; 2015. Exercise and the regulation of hepatic metabolism; pp. 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. 2009;37232:11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]