Abstract
Electrokinetic transport of ions between electrolyte solutions and ion permselective solid media governs a variety of applications, such as molecular separation, biological detection, and bioelectronics. These applications rely on a unique class of materials and devices to interface the ionic and electronic systems. The devices built on ion permselective materials or micro-/nanofluidic channels are arranged to work with aqueous environments capable of either manipulating charged species through applied electric fields or transducing biological responses into electronic signals. In this review, we focus on recent advances in the application of electrokinetic ion transport using nanofluidic and membrane technologies. We start with an introduction into the theoretical basis of ion transport kinetics and their analogy to the charge transport in electronic systems. We continue with discussions of the materials and nanofabrication technologies developed to create ion permselective membranes and nanofluidic devices. Accomplishments from various applications are highlighted, including biosensing, molecular separation, energy conversion, and bio-electronic interfaces. We also briefly outline potential applications and challenges in this field.
I. INTRODUCTION
There has been increasing interest in developing micro-/nanofluidic systems and ion permselective devices to manipulate ion transport in a manner analogous to the function of solid state electronic devices, such as diodes and transistors. The developments were driven by the rising demand for a better approach to interfacing electronics to a biological system which builds the basis for analytical techniques and biomedical devices. The tremendous advance of solid-state electronics in the past few decades has enabled high-performance and highly integrated electronics, resulting in rapid progress in computer and telecommunication technologies. While electronic devices are routed to form circuitries mostly for computing and signal processing, ionic devices can be leveraged to interface ions and molecules in aqueous media to implement biochemical analysis and processing. The permselectivity of nanofluidic and membrane devices allows modulation of the ionic conductance with respect to the surrounding fixed charges on the devices and the applied external electric field. The device can be exploited to realize various intriguing electrokinetic phenomena, such as ion current rectification, field-enhanced water dissociation, ion depletion, and ion gating. Such unique electrokinetic responses lead to developments of a new class of biosensors, molecular separation techniques, and ionic circuitries. The desire to understand the physiology of neural systems and the mechanism under biological computing and memory has also motivated research in the electrokinetics of ion permselective media and development of ionic devices to mimic the functionalities of neural components. The device made to transduce ionic signals in biology to electronic signals in solid-state electronics and vice versa will open up a variety of applications that require communications between biological systems and machines. An example is a therapeutic platform that produces electrical stimulations or drug delivery to enable therapies for disorders related to dysfunctional neural signaling.
The fundamental theory of the electrokinetic flow and ion transport in micro- and nanofluidics has been widely discussed in various review articles and books. In this review, we introduce the basics of ionic transport behavior and outline exciting developments in the field of ionic devices and their applications, including biosensing, molecular separation, biologically inspired devices, and bio-machine interface technology. This review does not cover the complete field of nanofluidic and membrane technologies. However, the discussion here would provide insight into the recent developments and the future potential applications of electrokinetic ion transport to a wide range of diverse fields.
II. THEORETICAL BASIS
Ions and electrons resemble each other in several aspects described by similar physical models. In diluted conditions, both obey Maxwell-Boltzmann statistics described in the context of kinetic theory for dilute gases. The transport kinetics of ions and electrons are driven by the gradient of electrochemical potential or equivalently the gradient of quasi-Fermi energy in the solid state. The gradient of electrochemical potential involves the contributions of both the carrier concentration gradient that induces diffusion flux and the potential gradient that results in migrative flux. The combination of these two fluxes can be modeled by the Nernst-Planck equation given by
| (1) |
with , , and being the diffusion coefficient, the molar concentration, and the charge number of i-th charge species, respectively. , , , and are the electrical potential, the universal gas constant, the Faraday constant, and the absolute temperature. Note that the Nernst-Planck equation is only valid for the system with dilute species. The contribution of convective flow can be neglected in nanofluidics, membranes, and solid-state materials that typically have negligible body flow velocity. The overall transport phenomena can be described by a electrodiffusion model that couples the Nernst-Planck equation and the Poisson equation which relates the electrical potential to the charge density (in C/m3).
| (2) |
where is the permittivity of the medium. The model serves as a theoretical basis for investigating charge transport in both ionic and electronic systems.1 Also, the electrons and holes in semiconductors have a strong similarity to the excess and deficiency of protons in water corresponding to acidic and basic solutions.2,3 From the device structure aspect, both electronic and ionic devices are made of materials that carry fixed charges to control the polarity of the conductive mobile charges through electroneutrality. In semiconductor devices, ionized dopants serve as the fixed charges that determine the type of majority carrier, yielding an extrinsic n-type or p-type semiconductor. On the other hand, ionic media have the fixed charge fulfilled by the ionized chemical groups carried by the polymer backbone of membranes or by the surface of nanochannels. The ionic media need to operate with a proper electrolyte concentration to ensure the transport of unipolar ions, i.e., conducting only cations or anions. The ion charge selectivity or ion permselectivity occurs when the pore size or the inter-charge distance in the membrane is comparable or smaller than the Debye screening length
| (3) |
where the symbols are defined as before. In this condition, the polarity and concentration of the conductive ions will be determined by the type and density of fixed charge on the ion permselective media. Both ion and electron conductive media can be arranged to deplete or enrich mobile charges under an external electric field. In semiconductor devices, the effect leads to a significant change in its electrical conductivity and is realized by a diode that rectifies electric current or a transistor that serves as a switch for logic circuit and signal processing. Similar electrical properties can be carried out by manipulating ion transport in the ion permselective media to create ionic rectification and field-effect ionic devices. Instead of being used for computing or signal processing, ionic devices manage the electrokinetic transport of ions and molecules to facilitate impurity ion removal, the concentration of charged molecules, water dissociation, and biomolecular detection.
It is worth noting that the velocity of the ion transport in ionic systems is limited by the double layer relaxation , with and D being the Debye length and diffusion coefficient of ions, much slower than the dielectric relaxation in solid state electronic materials. Because of the slow diffusion and electrophoretic transport of ions, it takes a longer time for ions to redistribute in response to the change in external electric fields. Ionic devices constructed by ion exchange membranes and nanofluidic channels tend to operate in a transient regime dominated by the nonequilibrium effect that leads to hysteretic current-voltage (I-V) characteristics.4–8 The transient hysteretic I-V curve reflects the slow evacuation of ions from the charged membranes until the ions are depleted in the junction region to create a high resistance. The size of the hysteretic loop depends on the quantity of ions the electric field needs to drive and how far from equilibrium the device is operated. The former is determined by the charge densities in the device and the electrolyte concentration, while the latter can be controlled by the voltage scan rate and range. The difference in charge transport speed leads to discrepant strategies for signal transmission in electronic and ionic systems. Electronic systems even operated at a high speed rely on steady-state electric signals for information transmission and processing. In biological systems, the signal propagation in neural networks requires highly dynamic ion flux across axon membranes to establish action potentials.
III. MATERIALS AND FABRICATION TECHNOLOGIES
Charge selectivity of ion permselective media is usually achieved by using ion-exchange membranes and nanofluidic channels. Ion-exchange membranes are polymeric frameworks containing highly concentrated charged chemical groups, such as sulfonate or ammonium groups. The chemical groups tend to ionize around neutral pH which allows the conduction of counter-ions. The membrane made to contain ionophores selectively binds and transports specific ion species to offer ion permselectivity. The polymeric framework can be synthesized by using hydrogels to carry the chemical groups. Hydrogels are hydrophilic crosslinked three-dimensional polymeric frameworks capable of holding large amounts of water. They are synthesized through polymerization of monomers using cross-linking reactions. Depending on the chemical groups available on the polymer backbone and their crosslinking density, the structure provides desired ionized charges, chemical functionalities, porosities, and mechanical strength. Hydrogels are typically obtained from the natural forms including proteins (e.g., collagen, fibrin, and gelatin) and polysaccharides (e.g., agarose, alginate, hyaluronic acid, and starch). They can also be derived synthetically as poly(acrylic acid), polyethylene glycol, and synthetic cellulose. The properties of synthetic hydrogels can be easily controlled by crosslinking with additional monomers of specific functionalities through a chemical reaction, ultraviolet irradiation, or thermal treatment. The controllable fashion facilitates their integration with microfluidic systems to implement miniaturized devices for sensing, biomolecular separation, chemical delivery, and tissue engineering.9–11 A variety of inorganic membranes can be produced to support ion permselectivity. Solid-state membranes, such as anodic aluminum oxide (AAO), are prepared by anodization of aluminum thin films or aluminum foils to create high-density well-ordered through-holes with the pore size controllable by the anodization condition. The alumina surface of AAO membranes can be further functionalized with charged chemical groups to enable ion permselectivity. An alternative method to prepare nanoporous membranes relies on the assembly of nanoparticles, such as silica colloidal nanoparticles, into a closely packed face-center cubic lattice structure.12–14 The pore size of the membrane is determined by the gap enclosed by the neighboring three nanoparticles and is adjustable by the particle size. The silica nanoparticle contains inherently negative charge and can be functionalized to comprise opposite charge polarity to support the permselectivity of the membrane.15,16
Nanofluidic channels and nanopores built on solid materials are the alternative approaches to carry out charge-selective ion transport. In a nanochannel with the size comparable to or less than the Debye screening length, the surface charges on the channel walls determine the charge polarity and concentration of the conducting ions. The nanochannel structures can be fabricated by using a conventional semiconductor process, including etching and glass bonding or removal of a nanometer-thick sacrificial layer encapsulated in a dielectric material. Nanoimprint lithography (NIL) enables high-throughput fabrication of nanoscale pattern transfer without complicated electron-beam lithography or photolithography. The nanochannels can be fabricated through the NIL followed by dry etching to form nanochannels on a glass or silicon substrate.17,18 The focused ion beam (FIB) milling technique was applied to achieve planar nanochannels with a width below 5 nm on quartz substrates.19 Tong et al. utilized the same FIB technique to fabricate cylindrical nanopores through a silicon nitride thin film.20 Siwy demonstrated the use of the ion track etching method, which passes energetic heavy ions through a polymer film [e.g., polyethylene terephthalate (PET) membrane] or a solid-state thin film (e.g., silicon nitride), to drill nanopores with a controllable pore size and channel geometry, such as an asymmetric corn-shaped nanopore.21–23 One can introduce charges to the surface of the nanochannel or nanopore through chemical functionalization of charged molecules or polymers.24–26 The unique isoelectric point of different solid oxide surfaces can also provide an inherent surface charge in the channel.27,28 The solid-state nanochannels and nanopores provide a well-defined geometry and robust mechanical property. However, it is quite challenging to fabricate a nanochannel with a diameter around 1 nm which is required to hold sufficient ion selectivity under physiological ionic strength (100–200 mM). Also, nanofluidic channels or nanopores deliver only nanoamperes of ion current, which may limit their utility.
As opposed to the solid-state nanochannels, ion-exchange membranes hold up high charge density on their polymer network, which allows supporting a strong charge selectivity even at a high ionic strength of greater than 1 M. The membrane-based devices are configured to have a large cross-sectional area to permit large ion flux. More details related to nanofluidic device fabrication technologies can be found in other review articles.29–32
IV. APPLICATIONS
Integrating ion-conductive media with microfluidic systems allows various applications on a miniaturized scale. We discuss the recent efforts toward ionic devices for application in several analytical and separation processes to sustain the throughput such as microfluidic pH control, free-flow electrophoretic chips for high-throughput biomolecule separation, desalination, and energy conversion based on reverse electrodialysis (RED).
A. Ion rectification and water splitting—Applications in ionic circuits and microfluidic pH control
Biological ion channels support ion permselectivity and unidirectional ion flux that play key roles in the transduction of neuronal signals. The unique property has attracted interest from researchers to explore artificial materials to resemble the functionality since the fifties of the last century. The resembling of the unidirectional ion transport was first attempted by demonstrating ion rectification in electrolyte solutions using a bipolar membrane composed of cation- and anion- exchange membranes joined in series.33 Each of the ion exchange membranes supports unipolar ions of opposite charges forming a charge distribution analogous to a semiconductor pn junction diode. The bipolar membrane exhibits similar rectifying ion current-voltage (I-V) characteristics as a semiconductor diode. Its I-V curve (Fig. 1) shows a high ion conductance with a positive potential applied to the cation-exchange membrane (CEM) and a saturated ion current when reversing the potential bias. The distinct ion conductance at opposite potential biases is attributed to the accumulation and depletion of ions inside the membrane in response to the direction of the applied electric field across the bipolar membrane. Rectified ion current has later been investigated using nanochannels or nanopores with breaking symmetry.34 In general, the devices that support asymmetric charge selectivity will lead to the asymmetric ion conductance and their configurations fall into at least three categories or their combinations: asymmetric surface charge26,27,35,36 asymmetric electrolyte conditions (e.g., ionic strength, pH, viscosity, and ion mobility),37,38 and asymmetric channel geometry.21–23,39,40 The diode-like behavior of hydrogel bipolar membranes was utilized to demonstrate ionic logic circuits.41 Kim et al. created an ionic junction field-effect transistor by placing a pair of cation-exchange hydrogel and anion-exchange hydrogel on both sides of a microfluidic channel.42 The device was designed to gate the ion conductance of the microchannel by inducing ion depletion upon the application of a voltage across the ion-exchange hydrogels. Sun managed the ion concentration polarization effect in membrane-integrated microfluidic channels to generate transistor-like switching characteristics with high ion current flux.43 The devices may serve as the building block for an ionic circuit platform.
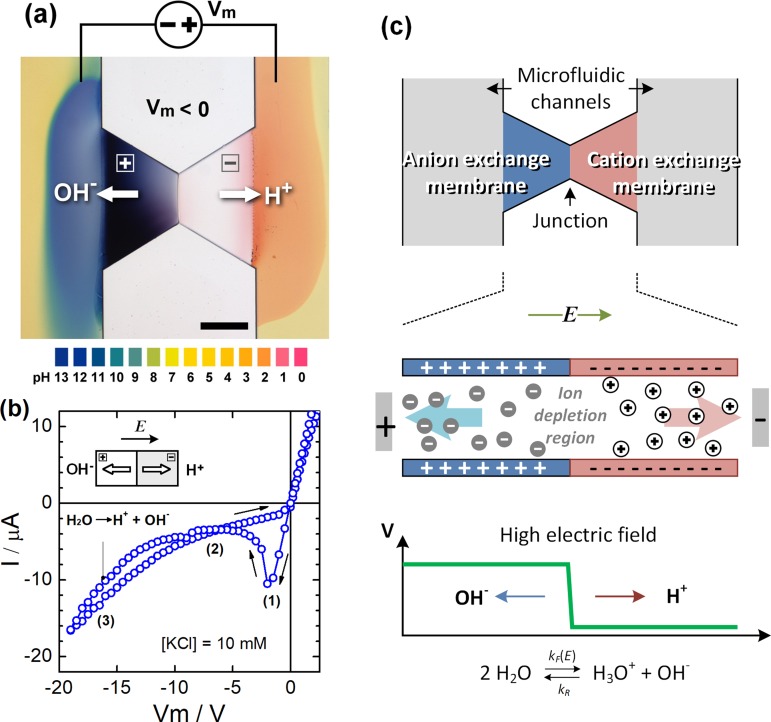
FIG. 1.
(a) Microscopy image of a reverse-biased bipolar membrane in the microfluidic channel that produces local pH changes through field-enhanced water splitting. (b) I-V characteristic of the bipolar membrane showing ionic rectification and water dissociation. (c) Schematics of the ion distribution and electric-field profile in a reverse-biased bipolar membrane. Reproduced with permission from Biomicrofluidics 5, 046502 (2011). Copyright 2011 AIP Publishing LLC.48
Another function that makes the bipolar membranes or ionic diodes useful is that one can apply an even greater reverse bias to enhance dissociation of water. In this condition, the large electric field depletes mobile ions from the junction of the two ion-exchange layers, forming a low ion conductive region with a thickness of a few Debye lengths. The entire applied voltage drops across such a short distance, creating a strong local electric field of the order of MV/cm. The high electric field increases the water dissociation rate constant, while the recombination rate remains unchanged according to Onsager's theory of the second Wien effect,44 which promotes strong splitting of water into protons and hydroxide ions which can be resolved in the local pH change shown in Fig. 1.45–48 In addition to high electric fields, it was suggested that water splitting can be elevated by protonation and deprotonation of the charged groups in the ion-exchange membranes.49–52
Field-enhanced water dissociation can be regulated by the voltage applied across a bipolar membrane. The effect was applied to produce precise pH regulation in microfluidic environments. The microfluidic pH control device called the pH actuator relies on the integration of hydrogel-based bipolar membranes in microfluidic devices.48 As shown in Fig. 2(a), two bipolar membranes are polymerized on the microfluidic chip to sandwich a channel; one serves as a proton pump, and the other serves as a hydroxide ion pump. Controlling the voltages across the membranes determines the amount of H+ and OH- ions injected into the stream which regulates its pH level without diluting the solution. The pH actuation technique is accompanied by specific microfluidic designs to generate constant pH levels or pH gradients across microfluidic channels. The method allows pH adjustment at the microscale without the electrolysis of water which is disadvantageous in the production of air bubbles. The technique was further utilized to demonstrate microfluidic free-flow isoelectric focusing (FF-IEF) for protein separation.53 The FF-IEF chip utilizes the pH actuator to generate a steady pH gradient in a downstream separation channel, Fig. 2(b). The pH actuation technique simplifies the IEF separation by providing stable pH gradients without an additional setup to generate multiple buffer inflows necessary in the conventional IEF process.
FIG. 2.
(a) Integrated bipolar membrane generates enhanced water dissociation for electrical pH control in microfluidic devices. Reproduced with permission from Biomicrofluidics 5, 046502 (2011). Copyright 2011 AIP Publishing LLC.48 (b) On-chip free-flow isoelectric focusing (FF-IEF) utilizes the integrated bipolar membrane to produce the pH gradient in the downstream separation chamber for protein separation. Reproduced with permission from Lab Chip 7, 979 (2014). Copyright 2014 The Royal Society of Chemistry.53
B. Ion concentration polarization for molecular preconcentration, water purification, and biosensing
Ion exchange membranes exhibit a nonlinear current-voltage (I-V) behavior characterized by three regimes as shown in Fig. 3(a).54–58 They are (1) Ohmic regime with a linear I-V relation at a low voltage; (2) limiting regime originated from an ion concentration polarization (ICP) effect in which ion depletion occurs at one side of the membrane due to the limited ion transport and results in a reduced ion conductance; and (3) overlimiting regime at a large voltage where the ion conductance increases due to multiple mechanisms, including the electro-driven vortices that destroy the resistive ion depletion zone or enhance the ion flux into the membrane and dissociation of water into protons and hydroxide ions induced by a high electric field. Enlarging the size of the microchannel connecting to the membrane was also found to induce electro-osmotic flow and electro-osmotic instability which lead to the overlimiting current.59 The ICP effect can be used to generate an ion depletion zone extended over a microfluidic channel. The ion depletion blocks the inflow of the charged species and, therefore, concentrates them at the edge of the ion-depleted zone [Fig. 3(b)].60,61 Han's group has demonstrated the use of nanochannels and ion-exchange membranes to produce ICP in microfluidics for efficient concentration of analytes.62–65 The ICP technique was also employed for desalination in microfluidics.66 A cation exchange membrane is placed at the entrance of one of the bifurcated channels. To desalinate the inflow, the membrane is biased in a way to create the ion depletion zone around the entrance to gate the ions from passing through. As a result, it forms a desalination channel and a brine channel. The microfluidic channel with a size on the order of hundred micrometers to millimeters is required to avoid turbulence flows that can disturb the ion depletion layer. This approach remains challenging because of its lack of feasible scale-up strategy, limited throughput, and possible contamination of chlorine species with desalted water. Another type of ICP desalination technique shown in Fig. 3(c) utilizes unipolar ion conduction to enhance salt removal efficiency and parallel channels to increase the process throughput.67,68 The current electrodialysis-based desalination method relies on both cation- and anion- exchange membranes to simultaneously remove both cations and anions in water. However, the difference in the diffusivities of both ions reduces the overall efficiency of desalination under a given current. An ICP desalination based on unipolar ion conduction conducting only cations or anions using the unipolar ion exchange membrane stack was found to save power consumption by ∼50% in the overlimiting regime, compared to conventional electrodialysis.
FIG. 3.
(a) Nonlinear I-V characteristics of an ion exchange membrane. (b) Ion concentration polarization (ICP) fulfilled by membrane-integrated microfluidics for preconcentration of molecules. Reproduced with permission from Top. Curr. Chem. 304, 153 (2011). Copyright 2011 Springer Nature.60 (c) Micro-ICP desalination platform formed by bounding two CEMs and two electrodes to a PDMS channel that bifurcates into desalted and brine downstream flows. Reproduced with permission from Sci. Rep. 6, 25349 (2016). Copyright 2016 Springer Nature 67 (d) Nucleic acid sensing platform utilizes the integrated membrane for DNA sensing which relies on the change in its nonlinear I-V characteristics in response to the binding of target DNA on the membrane surface. Reproduced with permission from Talanta 145, 35 (2015), and Biosens. Bioelectron. 60, 92 (2014). Copyright 2014 and 2015 Elsevier.69,72
The ion depletion and electro-driven vortices accountable for the limiting current and overlimiting regimes are sensitive to the environment in the vicinity of the membrane surface. The presence of large molecules on the membrane surface tends to eliminate the vortices and decreases the overlimiting current. The effect is more observable if the molecules have opposite charge to that on the membrane. For instance, the attachment of negatively charged DNA molecules to a positively charged anion-exchange membrane suppresses the development of ion depletion and vortices. Chang's group applied the electrokinetic property in the overlimiting regime for DNA sensing.69–73 The DNA sensor consists of an anion-exchange membrane with DNA probes functionalized on the depletion side of the membrane to provide selective detection. A nucleic acid sensing platform [Fig. 3(d)] was demonstrated by integrating all these ion-exchange membranes for molecular concentration and detection on a single chip. The platform was featured by a three-dimensional architecture in which the ion-exchange membranes were installed vertically to interface the microfluidic network from the top. The integration allows precise control of all the membranes on the chip to perform preconcentration of analyte and sensing by external electronic instrumentation.
C. Transistor-like switching
A field-effect nanofluidic transistor (FET) uses a gate electrode to control the ion conductance, which has a function analogous to a semiconductor field-effect transistor. The applied gate electrode modulates the surface potential of the channel wall and therefore the concentration of the majority of counterions in the channel. The structure of a nanofluidic FET can be a gated nanochannel74–76 or a gated nanopore which has the gate electrode embedded in sub-10-nm-sized nanopores.77,78 Researchers also demonstrated electrical gating of proton transport in aligned mesoporous silica thin films.79 The gating function enables control of molecular flows. For example, controlling the translocation of biomolecules, such as DNA and proteins, has been reported to improve the temporal resolutions in biomolecular imaging or DNA detection80,81 The confined geometry enabled by nanochannels has facilitated profiling of specific signaling proteins from individual cells.82 Protein analysis has been challenging as proteins tend to undergo large rearrangements from one conformation to another associated with the charged surface. Gated nanochannels can be used to direct flow control over protein analytes serving as a building block of integrated circuits for analysis of single cell contents.83 The small-volume flow control by the nanochannel also enables high-precision control in drug delivery.84 Like solid-state FETs, nanofluidic FETs also suffer the gate leakage current through the isolating dielectric layer which deteriorates the efficiency of the gate control. To prevent the gate leakage current, it is required to fabricate the channel structure using a high-quality dielectric material which may be formed by thermal oxidation or chemical vapor deposition and high-temperature annealing. Another fundamental issue that limits the performance of nanofluidic FET is the high dielectric constant of the aqueous solution in the channel. The field effect can manipulate mobile ions more efficiently when the dielectric constant of the gate insulating layer is much larger than that of the solution in the channel. With the dielectric constants of water being about 80 much larger than that of silica channels (i.e., 3–4), most of the gate potential drops across the low-k silica insulating layer leaving a very weak electric field in aqueous solution for ion control.
D. Memory effects
The hysteretic ion current observed in ionic devices can result from the transient process at a nonequilibrium condition discussed previously or a memory property of the material associated with the state and history of the device. The memory effect involves the changes in the device material property upon operation, such as redistribution of fixed charges or formation of a new material phase in the device. The device operating with such changes can function as a memristor (two-terminal “memory resistors”) or a memristive device. A memristor retains the internal resistance state according to the history of applied voltage and current. The transitions between distinct resistance states account for the switching mechanism of the device. One of the fingerprints of a memristive device is the self-crossing (pinched) hysteresis loop in the loci of the I-V response (Fig. 4). The change in the slope of the pinched hysteresis curves represents switching between different resistance states. A memristor is a promising device in neuromorphic applications as it behaves similar to a synapse. The device resistance is incrementally adjusted by the external electrical signal and can be used to implement learning and memory effects. It will find applications as diverse as nanoscale memory, sensors, and a new type of computer based on neuromorphic architecture. The neuromorphic system requires high-density memristors to form an artificial synaptic network to achieve low-power and high-efficiency computation.
FIG. 4.
(a) Fluidic-based ion memristor utilizes oxidization of the silicon electrode in the electrolyte to generate the memristive effect. (b) I-V characteristics and bistable resistive-state switching of an ion memristor. Reproduced with permission from Gongchen et al., Small 11, 5206 (2015). Copyright 2015 John Wiley and Sons.87
One type of solid-state memristor consists of a thin metal oxide layer sandwiched by metal electrodes. The structure enables resistive switching by controlling the growth of the nanoscale conductive filament across the metal oxide layer under voltage biases. An alternative material is based on soft materials in which polyelectrolyte-doped hydrogels are sandwiched with liquid metal electrodes.85 The device operates based on ion conductance in aqueous systems rather than conventional electron transport in solid state materials. The materials of the device are biocompatible and could be used to interface live neurons and biological tissues for in vivo operations. Researchers have been exploring different types of ionic memristors. For instance, a mixture of ionic liquid and water through a conical nanopore was found to exhibit memristive characteristics.86 Another class of ionic memristors called the fluidic-based ion memristor utilizes a silicon microelectrode in contact with an electrolyte solution to realize the resistive switching.87 As shown in Fig. 4(a), the memristive effect is due to anodic oxidation and cathodic deoxidation of the silicon microelectrode in an aqueous environment. The resistance of the silicon–electrolyte interface increases with the formation of silicon oxide at the interface with respect to the external voltage. The device is robust at high ionic strength. The ionic latch based on the memristor was demonstrated to store and sample electrical signals from the fluidic ionic system [Fig. 4(b)]. The function could assist multiplexed biosensing in providing nonvolatile memory and data acquisition of random transient signals from different sensors.
E. Energy conversion
Reverse electrodialysis (RED) converts the free energy from two salt solutions of different concentrations separated by an ion-exchange membrane. The electric potential established across the membrane is determined by the salinity ratio of two salt solutions.88–91 In general, the RED stack does not require any electrode for the conversion between electron current and ion current, different from the conventional electronic power sources, such as a battery or potentiostat.92 Han et al. demonstrated ionic circuits directly powered by a miniaturized RED stack [Fig. 5(a)]. The circuits built on ionic diodes composed of a pair of positively charged poly(diallyldimethylammonium chloride) (pDADMAC) and negatively charged poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (pAMPSA) polymerized in a microchip. In this ionic system, ionic diodes were connected through microfluidic channels to form a logic gate circuit. The ionic circuit and the RED were connected via electrolyte-filled tubings to establish ion conduction. The circuit was fully driven by the ionic current and voltage produced by the salinity gradient power in the RED. To adjust the voltage applied to the circuit, one can pinch the flexible tubing to control the cross-sectional area of the ion flux that resembles the function of a variable resistor. Figure 5(b) shows an ionic OR logic gate constructed by two ionic diodes.
FIG. 5.
(a) Ionic circuit powered by a reverse electrodialysis (RED) cell. (b) Ionic OR circuit with two ionic diodes formed by the connection of a pAMPSA cation exchange hydrogel and a pDADMAC anion exchange hydrogel. The input voltage was maintained at 3.1 V from RED. The digital output voltage corresponding to the bias across the ionic diode can be visualized by the fluorescence intensity of the pDADMAC stained by anionic fluorescein. The dark fluorescence indicates a forward-biased ionic diode. Reproduced with permission from Han et al., Sci. Rep. 7, 14068 (2017). Copyright 2017 Springer Nature.92
F. Ion-electron hybrid devices for the bio-machine interface
The emerging technology of wearable electronics and distributed electronics in the body may benefit medical diagnosis and therapeutics in providing real-time physiological monitoring and adaptive drug delivery. The development will enable therapies for disorders related to dysfunctional neural signalings, such as neuropathic pain,93 spinal cord injury,94 and Parkinson's disease.95 The technology requires a class of hybrid devices composed of biocompatible materials and capable of transducing ionic signals in biology to electronic signals in solid-state electronics and vice versa to communicate between biological systems and machines. The device will be used to detect the ionic signals associated with physiological states in biological systems and convert them into electronic readout for further data processing. Contrariwise, the device will transduce electrical signals to chemical or charge stimuli to regulate the physiology of cells, tissues, and organs.
An electrode interface is a typical transducer that manages potentials or currents between the electrons in the electronic circuits and the ions of biological tissue. The transduction can take place by charging the electric double layer at the electrode-solution interface or by undergoing electrochemical reactions of the species in the electrolyte. Another type of device can be made of the combination of an ion permselective polymer and a semiconductor. Several organic polymers that are conductive to both electrons and ions are promising materials for the biological-electronic interface.
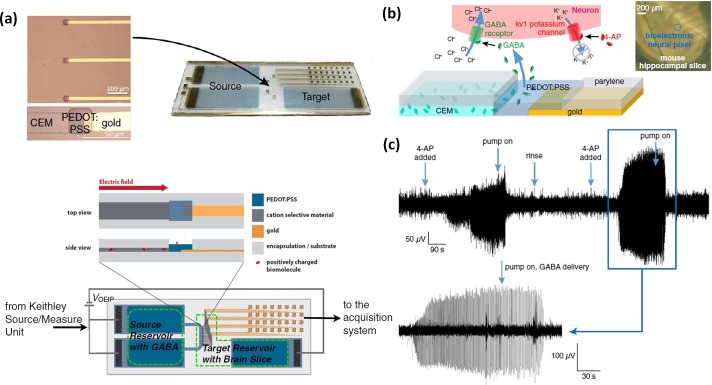
The hybrid conductive materials can be realized by incorporating ion exchange membranes with electronically and ionically conducting polymers, e.g., poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT:PSS). The PEDOT:PSS coated electrode allows reversible electron-to-ion conversion between the electrolyte and the electrode through redox reaction of the PEDOT polymer. Based on this material, Tybrandt et al.96,97 developed ionic bipolar junction transistors to demonstrate actively modulated delivery of the neurotransmitter glutamic acid. Gabrielsson made an ionic bridge rectifier by combining four bipolar membranes and PEDOT:PSS to convert an AC ionic current into a DC ionic current output.98 The ionic circuit was used to implement the electrophoretic delivery of neurotransmitters. Jonsson et al. used cation-exchange membranes and PEDOT:PSS to develop bioelectronic neural pixels to detect and record neural activity and deliver inhibitory neurotransmitters to the same sites as the recordings with feedback control.99 As shown in Fig. 6, the bioelectronic interfacing device electrophoretically delivers positively charged chemical stimulus through an organic electronic ion pump (OEIP) composed of a cation-exchange membrane (CEM) channel and a PEDOT:PSS outlet without fluid flows. The same PEDOT:PSS electrode detects the local neuronal response regarding ion fluxes in response to the chemical stimulation. The device shows the ability of both ionic signal detection and drug delivery, capable of obtaining the local therapeutic effects in the region of treatment. The device could be the foundation of a system for epilepsy treatment that detects an epileptic seizure at an early stage by delivering inhibitory neurotransmitters. The feedback control allows stopping seizures with a minimal amount of drug because the drug release could be efficiently regulated in response to the inhibitory effect measured in real time.
FIG. 6.
(a) Bioelectronic neural pixel for both drug delivery and neuronal signal recording. (b) A neural pixel has an organic electronic ion pump (OEIP) composed of the cation-exchange membrane (CEM) and conductive polymer PEDOT:PSS that conducts and delivers positively charged inhibitory neurotransmitter, γ-aminobutyric acid (GABA), to the local neuron of a mouse hippocampal. The same PEDOT:PSS electrode measures the local neuronal response in terms of ion fluxes. (c) Epileptiform activity of a mouse hippocampal preparation recorded from a single pixel before and during GABA delivery to the same pixel. Reproduced with permission from Jonsson et al., Proc. Natl. Acad. Sci. U.S.A. 113, 9440 (2016). Copyright 2016 United States National Academy of Sciences.99
V. OUTLOOK AND CONCLUSIONS
There is a fundamental difference between ionic systems and electronic systems in materials and fabrication technology. The aqueous media or polymeric materials required to support ion transport raise the challenge of device manufacturing and packaging that limit the device robustness and scaling capability. Although it is possible to mimic the operation of electronic devices using ionic systems, the solid-state materials are still superior in terms of operation speed, integration density, reliability, and cost-effectiveness. For ionic devices, one challenge arises from the need for dense packaging of the components to achieve the volume comparable to a biological neural system. This implies that a new set of materials and fabrication technologies is required to have many of the advantages of the biologically inspired computation but are easier to construct. The ionic devices made of soft and biocompatible materials will find more valuable applications in biological processing, biosensing, and the interface between electronic devices and biological systems. The bio-machine interface provides a bidirectional communication required to monitor the neuronal activity in the brain and to influence them by chemical stimulation to restore sensory functions for paralyzed individuals or for patients with motor disorders, such as Parkinson's disease.100 Ionic devices could potentially provide a stable and effective interface to mediate the soft, ion-rich neural tissue with the rigid, solid-state microelectronics and the instruments for signal readout, analysis, or feedback control for drug delivery.
ACKNOWLEDGMENTS
The author acknowledges partial financial support from the National Science Foundation (#1512816).
References
- 1. Van Roosbroeck W. V., Bell Syst. Tech. J. 29, 560 (1950). 10.1002/j.1538-7305.1950.tb03653.x [DOI] [Google Scholar]
- 2. Reiss H., J. Chem. Phys. 21, 1209 (1953). 10.1063/1.1699165 [DOI] [Google Scholar]
- 3. Shockley W., Nobel Lectures, Physics 1942–1962 ( Elsevier Publishing, Amsterdam, 1964), Vol. 344. [Google Scholar]
- 4. Mafe S., Manzanares J., and Ramirez P., Phys. Rev. A 42, 6245 (1990). 10.1103/PhysRevA.42.6245 [DOI] [PubMed] [Google Scholar]
- 5. Manzanares J., Murphy W., Mafe S., and Reiss H., J. Phys. Chem. 97, 8524 (1993). 10.1021/j100134a023 [DOI] [Google Scholar]
- 6. Cayre O. J., Chang S. T., and Velev O. D., J. Am. Chem. Soc. 129, 10801 (2007). 10.1021/ja072449z [DOI] [PubMed] [Google Scholar]
- 7. Han J. H., Kim K. B., Bae J. H., Kim B. J., Kang C. M., Kim H. C., and Chung T. D., Small 7, 2629 (2011). 10.1002/smll.201100827 [DOI] [PubMed] [Google Scholar]
- 8. Conroy D., Craster R., Matar O., Cheng L.-J., and Chang H. C., Phys. Rev. E 86, 056104 (2012). 10.1103/PhysRevE.86.056104 [DOI] [PubMed] [Google Scholar]
- 9. Drury J. L. and Mooney D. J., Biomaterials 24, 4337 (2003). 10.1016/S0142-9612(03)00340-5 [DOI] [PubMed] [Google Scholar]
- 10. Chung B. G., Lee K. H., Khademhosseini A., and Lee S. H., Lab Chip 12, 45 (2012). 10.1039/C1LC20859D [DOI] [PubMed] [Google Scholar]
- 11. Slaughter B. V., Khurshid S. S., Fisher O. Z., Khademhosseini A., and Peppas N. A., Adv. Mater. 21, 3307 (2009). 10.1002/adma.200802106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newton M., Bohaty A., White H. S., and Zharov I., J. Am. Chem. Soc. 127, 7268 (2005). 10.1021/ja043275d [DOI] [PubMed] [Google Scholar]
- 13. Choi E., Kwon K., Kim D., and Park J., Lab Chip 15, 168 (2015). 10.1039/C4LC01031K [DOI] [PubMed] [Google Scholar]
- 14. Choi E., Wang C., Chang G. T., and Park J., Nano Lett. 16, 2189 (2016). 10.1021/acs.nanolett.5b04246 [DOI] [PubMed] [Google Scholar]
- 15. Zharov I. and Khabibullin A., Acc. Chem. Res. 47, 440 (2014). 10.1021/ar400157w [DOI] [PubMed] [Google Scholar]
- 16. Schepelina O. and Zharov I., Langmuir 23, 12704 (2007). 10.1021/la702008j [DOI] [PubMed] [Google Scholar]
- 17. Guo L. J., Cheng X., and Chou C.-F., Nano Lett. 4, 69 (2004). 10.1021/nl034877i [DOI] [Google Scholar]
- 18. Liang X., Morton K. J., Austin R. H., and Chou S. Y., Nano Lett. 7(12), 3774 (2007). 10.1021/nl072253x [DOI] [PubMed] [Google Scholar]
- 19. Menard L. D. and Ramsey J. M., Nano Lett. 11, 512 (2011). 10.1021/nl103369g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong H. D., Jansen H. V., Gadgil V. J., Bostan C. G., Berenschot E., van Rijn C. J. M., and Elwenspoek M., Nano Lett. 4, 283 (2004). 10.1021/nl0350175 [DOI] [Google Scholar]
- 21. Siwy Z. S., Adv. Funct. Mater. 16, 735 (2006). 10.1002/adfm.200500471 [DOI] [Google Scholar]
- 22. Wei C., Bard A. J., and Feldberg S. W., Anal. Chem. 69, 4627 (1997). 10.1021/ac970551g [DOI] [Google Scholar]
- 23. Umehara S., Pourmand N., Webb C. D., Davis R. W., Yasuda K., and Karhanek M., Nano Lett. 6, 2486 (2006). 10.1021/nl061681k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yameen B., Ali M., Neumann R., Ensinger W., Knoll W., and Azzaroni O., J. Am. Chem. Soc. 131, 2070 (2009). 10.1021/ja8086104 [DOI] [PubMed] [Google Scholar]
- 25. Harrell C. C., Kohli P., Siwy Z., and Martin C. R., J. Am. Chem. Soc. 126, 15646 (2004). 10.1021/ja044948v [DOI] [PubMed] [Google Scholar]
- 26. Vlassiouk I. and Siwy Z. S., Nano Lett. 7, 552 (2007). 10.1021/nl062924b [DOI] [PubMed] [Google Scholar]
- 27. Yan R., Liang W., Fan R., and Yang P., Nano Lett. 9, 3820 (2009). 10.1021/nl9020123 [DOI] [PubMed] [Google Scholar]
- 28. Cheng L.-J. and Guo L. J., ACS Nano 3, 575 (2009). 10.1021/nn8007542 [DOI] [PubMed] [Google Scholar]
- 29. Duan C., Wang W., and Xie Q., Biomicrofluidics 7, 026501 (2013). 10.1063/1.4794973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia D., Yan J., and Hou S., Small 8, 2787 (2012). 10.1002/smll.201200240 [DOI] [PubMed] [Google Scholar]
- 31. Ha D., Hong J., Shin H., and Kim T., Lab Chip 16, 4296 (2016). 10.1039/C6LC01058J [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z., Wen L., and Jiang L., Chem. Soc. Rev. 47, 322 (2018). 10.1039/C7CS00688H [DOI] [PubMed] [Google Scholar]
- 33. Lovrecek B., Despic A., and Bockris J., J. Phys. Chem. 63, 750 (1959). 10.1021/j150575a030 [DOI] [Google Scholar]
- 34. Cheng L.-J. and Guo L. J., Chem. Soc. Rev. 39, 923 (2010). 10.1039/B822554K [DOI] [PubMed] [Google Scholar]
- 35. Karnik R., Duan C., Castelino K., Daiguji H., and Majumdar A., Nano Lett. 7, 547 (2007). 10.1021/nl062806o [DOI] [PubMed] [Google Scholar]
- 36. Ali M., Ramirez P., Mafe S., Neumann R., and Ensinger W., ACS Nano 3, 603 (2009). 10.1021/nn900039f [DOI] [PubMed] [Google Scholar]
- 37. Cheng L.-J. and Guo L. J., Nano Lett. 7, 3165 (2007). 10.1021/nl071770c [DOI] [PubMed] [Google Scholar]
- 38. Qiu Y., Lucas R. A., and Siwy Z. S., J. Phys. Chem. Lett. 8, 3846 (2017). 10.1021/acs.jpclett.7b01804 [DOI] [PubMed] [Google Scholar]
- 39. Jung J. Y., Joshi P., Petrossian L., Thornton T. J., and Posner J. D., Anal. Chem. 81, 3128 (2009). 10.1021/ac900318j [DOI] [PubMed] [Google Scholar]
- 40. Yan Y., Wang L., Xue J., and Chang H. C., J. Chem. Phys. 138, 044706 (2013). 10.1063/1.4776216 [DOI] [PubMed] [Google Scholar]
- 41. Han J. H., Kim K., Kim H., and Chung T., Angew. Chem., Int. Ed. 48, 3830 (2009). 10.1002/anie.200900045 [DOI] [PubMed] [Google Scholar]
- 42. Kwang B. K., Han J. H., Kim H. C., and Chung T. D., Appl. Phys. Lett. 96, 143506 (2010). 10.1063/1.3389492 [DOI] [Google Scholar]
- 43. Gongchen S., Satyajyoti S., and Chang H. C., Lab Chip 16, 1171 (2016). 10.1039/C6LC00026F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Onsager L., J. Chem. Phys. 2, 599 (1934). 10.1063/1.1749541 [DOI] [Google Scholar]
- 45. Strathmann H., Krol J. J., Rapp H.-J., and Eigenberger G., J. Membr. Sci. 125, 123 (1997). 10.1016/S0376-7388(96)00185-8 [DOI] [Google Scholar]
- 46. Mafe S. and Ramfrez P., Acta Polym. 48, 234 (1997). 10.1002/actp.1997.010480702 [DOI] [Google Scholar]
- 47. Simons R. and Khanarian G., J. Membr. Biol. 38, 11–30 (1978). 10.1007/BF01875160 [DOI] [Google Scholar]
- 48. Cheng L.-J. and Chang H. C., Biomicrofluidics 5, 046502 (2011). 10.1063/1.3657928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rubinstein I., Wrshawsky A., Schechtman L., and Kedem O., Desalination 51, 55 (1984). 10.1016/0011-9164(84)85052-3 [DOI] [Google Scholar]
- 50. Simons R., Desalination 28, 41 (1979). 10.1016/S0011-9164(00)88125-4 [DOI] [Google Scholar]
- 51. Simons R., Electrochim Acta 29, 151 (1984). 10.1016/0013-4686(84)87040-1 [DOI] [Google Scholar]
- 52. Simons R., Electrochim. Acta 30, 275 (1985). 10.1016/0013-4686(85)80184-5 [DOI] [Google Scholar]
- 53. Cheng L.-J. and Chang H. C., Lab Chip 7, 979 (2014). 10.1039/c3lc51023a [DOI] [PubMed] [Google Scholar]
- 54. Kooistra W., Desalination 2, 139 (1967). 10.1016/S0011-9164(00)84131-4 [DOI] [Google Scholar]
- 55. Frilette V. J., J. Phys. Chem. 61, 168 (1957). 10.1021/j150548a010 [DOI] [Google Scholar]
- 56. Rubinstein I. and Shtilman L., J. Chem. Soc., Faraday Trans. 2, 231 (1979). 10.1039/f29797500231 [DOI] [Google Scholar]
- 57. Barragan V. M. and Ruiz-Bauza C., J. Colloid Interface Sci. 205, 365 (1998). 10.1006/jcis.1998.5649 [DOI] [PubMed] [Google Scholar]
- 58. Chang H. C., Yossifon G., and Demekhin E. A., Annu. Rev. Fluid Mech. 44, 401 (2012). 10.1146/annurev-fluid-120710-101046 [DOI] [Google Scholar]
- 59. Dydek E. V., Zaltzman B., Rubinstein I., Deng D. S., Mani A., and Bazant M. Z., Phys. Rev. Lett. 107, 118301 (2011). 10.1103/PhysRevLett.107.118301 [DOI] [PubMed] [Google Scholar]
- 60. Senapati S., Basuray S., Slouka Z., Cheng L.-J., and Chang H. C., Top. Curr. Chem. 304, 153 (2011). 10.1007/978-3-642-23050-9 [DOI] [PubMed] [Google Scholar]
- 61. Slouka Z., Senapati S., and Chang H. C., Annu. Rev. Anal. Chem. 7, 317 (2014). 10.1146/annurev-anchem-071213-020155 [DOI] [PubMed] [Google Scholar]
- 62. Wang Y. C., Stevens A. L., and Han J. Y., Anal. Chem. 77, 4293 (2005). 10.1021/ac050321z [DOI] [PubMed] [Google Scholar]
- 63. Lee J. H., Song Y. A., and Han J. Y., Lab Chip 8, 596 (2008). 10.1039/b717900f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu V., Song Y. A., and Han J. Y., Lab Chip 10, 1485 (2010). 10.1039/b923214a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim P., Kim S. J., Han J., and Suh K. Y., Nano Lett. 10, 16 (2010). 10.1021/nl9023319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim S. J., Ko S. H., Kang K. H., and Han J., Nat. Nanotechnol. 5, 297 (2010). 10.1038/nnano.2010.34 [DOI] [PubMed] [Google Scholar]
- 67. Kwak R., Pham V. S., Kim B., Chen L., and Han J., Sci. Rep. 6, 25349 (2016). 10.1038/srep25349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim B., Kwak R., Kwon H. J., Pham V. S., Kim M., Al-Anzi B., Lim G., and Han J., Sci. Rep. 6, 31850 (2016). 10.1038/srep31850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Slouka Z., Senapati S., Shah S., Lawler R., Shi Z., Stack M. S., and Chang H.-C., Talanta 145, 35 (2015). 10.1016/j.talanta.2015.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Slouka Z., Senapati S., Yan Y., and Chang H. C., Langmuir 29, 8275 (2013). 10.1021/la4007179 [DOI] [PubMed] [Google Scholar]
- 71. Taller D., Richards K., Slouka Z., Senapati S., Hill R., Go D. B., and Chang H. C., Lab Chip 15, 1656 (2015). 10.1039/C5LC00036J [DOI] [PubMed] [Google Scholar]
- 72. Senapati S., Slouka Z., Shah S. S., Behura S. K., Shi Z., Stack M. S., Severson D. W., and Chang H. C., Biosens. Bioelectron. 60, 92 (2014). 10.1016/j.bios.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Egatz-Gomez A., Wang C., Klacsmann F., Pan Z., Marczak S., Wang Y., Sun G., Senapati S., and Chang H. C., Biomicrofluidics 10, 032902 (2016). 10.1063/1.4948525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karnik R., Fan R., Yue M., Li D., Yang P., and Majumdar A., Nano Lett. 5, 943 (2005). 10.1021/nl050493b [DOI] [PubMed] [Google Scholar]
- 75. Liu Y. and Yobas L., ACS Nano 10, 3985 (2016). 10.1021/acsnano.6b00610 [DOI] [PubMed] [Google Scholar]
- 76. Guan W., Fan R., and Reed M. A., Nat. Commun. 2, 506 (2011). 10.1038/ncomms1514 [DOI] [PubMed] [Google Scholar]
- 77. Gracheva M. E., Vidal J., and Leburton J. P., Nano Lett. 7, 1717 (2007). 10.1021/nl0707104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nam S. W., Rooks M. J., Kim K. B., and Rossnagel S. M., Nano Lett. 9, 2044 (2009). 10.1021/nl900309s [DOI] [PubMed] [Google Scholar]
- 79. Fan R., Huh S., Yan R., Arnold J., and Yang P., Nat. Mater. 7, 303 (2008). 10.1038/nmat2127 [DOI] [PubMed] [Google Scholar]
- 80. Paik K.-H., Liu Y., Tabard-Cossa V., Waugh M. J., Huber D. E., Provine J., Howe R. T., Dutton R. W., and Davis R. W., ACS Nano 6, 6767 (2012). 10.1021/nn3014917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kurz V., Tanaka T., and Timp G., Nano Lett. 14, 604 (2014). 10.1021/nl403789z [DOI] [PubMed] [Google Scholar]
- 82. Duncombe T. A., Tentori A. M., and Herr A. E., Nat. Rev. Mol. Cell Biol. 16, 554 (2015). 10.1038/nrm4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Karnik R., Castelino K., and Majumdar A., Appl. Phys. Lett. 88, 123114 (2006). 10.1063/1.2186967 [DOI] [Google Scholar]
- 84. Boukany P. E., Morss A., Liao W.-C., Henslee B., Jung H., Zhang X., Yu B., Wang X., Wu Y., Li L., Gao K., Hu X., Zhao X., Hemminger O., Lu W., Lafyatis G. P., and Lee L. J., Nat. Nanotechnol. 6, 747 (2011). 10.1038/nnano.2011.164 [DOI] [PubMed] [Google Scholar]
- 85. Koo H. J., So J. H., Dickey M. D., and Velev O. D., Adv. Mater. 23, 3559 (2011). 10.1002/adma.201101257 [DOI] [PubMed] [Google Scholar]
- 86. Sheng Q., Xie Y., Li J., Wang X., and Xue J., Chem. Commun. 53, 6125 (2017). 10.1039/C7CC01047H [DOI] [PubMed] [Google Scholar]
- 87. Gongchen S., Slouka Z., and Chang H. C., Small 11, 5206 (2015). 10.1002/smll.201501229 [DOI] [PubMed] [Google Scholar]
- 88. Hwang J., Sekimoto T., Hsu W.-L., Kataoka S., Endob A., and Daiguji H., Nanoscale 9, 12068 (2017). 10.1039/C7NR04387B [DOI] [PubMed] [Google Scholar]
- 89. Zhang Z., Kong X.-Y., Xiao K., Liu Q., Xie G., Li P., Ma J., Tian Y., Wen L., and Jiang L., J. Am. Chem. Soc. 137, 14765 (2015). 10.1021/jacs.5b09918 [DOI] [PubMed] [Google Scholar]
- 90. Veerman J., Saakes M., Metz S., and Harmsen G., J. Membr. Sci. 327, 136 (2009). 10.1016/j.memsci.2008.11.015 [DOI] [Google Scholar]
- 91. Post J. W., Veerman J., Hamelers H. V. M., Euverink G. J. W., Metz S. J., Nymeijer K., and Buisman C. J. N., J. Membr. Sci. 288, 218 (2007). 10.1016/j.memsci.2006.11.018 [DOI] [Google Scholar]
- 92. Han S. H., Kwon S.-R., Baek S., and Chung T. D., Sci. Rep. 7, 14068 (2017). 10.1038/s41598-017-14390-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Costigan M., Scholz J., and Woolf C. J., Annu Rev Neurosci. 32, 1 (2009). 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ho C. H., Triolo R. J., Elias A. L., Kilgore K. L., DiMarco A. F., Bogie K., Vette A. H., Audu M. L., Kobetic R., Chang S. R., Chan K. M., Dukelow S., Bourbeau D. J., Brose S. W., Gustafson K. J., Kiss Z. H. T., and Mushahwar V. K., Phys. Med. Rehabil. Clin. North Am. 25, 631 (2014). 10.1016/j.pmr.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Benabid A. L., Chabardes S., Mitrofanis J., and Pollak P., Lancet Neurol. 8, 67 (2009). 10.1016/S1474-4422(08)70291-6 [DOI] [PubMed] [Google Scholar]
- 96. Tybrandt K., Larsson K. C., Richter-Dahlfors A., and Berggren M., Proc. Natl. Acad. Sci. U.S.A. 107, 9929 (2010). 10.1073/pnas.0913911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tybrandt K., Gabrielsson E. O., and Berggren M., J. Am. Chem. Soc. 133, 10141 (2011). 10.1021/ja200492c [DOI] [PubMed] [Google Scholar]
- 98. Gabrielsson E. O., Janson P., Tybrandt K., Simon D. T., and Berggren M., Adv. Mater. 26, 5143 (2014). 10.1002/adma.201401258 [DOI] [PubMed] [Google Scholar]
- 99. Jonsson A., Inal S., Uguz I., Williamson A. J., Kergoat L., Rivnay J., Khodagholy D., Berg M., Bernard C., Malliaras G. G., and Simona D. T., Proc. Natl. Acad. Sci. U.S.A. 113, 9440 (2016). 10.1073/pnas.1604231113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rivnay J., Wang H., Fenno L., Deisseroth K., and Malliaras G. G., Sci. Adv. 3, e1601649 (2017). 10.1126/sciadv.1601649 [DOI] [PMC free article] [PubMed] [Google Scholar]