Abstract
Objective
To determine whether quantified pathologic response assessed as a percentage of residual tumor cells predicts recurrence-free survival (RFS) in patients with rectal cancer.
Methods
We studied 251 patients with rectal adenocarcinoma treated with neoadjuvant chemoradiation and radical resection. Quantified pathologic response was defined as an estimated proportion of residual cancer cells in relation to the tumor bed: complete, no residual cancer cells; near complete, ≤5%; major, >5% and <50%; and minor, ≥50%. The reproducibility of quantified pathologic response between 2 pathologists was assessed using tumors that did not show a complete response from 55 randomly selected patients.
Results
Pathologic response was complete in 21% of patients, near complete in 20%, major in 37%, and minor in 22%. Nineteen percent of patients had ypT0N0 disease, 27% ypT1-2N0, 21% ypT3-4N0, and 33% N+. The 5-year RFS rates by category of quantified pathologic response were as follows: complete, 95%; near complete, 88%; major, 69%; and minor, 61% (P < 0.001). Major and minor response, high histologic grade, and perineural invasion were significant predictors of decreased RFS in multivariate analysis. The 5-year RFS rates for patients with ypT3-4 or N+ disease were better for those with a near complete response (94%) than for those with a major (64%) or minor (61%) response (P < 0.02). Moderate to substantial agreement was observed between the 2 pathologists (κ = 0.72).
Conclusion
Quantified pathologic response is a predictor of RFS in patients with rectal adenocarcinoma and stratifies patients with high pathologic stage disease.
INTRODUCTION
Rectal cancer accounts for 28% of all cases of colorectal cancer, and an estimated 40,340 new patients will be diagnosed with rectal cancer in the United States in 2013.1 Most patients with rectal cancer present with locally advanced (stage II-III) disease, for which total mesorectal excision is the primary mode of therapy. The addition of preoperative chemoradiotherapy with fluoropyrimidine-based chemotherapy before total mesorectal excision improves oncologic outcomes in patients with locally advanced rectal cancer.2,3
Tumor regression after preoperative chemoradiotherapy for rectal cancer is an important secondary effect that can improve patient outcomes and the potential for achieving complete surgical resection.4 Multiple studies have demonstrated that total tumor regression or complete pathologic response to preoperative chemoradiotherapy is associated with improved outcome in patients with rectal cancer. The identification of complete pathologic response is increasingly being used to predict outcomes in patients with rectal cancer.5–7 However, complete pathologic response is demonstrated in only 15–20% of patients, necessitating additional categorization of pathologic response as a prognostic factor for most patients. Further classification of patients without a complete response may identify additional patients with improved outcomes and help differentiate them from those with an increased risk for recurrence. Subsequently this sub-grouping may improve the identification of patients who would benefit most from adjuvant chemotherapy or who may be eligible for subsequent treatment stratification.
Assessment of histopathologic response to chemoradiotherapy has been based on postoperative pathologic staging or tumor regression/pathologic response.8–13 Tumor regression/pathologic response is determined by the extent of replacement of the tumor bed with fibrosis and reduction in the number of tumor cells in the tumor bed. Previous studies of tumor regression/pathologic response either graded the fibrotic response or assessed the relative amount of residual tumor cells compared with fibrosis. However, these studies did not address the impact of variation in fibrotic response in different layers of the rectal wall on the measurement of pathologic response. Some of these studies included a category of no response, which is very rare in our and others’ experience. In addition, none of the previous studies examined the potential complementary value of pathologic response categorization with postoperative pathologic staging in predicting recurrence-free survival (RFS) in patients with rectal cancer.
Our group has previously demonstrated the prognostic relevance and reproducibility of pathologic response measured by quantitative assessment of residual tumor cell burden in esophageal cancer and hepatic colorectal metastases.14–16 However, this approach has not been tested in rectal cancer. In this large single-institutional study of locally advanced rectal cancer, we assessed the prognostic relevance of quantified pathologic response measured as residual tumor cell burden in surgically resected specimens of rectal cancer after neoadjuvant chemoradiation.
PATIENTS AND METHODS
Patients
We performed a retrospective cohort study of 251 patients with biopsy-proven, locally advanced (cT3-4 or cN+, staged by endorectal ultrasonography, computed tomography, or magnetic resonance imaging) rectal adenocarcinoma treated with preoperative chemoradiotherapy followed by radical resection at The University of Texas MD Anderson Cancer Center between 2000 and 2008. Patients were identified from our institutional colorectal cancer database and tumor registry. Patients with concurrent distant metastasis or concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, a need for emergency surgery, prior radiotherapy to the pelvis, or histology other than adenocarcinoma were excluded. The MD Anderson Institutional Review Board approved the study.
Clinical Staging, Treatment, and Pathologic Evaluation
In all patients, clinical stage was assessed by reviewing findings on endoscopic ultrasonography, magnetic resonance imaging, or computed tomography. All patients also underwent full colonoscopic evaluation to exclude additional tumors, as well as digital rectal examination and proctoscopy to determine the tumor location (distance from the anal verge). Neoadjuvant chemoradiotherapy consisted of a median radiation dose of 50.4 Gy and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent infusional fluorouracil or capecitabine). Surgery was typically performed 6 to 8 weeks following completion of chemoradiotherapy and consisted of low anterior resection, proctectomy with coloanal reconstruction, abdominoperineal resection, or multivisceral rectal resection using sharp mesorectal excision principles. Patients who underwent transanal local excision were excluded from our analysis. All medically fit patients were recommended to undergo adjuvant chemotherapy. The chemotherapy consisted of infusional fluorouracil or capecitabine for a period of 4 to 6 months. Oxaliplatin-containing regimens were introduced in 2003 at the treating physician’s discretion. In some cases, protocol-based concurrent chemotherapy included the addition of irinotecan or bevacizumab.
After resection, the specimen was meticulously examined and the entire tumor bed was embedded for hematoxylin and eosin staining and microscopic evaluation. Tumors were sampled for histopathologic examination using perpendicular sectioning of the epicenter of the tumor bed with part of the proximal and distal rectal wall in the same section. This technique was applied because in many cases it was essential to measure the distance between the tumor and the distal margin under the microscope. Most sections of the tumor bed contained both the center and the periphery of the tumor, and a couple of sections contained both sides (right and left or anterior and posterior) of the tumor bed. Each section of the tumor bed contained all layers of the rectal wall. The mesorectum was manually dissected, and lymph nodes were examined using 1 to 3 separate sections per node. A dedicated colorectal pathologist (A.A.) performed histopathologic assessment of all tumors in accordance with College of American Pathologists guidelines.17 Pathologic TNM stage was assessed as per the 7th edition of the American Joint Committee on Cancer staging guidelines.18 A margin was considered positive if tumor cells were present at or within 1 mm of the inked margin.
The extent of residual carcinoma for each tumor was assessed independently from nodal status by estimating the proportion of residual cancer cells in relation to the total tumor bed, as previously described.14–15 The tumor bed included grossly identifiable tumor or ulcer with mucosal congestion and fibrotic or edematous rectal wall. Histopathologic findings indicating the tumor bed included, tumor cells, areas of acellular mucin, chemoradiotherapy-related tissue injury with fibrocollagenous proliferation, vascular changes, mucosal ulcers, active chronic inflammation, and other reparative changes.
For each tumor, the percentage of the entire tumor bed occupied by cancer cells was estimated for each section, and the mean value of the individual section percentages was the percentage assigned to the tumor. In most cases, changes caused by chemoradiotherapy were patchy and sections with any chemoradiotherapy-related changes were included for estimation of residual tumor cell percentage. Because the percentage of tumor cells was assessed in all sections of the tumor bed regardless of the distribution of chemoradiotherapy-related changes, the tumor cell percentage was not dependent on the degree of fibrovascular change.
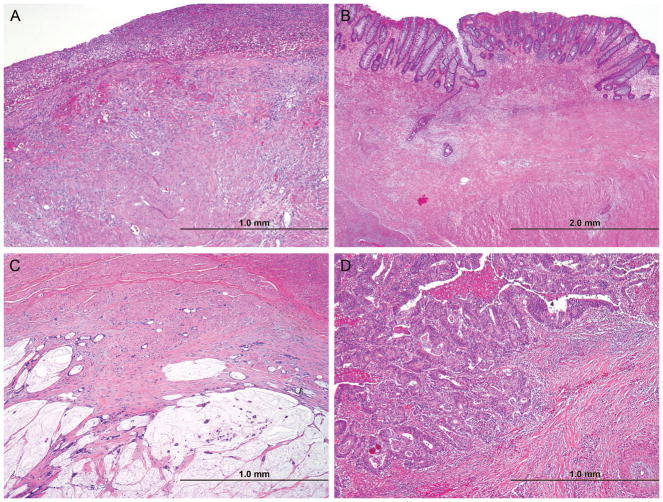
For the present study, 4 categories of quantified pathologic response were used: no residual cancer cells (complete pathologic response), up to 5% residual cancer cells remaining (near complete pathologic response), more than 5% and less than 50% residual cancer cells remaining (major pathologic response), and 50% or more residual cancer cells remaining (minor pathologic response; Figure 1).
FIGURE 1.
Photomicrographs showing representative examples of complete (A), near complete (B), major (C), and minor pathologic response (D). (Hematoxylin and eosin, magnification ×100.)
To assess the reproducibility of quantified pathologic response between 2 pathologists, slides from tumors that did not show a complete response from 55 randomly selected patients were independently reviewed by another pathologist (A.R.). The reviewing pathologists were blinded from the inclusion and exclusion criteria, treatment, study end point, and the 4 categories of residual tumor cell burden (pathologists reported only the percentage of residual tumor cells; categories were assigned later for analysis).
Postoperative follow-up consisted of routine physical examination and carcinoembryonic antigen measurement every 3 to 6 months, along with rectal examination or proctoscopy and cross-sectional imaging every 6 to 12 months for 5 to 7 years.
Statistical Analysis
Nonparametric data were expressed as medians and interquartile ranges and compared using the Kruskal-Wallis exact test. Follow-up duration was calculated using the reverse Kaplan-Meier method. RFS, defined as local or systemic recurrence or death, was calculated using the Kaplan-Meier method and compared using the log-rank test. Cases were censored if the outcome was a result of something other than recurrence (both local and distant) or death or if the patient was alive at the last follow-up. Univariate and multivariate analyses to identify predictors of survival were performed using the Cox proportional hazards regression model. Because ypT stage was highly correlated with the quantified pathologic response variable, it was not included in the multivariate analysis. Cohen’s Kappa was used to measure agreement between the 2 raters for quantified pathologic response category, as suggested by Landis and Koch.19 Because the quantified pathologic response categories are ordinal, weighted Kappa was used to assess disagreements, where a weight of 1.0, 0.5, and 0.0 indicates perfect agreement, partial agreement, and disagreement, respectively. We also evaluated the residual tumor cell category in a continuous form using linear regression for better visualization of the agreement pattern. The amount of variance of the dependent variable explained by the explanatory variable was determined by R2. P < 0.05 was considered statistically significant in all analyses. Analyses were performed using Stata MP, version 11.0 (College Station, TX).
RESULTS
Patient and Tumor Characteristics
A total of 157 men (63%) and 94 women (37%) were included in the analysis. The median age was 55 years (interquartile range [IQR], 49 to 65 years). The median tumor distance from the anal verge was 6 cm (IQR, 3 to 8 cm). Most tumors were cT3 on preoperative evaluation (n = 205, 82%). All patients underwent total or tumor-specific mesorectal excision depending on the extent of the tumor. One hundred three patients (41%) underwent low anterior resection, 81 patients (32.3%) underwent proctectomy with coloanal anastomosis, 61 patients (24.3%) underwent abdominoperineal resection, and 6 patients (2.4%) underwent other procedures. One hundred eighty-six patients (74%) had a sphincter-preserving operation.
Pathologic classification was ypT0N0 in 47 patients (18.7%), ypT1-2N0 in 68 patients (27.1%), ypT3-4N0 in 53 patients (21.1%), and any ypT with pN+ in 83 patients (33.1%). Patients with higher ypT designations following chemoradiotherapy were more likely to have positive ypN status (P < 0.001). Lymphovascular invasion was noted in 52 patients (20.7%), and perineural invasion was noted in 26 patients (10.4%). Nine patients (3.6%) had positive radial margins, and 2 (0.8%) had microscopically positive distal margins.
Patient and Tumor Characteristics by Quantified Pathologic Response
As shown in Table 1, complete pathologic response was observed in 53 patients (21.1%), near complete pathologic response in 49 patients (19.5%), major pathologic response in 94 patients (37.4%), and minor pathologic response in 55 patients (21.9%). There were no significant differences in age, sex, tumor distance from the anal verge, or radiation dose among the categories of quantified pathologic response. However, perineural and lymphovascular invasion were more common and ypT and ypN designations were higher in patients with major and minor pathologic response.
TABLE 1.
Baseline Characteristics of Patients with Rectal Cancer by Quantitative Pathologic Response* to Neoadjuvant Chemoradiation†
| Characteristic | Complete Pathologic Response (n=53) | Near Complete Pathologic Response (n=49) | Major Pathologic Response (n=94) | Minor Pathologic Response (n=55) | P |
|---|---|---|---|---|---|
| Age at diagnosis, years | 0.14 | ||||
| ≤60 | 13 (24.5) | 17 (34.7) | 26 (27.7) | 13 (23.6) | |
| 61–75 | 22 (41.5) | 26 (53.1) | 43 (45.7) | 32 (58.2) | |
| >75 | 18 (34) | 6 (12.2) | 25 (26.6) | 10 (18.2) | |
| Sex | 0.12 | ||||
| Male | 29 (54.7) | 26 (53.1) | 63 (67) | 39 (70.9) | |
| Female | 24 (45.3) | 23 (46.9) | 31 (33) | 16 (29.1) | |
| Distance of tumor from anal verge, cm | 0.15 | ||||
| ≤5 | 7 (13.2) | 3 (6.1) | 5 (5.3) | 4 (7.3) | |
| 6–10 | 17 (32.1) | 22 (44.9) | 45 (47.9) | 23 (41.8) | |
| >10 | 28 (52.8) | 24 (49) | 37 (39.4) | 27 (49.1) | |
| Unknown | 1 (1.9) | 0 (0) | 7 (7.4) | 1 (1.8) | |
| Clinical T designation | 0.51 | ||||
| cT1/cT2 | 4 (7.5) | 5 (10.2) | 3 (3.2) | 3 (5.5) | |
| cT3 | 46 (86.8) | 39 (79.6) | 77 (81.9) | 43 (78.2) | |
| cT4 | 3 (5.7) | 3 (6.1) | 10 (10.6) | 5 (9.1) | |
| Unknown | 0 (0) | 2 (4.1) | 4 (4.3) | 4 (7.3) | |
| Clinical N designation | 0.27 | ||||
| cN0 | 17 (32.1) | 17 (34.7) | 38 (40.4) | 22 (40) | |
| cN1/cN2 | 35 (66) | 29 (59.2) | 50 (53.2) | 26 (47.3) | |
| Unknown | 1 (1.9) | 3 (6.1) | 6 (6.4) | 7 (12.7) | |
| Tumor grade | 0.96 | ||||
| Low | 46 (86.8) | 41 (83.7) | 81 (86.2) | 47 (85.5) | |
| High | 6 (11.3) | 7 (14.3) | 12 (12.8) | 8 (14.5) | |
| Unknown | 1 (1.9) | 1 (2) | 1 (1.1) | 0 (0) | |
| Perineural invasion | <0.01 | ||||
| No | 38 (71.7) | 41 (83.7) | 70 (74.5) | 39 (70.9) | |
| Yes | 0 (0) | 1 (2) | 15 (16) | 10 (18.2) | |
| Unknown | 15 (28.3) | 7 (14.3) | 9 (9.6) | 6 (10.9) | |
| Lymphovascular invasion | <0.01 | ||||
| No | 38 (71.7) | 43 (87.8) | 64 (68.1) | 27 (49.10 | |
| Yes | 0 (0) | 1 (2) | 25 (26.6) | 26 (47.3) | |
| Unknown | 15 (28.3) | 5 (10.2) | 5 (5.3) | 2 (3.6) | |
| ypT designation | <0.01 | ||||
| 0 | 53 (100) | 0 (0) | 0 (0) | 0 90) | |
| 1 | 0 (0) | 19 (38.8) | 7 (7.4) | 1 (1.80 | |
| 2 | 0 (0) | 16 (32.70 | 31 (33) | 12 (21.8) | |
| 3 | 0 (0) | 12 (24.5) | 50 (53.20 | 40 (72.7) | |
| 4 | 0 (0) | 2 (4.1) | 6 (6.4) | 2 (3.6) | |
| ypN designation | <0.01 | ||||
| 0 | 47 (88.7) | 39 (79.6) | 57 (60.6) | 25 (45.5) | |
| 1 | 5 (9.4) | 9 (18.4) | 28 (29.8) | 25 (45.5) | |
| 2 | 1 (1.9) | 1 (2) | 9 (9.6) | 5 (9.1) | |
| Adjuvant chemotherapy | 0.22 | ||||
| No | 5 (9.4) | 2 (4.1) | 14 (14.9) | 8 (14.5) | |
| Yes | 48 (90.6) | 47 (95.9) | 80 (85.1) | 47 (85.5) | |
| Surgical margin status | 0.48 | ||||
| R0 | 41 (77.4) | 40 (81.6) | 69 (73.4) | 40 (72.7) | |
| R1 | 0 (0) | 1 (2) | 6 (6.4) | 2 (3.6) | |
| Unknown | 12 (22.6) | 8 (16.3) | 19 (20.2) | 13 (23.6) | |
| Type of surgery | 0.08 | ||||
| Abdominoperineal resection | 7(13.5) | 9(18) | 26(27.7) | 19(34.5) | |
| Coloanal anastomosis | 24(46.2) | 14(28) | 27(28.7) | 16(29.1) | |
| Low anterior resection | 19(36.5) | 27(54) | 39(41.5) | 18(32.7) | |
| Other | 2(3.8) | 0(0) | 2(2.1) | 2(3.6) | |
| Median radiation dose (interquartile range), Gy | 50.4 (46–50.4) | 50.4 (50.4–52.5) | 50.4 (50.4–50.5) | 50.4 (45–50.5) | 0.473 |
| Median follow-up duration (interquartile range), months‡ | 76 (53–98) | 65 (42–77) | 67 (50–83) | 75 (53–93) | 0.07 |
Complete response, no residual tumor cells; near complete pathologic response, ≤5% residual tumor cells; major response, >5% and <50% residual tumor cells; and minor response, ≥50% residual tumor cells.
Values in table are number of patients (percentage) unless otherwise indicated. Boldface indicates variables that are statistically significant.
Determined using the reverse Kaplan-Meier method.
Recurrence-free Survival
The median duration of follow-up for RFS was 70 months (IQR, 50 to 86 months). For the entire study population, the cumulative 3-year and 5-year RFS rates were 81.0% (95% confidence interval: 75.5–85.3) and 76.5% (95% confidence interval: 70.6–81.4), respectively.
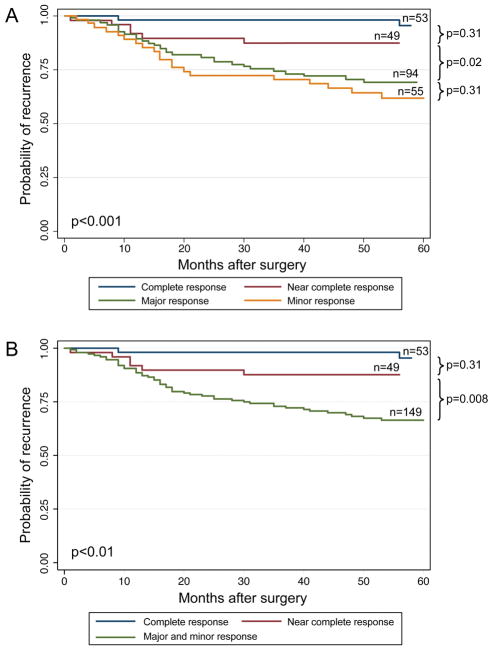
As shown in Figure 2, the cumulative 3-year and 5-year RFS rates, respectively, by categories of quantified pathologic response were as follows: complete response, 98.1% and 95.5%; near complete response, 87.3% and 87.3%; major response, 74.2% and 69.1%; minor response, 70.4% and 61.8% (P < 0.001). In subgroup analysis, differences in RFS between the complete and near complete response groups were not significant (P = 0.31). Additionally, differences in RFS were not observed between the major and minor response groups (P = 0.31) which had nearly overlapping RFS curves. However, significant differences were observed between the near complete and major response groups (P = 0.02). The RFS curves with the major and minor response groups combined are shown in Figure 2B.
FIGURE 2.
Recurrence-free survival curves (2A) and (2B) for categories of quantified pathologic response.
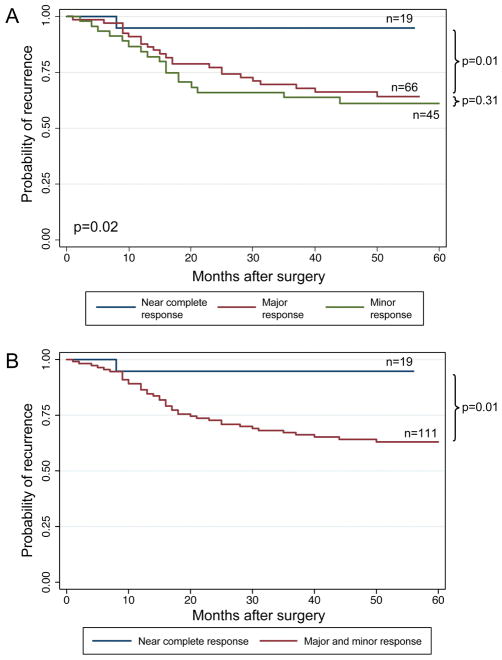
RFS curves for the subgroup of patients with ypT3-T4 or N+ disease are shown graphically in Figure 3. The cumulative 3-year and 5-year RFS rates, respectively, by categories of quantified pathologic response in this subgroup were as follows: near complete response, 94.7% and 94.7%; major response, 69.5% and 64.2%; minor response, 63.7% and 61.1%. Differences in RFS by category of response were significant (P = 0.02). In subgroup analysis, differences in RFS between the near complete and major response groups were significant (P = 0.02), but no significant differences were observed between the major and minor response groups (P = 0.31). The RFS curves with the major and minor response groups combined are shown in Figure 3B.
FIGURE 3.
Recurrence-free survival curves (3A) and (3B) for categories of quantified pathologic response for patients with ypT3-4 or ypN+ disease.
Results of univariate and multivariate Cox regression analyses of the predictors of RFS are shown in Table 2. In univariate analysis, factors associated with reduced RFS were major or minor pathologic response, high histologic grade, presence of perineural invasion, presence of lymphovascular invasion, high ypT designation, node-positive disease, and positive surgical margin. In the multivariate analysis, major and minor pathologic response, high histologic grade, and perineural invasion remained significant predictors of reduced RFS. Adjuvant therapy was associated with increased RFS in both univariate and multivariate analysis.
TABLE 2.
Factors Associated with Recurrence-free Survival as Estimated by Cox Regression Analysis
| Characteristic | Univariate Results | Multivariate Results | ||
|---|---|---|---|---|
|
| ||||
| HR | 95% CI | HR | 95% CI | |
| Quantitative pathologic response* | ||||
| Complete | 1 | REF | 1 | REF |
| Near complete | 1.95 | 0.55–6.95 | 2.04 | 0.55–7.56 |
| Major | 5.00 | 1.75–14.2 | 3.15 | 1.01–9.87 |
| Minor | 6.63 | 2.28–19.2 | 4.37 | 1.33–14.3 |
| Age at diagnosis, years | ||||
| ≤60 | 1 | REF | 1 | REF |
| 61–75 | 1.57 | 0.81–3.05 | 1.74 | 0.87–3.50 |
| >75 | 1.60 | 0.75–3.38 | 1.98 | 0.86–4.56 |
| Sex | ||||
| Male | 1 | REF | 1 | REF |
| Female | 0.74 | 0.43–1.28 | 0.89 | 0.48–1.64 |
| Distance of tumor from anal verge, cm | ||||
| ≤5 | 1 | REF | 1 | REF |
| 6–10 | 2.77 | 0.65–11.67 | 2.92 | 0.63–13.4 |
| >10 | 2.31 | 0.54–9.76 | 2.03 | 0.43–9.45 |
| Unknown | 8.30 | 1.67–41.26 | 7.92 | 1.33–47.1 |
| Clinical T designation | ||||
| cT1/cT2 | 1 | REF | 1 | REF |
| cT3 | 0.73 | 0.29–1.85 | 0.61 | 0.21–1.74 |
| cT4 | 0.83 | 0.24–2.90 | 0.60 | 0.14–2.50 |
| Unknown | 1.50 | 0.40–5.62 | 1.07 | 0.20–5.62 |
| Clinical N designation | ||||
| cN0 | 1 | REF | 1 | REF |
| cN1/cN2 | 0.83 | 0.49–1.41 | 0.97 | 0.52–1.80 |
| Unknown | 1.27 | 0.48–3.32 | 0.60 | 0.17–2.12 |
| Tumor grade | ||||
| Low | 1 | REF | 1 | REF |
| High | 1.98 | 1.07–3.65 | 2.44 | 1.13–5.26 |
| Perineural invasion | ||||
| No | 1 | REF | 1 | REF |
| Yes | 2.72 | 1.42–5.19 | 2.44 | 1.13–5.26 |
| Unknown | 1.07 | 0..51–2.21 | 2.13 | 0.66–6.82 |
| Lymphovascular invasion | ||||
| No | 1 | REF | 1 | REF |
| Yes | 2.15 | 1.24–3.72 | 1.07 | 0.55–2.09 |
| Unknown | 0.78 | 0.30–2.01 | 0.71 | 0.17–2.94 |
| ypT designation† | ||||
| 0 | 1 | REF | - | - |
| 1 | 2.91 | 0.78–10.85 | - | - |
| 2 | 3.32 | 1.08–10.18 | - | - |
| 3 | 5.30 | 1.87–15.02 | - | - |
| 4 | 18.64 | 5.38–64.55 | - | - |
| ypN designation | ||||
| 0 | 1 | REF | 1 | REF |
| 1 | 2.11 | 1.23–3.63 | 1.49 | 0.80–2.79 |
| 2 | 2.85 | 1.25–6.48 | 1.67 | 0.62–4.49 |
| Adjuvant chemotherapy | ||||
| No | 1 | REF | 1 | REF |
| Yes | 0.44 | 0.24–0.82 | 0.41 | 0.19–0.88 |
| Surgical margin status | ||||
| R0 | 1 | REF | 1 | REF |
| R1 | 3.01 | 1.19–7.63 | 1.85 | 0.59–5.77 |
| Unknown | 1.11 | 0.59–2.08 | 1.22 | 0.62–2.43 |
HR, hazard ratio, CI, confidence interval. Boldface numbers indicate statistically significant variables.
Complete response, no residual tumor cells; near complete pathologic response, ≤5% residual tumor cells; major response, >5% and <50% residual tumor cells; and minor response, ≥50% residual tumor cells.
ypT stage could not be included in the multivariate analysis because ypT stage and quantitative pathologic response categories were interdependent.
Reproducibility of Measurement of Quantified Pathologic Response
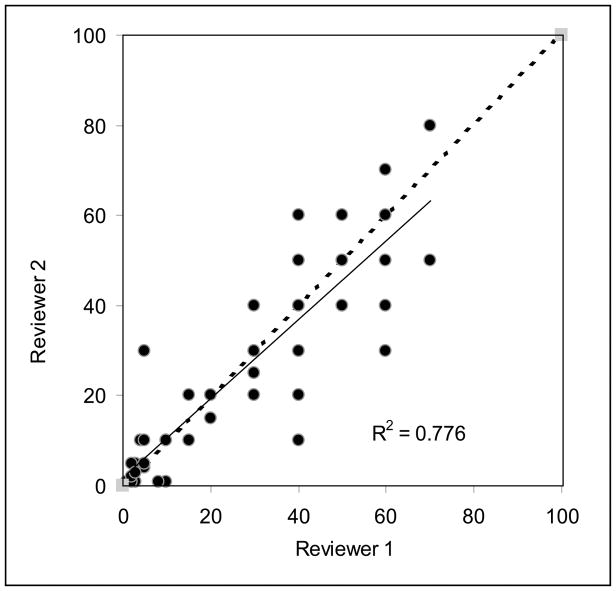
Both pathologists agreed with respect to categories of quantified pathologic response in 43 (78%) of 55 cases. The median difference between the measurements of percentage of residual tumor between 2 pathologists was 5%. Of the 14 patients classified by one pathologist as having a near complete response, 11 (79%) were classified as near complete response and 3 (21%) were classified as minor response by the other pathologist. Of the 23 patients classified by one pathologist as having a minor response, 19 (83%) were classified as minor response, 2 (9%) were classified as near complete response, and 2 (9%) were classified as major response by the other pathologist. Of the 17 patients classified by one pathologist as having a major response, 13 (76%) were classified as major response and 4 (24%) were classified as minor response by the other pathologist. Figure 4 shows a strong linear correlation between the 2 measurements of quantified pathologic response (R2 = 0.77). Moderate to substantial agreement was observed between the 2 pathologists for the categories of quantified pathologic response (κ = 0.72).
FIGURE 4.
Scatter plot showing the correlation between measurements of quantified pathologic response between 2 pathologists.
DISCUSSION
Results of the present study show that quantified pathologic response defined as the quantitatively assessed proportion of residual tumor cells is a predictor of RFS in patients with rectal cancer who are treated with neoadjuvant chemoradiotherapy followed by surgery. In addition, our findings show that quantified pathologic response further stratifies patients with high postoperative pathologic stage with respect to prognosis.
To the best of our knowledge, this is the first study from the United States demonstrating the prognostic significance of grading tumor regression/pathologic response in multivariate analysis in a large population of rectal cancer patients who were treated with neoadjuvant chemoradiation followed by surgical resection. Rodel et al8 described the prognostic significance of a 5-point grading system that was based on quantitation of fibrotic response in the tumor bed. This system showed significant correlation with overall survival outcome; grade 4 (complete regression) was associated with the longest overall survival rates (5-year overall survival rate, 86%), grades 0 (no response) and 1 (fibrosis in <25% of the tumor bed) were associated with the shortest overall survival rates (5-year overall survival rate, 63%), and grades 2 (fibrosis in ≥25–50% of the tumor bed) and 3 (fibrosis in >50% of the tumor bed) were associated with an intermediate overall survival outcome.
The American Joint Committee on Cancer/College of American Pathologists tumor regression grading system is based on the system originally described by Mandard et al20 in esophageal cancer and subsequently used by Ryan et al,21 Bouzourene et al,12 and Dworak et al22 in rectal cancer. This system does not require quantitative assessment of residual tumor cells and takes into account the density of tumor cells in association with the extent of fibrosis to grade the tumor regression. In our experience, the challenge with the tumor regression grading system is the very small number of patients in category 3, leading to a skewed distribution of patients in the other categories. In addition, we have observed variability of fibrotic response in different layers of the rectal wall. Uniform, easily identified fibrovascular changes are seen in the mucosa and submucosa. However, the muscularis propria shows minimal fibrosis, and fibrosis is patchy in perirectal soft tissue. In addition, methods based on assessing fibrosis do not account for acellular mucin, which is seen frequently in rectal cancer resection specimens. Fibrovascular response also likely varies among patients depending on their vascular health, serum cytokine levels, and inherent genetic variations in stromal cells. In the present study, quantified pathologic response was assessed by estimating the percentage of tumor cells in the entire tumor bed and was independent of degree of fibrosis.
The present study demonstrates the prognostic relevance of the near complete pathologic response category. Shia et al,23 in a univariate analysis of 69 patients, demonstrated a significant difference in patient outcome in those with more than 95% of the tumor replaced by fibrosis compared with those with <95% of the tumor replaced by fibrosis. In the present study, patients with the near complete response category demonstrated prolonged RFS even when the residual pathologic stage was advanced. The median 5-year RFS rate for patients with a near complete pathologic response was significantly higher than for patients with a major or minor response among all patients and patients with ypT3-4 or N+ stage. No significant differences in RFS were observed between the complete response and near complete response groups, although this may in part have been related to the low frequency of recurrence events in a relatively small cohort.
The addition of patients with near complete pathologic response to patients with complete pathologic response would expand the proportion of patients in the present study expected to have very good outcome after treatment of rectal cancer with neoadjuvant chemoradiotherapy to 41%. This group of patients who demonstrate a good response to treatment may be approached differently than the group of patients with major and minor response in terms of postoperative adjuvant chemotherapy and surveillance. In fact, the good responders may be most likely to benefit from continued 5-fluorouracil-based therapy and may not require as much intense surveillance as the major or minor response groups. Complete pathologic response has recently been accepted as a valid endpoint for phase III trials of treatments for breast cancer24 and has been recommended for use in trials of treatments for other solid tumors. Our findings suggest that for biomarker studies using pathologic response as an end point, it may be possible to consider the complete and near complete response groups together and compare them with the major or minor response groups.
This work builds on prior work from our group describing tumor response categories that are based on postoperative pathologic staging in a large cohort of patients undergoing multidisciplinary treatment for rectal cancer.13 Our findings in the present study indicate that the quantified pathologic response allows further stratification of patients on the basis of risk of recurrence among patients with high-stage disease (ypT3-T4 or N+), as described in our previous study.13 These findings are also complementary to our prior analysis, which showed that RFS rates in patients who had good response to neoadjuvant chemoradiation as evidenced by low postoperative pathology Tumor stage (ypT0-2), were not significantly different between patients with ypN0 and those with ypN+ disease.25
These findings require further validation, and additional insight may come from recently completed or ongoing studies, such as the intergroup PROSPECT study 26 or NSABP R-04 trial. Assessment of prognostic significance of quantified pathology response in patients of “Standard Arm” of PROSPECT trial may validate findings of present study which has similar treatment approach of preoperative radiation combined with 5FU/Capecitabine. Assessment of the quantified pathology response in “Selective Arm” will demonstrate impact of preoperative chemotherapy alone in achieving complete and near complete response and the additive effect of radiation in increasing the rate of achieving complete and near complete response. Application of this system of pathology response in patients enrolled in NSABPR-04 by combining complete and near complete response as good responders may facilitate in studying impact of oxaliplatin in preoperative chemoradiation for rectal cancer.
Limitations of our study include its retrospective nature, exclusion of residual tumor burden in regional lymph nodes, and the potential impact of variation in tumor cell density between the center and periphery of the tumor on measurement of quantified pathologic response. Most previous studies of tumor regression in rectal cancer and esophageal cancer assessed the tumor regression in the wall of the viscera and excluded the lymph nodes. However, a model that includes response in the rectal wall and lymph nodes and other pathologic parameters, similar to the model described by Symmans et al27 in breast cancer, may provide comprehensive risk assessment that is based on response to neoadjuvant therapy. The influence of tumor cell density variations was minimized in the present study by using a consistent approach of sampling in a single pathology laboratory. In previous studies involving esophageal cancer, we have shown a similar sampling technique with reproducible results for measuring pathologic response.28 The bias caused by the gradient of tumor cells between the center and periphery can be entirely excluded only by whole mount sections of the tumor bed, which is not routine practice for sampling rectal cancer in the United States.
In summary, we have described a new system of assessing response to chemoradiotherapy in rectal cancer that not only predicts RFS but also further stratifies patients with high postoperative pathologic stage with respect to RFS. In addition, our findings suggest that patients with a near complete pathologic response as described in this study may be considered together with patients with a complete pathologic response as patients with a good response to neoadjuvant therapy and excellent future prognosis. With validation in randomized datasets, quantified pathologic response may have implications for future study regarding intensity of postoperative chemotherapy and surveillance.
Acknowledgments
Source of Funding: Supported in part by Grant No. K07-CA133187 from the National Institutes of Health (NIH; G.J.C.) and Grant No. CA016672 from the NIH (MD Anderson’s Cancer Center Support Grant).
The authors thank Stephanie Deming and Erica Goodoff or editing and Kim-Anh Vu for assistance with images.
Footnotes
Presented at The United States and Canadian Academy of Pathology meeting, Baltimore, MD, on March 3, 2013.
References
- 1.American Cancer Society. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 3.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 4.Belluco C, De Paoli A, Canzonieri V, et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Ann Surg Oncol. 2011;18:3686–3693. doi: 10.1245/s10434-011-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas M, Nelemans PJ, Valentini V, et al. Long term outcome in patients with pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 6.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590–1598. doi: 10.1245/s10434-010-1506-1. [DOI] [PubMed] [Google Scholar]
- 7.Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09–01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 8.Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 9.Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236:75–81. doi: 10.1097/00000658-200207000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler JM, Warren BF, Mortensen NJ, et al. Quantification of histologic regression of rectal cancer after irradiation: a proposal for a modified staging system. Dis Colon Rectum. 2002;45:1051–1056. doi: 10.1007/s10350-004-6359-x. [DOI] [PubMed] [Google Scholar]
- 11.Huebner M, Wolff BG, Smyrk TC, et al. Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg. 2012;36:675–683. doi: 10.1007/s00268-011-1409-8. [DOI] [PubMed] [Google Scholar]
- 12.Bouzourene H, Bosman FT, Seelentag W, et al. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–1130. [PubMed] [Google Scholar]
- 13.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 15.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 16.Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31:58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 17.Washington K, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. doi: 10.5858/133.10.1539. Available at: http://www.cap.org/apps/cap.portal. [DOI] [PMC free article] [PubMed]
- 18.American Joint Committee on Cancer Staging Atlas. 7. New York: Springer; 2010. pp. 140–143. [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 20.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Ryan R, Gibbons D, Hyland JM, et al. Pathologic response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopath. 2005;47:141–6. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 22.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 23.Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28:215–23. doi: 10.1097/00000478-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Prowell T, Pazdur R. Pathologic complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 25.Park IJ, You YN, et al. Comparative analysis of lymph node metastases in patients with ypT0–2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56(2):135–141. doi: 10.1097/DCR.0b013e318278ff8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collette L, Bosset JF, et al. Patients with curative resection of cT3–4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25(28):4379–4386. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]
- 27.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;28:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 28.Wu TT, Chirieac LR, Abraham SC, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31(1):58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]