Abstract
Vascular aging fundamentally contributes to large and small vessel disease. Despite the importance of such changes for brain function, mechanisms that mediate such changes are poorly defined. We explored mechanisms that underlie changes with age, testing the hypothesis that Rho kinase (ROCK) plays an important role. In C57BL/6 mice, baseline diameter of isolated pressurized parenchymal arterioles were similar in adult (4–5 month) and old mice (22±1 month)(~15±1 microns). Endothelium-dependent dilation was impaired in old mice compared to adults in a pathway-specific manner. Vasodilation to NS-309 (which activates small- and intermediate-conductance Ca2+ activated K+ channels in endothelial cells) was intact, while endothelial nitric oxide (NO) synthase (eNOS)-mediated vasodilation was reduced by 60% or more, depending on the concentration (p<0.05). A similar reduction was present in basilar arteries. Inhibiting both ROCK isoforms with Y-27632 restored the majority of endothelial function in old mice. Because genetic background is a determinant of vascular disease, we performed similar studies using FVB/N mice. Endothelial dysfunction was seen with aging in both FVB/N and C57BL/6 mice, although the magnitude was increased almost 2-fold in the latter strain (p<0.05). In both strains of mice, age-induced endothelial dysfunction was reversed by inhibition of ROCK2 with SLX-2119. Thus, aging impairs endothelial function in both cerebral arteries and parenchymal arterioles, predominantly via effects on eNOS-dependent regulation of vascular tone. The magnitude of these changes was influenced by genetic background and mediated by ROCK2.
Keywords: endothelium, SK/IK channels, small vessel disease, nitric oxide
Introduction
Both large and small vessel disease contribute to strokes (ischemic and hemorrhagic), cognitive deficits, and other forms of neurological dysfunction. Most of these diseases have an aging component. Despite such relationships, relatively little is known regarding the biology that underlies vascular aging in brain. Therapeutically, our ability to slow or reverse the progression of vascular aging is limited due to poor understanding of mechanisms that control these processes.
Major mechanisms that contribute to regulation of cerebral blood flow (CBF) are impaired with aging. Effects of this impairment (along with other vascular alterations) include chronic reductions in CBF (hypoperfusion) that progress with age.1–3 This includes endothelium-dependent mechanisms, neurovascular coupling, and autoregulation.2–4 In addition to its impact on CBF, loss of endothelial health contributes to other aspects of vascular disease including loss of collateral vessels, platelet activation, atherosclerosis, accumulation of β-amyloid, and tauopathy.4–6 A common feature of vascular disease is loss of protective effects of nitric oxide (NO) produced by the endothelial isoform of NO synthase (eNOS).5, 7 The impact of such a loss is highlighted by the finding that endothelial dysfunction is an independent determinant of vascular events including stroke.8, 9
Based on this background, the present study had several goals. First, we examined effects of aging on parenchymal arterioles. Although a key target of small vessel disease, very little is known about specific changes that occur in this segment of the circulation with age or disease.4 We examined effects of aging on two endothelium-dependent pathways (eNOS-dependent and eNOS-independent) as well as myogenic tone. Second, we compared parenchymal arterioles to the basilar artery. Third, because genetic background can impact vascular disease,10, 11 we used mice on two defined backgrounds. Lastly, to further explore mechanisms that might be involved in vascular aging, we looked at the role of Rho kinase (ROCK) and its ROCK2 isoform on age-induced dysfunction. We found that aging impaired endothelial function in cerebral arteries and parenchymal arterioles via effects on select pathways. The magnitude of change, but not the underlying mechanism, varied with genetic background. These studies also provide the first evidence that ROCK2 is a key contributor to vascular dysfunction with aging.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Experimental animals
Protocols were approved by the University of Iowa and the University of Vermont Animal Care and Use Committees. We studied C57BL/6 and FVB/N mice fed standard chow and water ad libitum. Care and use of mice met the standards set by the National Institutes of Health for experimental animals. Details regarding the experimental procedures are presented in the online-only Date Supplement. Briefly, two segments of the vasculature were examined, looking at effects of aging, the role of ROCK, and the impact of genetic background.
Statistical analysis
All data are expressed as means±SE. Responses in parenchymal arteriole were expressed as percent change. Responses in basilar arteries were expressed as percent dilation (% of maximum), with 100% representing the difference between the resting value under basal conditions and the constricted value with U46619. Comparisons were made using a t-test or two-way analysis of variance followed by Bonferroni post-hoc test as appropriate. Statistical significance was accepted at P<0.05.
RESULTS
Effects of aging on parenchymal arterioles
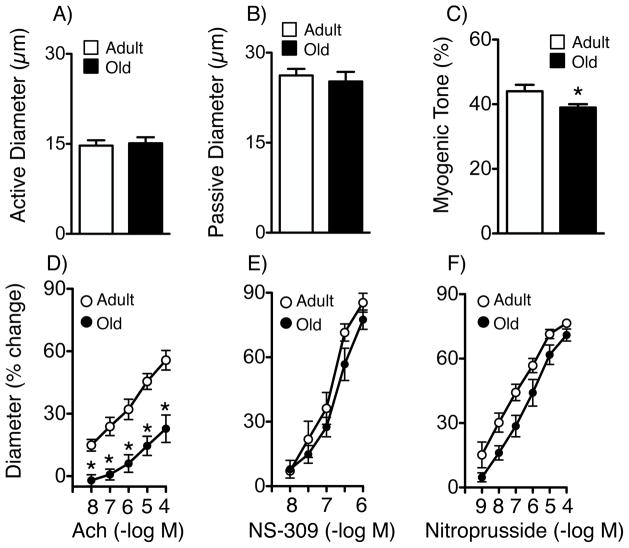
In C57BL/6 mice, baseline diameter of isolated parenchymal arterioles with active tone were similar in adult (4–5 mo of age) and old mice (22±1 mo)(14.7±0.9 and 15.1±1.1 μm in adult and old mice, respectively, p>0.05, Figure 1). Passive diameter in the same arterioles was 26.2±1.1 and 25.2±1.6 μm, respectively (Figure 1). Myogenic tone was 44±2% in adult mice and was reduced by 11.4% in arterioles from old mice (p<0.05, Figure 1).
Figure 1.
Active (A) and passive (B) diameter, myogenic tone (C), (A–C: young, n=15 arterioles from 15 mice; old, n=22 arterioles from 17 mice), responses to acetylcholine (Ach)(D, n=7–10), NS-309 (E, n=5–7) and nitroprusside (F, n=5) in adult and old mice. All data are mean±SE. * p<0.05 vs adult.
To determine the mediator of acetylcholine responses in parenchymal arterioles, we tested the role of NO using NG-nitro-L-arginine (L-NNA, 100 μmol/L), an inhibitor of NOS. Consistent with findings in human parenchymal arterioles,12 L-NNA nearly abolished acetylcholine-induced vasodilation (Figure S1). In contrast, NS-309 produces vasodilation by activation of small- and intermediate-conductance Ca2+ activated K+ channels (SK and IK, respectively) in endothelial cells.13, 14 The majority of the dilation of parenchymal arterioles to NS-309 was prevented by the combination of TRAM-34 (10 μmol/L) and UCL-1684 (0.1 μmol/L), IK and SK channel blockers, respectively (Figure S2A and B).
Because we did not observe any significant sex-dependent differences in dilation to acetylcholine or NS-309, responses from aged male and female mice were combined into one group. With aging, dilation of parenchymal arterioles to acetylcholine was reduced substantially, by 60% or more depending on the concentration (Figure 1). In contrast, vasodilation to NS-309 was not significantly affected (Figure 1). Arterioles dilated fully to the endothelium-independent agonists nitroprusside (Figure 1) and papaverine (Figure S3). We found previously that 0.1% DMSO (the concentration present in 1 μmol/L NS-309) only reduces tone in parenchymal arterioles by ~9%.14
Effects of aging on cerebral arteries: Role of ROCK
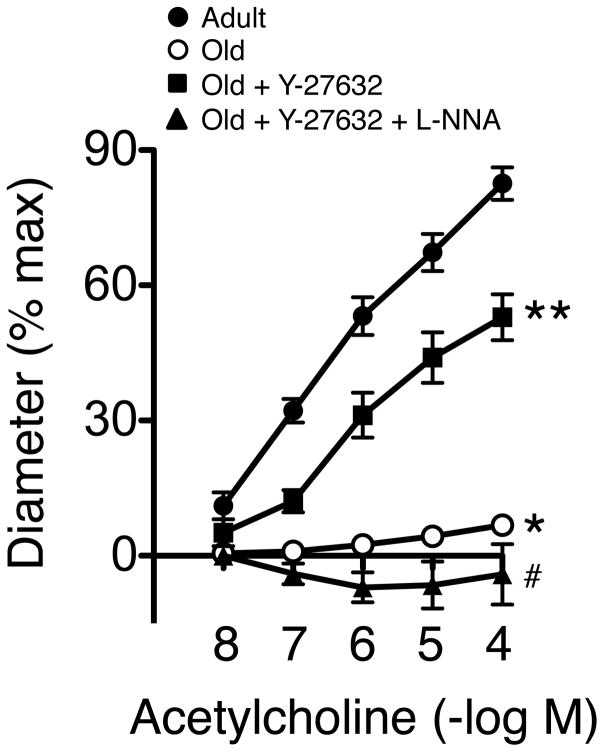
Similar to parenchymal arterioles, dilation of the basilar artery to acetylcholine was reduced by more than 90% in old mice (Figure 2). In examining mechanisms that might produce such effects, we tested the role of ROCK using Y-27632, an inhibitor of both ROCK1 and ROCK2.14 Acute treatment with Y-27632 (3 μmol/L) restored almost two-thirds of the vasodilator response to acetycholine in old C57BL/6 mice (Figure 2).
Figure 2.
Dilation of the basilar artery to acetylcholine in adult (5.6±0.4 mo of age, n=5) and old mice (21.2±0.2 mo, n=7) in the absence or presence of Y-27632 (n=7) or Y-27632 plus L-NNA (n=5). * p<0.05 vs adult; ** p<0.05 vs old; # p<0.05 vs old plus Y-27632.
Dilator responses of the basilar and middle cerebral arteries to acetylcholine are mediated by eNOS-derived NO.15, 16 Because ROCK exerts inhibitory effects on eNOS activity,14, 17 we determined if improved vasodilation to acetylcholine in arteries treated with Y-27632 was mediated by NO. In the presence of Y-27632 and L-NNA, dilation of arteries from old mice to acetycholine was essentially abolished (Figure 2), suggesting protective effects of Y-27632 involved restoration of NO-dependent signaling. These results are consistent with the concept that in the basilar artery, NO-independent mechanisms do not play much of a role in relation to acetylcholine-induced vasodilation.
Role of ROCK2 and the impact of genetic background
Both isoforms of ROCK are expressed in cerebral blood vessels,18 but surprisingly little is known regarding the relative contribution of each isoform.14 Thus, we tested the hypothesis that the ROCK2 isoform was involved in age-induced endothelial dysfunction. Because genetic background is a determinant of vascular function and may influence changes with aging, we performed the next series of studies using C57BL/6 and FVB/N mice, studying each strain at similar ages.
Baseline diameter of the basilar artery in adults was larger in FVB/N mice compared to C57BL/6 mice (by 18%, p<0.05)(Figures 3 and 4). With age, baseline diameter tended to increase in both strains but these differences were not significant (Figures 3 and 4)(p>0.05 vs adult). Baseline diameter was larger in old FVB/N compared to old C57BL/6 mice (by 12%, p<0.05). Based on two-way ANOVA, the dose response curve to acetycholine was not different in adult C57BL/6 versus adult FVB/N mice (Figure 5). In contrast, vasodilation to acetylcholine (1–100 μmol/L) was less in old C57BL/6 mice compared to old FVB/N (Figure 5). This difference was selective, as we observed full vasodilation to papaverine in both strains (Figures 3 and 4). Thus, aging reduced responses to acetylcholine in both strains, but the magnitude of reduction was greater in C57BL/6 mice. Despite this difference, SLX-2119 restored responses to acetylcholine in both stains of aged mice (Figures 3 and 4). SLX-2119 tended to increase vasodilator responses to acetylcholine in adult mice, but these effects were not statistically significant (Figures 3 and 4)(p>0.05).
Figure 3.
Baseline diameter of the basilar artery (A), responses to KCl (B), acetylcholine (Ach)(C) and papaverine (D) in adult (4.3±0.3 mo, n=5) and old (23.2±1.3 mo, n=7) C57BL/6 mice.
Figure 4.
Baseline diameter of the basilar artery (A), responses to KCl (B), acetylcholine (Ach)(C) and papaverine (D) in adult (4.2±0.3 mo, n=8) and old (22.3±0.1 mo, n=10) FVB/N mice.
Figure 5.
Responses of the basilar artery to acetylcholine in adult and old C57BL/6 and FVB/N mice. Ages and animal numbers of mice are provided in Figure 4.
DISCUSSION
There are several new findings in this study. First, brain parenchymal arterioles developed robust myogenic tone when pressured, results that are consistent with previous work in mouse, rat and human.12–14, 19 While aging was associated with a moderate reduction in myogenic tone, the mechanism that accounts for this change is unclear. Second, endothelium-dependent dilation in parenchymal arterioles was impaired with aging, but in an agonist-specific manner. Vasodilation to NS-309 was relatively intact while eNOS-mediated responses were substantially reduced. Third, similar to effects in parenchymal arterioles, aging produced endothelial dysfunction in a cerebral artery in both C57BL/6 and FVB/N mice. The magnitude of effect was greater in the C57BL/6 strain, revealing an influence of genetic background on cerebrovascular aging. Fourth, ROCK (specifically ROCK2) played a major role in age-induced endothelial dysfunction in both strains of mice. Conceptually, the results are important because in addition to providing new insight into effects of aging and underlying mechanisms in large and small vessels, they provide evidence some fundamental changes during aging are not fixed and may be reversed if the appropriate pathway is targeted.
Endothelial function in the aging brain
Endothelial dysfunction that worsens as aging progresses occurs in cerebral arteries in humans and animal models.16, 20–22 Similarly, impaired endothelium-dependent responses occur in arterioles on the brain surface (pial arterioles).23, 24 Measurements of local CBF 25, 26 suggest impairment of endothelial function extends deeper into the cortex. The results of the present study using pressurized parenchymal arterioles provide direct evidence in this regard.
We did not measure blood pressure in the current study. Previous studies found that blood pressure was not significantly altered in aged animals,23–25, 27, 28 while one study reported it was elevated modestly (~10 mmHg).29 Thus, it seems unlikely that changes in blood pressure made much of a contribution to endothelial dysfunction in the present study.
We detected a small reduction in responses to KCl and papaverine in the basilar artery with aging. While these differences were not statistically significant, we cannot exclude the possibility that changes in vascular mechanics contributed to this trend. Previous studies have described increased stiffness of cerebral arteries with aging.28, 29 Related to this point, parenchymal arterioles in old mice exhibited marked reductions in responses to acetylcholine but not papaverine. In mice, increased wall stiffness in cerebral arteries, but not parenchymal arterioles, was found with aging.29 Thus, substantial impairment of select vasodilator responses can occur with aging in the absence of a change in vascular distensibility.
Mechanisms of vascular aging
Although limited in number, some studies have examined mechanisms that contribute to cerebrovascular abnormalities with aging. In large arteries, mechanisms that have been implicated include angiotensin II (Ang II), oxidative stress (NADPH oxidase and the p66Shc adaptor protein), and poly-ADP-ribose polymerase.16, 21–23 Studies of pial arterioles and measurements of CBF suggest similar mechanisms contribute in the microcirculation.23, 25 A role for cyclooxygenase in microvascular dysfunction during aging has also been tested, with negative results.24
Pathway specific effects
The impact of aging on function of parenchymal arterioles has received very little study. In pial and parenchymal microvessels, changes in structure and permeability have been described.28–30 Consistent with our findings, Diaz-Otero et al found no change in passive diameter of brain parenchymal arterioles with aging.29 We now provide the first data related to age-induced changes in vascular function in pressurized parenchymal arterioles. Because mechanisms of endothelium-dependent dilation in parenchymal arterioles have unique features,19 it was difficult to predict whether changes would be similar to those in larger arteries upstream.16, 21–24 The finding that an eNOS-dependent response (acetylcholine-induced vasodilation) is substantially reduced with aging in parenchymal arterioles is consistent with data for larger arterioles and arteries. The finding that responses to NS-309 were largely preserved in parenchymal arterioles is interesting. Such results imply that despite reduced eNOS-dependent responses, the vasodilator pathway activated by NS-309 remains intact and thus might serve as a therapeutic target during aging, a condition in which chronic hypoperfusion is common and thought to contribute to changes in cognition.1, 2, 31 A limitation of the present study was that changes in membrane potential of endothelial cells were not measured directly in pressurized parenchymal arterioles.
To our knowledge, only one previous study looked at age-related effects on function of brain parenchymal arterioles. Using electrical field stimulation of brain slices to model neurovascular coupling,32 smaller increases in diameter of parenchymal arterioles occurred in old compared to adult mice. Mechanisms that contributed to these changes were not evaluated.
A limitation of the brain slice model is that vessels are not studied at constant pressure (or constant flow) or at optimal tension. A potential issue when studying isolated pressurized parenchymal arterioles relates to removal of vessels from the brain and possible interactions with perivascular cells. However, the current finding of endothelial dysfunction with aging is consistent with studies of pial arterioles in vivo and measurements of local CBF.23–26 Thus, the study of isolated parenchymal arterioles can be a good predictor of what occurs in vivo.
ROCK and vascular aging
In combination with RhoA, ROCK exerts effects in the vasculature, including effects on vessel tone.17 Within endothelial cells, ROCK inhibits activity of eNOS,17 an effect that would contribute to the progression of vascular disease. Although ROCK has been implicated in vascular disease,14, 17 there has been little attention to the possibility it contributes to changes with aging. ROCK promotes a senescent phenotype in cultured endothelium,33 while ROCK1 contributed to increased permeability in a model of aging, also based on cultured cells.34
Oxidative stress is a feature of vascular aging.2, 16, 21, 23, 25 In this context, interactions between RhoA/ROCK and reactive oxygen species have been described.17, 35 In many, but not all studies, Ang II activates ROCK.9, 14, 36 Thus, Ang II and oxidative stress may activate ROCK during aging. In addition, ROCK has inhibitory effects on eNOS, and loss of eNOS-derived NO may promote further ROS generation. Related to effects on eNOS, the NO component of endothelial function is progressively reduced with aging.16, 21, 26 The finding that inhibition of ROCK with Y-27632 substantially improved NO-mediated dilation in arteries from old mice is consistent with such a mechanism. Considering the lack of data in this area, it was difficult to predict which isoform of ROCK might be involved, but our data suggest that ROCK2 plays a major role. ROCK2 also contributes to endothelial abnormalities in a model of Ang II-induced vascular disease.14 Thus, ROCK2 may be a common underlying mechanism that affects endothelial cells in the face of major risk factors for vascular disease.
Impact of genetic background on vascular changes with aging
Genetic background influences major aspects of vascular biology.10, 37, 38 A limitation of most preclinical studies is that key observations are based solely on data from a single experimental strain.10, 39 Thus, an important issue which is often left unanswered is whether findings obtained are unique to a specific genetic background.
To enhance the current study, we performed studies using mice on two different genetic backgrounds. Because they are perhaps the most commonly used strain in cardiovascular and stroke related research, we used C57BL/6 mice. To determine if findings were unique to C57BL/6, we repeated some key experiments in FVB/N mice. FVB/N mice are much less susceptible to development of atherosclerosis compared to C57BL/6 mice,11 with differences in quantitative trait loci and lipid profiles. The severity of stroke and the subsequent immune response also differs between the strains.40 Considering these features, we felt a comparison of effects of aging on endothelial function in the two strains would be of interest. Responses of basilar arteries to acetylcholine were similar in adult C57BL/6 and FVB/N mice. However, while aging produced endothelial dysfunction in both strains, the effects were greater in magnitude in C57BL/6 mice. Despite this difference, the underlying mechanism was similar, as inhibition of ROCK2 restored endothelial function in both strains of mice.
Conclusions and Perspective
The basic biology of vascular aging has been greatly understudied relative to its clinical impact. When small vessel disease is taken into consideration, this gap becomes even greater. The present study provides new mechanistic insight into this area and supports several concepts. First, aged-induced endothelial dysfunction was present in cerebral arteries and small parenchymal arterioles. In both segments, eNOS-dependent responses were affected and may contribute to chronic reductions in CBF seen in aged humans and mice.1, 9, 31, 41 In addition to effects on vascular tone, eNOS deficiency (combined with aging) produces platelet dysfunction, infarction, and cognitive deficits.42 Second, in parenchymal arterioles, responses to NS-309 were intact suggesting an endothelium-dependent hyperpolarization mechanism was present and functional despite aging. Third, ROCK2 was implicated as an important contributor to vascular abnormalities, a potentially significant finding considering activity of ROCK has been positively associated with vascular events including stroke.43 Lastly, genetic background influences the magnitude of age-induced endothelial changes.
Supplementary Material
Novelty and Significance.
1. What Is New?
Endothelium-dependent dilation in parenchymal arterioles with myogenic tone was impaired with aging, but in an agonist-specific manner.
Similar to effects in parenchymal arterioles, aging produced endothelial dysfunction in a large cerebral artery in both C57BL/6 and FVB/N mice. The magnitude of the effect was greater in the C57BL/6 strain, revealing an influence of genetic background on a key element of vascular aging.
ROCK (specifically ROCK2) played a major role in age-induced endothelial dysfunction in both strains of mice, providing the first evidence that ROCK2 is a key contributor to vascular dysfunction with aging.
2. What Is Relevant?
Relatively little is known regarding the biology that underlies vascular aging, particularly in brain. Our ability to slow or reverse the progression of vascular aging is limited, in part due to poor understanding of underlying mechanisms.
Little is known regarding the impact of specific ROCK isoforms or genetic background in vascular aging.
Effects of aging on function of parenchymal arterioles have been largely unexplored.
The results provide evidence that some fundamental changes during aging are not fixed and may be reversed by targeting of appropriate pathways.
3. Summary
This study provides new mechanistic insight and supports several concepts. First, aged-induced endothelial dysfunction was present in cerebral arteries and parenchymal arterioles. In both segments of the vasculature, eNOS-dependent responses were affected and may contribute to chronic reductions in CBF seen with aging. Second, in parenchymal arterioles, the dilator pathway activated by NS-309 was present and functionally intact despite aging. Third, ROCK2 was implicated as an important contributor to vascular abnormalities, a potentially significant finding considering activity of ROCK has been positively associated with cardiovascular events including stroke. Lastly, genetic background influences the magnitude of age-induced endothelial changes.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Institutes of Health (HL-113863, NS-096465, HL-136636), the Department of Veterans Affairs (BX001399), and the Fondation Leducq (Transatlantic Network of Excellence). Post-doctoral Fellowship support was provided by the National Health and Medical Research Council of Australia (1053786).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faraci F. Cerebral vascular dysfunction with aging. In: Masoro EJAS, editor. Handbook of the Biology of Aging. Academic Press; 2011. pp. 405–418. [Google Scholar]
- 3.Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Res Rev. 2016;30:49–60. doi: 10.1016/j.arr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 4.De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Molec Neurobiol. 2016;36:241–258. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120:449–471. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katusic ZS, Austin SA. Endothelial nitric oxide: Protector of a healthy mind. Eur Heart J. 2014;35:888–894. doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emdin CA, Khera AV, Klarin D, et al. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation. 2018;137:222–232. doi: 10.1161/CIRCULATIONAHA.117.028021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraci FM. Protecting against vascular disease in brain. Am J Physiol. 2011;300:H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H, Lusis AJ. Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metabol. 2017;25:248–261. doi: 10.1016/j.cmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sontag TJ, Krishack PA, Lukens JR, Bhanvadia CV, Getz GS, Reardon CA. Apolipoprotein A-I protection against atherosclerosis is dependent on genetic background. Arterioscler Thromb Vasc Biol. 2014;34:262–269. doi: 10.1161/ATVBAHA.113.302831. [DOI] [PubMed] [Google Scholar]
- 12.Elhusseiny A, Hamel E. Muscarinic- but not nicotinic-acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: A possible role for the M5 receptor subtype. J Cerebral Blood Flow Metabol. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Dabertrand F, Kroigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks D, Brayden J, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci. 2015;112:E796–805. doi: 10.1073/pnas.1420765112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Silva TM, Kinzenbaw DA, Modrick ML, Reinhardt LD, Faraci FM. Heterogeneous impact of ROCK2 on carotid and cerebrovascular function. Hypertension. 2016;68:809–817. doi: 10.1161/HYPERTENSIONAHA.116.07430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- 16.Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age. 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. doi: 10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD, Faraci FM. Genetic interference with peroxisome proliferator-activated receptor γ in smooth muscle enhances myogenic tone in the cerebrovasculature via a Rho kinase-dependent mechanism. Hypertension. 2015;65:345–351. doi: 10.1161/HYPERTENSIONAHA.114.04541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatake K, Kakishita E, Wakabayashi I, Sakiyama N, Hishida S. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke. 1990;21:1039–1043. doi: 10.1161/01.str.21.7.1039. [DOI] [PubMed] [Google Scholar]
- 21.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol. 2009;296:H1914–1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Savarese G, Perrone-Filardi P, Luscher TF, Camici GG. Enhanced age-dependent cerebrovascular dysfunction is mediated by adaptor protein p66Shc. Intl J Cardiol. 2014;175:446–450. doi: 10.1016/j.ijcard.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: Role of oxidative stress. Microcirculation. 2008;15:225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 24.Mayhan WG, Faraci FM, Baumbach GL, Heistad DD. Effects of aging on responses of cerebral arterioles. Am J Physiol. 1990;258:H1138–1143. doi: 10.1152/ajpheart.1990.258.4.H1138. [DOI] [PubMed] [Google Scholar]
- 25.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cerebral Blood Flow Metabol. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 26.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag W, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinh QN, Drummond GR, Kemp-Harper BK, Diep H, De Silva TM, Kim HA, Vinh A, Robertson A, Cooper M, Mansell A, Chrissobolis S, Sobey CG. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging. 2017;9:1595–1606. doi: 10.18632/aging.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res. 1990;66:1747–1754. doi: 10.1161/01.res.66.6.1747. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol. 2016;310:H365–375. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knox CA, Yates RD, Chen I, Klara PM. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathol. 1980;51:1–13. doi: 10.1007/BF00688844. [DOI] [PubMed] [Google Scholar]
- 31.Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA. Cerebral perfusion and the risk of dementia: A population-based study. Circulation. 2017;136:719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 32.Balbi M, Ghosh M, Longden TA, Jativa Vega M, Gesierich B, Hellal F, Lourbopoulos A, Nelson M, Plesnila N. Dysfunction of mouse cerebral arteries during early aging. J Cerebral Blood Flow Metabol. 2015;35:1445–1453. doi: 10.1038/jcbfm.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usui S, Iso Y, Sasai M, Mizukami T, Mori H, Watanabe T, Shioda S, Suzuki H. Kisspeptin-10 induces endothelial cellular senescence and impaired endothelial cell growth. Clinical Sci. 2014;127:47–55. doi: 10.1042/CS20130505. [DOI] [PubMed] [Google Scholar]
- 34.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer C, Reinhart-King C. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simplicio JA, Hipolito UV, Vale GT, Callera GE, Pereira CA, Touyz RM, Tostes RC, Tirapelli C. Acute ethanol intake induces NADPH oxidase activation and RhoA translocation in resistance arteries. Arq Bras Cardiol. 2016;107:427–436. doi: 10.5935/abc.20160147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766. doi: 10.1161/HYPERTENSIONAHA.113.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasreen S, Nabika T, Shibata H, Moriyama H, Yamashita K, Masuda J, Kobayashi S. T-786C polymorphism in endothelial NO synthase gene affects cerebral circulation in smokers: Possible gene-environmental interaction. Arterioscler Thromb Vasc Biol. 2002;22:605–610. doi: 10.1161/01.atv.0000013286.60021.fe. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cerebral Blood Flow Metabol. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer CJ. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017;133:245–261. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HA, Whittle SC, Lee S, Chu HX, Zhang SR, Wei Z, Arumugam T, Vinh A, Drummond G, Sobey CG. Brain immune cell composition and functional outcome after cerebral ischemia: Comparison of two mouse strains. Front Cell Neurosci. 2014;8:365. doi: 10.3389/fncel.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz A, Lin A. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging. 2016;8:2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan XL, Xue YQ, Ma T, Wang X, Li JJ, Lan L, Malik K, McDonald M, Dopico A, Liao F. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener. 2015;10:24. doi: 10.1186/s13024-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension. 2014;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.