Abstract
Previous research has revealed that people can suppress salient stimuli that might otherwise capture visual attention. The present study tests between three possible mechanisms of visual suppression. According to first-order feature suppression models, items are suppressed on the basis of simple feature values. According to second-order feature suppression models, items are suppressed on the basis of local discontinuities within a given feature dimension. According to global-salience suppression models, items are suppressed on the basis of their dimension-independent salience levels. The current study distinguished among these models by varying the predictability of the singleton color value. If items are suppressed by virtue of salience alone, then it should not matter whether the singleton color is predictable. However, evidence from probe processing and eye movements indicated that suppression is possible only when the color values are predictable. Moreover, the ability to suppress salient items developed gradually as participants gained experience with the feature that defined the salient distractor. These results are consistent with first-order feature suppression models, and are inconsistent with the other models of suppression. In other words, people primarily suppress salient distractors on the basis of their simple features and not on the basis of salience per se.
Keywords: suppression, attentional capture, eye movements, visual attention
In daily life, people must frequently be alerted to important or dangerous information in the environment. To accomplish this, objects are often equipped with salient features, such as neon colors or flashing beacons. For example, bicyclists frequently wear brightly colored clothing and use flashing lights to attract the attention of busy drivers. This is based on an implicit assumption that salient stimuli will automatically attract an observer’s attention, even when the observer is not actively seeking these stimuli. However, researchers have actively debated whether salient signals automatically guide visual attention.
The field has traditionally been divided into to two competing theoretical camps, which make opposing predictions. Stimulus-driven theories propose that salient features automatically capture visual attention, regardless of the viewer’s goals (Franconeri & Simons, 2003; Theeuwes, 1992, 2010; Yantis & Jonides, 1984). Many studies use uniquely colored objects on a homogenous background (called color singletons) as the salient stimuli. Stimulus-driven theories garner support from studies using visual search tasks showing that the presence of salient stimuli can slow target detection (Theeuwes, 1992) and attract overt shifts of the eyes (Theeuwes, Kramer, Hahn, & Irwin, 1998).
Goal-driven theories, however, propose that visual attention is captured only by features that match the target of the search task (called an attentional set; Folk, Remington, & Johnston, 1992; Folk & Remington, 2010). These theories are supported by studies demonstrating that capture by salient items is contingent upon the item’s match with the feature used to locate the target (Bacon & Egeth, 1994; Folk, Leber, & Egeth, 2002; Folk & Remington, 1998; Lien, Ruthruff, Goodin, & Remington, 2008; Lien, Ruthruff, & Johnston, 2010). Goal-driven theorists reason that many supposed instances of “stimulus-driven” capture by color singletons results from the use of an attentional set that favors any type of feature singleton rather than a specific feature value (called singleton-detection mode; Bacon & Egeth, 1994).
At face value, these two theories make exactly opposite predictions about when to expect attentional capture, both in the laboratory and in daily life. However, substantial bodies of research have accumulated in favor of both theories, leaving the field in a quandary. The empirical stalemate has now lasted decades, and a resolution is badly needed: Attentional capture research has profound implications for the basic science of vision and is pertinent to the design of visual warning signals.
The Signal Suppression Hypothesis
One proposed reconciliation to the attentional capture debate is the signal suppression hypothesis (Sawaki & Luck, 2010). According to this hybrid model, all salient items produce a bottom-up salience signal that automatically attempts to attract spatial attention. However, this salience signal can be suppressed by a top-down inhibitory mechanism before attention shifts to the object generating the salience signal. Similar to stimulus-driven theories, this theory predicts that the most salient item in the visual field will capture attention by default, but only in the absence of top-down suppression. In the presence of top-down suppression, attentional capture can be avoided. Thus, as in goal-driven theories, the actual presence or absence of capture will depend on top-down attentional control. However, unlike goal-driven theories, the signal suppression hypothesis posits that salient items will capture attention even if they do not match the attentional set unless the suppression mechanism is used.
Initial evidence for suppression of salient stimuli came from an event-related potential (ERP) component called the distractor positivity (PD), which appears to reflect distractor suppression (Hickey, Di Lollo, & McDonald, 2009). Several studies showed that salient distractors elicit a PD component in the absence of behavioral capture (e.g., Eimer & Kiss, 2008; Gaspar & McDonald, 2014; Jannati, Gaspar, & McDonald, 2013; Sawaki & Luck, 2010, 2011). In most of these studies, however, the evidence for suppression was indirect: it is impossible to be certain that the ERP component observed in these experiments was the same as the PD component that reflects distractor suppression.
To provide converging evidence of suppression, Gaspelin, Leonard, and Luck (2015) used a novel behavioral task called the capture-probe paradigm (see also Kim & Cave, 1995). On most trials, participants performed a traditional capture task – they searched for a target shape (e.g., green diamond) and made a speeded response to the location of a dot within the target shape. An irrelevant color singleton could be present or absent. If this singleton captured attention, responses should be slower on trials where the singleton was present than on trials when it was absent (a singleton presence cost). On other trials, the search array appeared with probe letters superimposed over the stimuli, and participants performed a probe task in which they attempted to report as many of the probe letters as possible. The probability that the probe is reported at a given location can be used as an index of the degree of processing at that location. For example, probes presented at the target location were very likely to be reported. The key result was that, under conditions that elicited no singleton presence cost, participants were actually less likely to report letters at the singleton distractor location than at a typical nonsingleton distractor location. This result indicated that processing at the location of the salient item was suppressed.
Gaspelin, Leonard, and Luck (2017) provided converging evidence of suppression using a paradigm that measured overt eye movements. Participants performed a task that was nearly identical to the search trials in the aforementioned capture-probe study except that the search displays were modified to encourage eye movements and no probe stimuli were used. Consistent with the signal suppression hypothesis, participants’ first eye movements were less likely to land on singleton distractors than to land on nonsingleton distractors. In other words, overt shifts of visual attention to singleton distractors were suppressed below baseline levels. Similar results were reported in monkeys by Ipata, Gee, Gottlieb, Bisley, and Goldberg (2006).
Classes of Attentional Guidance Signals
Multiple sources of evidence now support the hypothesis of a mechanism that suppresses salient singleton distractors. However, it is not yet known how the visual system determines which items in the visual field should be suppressed, and the goal of the present study was to address this gap in knowledge. Before describing our specific hypotheses, however, we will first review the types of information that may be used to guide visual attention more generally.
The simplest possibility is that attention is guided by first-order features, which are the specific feature values of a single object (e.g., blue, horizontal, bright). Presumably, various dimensions of first-order features—such as color, orientation, and luminance—are represented in maps that indicate the locations of specific feature values in the visual field. These feature maps are constructed preattentively, allowing them to guide visual attention (Treisman & Gelade, 1980). First-order feature maps are a key component of virtually every model of visual attention (Itti & Koch, 2001; Luck, Girelli, McDermott, & Ford, 1997; Treisman & Gelade, 1980; Wolfe, Cave, & Franzel, 1989).
The visual system also represents second-order features, which are defined by relationships among the values within a first-order feature dimension (Cavanagh & Mather, 1989; Chubb & Sperling, 1989; Julesz, 1975). Feature discontinuities are a subclass of second-order features that have received considerable interest in attention research. They represent differences between the feature value of one object and nearby objects. For example, a color singleton is a particularly potent feature discontinuity that could potentially be used to guide attention, even if the observers do not know the first-order feature of the item for which they are searching (e.g., whether it is red among blue or green among yellow). Note that second-order feature discontinuities could be defined separately for each individual feature dimension. For example, color discontinuities could be represented separately from shape discontinuities. Second-order feature maps of feature discontinuities are proposed, most notably, by guided search models (Wolfe, 1994; Wolfe et al., 1989).
A third type of representation is a global-salience map, which combines information from multiple first- and second-order feature maps to provide a dimension-independent map of salience. Several models of visual attention posit the existence of such a dimension-independent salience map (Itti & Koch, 2001; Koch & Ullman, 1985; Treisman & Gelade, 1980; Treisman & Sato, 1990). The “activation map” in guided search models may also be viewed as a dimension-independent salience map (Wolfe, 1994; Wolfe et al., 1989).
Previous evidence strongly suggests that each of these three types of representations plays a role in guiding attention toward specific objects. For example, in visual search tasks, if the target color is known in advance, attention will be rapidly directed to objects containing that color, even if target-colored objects are not distinguished by second-order feature information or other bottom-up salience signals (Egeth, Virzi, & Garbart, 1984; Lien et al., 2008; Luck et al., 1997; Sun, Chubb, Wright, & Sperling, 2016; Woodman & Luck, 1999, 2003). Thus, first-order features can certainly guide attention. Furthermore, attention will be guided even more rapidly to an item defined by a particular color if that color is very different from the colors of the surrounding objects (Duncan & Humphreys, 1989; Julesz, 1975; Luck & Hillyard, 1994; Treisman & Gelade, 1980), providing evidence that second-order feature discontinuities can also guide attention. Other evidence suggests that attention can be guided by global salience, independent of both feature values and feature dimensions (Gottlieb, Kusunoki, & Goldberg, 1998; Itti & Koch, 2001).
Potential Mechanisms of Suppression
Because these three types of representations are used to guide visual attention toward specific objects, it is prima facie plausible that they could be used to guide attention away from specific objects. Moreover, as reviewed in this section, there is suggestive evidence that these representations can be used to guide suppression. However, the existing evidence is either indirect or incomplete. In the current study, therefore, we directly tested the role of each of these three types of representations in controlling the suppression of salient distractors. The three models are summarized in Table 1.
Table 1.
Potential Models of Suppression of Visual Attention
| Model | Description | Example | First-Order Feature Knowledge Required? |
|---|---|---|---|
| First-Order Feature Suppression | Visual system suppresses items defined by a specific feature value | “Suppress red items” | Yes |
| Second-Order Feature Suppression | Visual system suppresses items defined by local discontinuities within a specific feature dimension | “Suppress color singletons” | No |
| Global Salience Suppression | Visual system suppresses items defined by high salience, independent of specific feature values or dimensions | “Suppress any feature singleton” | No |
According to a first-order feature suppression model, the visual system can be set to ignore only specific feature values. For example, if a salient distractor is consistently red, participants may learn to suppress red items. Some evidence indirectly supports this possibility (Andrews, Watson, Humphreys, & Braithwaite, 2011; Arita, Carlisle, & Woodman, 2012; Cunningham & Egeth, 2016; Vatterott & Vecera, 2012; Watson & Humphreys, 1997; Woodman, Luck, & Schall, 2007). Notably, Vatterott & Vecera (2012) had participants search for a green circle amongst other green shapes and an irrelevant singleton distractor appeared on half of trials. Importantly, the color of the singleton remained constant over a block of trials and then changed for the next block (e.g., yellow in the first block, red in second block, blue in third block, etc.). In the first half of each block, there was a robust singleton presence cost, suggesting that the singleton captured attention. In the second half of each block, singleton presence costs were absent, suggesting that the singleton no longer captured attention. This was taken as evidence that, as participants gained experience with the particular feature value of color singleton, they were able to suppress items containing this feature value. This is an example of a first-order feature suppression model because participants required advance knowledge of the singleton color in order to suppress it. However, this study did not yield direct evidence of suppression per se: it simply yielded an absence of a singleton presence cost, which could mean that the singleton was treated equivalently with the nonsingleton distractor items rather than being suppressed.
According to a second-order feature suppression model, the visual system can be set to suppress local feature discontinuities on a specific feature dimension. For example, imagine a task where a participant is searching for a pop-out target defined by shape (e.g., a circle amongst diamonds), and a task-irrelevant color singleton is presented in an unpredictable color. To perform the task, participants might ignore feature discontinuities on the color dimension, but boost feature discontinuities on the shape dimension. Indeed, there is some evidence that visual attention can be selectively tuned to detect feature-discontinuities on a specific feature dimension (the dimensional weighting account; Found & Müller, 1996; Müller, Heller, & Ziegler, 1995; Müller, Reimann, & Krummenacher, 2003). For example, Müller, Heller, and Ziegler (1995) found that participants could rapidly detect feature discontinuities when the relevant feature dimension was known in advance (e.g., “find the orientation singleton”), but detection performance was poorer when the dimension of the feature discontinuity was unknown (e.g., “find the pop-out”). This is clear evidence that feature discontinuities may boosted on a specific feature dimension. However, it is unclear if visual attention can actually suppress feature discontinuities on a given feature dimension.
According to a global salience suppression model, the visual system can suppress the item with the strongest bottom-up salience signal (or any item that exceeds some threshold), irrespective of the features that produce that signal. Unlike the second-order feature suppression models mentioned above, global salience suppression models are completely blind to feature dimensions. In other words, the salience signal is a weighted sum across several dimensions (line orientation, color, shape, luminance) that is merged before it reaches the suppressive mechanism. This model also seems reasonable and is supported indirectly by previous research. For example, Sawaki and Luck (2010, Experiment 3) had participants search for a target letter (e.g., large A) amongst other letters and make a speeded button-press if it was present. On some trials, no target was presented and instead a color singleton distractor was presented. Importantly, the color of the target and singleton varied randomly from trial-by-trial in this experiment. The color singleton was a green singleton amongst red items on some trials, and it was a red singleton amongst green items on other trials. Thus, participants could not predict the upcoming singleton color. Nonetheless, the singleton elicited a significant PD component, suggesting that the singleton was suppressed even though participants had no foreknowledge of the singleton feature. However, this conclusion relies on the assumption that the PD component reflects suppression, which is not yet known with certainty to be true in all cases.
The Current Study
Each of these three models can explain the suppression of salient items observed in previous research (Gaspelin et al., 2015, 2017). In the current study, we distinguish between them by testing whether suppression requires advance knowledge of the salient item’s specific feature value. If so, this would effectively rule out the second-order suppression models and the global-salience suppression models, because both models predict that suppression of salient singletons can occur without foreknowledge of the upcoming singleton’s color value (see the rightmost column in Table 1). First-order feature suppression models uniquely predict that suppression of salient items is impossible without knowledge of the upcoming singleton’s color value.
We test the role of first-order feature knowledge by using converging methods that allow us to assess processing at each location. In Experiment 1, we use a capture-probe paradigm that is nearly identical to that used by Gaspelin et al. (2015, Experiment 4), except that we vary the stimulus colors randomly trial-by-trial. Experiment 2 replicates the findings of Experiment 1 with different stimuli and task timings. Experiment 3 extends these findings to overt shifts of visual attention, using an eyetracking paradigm similar to that used by Gaspelin et al. (2017, Experiment 3). Experiment 4 uses this same eyetracking paradigm to demonstrate that, as participants gain experience with a particular singleton feature value, they gradually learn to suppress that first-order feature value.
To preview the results, all four experiments provided strong evidence that first-order feature information is required to suppress singletons. Because Sawaki & Luck (2010) found that salient singleton distractors elicited a PD even when the target and singleton colors were unpredictable, we initially predicted that these experiments would support either the global-salience model or the second-order feature suppression model. However, we found evidence that in every case favored first-order feature suppression models and was inconsistent with global salience-based and second-order feature suppression models.
Experiment 1
In a previous study, Gaspelin et al. (2015, Experiment 4) used the capture-probe paradigm to demonstrate that people could suppress salient singletons when the singleton feature was predictable. The key finding was that participants were less likely to report probe letters at the singleton distractor location than at the nonsingleton distractor locations (called a probe suppression effect). The purpose of Experiment 1 was to determine whether this suppression effect remains when the singleton color is unpredictable. The method was identical to that of Gaspelin et al. (2015, Experiment 4), except that the singleton and nonsingleton colors swapped unpredictably from trial-to-trial.1 Both global salience models and second-order suppression models propose that people can suppress color singletons even if they do not know the first-order feature values of a given singleton. Thus, these models predict robust probe suppression effects. According to first-order feature suppression models, however, participants should not be able to suppress the singleton when they cannot predict its color. Thus, this model predicts that probe suppression effects should be eliminated in this experiment.
Methods
Participants
We chose an a priori sample size of 24 participants per experiment to match our prior experiments using the capture-probe paradigm (Gaspelin et al., 2015). After the completion of the experiment, we conducted a power analysis to determine whether our sample size had been adequate to detect the types of suppression effects we have observed in previous probe studies. We estimated the population effect size and standard deviation by pooling the probe suppression effects (see above) across participants from three previous experiments that were similar in methodology to the current experiment (Gaspelin et al., 2015, Experiments 2 – 4), yielding an N of 72 participants. The probe suppression effects in the pooled data were quite robust, with an effect size of dz = .970. Thus, to achieve a power of 95% and an alpha of 5% with this effect size, a sample size of 16 participants would be needed. Thus, our sample size of 24 participants was more than adequate to detect an effect of this magnitude. However, this effect size was estimated from a finite sample, and the actual population effect size may be substantially smaller than this estimate (leading to reduced power). We therefore computed a bootstrapped confidence interval on this effect size by sampling with replacement 10,000 times from the set of 72 participants (Efron & Tibshirani, 1993), which yielded a 95% confidence interval of dz = [.75, 1.24]. Thus, we can be 95% certain that the population effect size from these previous experiments is at least 0.75. With the sample size of 24 participants used in each of the present experiments, we had 94% power to detect an effect of this size. Thus, our power was quite high even with this very conservative estimate of the anticipated effect size.
The participants were University of California, Davis students who participated to receive course credit. One participant had abnormally low accuracy (more than 2.5 standard deviations from the group mean) and was replaced. Of the final sample participants, 21 were female and 3 were male. The mean age was 20.0 years. All participants reported (in every experiment reported here) had normal color vision as assessed by an Ishihara color vision test and had normal or corrected-to-normal visual acuity. This study received ethical approval from an institutional review board at the University of California, Davis.
Apparatus
Stimuli were presented using PsychToolbox (Brainard, 1997) on an HP ZR2440w LCD monitor with a black background that was placed at a viewing distance of 70 cm. A photosensor was used to measure the timing delay of the video system (32 ms), and this delay was subtracted from all latency values reported in this paper.
Stimuli & Procedure
The stimuli and procedure were identical to those in Gaspelin et al. (2015, Experiment 4), except that the singleton and nonsingleton colors swapped randomly trial-by-trial (see Figure 1). Each search display contained four shapes: a diamond (1.6° by 1.6°), a circle (1.4° diameter), a square (1.2° in width and height), and a hexagons (1.5° in width and height) drawn in green (30.5 cd/m2, x = .30, y = .61) or red (30.4 cd/m2, x = .64, y = .34). The target was always the diamond for half of the participants and always the circle for the other half. Each shape was centered 2.0° from fixation and contained a 0.2° black dot located 0.2° from either the left or right side of the shape. On probe trials, upper-case letters (0.8° tall) were presented in white (132.0 cd/m2) at the center of each shape using an Arial font, followed by # symbols as masks. A subsequent response screen contained all letters from the English alphabet in white. A gray fixation cross (30.3 cd/m2, 0.4° × 0/4°) was continuously visible except during the response screen and intertrial interval.
Figure 1.
Stimuli from Experiment 1, which were identical to those of Gaspelin et al. (2015, Experiment 4), except that the target and singleton color changed randomly trial-by-trial. On search trials, participants made a speeded button-press to the location of a dot (left or right) inside the target shape. On probe trials, letters appeared briefly at each search location and then were immediately masked. Participants reported as many letters as possible from this array, via mouse-click. In grayscale versions of this figure, red objects are outlined in solid lines and green objects are outlines in dotted lines.
On search trials (70% of trials), the task was to report whether the black dot was on the left or right side of the target shape (by pressing keys labeled “L” or “R” on the keyboard with their left hand). Target location and dot location varied randomly. All items were a single color on 50% of trials (red for half of the participants and green for the others), and one item was drawn in the other color on the remaining trials. The location of this color singleton distractor was random except that it was never the target location. Participants were told this and were encouraged to ignore the color singleton. Search trials began with a presentation of a blank screen for 500 ms followed by a fixation screen for 1000 ms. Next, the search array appeared until response. If participants took too long to respond (more than 2000 ms), a timeout display appeared with the text “Too Slow” for 500 ms. If the response was incorrect, a 200 Hz tone sounded for 500 ms.
On probe trials (30% of trials), a letter was presented inside each shape. The letters on a given trial were selected at random, without replacement, from the 26 letters of the English alphabet. On these trials, participants did not make a dot-location response but instead used the mouse to click on all letters on a response screen that they remembered seeing in the probe display (with no time pressure). The letter-probe array appeared for 100 ms. Next, to minimize any movement of spatial attention within iconic memory, the probe letters were immediately replaced with masks (“#” symbols) embedded inside the shapes for 500 ms (Loftus, Johnson, & Shimamura, 1985). Finally, the response screen appeared, and participants reported as many letters as they could remember (between 0 and 4) via mouse-click. Each letter in the response screen turned yellow when clicked, and the participant clicked a gray OK box (4.5 ° × 2.5°) when finished.
To achieve good performance in this paradigm, we have found that participants need substantial practice with the search task alone before the probe trials are added. Consequently, participants first practiced only the search task for two blocks of 48 trials. Then, participants practiced the combined capture-probe paradigm for two blocks of 48 trials. The main experiment consisted of 10 blocks of 48 trials, yielding 144 probe trials, 72 with and 72 without an irrelevant singleton. Participants received block-by-block feedback on mean response time (RT) and accuracy.
Analysis
Trials with an RT less than 200 ms or greater than 1,500 ms (0.9% of trials) were excluded from all search-task analyses. Additionally, trials with an incorrect response (2.2%) were excluded from search-task RT analyses. These trial-by-trial exclusion criteria were established a priori to match the methods used by Gaspelin et al. (2015), making it possible to directly compare results across studies.1 Mean accuracy for the search task was 97.8% (with the lowest accuracy being 94.7%).
We used t tests and ANOVAs for all statistical analyses. Because our data (especially the proportion correct values) may deviate significantly from normality, we additionally conducted a permutation test to compute a non-parametric p value for each t and F value (e.g., see Pitman, 1937; Welch, 1990). For the t tests and one-way ANOVAs, we created 10,000 permutations of the original data and conducted t or F tests on these permutations to obtain an empirical null distribution of the t or F statistic. For our 2 × 2 factorial ANOVAs, we reduced each main effect to a t test by averaging across the other dimension prior to permutation, and we reduced the interaction to a t test by computing a difference score for one dimension at each level of the other dimension, permuting the scores, and performing a t test comparing the two difference scores (Anderson & Braak, 2003). The resulting null distribution for a given test is the distribution of t or F values that would be expected to be obtained by chance alone. We then calculated the proportion of t or F values that were greater than the observed t or F value, which is the non-parametric p value for that test. We found that the permutation-based p values were quite close to the original p values from the parametric tests, and every permutation test in the present study produced the same accept/reject H0 decision as the corresponding parametric test. Thus, we report only the parametric p values.
We additionally report Cohen’s d as a measure of effect size for each experiment. We used ds for between-subject comparisons and dz for within-subject comparisons (for the exact formulas, see Lakens, 2013).
In cases where the absence of an effect was important for distinguishing between competing theories, we provide the Bayes factor corresponding to the t test (Rouder, Speckman, Sun, Morey, & Iverson, 2009), using the Jeffrey-Zellner-Siow prior on effect size with the default scale factor of 0.707. Bayes factors quantify the relative likelihood of obtaining the observed data under the null hypothesis compared to the alternative hypothesis, and they are equally well suited to quantifying the evidence for versus against the null hypothesis.
Results
Search Task Analysis
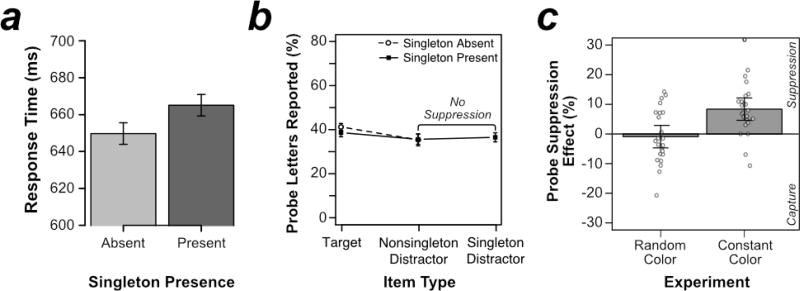
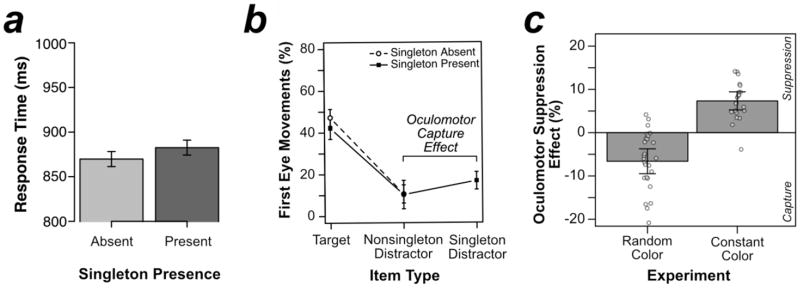
As shown in Figure 2a, responses in the search task were slower when the color singleton was present (665 ms) than when it was absent (650 ms), t(23) = 3.864, p < .001, d = .789. This 15-ms singleton presence cost may reflect genuine attentional capture by the color singleton; however, it may instead reflect a slowed decision about where to move attention rather than an allocation of attention to the location of the singleton (i.e., a filtering cost; Becker, 2007; Folk & Remington, 1998). Consequently, the probe trials are essential to provide a more conclusive answer about the allocation of visual attention to items in the search display (see below).
Figure 2.
Results from Experiment 1. (a) Mean response time (RT) from search trials. (b) Percentage of probe letters reported as a function of search-item type. Results are presented separately for trials on which the color-singleton distractor was present and trials on which it was absent. (c) Singleton suppression effects, calculated as probe report accuracy for the average of nonsingleton distractors minus singleton distractors, from Experiment 1 (random color) contrasted with Gaspelin et al. (2015, Exp. 4; constant color). Each white dot represents a unique participant’s probe suppression effect. Error bars in (a) and (b) represent the within-subjects 95% confidence interval (Cousineau, 2005; Morey, 2008). Error bars in (c) represent the between-subject 95% confidence intervals.
Error rates did not significantly differ between singleton-absent trials (2.2%) and singleton-present trials (2.1%), t(23) = .296, p = .770, d = .060.
Probe Task Analysis
Participants reported an average of 2.0 letters per trial, and 79% of these letters were actually present in the probe array. We calculated the proportion of probes that were reported at the target location, at the singleton location, and at each nonsingleton location. We then averaged across the nonsingleton locations to provide a “per location” measure of probe accuracy.
As shown in Figure 2b, probe letters inside the singleton distractor were approximately equally likely to be reported as probe letters inside nonsingleton distractor locations (means of 37% versus 36%, respectively). In other words, the probe suppression effect—calculated as probe report accuracy averaged across the nonsingleton distractor locations minus probe report accuracy at the singleton location—was −1% (see the leftmost bar in Figure 2c). A planned t test revealed no significant difference between these rates of report, t(23) = .491, p = .628, d = .100. If anything, the nonsignificant trend was opposite to the direction predicted by second-order and global salience suppression models – accuracy at the singleton location was slightly enhanced. Because conventional frequentist statistics cannot be used to draw strong conclusions about the lack of a difference between conditions, we computed the Bayes Factor for this comparison (Rouder et al., 2009). The Bayes Factor was 4.18 in favor of the null hypothesis, indicating that the data were considerably more consistent with the null hypothesis than with the alternative hypothesis.
We also examined how the presence or absence of the color singleton affected the report of probe letters at the target and nonsingleton-distractor locations. As shown in Figure 2b, participants were slightly more likely to report probe letters at the target location than probe letters at the nonsingleton distractor locations. However, the presence of a color singleton did not greatly impact target or distractor processing (e.g., see Gaspelin et al., 2015, Experiment 1, for an example of singleton presence harming detection of target probes). These effects were formally analyzed in a two-way analysis of variance (ANOVA) with factors of singleton presence (present vs. absent) and probe type (target vs. nonsingleton distractor). This analysis indicated that accuracy was significantly higher for probes at the target location (40%) than for probes at the nonsingleton-distractor locations (35%), F(1, 23) =18.734, p < .001, . This 5% difference reflects the attentional enhancement of the target stimulus. Note that experiments with longer exposure durations of the search array before the probe result in much larger target enhancement effects (see Experiment 2 or Gaspelin et al., 2015, Experiments 1 – 3). The analysis also indicated that there was no significant difference in probe report accuracy when the singleton was absent (38%) than when the singleton was present (37%), F(1, 23) = 2.373, p = .137, . Finally, the interaction of singleton presence and probe type was nonsignificant, F(1, 23) = 2.285, p = .144, .
We conducted planned t tests comparing probe report accuracy on singleton present trials and singleton absent trials. For probes at the target location, there was a trend for probe report accuracy to be higher on trials where the singleton was absent (41%) than trials where the singleton was present (39%), t(23) = 1.846, p = .078, d = .377. For probes at the nonsingleton distractor location, accuracy was virtually identical on trials where the singleton was present (36%) versus when it was absent (35%), t(23) = .234, p = .817, d = .048.
Across-Experiment Comparison
In the current experiment, we varied the singleton color randomly trial-by-trial (random-color), whereas Gaspelin et al. (2015, Experiment 4) used the same singleton color across the entire experimental session (constant-color). Given that these two experiments were identical except for the randomization of the singleton color, we directly compared performance on the probe task from the two experiments. In the present random-color experiment, we observed essentially no difference in performance between probes at the singleton and nonsingleton distractor locations. In the constant-color experiment (Gaspelin et al., 2015, Experiment 4), however, we observed an 8% suppression of performance for probes at the singleton location compared to probes at the nonsingleton distractor locations. These effects were formally analyzed in terms of probe suppression effects, calculated as probe report accuracy for nonsingleton distractors minus probe report accuracy for singleton distractors. As shown in Figure 2c, probe suppression effects were significantly larger when color was held constant (+8%) than when it varied trial-by-trial (−1%), t(46) = 3.59, p < .001, ds = 1.036. These results clearly support models of suppression based on the use of first-order feature values and are incompatible with global salience-based suppression models and second-order feature discontinuity suppression models.
Note that some individual participants exhibited substantial probe suppression effects, even though the mean across participants was near zero (see the single-participant values in Figure 2c). However, as described in the Supplementary Materials, we found no credible evidence that the data consisted of a mixture of some participants who were able to suppress the singletons and other participants who were captured by them.
Intertrial Priming Analysis
We also investigated the role of intertrial priming on attentional capture in the current experiment (Becker, 2010; Maljkovic & Nakayama, 1994). Although color could not be used to reliably locate the target in the present experiment, the target color on one trial may have automatically led to an implicit attentional set for that color on the next trial. Relatedly, the singleton color on one trial may have led to the automatic suppression of that color on the next trial. In both cases, this would cause participants to boost the relevant feature on the next trial.
We compared capture effects and probe effects on color repeat trials (e.g., green-red to green-red) and color swap trials (e.g., green-red to red-green). This analysis revealed that singleton presence costs and probe capture effects were large on color swap trials, confirming the effects of intertrial priming (see the Supplementary Material for more details). However, this analysis was underpowered because it involved dividing the already-small percentage of probe trials (30%) into two subsets of trials. Nonetheless, it does raise the possibility that the swapping of the target and singleton colors in the present experiment might have led to priming effects that artificially eliminated the ability to suppress the singleton color. This possibility is addressed in Experiment 2 and 4.
Discussion
In previous studies, we found strong probe suppression effects when we held the singleton color constant across the session (Gaspelin et al., 2015). The current experiment exactly replicated a previous experiment (Gaspelin et al., 2015, Experiment 4), but swapped the singleton and nonsingleton colors randomly trial-by-trial. Probe suppression effects were completely eliminated. This contradicts global salience suppression models and second-order feature discontinuity suppression models but is consistent with a first-order feature suppression model.2
Experiment 2
Experiment 2 replicated Experiment 1 with two changes designed to increase the possibility of observing probe suppression effects. First, we used four possible color configurations (green-pink, pink-green, blue-orange, and orange-blue). We reasoned that more variability in the colors might discourage participants from relying on specific color values and encourage them to use second-order feature discontinuities or overall salience levels to determine which item should be suppressed. Increasing the number of colors reduces the likelihood that the singleton color on a given trial would be the same as either the singleton color or the target color from the previous trial (and therefore reduced the impact of priming from the previous trial).
Second, we altered the timing of the probe trials to further encourage suppression. On probe trials, Experiment 2 provided a 100-ms preview of the search array before presenting the probe array. This has previously been shown to enhance target processing (e.g., Gaspelin et al., 2015), and it may also allow for increased sensitivity to probe suppression effects.
The predictions were identical to those of Experiment 1. According to global salience suppression models and second-order feature suppression models, participants will not require advance knowledge of the singleton color in order to suppress – thus, we should observe robust probe suppression effects. According to a first-order feature suppression model, however, participants require advance knowledge of the singleton color in order to suppress – thus, we should observe no probe suppression effects.
Methods
The methods were identical to those of Experiment 1, except for a few key changes. First, we ran a new set of 24 subjects on this experiment. This sample size was selected a priori to match the sample size of Experiment 1. One participant was replaced due to low accuracy (2.5 standard deviations below the group mean) and one participant was replaced due to slow overall response times (2.5 standard deviations above the group mean). In the final set of participants, 19 were female and 5 were male. The mean age was 20.5 years.
Search arrays were constructed from the following photometrically equiluminant colors: blue (30.5 cd/m2, x = .175, y = .175), orange (30.5 cd/m2, x = .175, y = .175), pink (30.7 cd/m2, x = .327, y = .199), and green (30.5 cd/m2, x = .293, y = .606). To assure that the singleton colors “popped out” of the display, each color was paired with an opposing color that was farthest away in hue within our color space (i.e., orange with blue and pink with green), yielding four color configurations depending on which color was the target and which item color was the singleton (green-pink, pink-green, orange-blue, blue-orange). The color configuration was chosen randomly trial-by-trial.
We also altered the timing on probe trials. Each probe trial began with the presentation of the search array alone for 100 ms. Next, the letter-probe array appeared for 100 ms and was immediately replaced with masks (“#”) embedded inside the shapes for 500 ms. This technique has been previously shown to lead to very high accuracy for the report of target probes, as well as large suppression effects (Gaspelin et al., 2015).
Results
Search Task Analysis
Trials with an RT less than 200 ms or greater than 1,500 ms (0.5% of trials) were excluded from all search-task analyses. Additionally, trials with an incorrect response (2.5%) were excluded from search-task RT analyses. Mean accuracy for the search task was 97.4% (with the lowest observed accuracy being 91.4%).
As shown in Figure 3a, responses in the search task were slightly slower when the color singleton was present (625 ms) than when it was absent (618 ms): a 7-ms singleton presence cost, t(23) = 2.066, p = .05, d = .422. There was not a significant difference in error rates on singleton-absent trials (2.6%) and singleton-present trials (2.6%), t(23) = .154, p = .879, d = .031. As in Experiment 1, these results suggest that, if anything, the color singleton weakly captured attention (but again, these small RT costs could reflect spatially nonspecific filtering costs, and the key predictions focus on the probe trials; e.g., see Folk & Remington, 1998).
Figure 3.
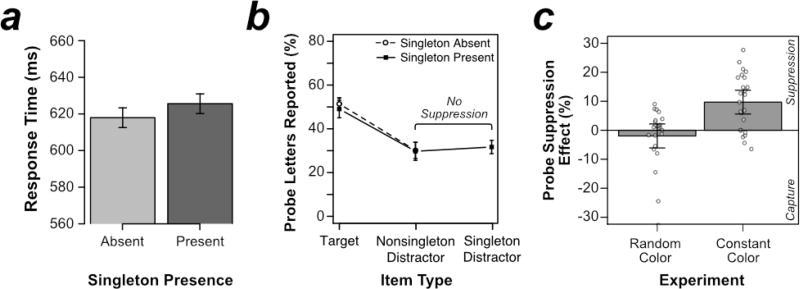
Results from Experiment 2, in which color was varied randomly trial-by-trial. (a) Mean response time (RT) from search trials. (b) Percentage of probe letters reported as a function of search-item type. Results are presented separately for trials on which the color-singleton distractor was present and trials on which it was absent. (c) Probe suppression effects, calculated as probe report accuracy for the average of nonsingleton distractors minus singleton distractors, from Experiment 2 (random color) contrasted with a supplemental comparison experiment that used a constant color. Each white dot represents a unique participant’s probe suppression effect and the shaded gray areas represent a smoothed density curve. Error bars in (a) and (b) represent the within-subjects 95% confidence interval. Error bars in (c) represent the between-subject 95% confidence intervals.
Probe Task Analysis
Participants reported an average of 1.6 letters per trial, and 89% of these letters were actually present in the probe array.
As in Experiment 1, the key question was whether probe processing would be suppressed at the singleton location. As shown in Figure 3b, probe report accuracy at the location of the singleton distractor (32%) was approximately equal to accuracy at the average of the nonsingleton distractor locations (30%), t(23) =.982, p = .336, d = .200. If anything, the nonsignificant trend was opposite to the direction predicted by second-order feature suppression and global salience suppression models. Participants were more likely to report the letter at the singleton location than at the nonsingleton locations. We again computed the Bayes factor for this comparison and found that it was 3.03 in favor of the null hypothesis.
We also examined how the presence or absence of the color singleton affected the report of probe letters at the target and nonsingleton-distractor locations. As shown in Figure 3b, participants were much more likely to report probe letters at the target location than probe letters at the nonsingleton distractor locations – unlike in Experiment 1. However, the presence of a color singleton did not greatly impact target or distractor processing (e.g., see Gaspelin et al., 2015, Experiment 1, for an example of singleton presence harming detection of target probes).
These effects were formally analyzed in a two-way analysis of variance (ANOVA) with factors of singleton presence (present vs. absent) and probe type (target vs. nonsingleton distractor). This analysis indicated that report accuracy was significantly higher for probes at the target location (50%) than for probes at the nonsingleton-distractor locations (31%), F(1, 23) = 63.133, p < .001, . This 19% difference reflects the attentional enhancement of the target stimulus. A planned t test revealed that this target enhancement was indeed greater in the current experiment with the delay between search array onset and probe array onset (19%) than in Experiment 1 with the search-combined-with-probe display (5%), t(46) = 5.757, p < .001, d = 1.662. The analysis also indicated a nonsignificant trend for probe report accuracy to be higher when the singleton was absent (41%) than when the singleton was present (40%), F(1, 23) = 3.069, p = .093, . Finally, the interaction of singleton presence and probe type was nonsignificant, F(1, 23) = .613, p = .442,
We conducted two planned t tests comparing processing at a given item’s location when the singleton was present versus absent. For probes at the target location, probe report accuracy was not significantly different on trials where the singleton was absent (51%) compared to trials where the singleton was present (49%), t(23) = 1.262, p = .220, d = .258. For probes at the nonsingleton distractor location, accuracy was virtually identical on trials where the singleton was present (30%) versus when it was absent (30%), t(23) = .445, p = .661, d = .091.
As in Experiment 1, we found no credible evidence that a subset of participants were able to suppress the singletons in this experiment (see Supplementary Materials).
Color Unprimed Trials Only
This experiment used four color configurations (green-pink, pink-green, blue-yellow, and yellow-blue). Thus, on half of trials, the current color configuration was constructed of a completely different color configuration than the previous trial (color unprimed trials).3 Weak versions of global salience-based/second-order feature discontinuity suppression models might predict large probe suppression effects, but only on color unprimed trials. In other words, participants may be able to suppress the salient item but only when these effects are not masked by intertrial priming.
To assess this possibility, we analyzed probe report accuracy on probe trials limiting the analysis to only unprimed trials. We found that participants were equally likely to report probes at the singleton location (31%) and at the nonsingleton locations (31%), t(23) = .230, p = .820, d = .047. This 0% suppression effect suggests that participants did not suppress the singleton location even on trials where the target and singleton colors had not been presented on the previous trial. This is inconsistent even with weak versions of the global salience and second-order feature suppression models in which salience-based suppression can be observed only in the absence of intertrial priming. However, the results are fully consistent with the first-order feature suppression model.
Some priming may still have been present from trials before the immediately preceding trial (e.g., trial t − 2), so this experiment cannot completely rule out any impact of priming on the lack of suppression. However, Experiment 4 will address this more definitively by using a procedure in which the target and singleton colors never switch roles.
Supplemental Comparison Experiment
We found no evidence of suppression in Experiment 2 when the singleton color was unpredictable. To be certain that the lack of suppression reflects the unpredictability of the singleton color and not some other feature of the experimental design, we conducted a comparison experiment that used the same methods and stimuli as Experiment 2 but held the singleton and nonsingleton colors constant across the entire experiment, giving participants an opportunity to use first-order feature suppression. For the sake of brevity, the details of this experiment are presented in Supplementary Materials.
Unlike Experiment 2, we found a significant probe suppression effect in this comparison experiment. Moreover, as shown in Figure 3c, the suppression in this comparison experiment (10%) was significantly greater than in Experiment 2 (−2%), t(46) = 4.142, p < .001, d = 1.19. Thus, suppression is possible when the singleton and nonsingleton colors are predictable and significantly reduced when they are unpredictable.
Discussion
Experiment 2 replicated the results of Experiment 1, with two key changes meant to encourage second-order or salience-based suppression: (a) we used four color configurations to increase the likelihood of suppression of feature discontinuities rather than specific color values, and (b) we increased the exposure duration prior to probe onset to provide more time for suppression to impact processing. Despite these changes, we still found no probe suppression effects. In other words, even when we established favorable conditions for global salience/second-order feature suppression models, participants still could not suppress color singletons. The present results are instead consistent with first-order feature suppression models.
Experiment 3
In Experiments 1 and 2, we used the capture-probe paradigm to assess suppression effects in covert visual attention. In Experiment 3, we provide converging evidence by assessing overt visual attention using an eye-tracking paradigm developed by Gaspelin et al. (2017). The general method was similar to that used in the search trials of Experiments 1 and 2 of the present study, except that the stimuli were modified to encourage eye movements. No probe trials were included. Instead, the landing position of the first eye movement on each trial was used to assess the attentional priority of the individual items within the search array. Previous research showed that, when the singleton color was predictable, initial eye movements were less likely to land on the singleton distractor than a baseline comparison (an oculomotor suppression effect; Gaspelin et al., 2017).
In Experiment 3, we replicated this paradigm except that we randomly swapped the singleton and nonsingleton colors trial-by-trial, as in Experiment 1. According to global salience and second-order feature models, oculomotor suppression of the singleton should still be robust: first eye movements should be less likely to land on the color singleton than on the nonsingleton distractors. According to first-order feature suppression models, however, oculomotor suppression effects should be eliminated because participants now have no foreknowledge of the upcoming singleton color.
Methods
Participants
We chose an a priori sample size of 28 participants, matching similar eye-tracking experiments from our lab (Gaspelin et al., 2017). After the completion of the experiment, we conducted a power analysis to determine whether our sample size had been adequate to detect the types of suppression effects we have observed in previous eye-tracking studies. We estimated the population effect size and standard deviation by pooling the oculomotor suppression effects (as defined above) across the participants in two previous experiments with similar methods (Gaspelin et al., 2017, Experiments 2–3), resulting in an N of 40. The oculomotor suppression effect was quite large: d = 1.63. Thus, to achieve a power of 95% and an alpha of 5% for this effect size, a sample size of 8 participants would be needed. Thus, our sample size of 28 was more than adequate to detect oculomotor suppression effects of the sort observed in our previous experiments. As in Experiment 1, we used a bootstrapping approach to estimate a confidence interval around this effect size. With 10,000 bootstraps, we obtained a 95% CI of [1.01, 2.41]. With the sample size of 28 participants used in each of the eye-tracking experiments, we had 99% power to detect an effect of this size.
One participant had abnormally low accuracy (more than 2.5 standard deviations from the group mean) and was replaced. Of the final sample of participants, 17 were female and 11 were male. The mean age was 20.9 years.
Apparatus
The stimulus presentation system was identical to that used in Experiments 1 and 2. An SR Research Eyelink 1000 desk-mounted system recorded eye position monocularly from the right eye at 500 Hz.
Stimuli & Procedure
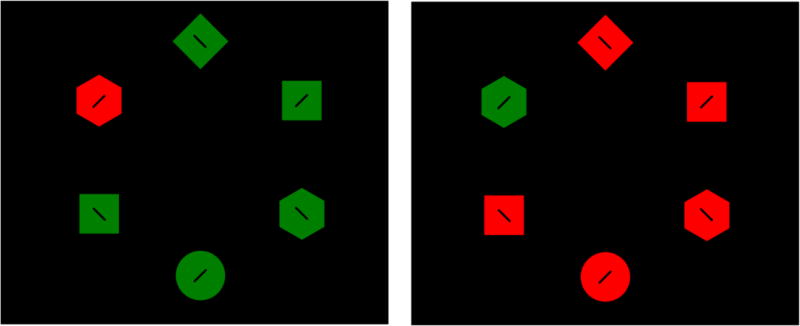
The stimuli and procedure were nearly identical to those used in Gaspelin et al. (2017, Experiment 3) and were analagous to those used in Experiments 1 and 2 of the present study except that the displays were adapted to measure eye movements (see Figure 4). Each search display contained 6 items distributed at equal distances around a notional circle with a radius of 4.5°. The individual stimuli were diamonds (0.8° by 0.8°), circles (0.9° diameter), squares (0.8° in width and height), and hexagons (0.8° in width and height) drawn in red (23.3 cd/m2, x = .65, y = .34) or green (23.3 cd/m2, x = .29, y = .63). Each shape contained a black line subtending 0.30° × 0.05° that was tilted 45° to the left or right. A gray fixation cross (23.3 cd/m2, 0.1° by 0.1°) appeared on a screen before the search array.
Figure 4.
Stimuli from Experiment 3, which were identical to those of Gaspelin et al. (2017, Experiment 3), except that the target and singleton color changed randomly trial-by-trial. The lines inside the shapes are increased in size for illustrative purposes – in the actual task, they were much smaller to strongly encourage overt eye movements. In grayscale versions of this figure, red objects are outlined in solid lines and green objects are outlines in dotted lines. Participants made a speeded button-press to the tilt of the line (left or right) inside the target shape.
The search target was defined as a specific shape (diamond or circle). The task was to report whether the line inside the target shape tilted to the left or right (by pressing gamepad buttons with the left or right index fingers). Pilot studies indicated that participants could not easily report the orientation of the target line without fixating it. Thus, our task implicitly required eye movements, but we did not explicitly instruct participants to move their eyes toward the target. Target location and target line tilt varied randomly.
The target and the nonsingleton distractors were green on half of trials and red on the other half (in unpredictable order). A color singleton was presented on 50% of trials and was always the opposite of the nonsingleton color. The location of this color singleton distractor was random except that it was never the target location. Participants were told this and were encouraged to ignore the color singleton.
Trials began with the presentation of a blank screen for 500 ms. This was followed by a fixation screen containing only the fixation point; this screen remained visible until the participant maintained fixation within a 1.5° radius of the fixation point for 100 ms. The search array then appeared and remained visible until response. If participants took too long to respond (more than 3000 ms), a timeout display appeared with the text “Too Slow” for 500 ms. If the response was incorrect, a 200 Hz tone sounded for 500 ms. The blank screen for the next trial then appeared immediately.
Participants practiced the search task for two blocks of 64 trials. The main experiment consisted of ten blocks of 64 trials, yielding 640 trials, 320 with and 320 without an irrelevant singleton. Note that this is nearly double the number of trials used in the previous study of Gaspelin et al. (2017), which was intended to maximize our ability to detect probe suppression effects. Participants received block-by-block feedback on mean response time (RT) and accuracy. At the beginning of each block, the eyetracker was calibrated using a nine-point calibration technique. During the main task, the eyetracker was recalibrated if a participant failed to fixate the central cross for more than 8 seconds at the beginning of a trial.
Analysis
The onset of a saccade was defined using a minimum eye velocity threshold of 30°/s and a minimum acceleration threshold of 9500°/s2. To classify the landing position of the first saccade on each trial, an annulus was defined that was centered on the fixation cross, with an inner radius of 1.5° and an outer radius of 7.5°. First saccades were defined as the first eye movement landing inside the annulus. The landing position was then classified by selecting the nearest search item. This technique effectively created wedge-shaped interest areas around each search item. Saccadic latency was measured as the start time of the first saccade that landed in the annulus.
We excluded trials with abnormal manual response times (less than 200 ms or greater than 2000 ms, accounting for 0.6% of trials), trials in which participants made no eye movement (0% of trials), and trials with abnormal saccade latencies (less than 50 ms or greater than 1000 ms, accounting for 1.2% of trials). Additionally, we excluded trials with manual response errors (0.8%) from all analyses except manual response error analyses. Altogether, 2.0% of trials were excluded. These trial-by-trial exclusion criteria were established a priori to match the methods of Gaspelin et al. (2017). Mean accuracy was 96.7% (with the lowest observed accuracy being 91.7%).
Results
Manual Responses
As shown in Figure 5a, manual responses in the search task were slower when the color singleton was present (883 ms) than when it was absent (870 ms): a 13-ms singleton presence cost, t(27) = 2.222, p = .035, d = .420. Because singleton presence costs could reflect spatially nonspecific filtering costs (Folk & Remington, 1998), the eyetracking data below will directly address whether overt visual attention was directed to the color singleton. Error rates were similar on singleton-absent trials (3.2%) and singleton-present trials (3.3%), t(27) = .172, p = .865, d = .032.
Figure 5.
Results from Experiment 3, in which color was held constant throughout the experimental session. (a) Mean response time (RT) on manual responses. (b) Percentage of first eye movements to each search-item type. Results are presented separately for trials on which the color-singleton distractor was present and trials on which it was absent. (c) Oculomotor suppression effects, calculated as the percentage of first eye movements to the average of nonsingleton distractors minus singleton distractors, from Experiment 3 contrasted with Gaspelin et al. (2017, Experiment 3) that used a constant color. Each white dot represents a unique participant’s oculomotor suppression effect and the shaded gray areas represent a smoothed density curve. Error bars in (a) and (b) represent the within-subjects 95% confidence interval. Error bars in (c) represent the between-subject 95% confidence intervals.
First Saccade Destination
If the singleton distractor captures the eyes, the initial saccade should be more likely to move to the singleton distractor than to the average of the nonsingleton distractors. We therefore compared the number of first saccades allocated to each search item. We divided the number of saccades allocated to nonsingleton distractors by the number of nonsingleton locations, providing a “per location” measure (to allow for a fair comparison of singletons and nonsingleton distractors). As can be seen in Figure 5b, first eye movements were much more likely to be directed toward the singleton distractor (17%) than toward the average nonsingleton distractor (10%): a 7% oculomotor capture effect, t(27) = 4.714, p < .001, d = .891. This indicates that the color singleton captured overt attention in this study, whereas our previous study found that overt attention was suppressed at the location of the color singleton when its color remained constant over trials (Gaspelin et al., 2017).
We also examined how the presence or absence of a singleton impacted first saccades to the target and nonsingleton distractor items. If the singleton captures the eyes, then the first saccade should be less likely to land on the target location. Indeed, first saccades were less likely to land in the target region when the singleton was present than when the singleton was absent (upper-left of Figure 5b). First saccades to the nonsingleton distractor locations were not influenced by the presence or absence of the color singleton.
Because saccades could be directed only to the target or to a nonsingleton distractor on singleton-absent trials, these two saccade destinations were not independent, making it inappropriate to conduct an ANOVA on the proportion of saccades that landed on these two locations. We therefore conducted a t test comparing the proportion of first saccades that were directed to the target on singleton-present versus singleton-absent trials. We found that the proportion of first saccades directed to the target location was significantly reduced (by 5%) on singleton-present trials compared to singleton-absent trials, t(27) = 4.143, p < .001, d= .783. These results provide additional evidence that first eye movements were captured by the irrelevant singleton, thereby decreasing the probability that the initial eye movement would be directed toward the target. Thus, participants were unable to suppress oculomotor capture by the color singleton when the specific color of this singleton varied unpredictably.
Across-Experiment Comparison
In the current experiment, we varied the color configuration randomly trial-by-trial (random color), whereas Gaspelin et al. (2017, Experiment 3) used the same color configuration across the entire experimental session (constant color). Given that these two experiments were identical except for the randomization of the color configuration and the number of trials, we directly compared first eye movements across experiments. In the present random-color experiment, first eye movements were more likely to land on the singleton than the nonsingleton distractors. In the prior constant-color experiment, however, first eye movements were less likely to land on the singleton than the average nonsingleton distractor. These effects were formally analyzed by comparing oculomotor suppression effects—calculated as the likelihood of first fixating nonsingleton distractors minus the likelihood of first fixating singleton distractors—across experiments. As shown in Figure 5c, oculomotor suppression effects were significantly larger when the singleton color was held constant (+7%) than when it varied randomly trial-by-trial (−7%), t(46) = 7.483, p < .001, d = 2.191.
Saccadic Latencies
The three suppression models do not make any clear predictions about saccadic latencies. However, for the sake of completeness, a table of saccadic latencies has been included in the Supplementary Materials. Note that the previous Gaspelin et al. (2017) eye-tracking study used saccadic latencies to rule out rapid disengagement models of suppression, in which an initial capture of attention by the singleton is rapidly followed by suppression. We found no oculomotor suppression in the current experiment, so there was no need to rule out rapid disengagement models.
Color Swap Trials vs. Color Repeat Trials
We also investigated the role of intertrial priming on attentional capture in the current experiment (Maljkovic & Nakayama, 1994). When the singleton color was the target color from the previous trial (color swap trial), priming should lead to particularly large capture effects. When the singleton color was repeated from the previous trial (color repeat trial), however, any priming-induced capture effects should be eliminated. To investigate this, we separated the data into color swap and color repeat trials (as in Experiment 1). We then compared singleton presence costs and oculomotor suppression effects on color swap and color repeat trials. Notably, there were robust singleton presence costs and oculomotor capture effects on color swap trials, but there was still no evidence of oculomotor suppression (see Supplementary Materials for details).
Discussion
In a previously published eye-tracking study, we found that people can suppress oculomotor capture by color singletons when the color configuration remained constant across the session (Gaspelin et al., 2017). Experiment 3 used nearly identical methods, except that the singleton and nonsingleton colors varied randomly trial-by-trial. Here, the singleton was not suppressed — in fact, we found evidence that the singleton actually captured attention. These findings are incompatible with global salience-based models and second-order suppression models, but they are consistent with a first-order feature suppression model.
Experiment 4
Experiments 1–3 provided clear evidence that participants cannot suppress color singletons without first-order feature knowledge about an upcoming search display. All of these experiments used a similar tactic: we varied the upcoming singleton and nonsingleton colors randomly trial-by-trial, which prevents suppression based upon first-order features. We have assumed that the lack of suppression effects in those experiments reflected the unpredictability of the singleton color, but they could instead reflect the unpredictability of the nonsingleton color. In Experiment 4, we keep the nonsingleton color constant and use a somewhat different approach to varying the singleton color.
Specifically, we used an approach pioneered by Vatterott and Vecera (2012), which examines whether participants can learn to suppress a given singleton color over a period of many trials. In that study, the color of the singleton distractor remained constant within a block but changed between blocks. The target color was constant throughout the experimental session. Manual RT data indicated that the singletons captured attention during the first half of a given block but not during the second half of the block. This suggested that participants were learning to suppress the color of the distractor over the course of a block. However, no evidence of suppression per se was obtained. The goal of the present study was to combine this approach with the eye-tracking methods of Experiment 3 to determine more conclusively whether participants learn to suppress the singleton color over the course of a block.
The color of the nonsingleton items (including the target) remained constant. The singleton color, however, was blocked into sets 120 trials (e.g., pink for the first 120 trials, green for the next 120 trials, etc.). First-order feature suppression models predict that, when the singleton color changes, oculomotor suppression effects should be temporarily disrupted until the participant can learn to suppress the new color. Second-order feature suppression models and global-salience models, however, predict that changing feature value of the color singleton should not matter – oculomotor suppression effects should remain robust when the singleton color changes from one block to the next.
We chose to examine suppression using the eyetracking methods of Experiment 3 rather than using the capture-probe paradigm used in Experiments 1 and 2. The present experiment requires dividing the trials into separate bins for different periods of time following a change in the singleton color. Because the probe paradigm provides data about suppression on only the 30% of trials that contain probes, it would have been difficult to subdivide the data further and still obtain reliable estimates of probe processing at each location. By contrast, the eye tracking paradigm yields useful data on every trial. In general, we find much larger effect sizes with the oculomotor measures than with the probe suppression effects, as indicated by the power analyses described for Experiments 1 and 3.
Methods
All methods were identical to those of Experiment 3, except for the following changes. First, we ran a new sample of 28 participants, matching the sample size in Experiment 3 and our previous eye-tracking studies. Of these participants, 19 were female and 9 were male. The mean age was 20.2 years.
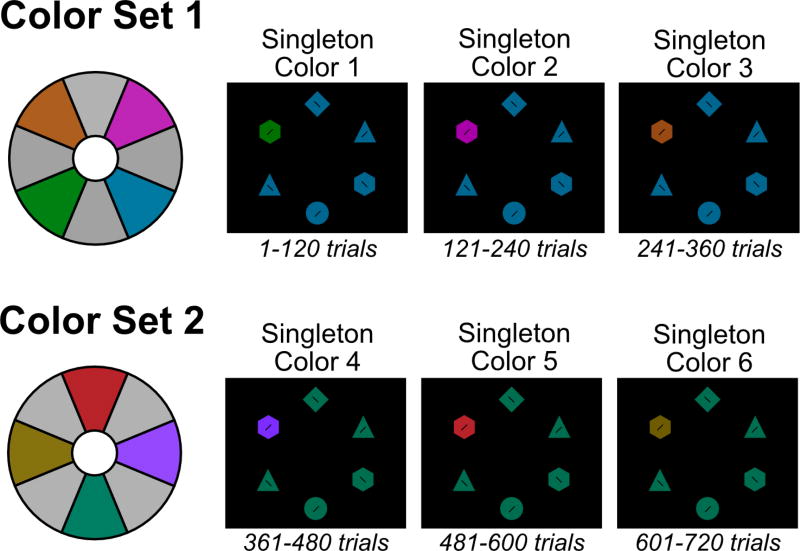
Second, we carefully selected two new sets of photometrically isoluminant colors (see Figure 6). The first set was orange (18.6 cd/m2, x = .55, y = .50), pink (18.4 cd/m2, x = .33, y = .17), green (18.2 cd/m2, x = .29, y = .63), and blue (18.7 cd/m2, x = .19, y = .26). The second set was teal (18.6 cd/m2, x = .243, y = .453), brown (18.3 cd/m2, x = .46, y = .50), red (18.1 cd/m2, x = .60, y = .34), and purple (18.6 cd/m2, x = .21, y = .11). The colors within a set were chosen to be maximally different in hue. Participants performed two parts of the experiment, one with each color set. The nonsingleton color remained constant for a given color set, but the singleton color varied from block to block within a set. This made it possible to increase the number of different singleton colors that could be tested in a given participant while maintaining high levels of color discriminability between the target and singleton colors within a given trial block.
Figure 6.
Stimuli from Experiment 4. Two color sets (1 and 2) were constructed from 8 photometrically isoluminant colors. Note that the colors shown here may look quite different from the actual colors because of variations in how colors are rendered on different devices. From color set 1, a target color was randomly selected (e.g., blue) and the remaining colors became potential singleton colors that were blocked for 120 trials. Next, from color set 2, a new target color was randomly selected (e.g., teal) and the remaining colors became potential singleton colors. Half of the participants used color set 1 for the first half of the experiment and color set 2 for the second half, and this was reversed for the remaining participants.
The order of color sets was counterbalanced across subjects. At the beginning of each color set, one color was randomly assigned as the target color (e.g., blue) and the other colors became possible singleton colors (e.g., pink, green, and orange). The task was divided into blocks of 120 trials, with a short rest period halfway through each block. The nonsingleton color remained constant for three blocks, but a different singleton color was chosen at random (without replacement) from the set possible singleton colors for each of these three blocks. After these three blocks, the colors switched to the second color set – again, one color was randomly assigned as the nonsingleton color (e.g., teal) and the other colors became possible singleton colors (e.g., red, purple, and brown). At the beginning of each block (and after the mid-block rest), participants were shown the upcoming singleton color and were explicitly instructed to ignore that color. Finally, we replaced the square distractors with triangles, because previous experiments revealed that in the diamond target condition, square distractors elicited a large proportion of first fixations.
For the analyses, each block of 120 trials was divided into quarter-blocks of 30 trials so that we could examine how performance changed over the course of a block. This “bin size” of 30 trials was based on the bin size in Vatterott and Vecera (2012) and in previous eye-tracking studies conducted in our laboratory (Gaspelin, Leonard, & Luck, 2017; Leonard & Luck, 2011). We then pooled the data for a given quarter-block across the remaining four singleton colors (the second two singleton colors for each color set), to increase statistical power. The first block from each color set was excluded to ensure that participants had plenty of opportunity to form a template of the target color for that color set.
Results
Manual Responses by Pooled Quarter-Block
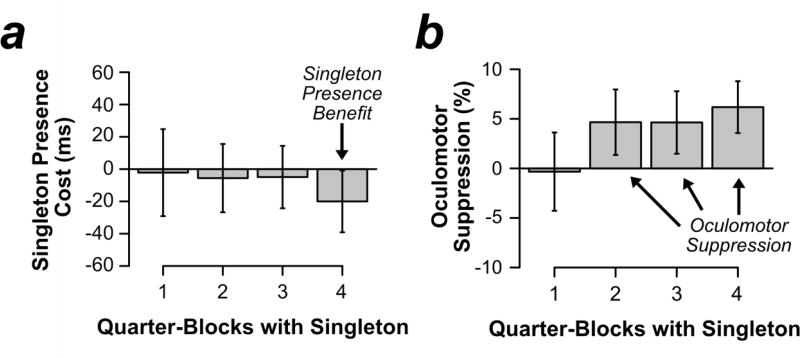
Table 2 shows overall manual RT as a function of singleton presence and pooled quarter-block. Figure 7a shows singleton presence costs as a function of pooled quarter-block. In the final quarter-block, participants were actually faster when the singleton was present than when it was absent: a singleton presence benefit. Manual RTs were analyzed in a two-way repeated measures ANOVA with the factors quarter-block (1, 2, 3, 4) and singleton presence (present vs. absent). To avoid issues of sphericity, all reported p values were Greenhouse-Geisser corrected for ANOVAs with more than two levels of a given factor (which did not apply in Experiments 1–3). Manual responses were generally slower in the early quarter-blocks (808 ms and 785 ms) than the late quarter-blocks (766 ms and 780 ms), F(3, 81) = 10.002, p < .001, . Manual RTs were not significantly different for singleton present trials (781 ms) and singleton absent trials (789 ms), F(1, 27) = 1.153, p = .292, . A small singleton presence benefit (negative singleton presence cost) emerged in the final quarter block (see Figure 7a), but the main effect of quarter block was not significant, F(3, 81) = .870, p = .453, . Planned t tests compared mean RT on singleton present and singleton absent trials in each quarter-block bin. There was no nonsignificant singleton presence cost or benefit in each of the first three quarter blocks, but the singleton presence benefit was significant in the final quarter block: quarter block 1, t(27) = .166, p = .870, d = .031; quarter block 2, t(27) = .541, p = .593, d = .102; quarter block 3, t(27) = .522, p = .606, d = .099; quarter block 4, t(27) = 2.150, p = .041, d = .406.
Table 2.
Manual Response Time and Error Rates by Singleton Presence and Hemiblock
| Hemiblock | Singleton Present |
Singleton Absent |
Singleton Presence Cost |
|---|---|---|---|
| 1 | 807 (2.0) | 809 (1.5) | −2 |
| 2 | 783 (2.4) | 788 (2.5) | −6 |
| 3 | 763 (2.1) | 768 (2.2) | −5 |
| 4 | 770 (1.5) | 790 (1.9) | −20 |
Note. Singleton presence costs were calculated as mean RT on singleton present trials minus mean RT on singleton absent trials. Mean error rates are shown in parentheses.
Figure 7.
Results from Experiment 4 as a function of quarter-block pooled across the four singleton colors (quarter-block 1 = 1–30 trials, quarter-block 2 = 31–60 trials, quarter-block 3 = 61–90 trials, and quarter-block 4 = 91–120 trials). (a) Singleton presence costs on manual responses, calculated as mean RT on singleton-present trials minus singleton-absent trials. (b) Oculomotor suppression effects, calculated as percentage of first eye movements to the average of nonsingleton distractors minus first eye movements to singleton distractors. Error bars reflect the within-subject 95% confidence intervals.
Given the nonsignificant ANOVA but the presence of a significant singleton presence benefit in the final quarter block, these results are weakly consistent with the first-order feature suppression models. However, singleton presence costs/benefits on manual RT are a poor measure of suppression because they may be contaminated by filtering costs (Folk & Remington, 1998), differences in overall RT (Gaspelin, Margett-Jordan, & Ruthruff, 2014), and dwell time after initial capture (Gaspelin, Ruthruff, & Lien, 2016). The eyetracking data in the following section will directly address whether overt visual attention was directed to the color singleton.
The same ANOVA was conducted on mean error rates (see Table 2). There was a trend for error rates to shrink across quarter-blocks, F(3, 81) = 2.592, p = .068, . There was no significant difference in accuracy on singleton present and absent trials, F(1, 27) = .0002, p = .988, . The interaction of singleton presence and quarter-block was also nonsignificant, F(3, 81) = .536, p = .637, .
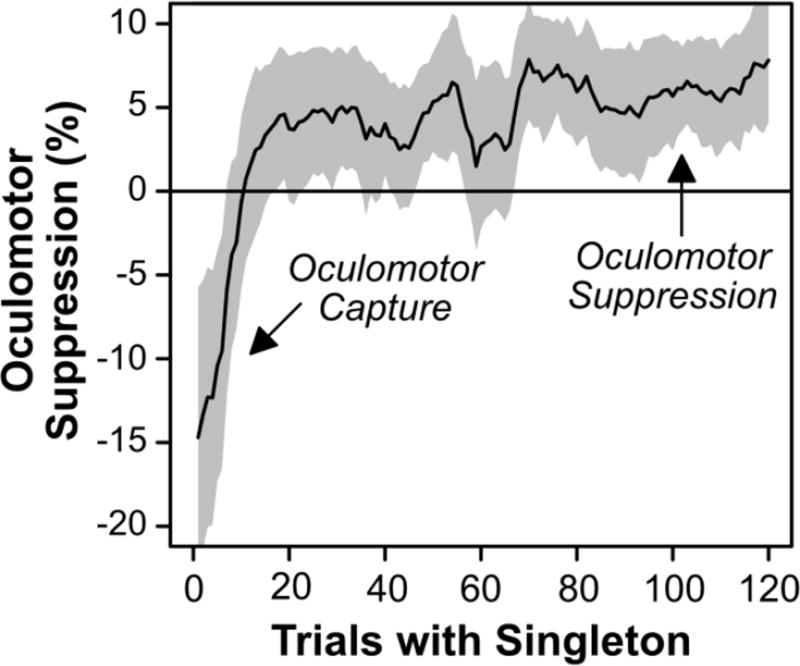
First Saccade Destination by Pooled Quarter-Block
The landing position of first saccades separated by singleton presence and pooled quarter-block are shown in Table 3. Oculomotor suppression effects, calculated as the percentage of first saccades to the average of the nonsingleton distractors minus percentage of first saccades to the singleton distractor, are shown in Figure 7b. As can be seen, oculomotor suppression effects were absent in the first quarter-block (−0.3%). However, oculomotor suppression effects were robust in the final quarter-block (6.1%). We formally analyzed these oculomotor suppression effects in a one-way repeated measures ANOVA with the factor pooled quarter-block (1, 2, 3, 4). This revealed that suppression effects changed significantly across the quarter-blocks, F(3, 81) = 7.941, p < .001, η2 = .227. Planned t tests assessed the statistical significance of each oculomotor suppression effect at each quarter-block by comparing the percentage of first saccades to land on nonsingleton distractors with the percentage of first saccades to land on singletons. In this first quarter-block, there was no oculomotor suppression effect (−0.3%), t(27) = .171, p = .866, d = .032. Suppression effects were significant, however, in the second quarter-block (4.7%), t(27) = 2.894, p = .007, d = .547, third quarter-block (4.6%), t(27) = 3.017, p = .006, d = .570, and fourth quarter-block (6.2%), t(27) = 4.851, p < .001, d = .917. These results are consistent with a model in which suppression builds up as participants gain experience with the singleton color value. We provide a more fine-grained analysis of the time course of suppression in a later section.
Table 3.
First Eye Movement Landing Destination by Singleton Presence, Item Type, and Hemiblock
| Singleton Absent | Singleton Present | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hemiblock | Target | Nonsingleton Distractor |
Target | Nonsingleton Distractor |
Singleton Distractor |
Suppression Effect |
| 1 | 38.4% | 12.3% | 38.6% | 12.2% | 12.5% | −0.3% |
| 2 | 43.8% | 11.2% | 40.8% | 12.8% | 8.1% | 4.7% |
| 3 | 44.5% | 11.1% | 41.7% | 12.6% | 8.0% | 4.6% |
| 4 | 43.7% | 11.3% | 45.5% | 12.1% | 6.0% | 6.1% |
Note. Suppression effects were calculated as percentage of first fixations to the nonsingleton distractor minus the percentage of first fixations to the singleton distractor on singleton present trials.
Oculomotor Suppression Effects Interrupted by Singleton Color Switch
The key comparison in this experiment was to see if changing the singleton color temporarily eliminated oculomotor suppression effects, which requires comparing the amount of suppression at the end of one block with the amount of suppression at the beginning of the next block. Table 4 shows probe suppression effects for each color switch (e.g., the final quarter-block of Singleton Color 1 to the first quarter-block of Singleton Color 2). The switch from Singleton Color 3 to Singleton Color 4 was excluded, because the relevant target color also changed (refer to Figure 6). Oculomotor suppression effects were clearly reduced each time the singleton color changed its color value. To improve statistical power, we pooled the oculomotor suppression effects across all four of these singleton-color switches. The pooled values (see bottom row of Table 4) show that oculomotor suppression effects were reduced from 5.8% in the pre-switch quarter-block to −0.1% in the post-switch quarter-block, t(27) = 3.262, p = .003, d = .616.
Table 4.
Reduction in Suppression Effects to Color Singletons for Each Color Switch (from the last quarter-block before a switch to the first quarter-block after a switch)
| Singleton Colors | Pre-Switch Suppression Effect |
Post-Switch Suppression Effect |
Suppression Effect Reduction |
|---|---|---|---|
| Color 1 to Color 2 | 5.7% | −4.2% | 9.9% |
| Color 2 to Color 3 | 5.4% | 2.4% | 3.0% |
| Color 4 to Color 5 | 4.5% | 0.0% | 4.5% |
| Color 5 to Color 6 | 7.4% | 1.5% | 6.0% |
|
| |||
| Pooled | 5.8% | −0.1% | 5.9% |
Note. Suppression effects were calculated as the percentage of first eye movements to nonsingleton distractors minus the those to singleton distractors. Suppression effect reduction was calculated a pre-switch suppression minus post-switch suppression. Pooled analyses averaged the four singleton-color switches for added statistical power.
Running Averages of Oculomotor Suppression Effects
We also developed a more continuous measure of changes in singleton processing over the course of each block. We first pooled the data across the four color sets (excluding the first block for a given color set), just as before. We then calculated a running average of oculomotor capture effects across sets of 11 consecutive trials (trial t−5 through trial t+5). Because the data were pooled across four blocks of trials, each time point was an average computed from 44 trials (i.e., 4 color sets × 11 trials). Oculomotor suppression effects were calculated just as they were in our previous analyses – by subtracting the percentage of first saccades allocated to the average of the nonsingleton distractors minus the percentage of first saccades allocated to the singleton.
As can be seen in Figure 8, oculomotor suppression effects gradually increased as participants gained experience with a specific singleton color value. At the beginning of a block, immediately after a change in the singleton color, first saccades were biased toward the singleton distractors compared to nonsingleton distractors (oculomotor capture). However, as participants gained experience with a given singleton color value, they gradually became biased away from the singleton distractors (oculomotor suppression). We performed t tests at each time point comparing the percentage of first fixations to the singleton and the average nonsingleton. As can be seen by the confidence intervals (shaded gray), both the initial oculomotor capture effect and final oculomotor suppression effect were highly significant.
Figure 8.
Running averages of oculomotor suppression effects. Running averages were computed across 11 trials (trial t − 5 through trial t + 5). Oculomotor capture was calculated as the percentage of first saccades to the average of the nonsingleton distractors minus the percentage of first saccades to singleton distractors. Shaded gray regions reflect the within-subject 95% confidence intervals.
These timecourse analyses involved a large number of statistical tests, but typical corrections for multiple comparisons would be overly conservative given that these tests were not independent. For this reason, we conducted an additional analysis that provides a better correction for multiple comparisons. Specifically, we used a nonparametric permutation-based analysis that was originally developed to assess timecourse differences between event-related potentials (Groppe, Urbach, & Kutas, 2011; Maris & Oostenveld, 2007), but has been adapted to look at timecourse differences in eye movements (Gaspelin et al., 2017; Oakes, Baumgartner, Barrett, Messenger, & Luck, 2013). This approach asks whether the observed length of a run of significant t values is greater than expected by chance. A permutation analysis is conducted in which the location labels for the singleton and nonsingleton items are randomly permuted trial-by-trial prior to the analyses, and this is repeated 1,000 times to yield an empirical null distribution of the number of consecutive significant differences that would be expected by chance. If the observed number of consecutive significant differences is beyond the 95th percentile of this null distribution, then that period of differences is considered statistically significant. Indeed, the observed run length of the oculomotor suppression was well above the 95th percentile of the null distribution (which was 9 consecutive points).
Saccadic Latencies
None of the three models tested in this paper make explicit predictions about saccadic latencies. But, for the sake of completeness and for comparison with our previous eyetracking studies (Gaspelin et al., 2017), we have included analyses of saccadic latencies in the Supplementary Material.
Discussion
Participants in this experiment performed the same eyetracking task used in Experiment 3, but the singleton color changed after every block of 120 trials. The singleton captured overt attention for the first ~5 trials following a change in the color of the singleton, but by the end of a block it was suppressed. This is exactly what would be expected if participants learned to suppress a given singleton color value and were briefly unable to suppress the singletons after a color change. By contrast, second-order and global salience suppression models would predict no impairment in singleton suppression when the singleton’s color changes.
General Discussion
Previous research has demonstrated that salient items can be suppressed below baseline levels of processing (Gaspelin, Leonard, & Luck, 2015, 2017). However, the precise nature of this suppressive mechanism was unclear. In the current study, we tested between three classes of models (Table 1). According to global-salience suppression models and second-order feature models, people require no foreknowledge about specific feature values of salient items to suppress them. Rather people can simply suppress salient items on the basis of their bottom-up salience signal within a priority map or on the basis of a local feature discontinuity, respectively. According to first-order suppression models, however, people require foreknowledge of the specific feature values of the salient items to suppress them. To our knowledge, no previous studies have directly attempted to distinguish among these models. The few relevant studies that have come close have provided mixed results (Arita et al., 2012; Vatterott & Vecera, 2012; Sawaki & Luck, 2010; Woodman & Luck, 2007). Problematically, none of these studies used measures that can directly compare the processing at the singleton location to the processing at the nonsingleton distractor locations, which is necessary for drawing strong conclusions about suppression. The present study was conducted to provide such measures.
Evidence for First-Order Suppression
Experiment 1 used a variation on the capture-probe paradigm, in which robust suppression effects were previously observed when the singleton color was predictable (e.g., Gaspelin et al., 2015). Here, we made the colors unpredictable to determine if suppression is possible when the features of the singleton are unknown. This eliminated the probe suppression effect – participants were equally likely to report letters at the singleton and nonsingleton distractor locations. These results are consistent with first-order feature suppression models, but are inconsistent with global salience suppression and second-order feature suppression models. Experiment 2 replicated this basic result in an experimental design with greater color variability and with timing parameters that provided more opportunity to observe suppression if it was present.
Experiment 3 provided converging evidence using measures of overt attention. The search task was nearly identical to that used in Experiments 1 and 2, except for small changes designed to encourage the use of eye movements. A previous study using this eye-tracking technique showed that the initial eye movement on a given trial avoided the color singleton when its color was predictable (Gaspelin et al., 2017, Experiment 3). We replicated the design of this experiment, except that we varied the colors of the singleton and nonsingleton randomly trial-by-trial. Oculomotor suppression effects were completely eliminated. If anything, the color singleton now captured overt attention. These findings provide converging evidence in favor of first-order feature suppression models and against global salience/second-order feature suppression models.
Experiment 4 provided further evidence for the importance of first-order features in singleton suppression. The nonsingleton color remained constant over multiple blocks, but the singleton color changed from block to block (as in the previous RT study of Vatterott & Vecera, 2012). First-order suppression models predict that suppression should be temporarily disrupted when the singleton changes to a new color. Second-order suppression models and global salience models, however, both predict that changes in the specific color of the singleton should not disrupt suppression because suppression in these models is not based on the first-order color of the singleton. The data clearly supported first-order suppression models: Suppression was disrupted for several trials after a change in the color of the singleton. As participants gained experience with the new color value, suppression returned. Running averages across trials suggested that attention was captured by the singleton for approximately 5 trials after a change in the color of the singleton, followed by a statistically significant suppression after several trials with a given singleton color. This pattern directly contradicts second-order and global salience models, which posit no role of the singleton’s first-order features. The results, instead, support first-order feature suppression models.
Sawaki and Luck (2010, Experiment 3) reached the opposite conclusion of these experiments, albeit using the PD component to index suppression. In that study, the color of the singleton and nonsingleton colors varied randomly trial-by-trial (analogous to our Experiment 1), yet the singleton elicited a robust PD component, which was consistent with global salience/second-order feature suppression models. We fully expected to find analogous results in the present behavioral experiments, but these four experiments consistently demonstrated that first-order information was necessary for suppression. One possibility is that the PD may reflect a suppressive mechanism that impacts some aspects of behavior and not others, but further research is needed to resolve this discrepancy.
A more general possibility is that some suppression can be achieved without foreknowledge of the singleton’s upcoming feature value, but that this suppression is too weak to suppress the singleton below baseline (i.e., below the level of the nonsingleton distractors). In other words, second-order feature suppression or global salience suppression mechanisms may have been operating in the present experiments to reduce the amount of attention capture even if they were unable to eliminate the capture or suppress the singleton below baseline. Thus, although the present results demonstrate that first-order suppression mechanisms exist and are very powerful, we cannot entirely rule out the existence of weaker second-order feature suppression and global salience suppression mechanisms. Relatedly, it is also plausible that, with extensive practice or training, participants could eventually learn to suppress singletons based upon their salience alone (and would require no first-order feature information about the singletons). Future research should investigate these issues.
Distractor Suppression or Target Upweighting?
Although the results of the present study and our previous studies (Gaspelin et al., 2015, 2017) are naturally explained by a first-order suppression model, they can also be explained by an alternative model that relies on first-order features but does not involve suppression per se. Such a model would be identical to Wolfe’s guided search model (Wolfe, 1994; Wolfe et al., 1989) and similar to many other models of visual search (Treisman & Gelade, 1980). According to this alternative explanation, visual attention boosts the search priority of items with target-matching features, including the nonsingleton distractors, and the singleton appears to be suppressed only by comparison with these higher-priority items. We call this target-feature upweighting (see Bichot, Rossi, & Desimone, 2005) to highlight the distinction between this mechanism and mechanisms that involve directly suppressing the distractor features.
In our experiments with a predictable singleton color, the color of the target (and the nonsingleton distractors) was also highly predictable. If participants increase the priority of the target color, this will also increase the priority of the nonsingleton distractors (which have the same color as the target) relative to the singleton distractor. As a result, performance at the singleton location will be reduced compared to performance at the nonsingleton locations, creating the appearance of suppression at the singleton location when in fact it is a result of upweighting the target color (and hence the nonsingleton distractor color). Upweighting-based models could also explain speeded RTs on singleton-present trials compared to singleton-absent trials – fewer items are boosted on trials when the singleton is present, speeding target detection.
The results of Experiment 4 are not as easily explained by target-feature upweighting. After all, suppression was momentarily disrupted by changing the singleton color, even when the relevant, to-be-upweighted color was held constant, which is most naturally explained by a mechanism that directly suppresses the singleton color. However, target-feature upweighting could still explain these results if we assume that participants boosted color values that were close to the target color value but were slightly shifted away from the singleton color value. For example, when searching for a blue target and avoiding green singletons, participants may boost a relevant feature value somewhere between blue and pink (see the color wheel at the top of Figure 6). This is quite plausible, because psychophysical and neuroimaging data indicate that people will use a template that is shifted away from the true target value to increase discriminability between the target and the likely distractors (e.g., see Navalpakkam & Itti, 2007; Scolari & Serences, 2009). Thus, although the results of Experiment 4 are most naturally explained by suppression of the singleton color, we cannot rule out target-feature upweighting.
It should be noted that singleton suppression and target-feature upweighting are not necessarily mutually exclusive, and this may turn out to be an empty theoretical distinction. Some existing evidence suggestions that the visual system may simultaneously suppress distractor features and enhance target features to guide search (e.g., see Andersen & Müller, 2010; Navalpakkam & Itti, 2007). For example, Andersen and Müller had participants search displays of randomly moving blue and red dots to detect brief intervals of coherent motion. At the beginning of a trial, the fixation cross turned either blue or red to denote which color to monitor for coherent motion. The authors recorded steady state evoked potentials (SSVEPs) independently from the blue and red stimuli, using the SSVEP amplitude prior to the color cue as a neutral baseline for comparison. After the appearance of the color cue, there was both an increase in the SSVEP amplitude for the relevant color and a decrease in the SSVEP amplitude for the ignored color, indicating concurrent suppression and enhancement.
The idea that first-order features can be used to guide visual attention is not new. According to guided search models, the visual system parses a scene into a series of low-level feature maps (Wolfe, 1994, 2006; Wolfe et al., 1989). These maps are used to generate an activation map, whereby items in the visual field are assigned a weight denoting attentional priority. Critically, this guidance map is constructed preattentively (i.e., in parallel with no attentional restrictions). Traditionally, guided search models have traditionally been couched in terms of upweighting – relevant features are boosted. However, guided search models could easily accommodate the burgeoning evidence for suppression by positing that simple features can guide attention via both upweighting and downweighting. Indeed, when Treisman’s feature integration theory was updated to take into account the evidence for guided search, the revised theory proposed that irrelevant features were downweighted (Treisman & Sato, 1990).
Other Classes of Salient Stimuli
Almost all of the research on the suppression of attentional capture has used one class of salient stimuli: color singletons (e.g., Gaspar & McDonald, 2014; Gaspelin et al., 2015, 2017; Sawaki & Luck, 2010; Vatterott & Vecera, 2012). It is unclear if other types of salient stimuli, such as objects that appear suddenly (called abrupt onsets), can be suppressed. Much evidence suggests that onsets can capture attention under circumstances where color singletons cannot (Franconeri & Simons, 2003; Jonides & Yantis, 1988). Furthermore, some of the evidence supporting goal-driven accounts claiming that abrupt onsets do not capture attention may have been flawed (Folk & Remington, 2015; Gaspelin et al., 2016).
Conclusions
In summary, the current experiments demonstrate that our previously-observed attentional suppression effects (e.g., Gaspelin et al., 2015, 2017) critically depend on foreknowledge of the first-order feature values in the search display. Without foreknowledge of the upcoming singleton color value, participants cannot bias attentional allocation away from that item. This directly contradicts models proposing that salient items can be suppressed on the basis of a feature-independent salience signal but is consistent with first-order suppression models, consistent with decades-old models of visual search (Wolfe et al., 1989; Treisman & Sato, 1990).
Supplementary Material
Public Significance Statement.
This study demonstrates people can suppress attentional allocation to salient items, such as brightly colored objects. Importantly, this ability to suppress attentional allocation to salient objects develops gradually as people gain experience with particular features of those objects.
Acknowledgments
This study was made possible by National Research Service Award F32EY024834 to N.G. from the National Eye Institute and by NIH Grants R01MH076226 and R01MH065034 to S.J.L.
Footnotes
Using a more lenient exclusion criterion (e.g., 200 ms to 2000 ms) did not alter any of the main findings in this paper.
In this experiment, both the singleton and nonsingleton colors were unpredictable, which raises the possibility that the effects were driven by the unpredictability of the nonsingleton color rather than the singleton color. Experiment 4 shows that suppression is disrupted when the singleton color changes even when the nonsingleton color is held constant, indicating that the key factor is the predictability of the singleton color.
The reader may notice that on singleton-absent trials, only one color from a pair was presented. We still classified each trial as belonging to a color pair, regardless of whether the singleton was presented. For example, we removed trials where the target color was pink and then was green. We did this to take extra precaution because participants may implicitly learn the color pairs to the point that presenting one color causes them to suppress another.
References
- Andersen SK, Müller MM. Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature-selective attention. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13878–82. doi: 10.1073/pnas.1002436107. http://doi.org/10.1073/pnas.1002436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Braak CT. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation. 2003;73:85–113. [Google Scholar]
- Andrews LS, Watson DG, Humphreys GW, Braithwaite JJ. Flexible feature-based inhibition in visual search mediates magnified impairments of selection: Evidence from carry-over effects under dynamic preview-search conditions. Journal of Experimental Psychology: Human Perception and Performance. 2011;37(4):1007–1016. doi: 10.1037/a0023505. http://doi.org/10.1037/a0023505. [DOI] [PubMed] [Google Scholar]
- Arita JT, Carlisle NB, Woodman GF. Templates for rejection: Configuring attention to ignore task-irrelevant features. Journal of Experimental Psychology: Human Perception and Performance. 2012;38(3):580–584. doi: 10.1037/a0027885. http://doi.org/10.1037/a0027885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Perception & Psychophysics. 1994;55(5):485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Becker SI. Irrelevant singletons in pop-out search: Attentional capture or filtering costs? Journal of Experimental Psychology: Human Perception and Performance. 2007;33(4):764–787. doi: 10.1037/0096-1523.33.4.764. http://doi.org/10.1037/0096-1523.33.4.764. [DOI] [PubMed] [Google Scholar]
- Becker SI. Oculomotor capture by colour singletons depends on intertrial priming. Vision Research. 2010;50(21):2116–2126. doi: 10.1016/j.visres.2010.08.001. http://doi.org/10.1016/j.visres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science (New York, N.Y.) 2005;308(5721):529–534. doi: 10.1126/science.1109676. http://doi.org/10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: The long and short of it. Spatial Vision. 1989;4(2):103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Two motion perception mechanisms revealed through distance- driven reversal of apparent motion. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2985–2989. doi: 10.1073/pnas.86.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CA, Egeth HE. Taming the White Bear. Psychological Science. 2016;27(4):476–485. doi: 10.1177/0956797615626564. http://doi.org/10.1177/0956797615626564. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychological Review. 1989;96(3):433–458. doi: 10.1037/0033-295x.96.3.433. http://doi.org/10.1037/0033-295X.96.3.433. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall/CRC; 1993. [Google Scholar]
- Egeth HE, Virzi Ra, Garbart H. Searching for conjunctively defined targets. Journal of Experimental Psychology. Human Perception and Performance. 1984;10(1):32–39. doi: 10.1037//0096-1523.10.1.32. http://doi.org/10.1037/0096-1523.10.1.32. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Involuntary attentional capture is determined by task set: Evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 2008;20(8):1423–1433. doi: 10.1162/jocn.2008.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics. 2002;64(5):741–753. doi: 10.3758/bf03194741. http://doi.org/10.3758/BF03194741. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW. Selectivity in distraction by irrelevant featural singletons: Evidence for two forms of attentional capture. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(3):847–858. doi: 10.1037//0096-1523.24.3.847. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW. A critical evaluation of the disengagement hypothesis. Acta Psychologica. 2010;135(2):103–105. doi: 10.1016/j.actpsy.2010.04.012. http://doi.org/10.1016/j.actpsy.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW. Unexpected Abrupt Onsets Can Override a Top-Down Set for Color Unexpected Abrupt Onsets Can Override a Top-Down Set for Color. Journal of Experimental Psychology : Human Perception and Performance. 2015 doi: 10.1037/xhp0000084. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18(4):1030–1044. http://doi.org/10.1037/0096-1523.18.4.1030. [PubMed] [Google Scholar]
- Found A, Müller HJ. Searching for unknown feature targets on more than one dimension: Investigating a “dimension-weighting” account. Perception & Psychophysics. 1996;58(1):88–101. doi: 10.3758/bf03205479. http://doi.org/10.3758/BF03205479. [DOI] [PubMed] [Google Scholar]
- Franconeri SL, Simons DJ. Moving and looming stimuli capture attention. Perception & Psychophysics. 2003;65(7):999–1010. doi: 10.3758/bf03194829. [DOI] [PubMed] [Google Scholar]
- Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. The Journal of Neuroscience. 2014;34(16):5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. http://doi.org/10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, Luck SJ. Direct Evidence for Active Suppression of Salient-but-Irrelevant Sensory Inputs. Psychological Science. 2015;22(11):1740–1750. doi: 10.1177/0956797615597913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, Luck SJ. Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, & Psychophysics. 2017:1–18. doi: 10.3758/s13414-016-1209-1. http://doi.org/10.3758/s13414-016-1209-1. [DOI] [PMC free article] [PubMed]
- Gaspelin N, Margett-Jordan T, Ruthruff E. Susceptible to distraction: Children lack top-down control over spatial attention capture. Psychonomic Bulletin & Review. 2014;22(2):461–468. doi: 10.3758/s13423-014-0708-0. http://doi.org/10.3758/s13423-014-0708-0. [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Ruthruff E, Lien M. The Problem of Latent Attentional Capture : Easy Visual Search Conceals Capture by Task-Irrelevant Abrupt Onsets. Journal of Experimental Psychology : Human Perception and Performance. 2016 doi: 10.1037/xhp0000214. http://doi.org/10.1037/xhp0000214. [DOI] [PMC free article] [PubMed]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391(6666):481–4. doi: 10.1038/35135. http://doi.org/10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Groppe D, Urbach T, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology. 2011;141(4):520–529. doi: 10.1111/j.1469-8986.2011.01273.x. http://doi.org/10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience. 2009;21(4):760–775. doi: 10.1162/jocn.2009.21039. http://doi.org/10.1162/jocn.2009.21039. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Gottlieb J, Bisley JW, Goldberg ME. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nature Neuroscience. 2006;9(8):1071–1076. doi: 10.1038/nn1734. http://doi.org/10.1038/nn1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews. Neuroscience. 2001;2(3):194–203. doi: 10.1038/35058500. http://doi.org/10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Jannati A, Gaspar JM, McDonald JJ. Tracking target and distractor processing in fixed-feature visual search: evidence from human electrophysiology. Journal of Experimental Psychology. Human Perception and Performance. 2013;39(6):1713–30. doi: 10.1037/a0032251. http://doi.org/10.1037/a0032251. [DOI] [PubMed] [Google Scholar]
- Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Perception & Psychophysics. 1988;43(4):346–354. doi: 10.3758/bf03208805. [DOI] [PubMed] [Google Scholar]
- Julesz B. Experiments in the visual perception of texture. Scientific American. 1975;232(4):34–43. doi: 10.1038/scientificamerican0475-34. http://doi.org/10.1038/scientificamerican0475-34. [DOI] [PubMed] [Google Scholar]
- Kim M-S, Cave KR. Spatial Attention in Visual Search for Features and Feature Conjunctions. Psychological Science. 1995;6(6):376–380. http://doi.org/10.1111/j.1467-9280.1995.tb00529.x. [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Human Neurobiology. 1985 http://doi.org/10.1016/j.imavis.2008.02.004. [PubMed]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013 Nov;4:1–12. doi: 10.3389/fpsyg.2013.00863. http://doi.org/10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber AB, Egeth HE. Attention on autopilot: Past experience and attentional set. Visual Cognition. 2006;14(4–8):565–583. [Google Scholar]
- Leonard CJ, Luck SJ. The role of magnocellular signals in oculomotor attentional capture. Journal of Vision. 2011;11(13):11–11. doi: 10.1167/11.13.11. http://doi.org/10.1167/11.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien M-C, Ruthruff E, Goodin Z, Remington RW. Contingent attentional capture by top-down control settings: Converging evidence from event-related potentials. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(3):509–530. doi: 10.1037/0096-1523.34.3.509. http://doi.org/10.1037/0096-1523.34.3.509. [DOI] [PubMed] [Google Scholar]
- Lien M-C, Ruthruff E, Johnston JC. Attentional capture with rapidly changing attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 2010;36(1):1–16. doi: 10.1037/a0015875. http://doi.org/10.1037/a0015875. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Johnson CA, Shimamura AP. How much is an icon worth? Journal of Experimental Psychology: Human Perception and Performance. 1985;11(1):1–13. doi: 10.1037//0096-1523.11.1.1. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: an ambiguity resolution theory of visual selective attention. Cognit Psychol. 1997;33(1):64–87. doi: 10.1006/cogp.1997.0660. http://doi.org/10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994 doi: 10.1111/j.1469-8986.1994.tb02218.x. http://doi.org/10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Memory & Cognition. 1994;22(6):657–672. doi: 10.3758/bf03209251. http://doi.org/10.3758/BF03209251. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. http://doi.org/10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Heller D, Ziegler J. Visual search for singleton feature targets within and across feature dimensions. Perception & Psychophysics. 1995;57(I):1–17. doi: 10.3758/bf03211845. http://doi.org/10.3758/BF03211845. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Reimann B, Krummenacher J. Visual search for singleton feature targets across dimensions: Stimulus- and expectancy-driven effects in dimensional weighting. Journal of Experimental Psychology: Human Perception and Performance. 2003;29(5):1021–1035. doi: 10.1037/0096-1523.29.5.1021. http://doi.org/10.1037/0096-1523.29.5.1021. [DOI] [PubMed] [Google Scholar]
- Navalpakkam V, Itti L. Search Goal Tunes Visual Features Optimally. Neuron. 2007;53(4):605–617. doi: 10.1016/j.neuron.2007.01.018. http://doi.org/10.1016/j.neuron.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Baumgartner HA, Barrett FS, Messenger IM, Luck SJ. Developmental changes in visual short-term memory in infancy: Evidence from eye-tracking. Frontiers in Psychology. 2013 Oct;4:1–13. doi: 10.3389/fpsyg.2013.00697. http://doi.org/10.3389/fpsyg.2013.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Cross-dimensional interaction and texture segregation. Perception & Psychophysics. 1988;43(4):307–318. doi: 10.3758/bf03208800. [DOI] [PubMed] [Google Scholar]
- Pitman EJG. Significance Tests Which May be Applied to Samples From any Populations. Supplement to the Journal of the Royal Statistical Society. 1937;4(1):119–130. [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review. 2009;16(2):225–237. doi: 10.3758/PBR.16.2.225. http://doi.org/10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception, & Psychophysics. 2010;72(6):1455–1470. doi: 10.3758/APP.72.6.1455. http://doi.org/10.3758/APP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. Active suppression of distractors that match the contents of visual working memory. Visual Cognition. 2011;19(7):956–972. doi: 10.1080/13506285.2011.603709. http://doi.org/10.1080/13506285.2011.603709.Active. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari M, Serences JT. Adaptive Allocation of Attentional Gain. Journal of Neuroscience. 2009;29(38):11933–11942. doi: 10.1523/JNEUROSCI.5642-08.2009. http://doi.org/10.1523/JNEUROSCI.5642-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Chubb C, Wright CE, Sperling G. Human attention filters for single colors. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(43):E6712–E6720. doi: 10.1073/pnas.1614062113. http://doi.org/10.1073/pnas.1614062113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Perception & Psychophysics. 1992;51(6):599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychologica. 2010;135(2):77–99. doi: 10.1016/j.actpsy.2010.02.006. http://doi.org/10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE. Our eyes do not always go where we want them to go: Capture of the eyes by new objects. Psychological Science. 1998;9(5):379–385. [Google Scholar]
- Treisman A, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12(1):97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Treisman A, Sato S. Conjunction Search Revisited. Journal of Experimental Psychology: Human Perception and Performance. 1990;75(3):459–478. doi: 10.1037//0096-1523.16.3.459. http://doi.org/10.1037/0096-1523.16.3.459. [DOI] [PubMed] [Google Scholar]
- Vatterott DB, Vecera SP. Experience-dependent attentional tuning of distractor rejection. Psychonomic Bulletin & Review. 2012;19(5):871–8. doi: 10.3758/s13423-012-0280-4. http://doi.org/10.3758/s13423-012-0280-4. [DOI] [PubMed] [Google Scholar]
- Watson DG, Humphreys GW. Visual marking: prioritizing selection for new objects by top-down attentional inhibition of old objects. Psychological Review. 1997;104(1):90–122. doi: 10.1037/0033-295x.104.1.90. http://doi.org/10.1037/0033-295X.104.1.90. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Construction of permutation tests. Journal of the American Statistical Association. 1990;85(411):693–698. doi: 10.1080/01621459.1990.10474917. http://doi.org/doi: 10.2307/2290004. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychonomic Bulletin & Review. 1994;1(2):202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 4.0. Integrated Models of Cognitive Systems. 2006;(3):99–120. http://doi.org/10.1007/978-94-011-5698-1_30.
- Wolfe JM, Cave KR, Franzel SL. Guided search: An alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human Perception and Performance. 1989;15(3):419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400(6747):867–869. doi: 10.1038/23698. http://doi.org/10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology. Human Perception and Performance. 2003;29(1):121–138. doi: 10.1037//0096-1523.29.1.121. http://doi.org/10.1167/1.3.103. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ, Schall JD. The role of working memory representations in the control of attention. Cerebral Cortex. 2007;17(SUPPL 1) doi: 10.1093/cercor/bhm065. http://doi.org/10.1093/cercor/bhm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: Evidence from visual search. Journal of Experimental Psychology: Human Perception and Performance. 1984;10(5):601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.