Abstract
Objectives
To compare bleeding and clinical events of patients with stable angina or silent ischemia undergoing percutaneous coronary intervention (PCI) treated with unfractionated heparin (UFH) or bivalirudin.
Background
Few direct comparisons between UFH monotherapy vs. bivalirudin exist for patients with stable ischemic heart disease undergoing PCI.
Methods
A prospective, investigator-initiated, single-center, single-blinded, randomized trial of UFH vs. bivalirudin was conducted. The primary endpoint was all bleeding (major and minor) from index-hospitalization to 30 days post discharge. Secondary endpoints included major adverse cerebral and cardiovascular events (MACCE) and net adverse clinical events (NACE).
Results
Two-hundred-sixty patients were randomized for treatment with either UFH (n=123) (47%) or bivalirudin (n=137) (53%) There were no significant differences in baseline clinical and angiographic characteristics between the two groups. Primary endpoint was similar in both groups (10.9% with bivalirudin vs. 7.3% with UFH [p=0.31]). Major bleeding rates were 5.8% and 2.4%, respectively (p=0.17). There was a higher MACCE (3.5% vs. 0%, p=0.03) and NACE (8.8% vs. 2.4%, p=0.03) rate with bivalirudin compared to UFH, respectively. Bivalirudin had increased odds of NACE (OR = 3.65, 95% CI: 1.00–13.3.6). Death and stent thrombosis rates were low and similar in both groups. Radial access was associated with fewer bleeding events compared to femoral access but not statistically significant (p=0.29).
Conclusions
Among patients with stable angina or silent ischemia, there was no difference between UFH and bivalirudin in bleeding rates up to 30-days post-PCI. MACCE and NACE were higher among the bivalirudin group. Radial access was associated with a numerically lower rate of bleeding compared with femoral access.
INTRODUCTION
The current anticoagulation strategy in patients with stable coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) varies widely based on operator and regional preferences.1 Contemporary practice for these procedures includes the combined administration of oral anti-platelet agents and intravenous anticoagulation with either unfractionated heparin (UFH) or bivalirudin.2 Current guidelines list both of these anticoagulants as having a Class I indication for patients undergoing PCI.2, 3
Although there are several studies and meta-analyses that have compared the use of UFH with bivalirudin in patients with acute coronary syndrome (ACS) undergoing PCI, there is limited data comparing clinical outcomes in patients with stable CAD undergoing PCI.4, 5 In the Intracoronary Stenting and Antithrombotic Regimen – Rapid Early Action for Coronary Treatment 3 trial (ISAR-REACT 3), patients with stable and unstable angina undergoing PCI had significantly higher major and minor bleeding events with UFH.6 However, the dose of UFH administered through this trial was substantially higher than in contemporary practice (140 units/kg given as a bolus), which may have contributed to the increased bleeding rates.
The influence of the arterial access site on bleeding complications is increasingly scrutinized. Meta-analysis conducted by Mina et. al., suggested bivalirudin was associated with fewer bleeding events only when femoral access was employed, whereas radial access bleeding was only reduced with the utilization of UFH among patients with ACS.7 Most recently, the large scale randomized superiority trial Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX Access) compared radial to femoral approaches in 8,404 ACS patients undergoing PCI. Radial access significantly reduced NACE by way of lower all-cause mortality and major bleeding.8 Additionally, other contemporary trials have reported on the impact of access sites in patients undergoing primary PCI.9, 10 The aim of the present study was to compare bivalirudin with UFH in patients with stable CAD undergoing PCI on dual anti-platelet therapy (DAPT). An exploratory sub-analyses was also conducted to assess the impact of transradial vs transfemoral access.
METHODS
Study design and population
The STable Angina Therapy with bivalirudin or Unfractionated heparin for patientS undergoing Percutaneous Coronary Intervention study (STATUS-PCI) (NCT01464671) was a prospective, randomized, single center trial that compared bivalirudin with UFH in patients with stable angina or silent ischemia undergoing PCI. The study was reviewed and approved by the Stony Brook University Institutional Review Board and was supported by grant M01-RR-107-10 (Clinical Research Center) from the National Institutes of Health (Bethesda, MD). This grant provided support staff for statistical analysis and informatics systems applications as well as nursing staff to perform telephone follow-up. The study was also partially supported by a grant from The Medicines Company Inc. (Parsippany, NJ) that supplied bivalirudin for those subjects randomized to the bivalirudin arm. The company had no involvement in any aspect of trial design, conduct, data analysis or reporting. An independent data safety and monitoring board provided appropriate oversight and monitoring of the conduct of the study.
Eligible patients presenting to our institution during the recruitment period from December 2009 until June 2013 were screened for enrollment (Figure 1). Patients eligible for study participation had to be ≥18 years of age and scheduled for elective cardiac catheterization for evaluation of CAD with either stable angina or silent ischemia on prior non-invasive testing. Patients were excluded if they had known intolerance, hypersensitivity, or contraindication to DAPT, bivalirudin or UFH. Patients presenting with an acute coronary syndrome, hemodynamic instability or cardiogenic shock were also excluded from the study. All subjects underwent cardiac catheterization performed as per the standard of care practices. Upon determining appropriateness for PCI by the treating physician, the un-blinded study coordinator enrolled each subject into the study.
Figure 1.
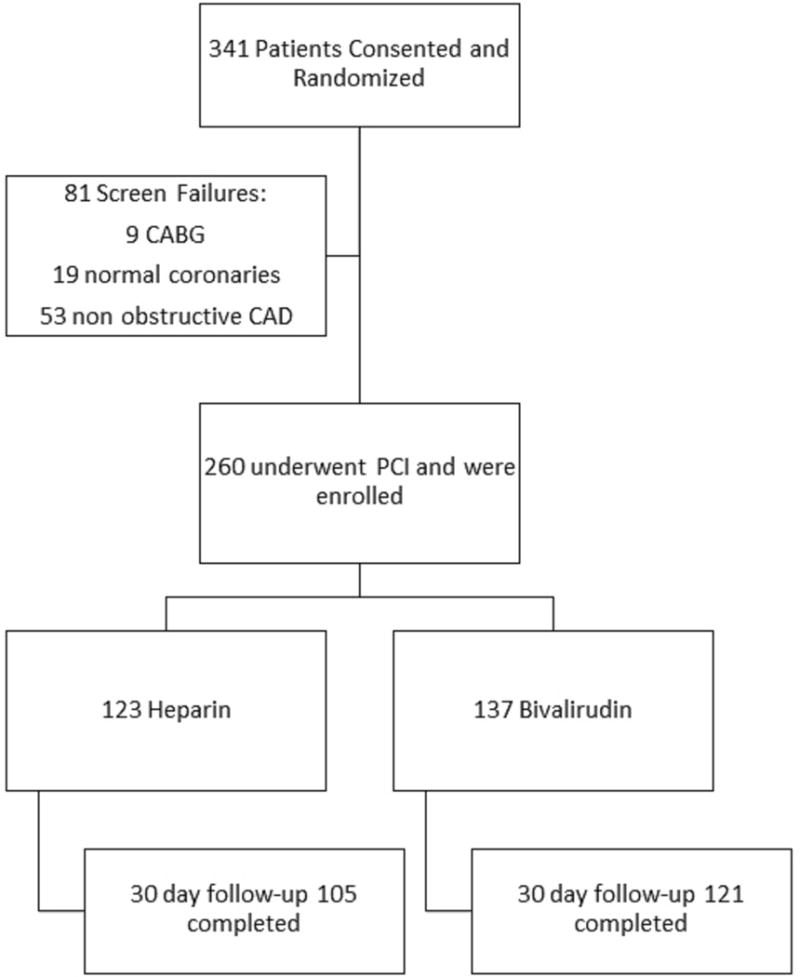
Flow of patients screened and enrolled within the STATUS PCI study.
Subjects were randomly allocated in a 1:1 fashion to either bivalirudin or UFH prior to intervention. The randomization scheme was by block permutation via a computerized software application with assignment of hundreds of unique numerical identifiers charted in varying permutated blocks to correspond in a random 1:1 fashion with both study drug-arms. Each subject was blinded to their study drug throughout their enrollment. In addition, all personnel obtaining the follow-up patient evaluations for all clinical endpoints were blinded to the treatment assignment. Only the un-blinded study coordinators as well as the treating physician were aware of the subjects’ treatment allocation.
Procedures
All patients underwent coronary angiography for evaluation of obstructive CAD; no study-related restrictions were imposed on the conduction of the angiogram or PCI. All enrolled subjects received DAPT either prior to the procedure or at the time of PCI with a loading dose of 600 mg clopidogrel or 60 mg prasugrel, or 180mg ticagrelor as per current guidelines. Among patients in the UFH arm: 93.5% were treated with clopidogrel, 5% were treated with prasugrel, and 1.5% were treated with ticagrelor. Whereas among patients in the bivalirudin arm: 89% were treated with clopidogrel, 8.8% were treated with prasugrel, and 2.25% were treated with ticagrelor. Although no subjects received glycoprotein IIb-IIIa inhibitors, their use during and after the PCI was left to the discretion of the treating interventional cardiologist as a bail-out option. Decision to utilize radial or femoral access was also left to the discretion of the treating interventional cardiologist.
Weight-adjusted UFH was administered as an intravenous bolus (70 IU/kg) to achieve an activated clotting time (ACT) of 250 to 300 seconds with the HemoTec® device. ACT was checked following administration of the bolus and every 20 minutes during the procedure to ensure that the ACT was at the target level. Bivalirudin was administered as an intravenous bolus (0.75 mg/kg) followed by an infusion (1.75 mg/kg/h) for the remaining duration of the PCI. ACT levels were checked after the bolus administration of bivalirudin to ensure that the ACT was >250 sec. An additional bolus was given if the ACT was <250 sec. In patients with creatinine clearance <30 mL/min, bivalirudin infusion rate was reduced to 1.0 mg/kg/h; the infusion was further reduced to 0.25 mg/kg/h for patients on hemodialysis.
Sheath removal was performed 2 hours after discontinuation of the bivalirudin infusion or when the ACT was <180 seconds in patients randomized to UFH. Femoral and radial access sites were monitored by blinded study coordinators immediately after the procedure, before and after sheath removal and prior to discharge.
Each subject was evaluated at the index hospitalization and at 30 days after. Data was collected by blinded study personnel at each follow-up encounter. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Stony Brook University.11 Investigators conducted random audits against the data at different time points during the study’s progress to crosscheck collected data. All primary and secondary endpoints were adjudicated by members of an independent Clinical Events Committee, who were blinded to treatment assignments. Safety endpoints were monitored by members of a Data Safety and Monitoring Board who had full access to all aspects of the study data and documentation.
Study outcomes and statistical analysis
The primary endpoint of the study was cumulative bleeding occurrence within 30 days of index hospitalization as defined by the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial definition.12 Major bleeding was defined as intracranial, intraocular, or retroperitoneal hemorrhage, clinically overt blood loss resulting in a decrease in Hg of more than 3 g/dL, any decrease in Hg of more than 4 g/dL, or transfusion of 2 or more units of packed RBC or whole blood. Minor bleeding was defined as clinically overt bleeding that did not meet criteria for major bleeding (i.e., all other). Secondary endpoints included Major Adverse Cerebral and Cardiovascular Events (MACCE, defined as all-cause mortality, MI, target vessel revascularization [TVR], and cerebral vascular accident [CVA]), stent thrombosis, and net adverse clinical events (NACE, define as all MACCE and major bleeding events) up to 30 days after index hospitalization. Based on power calculations using a significance level of >0.05 and a power of 0.80, a total of 776 patients would be needed, 388 patients in each group (with continuity correction) were required to show a 50% relative decrease in the primary endpoint. This assumed a 12% rate of bleeding in the control arm (i.e. UFH).13
An exploratory sub-analyses of access site was conducted after the completion of study enrollment. Utilization of transradial or transfemoral access site was not part of the randomization process and was left to the discretion of the treating physician cardiologist. No patients were converted from radial to femoral access (or vice-versa).
Chi-squared was used when applicable to compare differences in categorical variables, with Fisher’s exact test utilized in scenarios of sample sizes less than 5. The student’s t-test was used for continuous variables when applicable. STATA 12 (StataCorp LP, College Station, TX) was used for data analysis and a two-tailed p-value of 0.05 was regarded as statistically significant. Multivariable logistic regression analyses was performed to determine predictors for all bleeding, major bleeding, minor bleeding, MACCE, and NACE among all patients randomized to UFH or bivalirudin. Predictor variables included drug randomization arm, access site, and all factors that had a p-value <0.1 in the univariate models. Odds ratios (OR) and 95% confidence intervals (95% CI) were reported. All analysis was conducted according to an intention-to-treat methodology.
RESULTS
Between December 8, 2009 and June 26, 2013, a total of 341 patients were consented and enrolled in the study. Of these, 81 patients (23.8%) failed screening mainly because they did not undergo PCI. Two-hundred-sixty enrolled patients underwent PCI and were randomized for treatment with either UFH (n=123) (47%) or bivalirudin (n=137) (53%) (Figure 1). On December 19, 2013, after interim analysis of the data, the DSMB concurred and recommended to stop recruitment for futility. The DSMB did not identify any safety concerns for the patients enrolled in the trial and while further review of the data was conducted, the Institutional Review Board was informed that recruitment in the trial was suspended.
Clinical and Angiographic Characteristics
There were no significant differences between the baseline demographic characteristics of patients enrolled in the two treatment groups (Table 1). A lower left ventricular ejection fraction (52.4% vs 56.6%, p=0.008) was observed among patients randomized to UFH (Table 2). Femoral access was more commonly used in both groups (71.5% and 77.8%, respectively), with near concordant closure device rates. There were no significant differences in angiographic and lesion characteristics between the two groups (Table 2). The left anterior descending artery and its branches were the most commonly treated coronary territories, followed by the left circumflex artery and right coronary artery.
Table I.
Baseline Demographics of Study Population
| Heparin (n =123) |
Bivalirudin (n =137) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years ± SD) | 62.4 ± 9.6 | 62.3 ± 10.1 | 0.968 |
| BMI (± SD) | 31.2 ± 5.2 | 30.4 ± 5.2 | 0.228 |
| Males | 97 (78.9%) | 106 (77.4%) | 0.702 |
| Race (%) | 0.629 | ||
| Whites | 118 (95.9%) | 132 (96.4%) | 0.557 |
| Hypercholesterolemia | 106 (86.2%) | 117 (85.4%) | 0.821 |
| Hypertension | 112 (91.1%) | 117 (85.4%) | 0.336 |
| Family history of premature CAD | 52 (42.2%) | 60 (43.8%) | 0.334 |
| Active or Former Tobacco Use | 82(66.7) | 91(66.4%) | 0.91 |
| Diabetes Mellitus | 53(43.1) | 52 (37.7%) | 0.400 |
| Previous TIA/CVA | 6 (4.9%) | 8 (5.8%) | 0.732 |
| COPD | 10 (8.1%) | 19 (13.9%) | 0.137 |
| PVD | 4 (3.3%) | 6 (4.4%) | 0.637 |
| CKD | 6 (4.9%) | 14 (10.2%) | 0.111 |
| History of CHF | 12 (9.8%) | 7 (5.4%) | 0.174 |
| Previous MI | 49 (39.8%) | 50 (36.5%) | 0.927 |
| Previous PCI (multiple criteria apply per subject) | 86 (69.9%) | 82 (59.9%) | 0.090 |
| National Cardiovascular Data Registry® CathPCI Bleeding Risk Score | 1.4% ± 1.2 | 1.6% ± 1.8 | 0.173 |
Table II.
Angiographic characteristics
| Heparin (n=123) |
Bivalirudin (n=137) |
p-value | |
|---|---|---|---|
| Left Ventricular Ejection Fraction (± SD) | 52.4% (± 11.4) | 56.6% (±10.7) | 0.008 |
| Access Site | |||
| Radial A. | 35 (28.5%) | 32 (23.7%) | 0.392 |
| Femoral A. | 88 (71.5%) | 105 (77.8%) | 0.253 |
| Closure device was used | 62 (70.5%)* | 75 (71.4%)* | 0.447 |
| Vessels Treated | |||
| Left Main | 4 (3.3%) | 9 (6.7%) | 0.209 |
| Left Anterior Descending | 46 (37.4%) | 65 (47.4%) | 0.082 |
| Left Circumflex | 43 (35.0%) | 44 (32.6%) | 0.699 |
| Right Coronary | 42 (34.1%) | 44 (32.6%) | 0.802 |
| Arterial and Venous Graft | 9 (7.3%) | 4 (3.0%) | 0.355 |
| Lesion Characteristics | |||
| Calcified Lesion | 14 (11.4%) | 15 (10.9%) | 0.950 |
| Tortuosity | 7 (5.7%) | 3 (2.2%) | 0.151 |
| Bifurcation | 26 (21.1%) | 36 (26.7%) | 0.297 |
| Drug-Eluting Stent | 99 (80.5%) | 116 (84.6%) | 0.252 |
Note: Closure device percentage is derived from femoral access only.
Bleeding Events
A baseline National Cardiovascular Data Registry® (NCDR) CathPCI Bleeding Risk Score percent was calculated for all patients in the study.14 There was no significant difference in baseline bleeding risk scores between patients randomized to UFH or bivalirudin (1.4% ± 1.2 vs 1.6% ± 1.8, respectively, p=0.17) (Table 1). From index-hospitalization up to 30 days follow-up there was no difference in the primary endpoint of the study, which occurred in 7.3% of patients randomized to UFH and 10.9% of patients randomized to bivalirudin (p=0.31) (Table 3). Major bleeding rates from index-hospitalization up to 30 days follow-up was similar in the UFH and bivalirudin groups (2.4% and 5.8% respectively, p=0.174) (Table 3).
Table III.
Bleeding Events by Treatment Group (Heparin vs Bivalirudin) Primary Endpoint: Index up to 30 days post-procedure
| Patients in Heparin Group (n = 123) |
Patients in Bivalirudin Group (n= 137) |
p-value | |
|---|---|---|---|
| All bleeding (Primary Endpoint)± | 9 (7.3%) | 15 (10.9%) | 0.312 |
| Major Bleeding* | 3 (2.4%) | 8 (5.8%) | 0.174 |
| Minor Bleeding¥ | 8 (6.5%) | 12 (8.8%) | 0.496 |
| Retroperitoneal bleed | 0 (0.0%) | 1 (0.73%) | 0.513 |
| Gastrointestinal bleed | 2 (1.63%) | 5 (3.65%) | 0.447 |
| Intracranial bleed | 0 (0.0%) | 1 (0.73%) | 0.513 |
| Tamponade | 0 (0.0%) | 0 (0.0%) | - |
| Hemoptysis | 0 (0.0%) | 1 (0.73%) | 0.513 |
| Epistaxis | 1 (0.81%) | 3 (2.19%) | 0.576 |
| Hematuria unassociated with impact/trauma | 0 (0.0%) | 1 (0.73%) | 0.513 |
| Hematoma of ≥ 5 cm in Diameter | 1 (0.8%) | 2 (1.5%) | 0.918 |
| Surgical or endoscopic management of bleed | 0 (0.0%) | 0 (0.0%) | - |
| Transfusion of blood | 1 (0.81%) | 0 (0.0%) | 0.375 |
| Other bleeding | 6 (4.88%) | 5 (3.65%) | 0.113 |
All bleeding was counted as a single composite event per patient regardless if they had different forms of bleeding
Major bleeding is defined as: intracranial, intraocular, or retroperitoneal hemorrhage, clinically overt blood loss resulting in a decrease in Hg of more than 3 g/dL, any decrease in Hg of more than 4 g/dL, or transfusion of 2 or more units of packed red blood cells or whole blood.
Minor bleeding is defined as: clinically overt bleeding that did not meet criteria for major bleeding (i.e., all other).
Patients treated via femoral access had numerically higher bleeding rates which was not statistically significant (p=0.285). There was also no significant difference between the UFH and bivalirudin group among patients treated via femoral vs. radial access (Figure 2).
Figure 2.
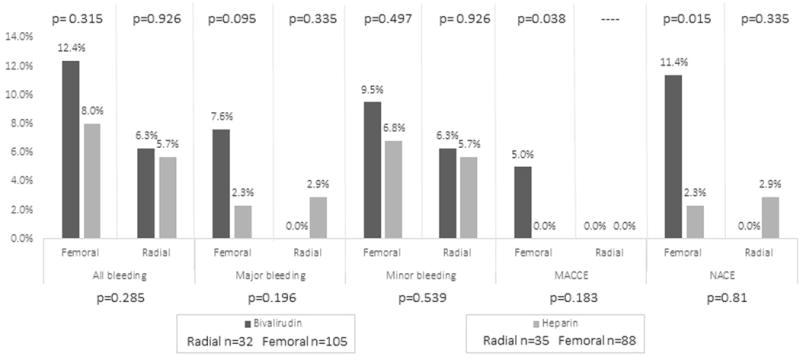
Clinical events by access site from index- hospitalization to 30-days follow-up for patients randomized to UFH or bivalirudin.
Clinical Events
Bivalirudin compared with UFH resulted in significantly increased MACCE rates (3.5% vs. 0%, p=0.032). All of these events were accrued among patients treated with bivalirudin via the femoral access, predominantly caused by MI and cardiac death (Figure 2). However, statistically there was no significant difference in MACCE between access sites (p=0.183, Figure 2). Stent thrombosis rates were low and similar in both groups. Two patients in the bivalirudin cohort and none in UFH cohort experienced early-stent thrombosis from index-hospitalization to 30-days (1.46% vs. 0%, p=0.50).
NACE occurred more frequently among patients randomized to bivalirudin (8.8% vs. 2.4%, p=0.029). When assessed by access site, femoral approach demonstrated higher rates of NACE among the bivalirudin cohort compared to UFH (11.4% vs. 2.3%, p=0.015, Figure 2). There was no overall difference between femoral and radial approach in regard to NACE (p=0.810) (Figure 2).
Predictors of bleeding, MACCE, and NACE
Multivariate logistic regression analyses of different composite bleeding endpoints showed that femoral access (OR = 1.79, 95% CI: 0.58–5.46) and radial access (OR = 0.55, 95% CI: 0.18–1.70) were not independently associated factors for all bleeding events (Table 4). The presence of bivalirudin was noted to be predictive of NACE (OR = 3.65, 95% CI: 1.00–13.3.6) at 30 days whereas femoral access was not associated with NACE (OR = 4.92, 95% CI: 0.63 – 38.54) (Table 4).
Table IV.
Multivariate Regression Models of 30 Day Predictors for: Total Bleeding, Major Bleeding, Minor Bleeding, MACCE, and NACE
| Odds Ratio | 95% Confidence Interval | |
|---|---|---|
| TOTAL BLEEDING: | ||
| Previous Percutaneous Coronary Intervention | 0.76 | 0.32 – 1.82 |
| Femoral Access | 1.79 | 0.58 – 5.46 |
| Radial Access | 0.55 | 0.18 – 1.70 |
| Bivalirudin | 1.49 | 0.62 – 3.55 |
| MAJOR BLEEDING: | ||
| Previous Percutaneous Coronary Intervention | 0.67 | 0.20 –2.30 |
| Femoral Access | 3.52 | 0.44 –28.20 |
| Bivalirudin | 2.32 | 0.60 – 9.02 |
| MINOR BLEEDING: | ||
| Previous Percutaneous Coronary Intervention | 1.05 | 0.40 – 2.74 |
| Femoral Access | 1.39 | 0.45 – 4.34 |
| Bivalirudin | 1.36 | 0.54 – 3.48 |
| MACCE: | ||
| Previous Percutaneous Coronary Intervention | 2.45 | 0.26 – 22.76 |
| Femoral Access | omitted* | |
| Bivalirudin | omitted* | |
| NACE: | ||
| Previous Percutaneous Coronary Intervention | 0.86 | 0.29 – 2.55 |
| Femoral Access | 4.92 | 0.63 – 38.54 |
| Bivalirudin | 3.65 | 1.00 – 13.36 |
Bivalirudin and access site were omitted from MACCE multivariate analyses as all events of MACCE at 30 days were among the bivalirudin group treated by femoral access
DISCUSSION
The results of STATUS-PCI study showed that in patients with stable CAD undergoing PCI with either UFH or bivalirudin and contemporary use of DAPT: 1) there was no significant difference in bleeding complications at 30 days; 2) there was a significant increase in MACCE and NACE rates among patients treated with bivalirudin; 3) bivalirudin was associated with increased odds for NACE in multivariate predictor modeling; 4) radial access was associated with a numerically lower rate of bleeding complications when compared with femoral access; and 5) radial access and heparin use in multivariate modelling demonstrated the most favorable odds for decreasing bleeding.
Despite its limitations, UFH it still considered a Class I indication for anticoagulation in patients undergoing PCI.15 Recent meta-analyses have demonstrated similar MACE and NACE rates between bivalirudin and UFH (particularly when accounting for concomitant GP IIb/IIIa inhibitors) among higher acuity ACS patients undergoing PCI via transradial approach, and using more potent antiplatelet therapy.7, 16, 17 However, previous randomized trials that enrolled mainly patients with ACS demonstrated a significant reduction of access site and non-access site bleeding complications in patients treated with bivalirudin as compared with patients treated with UFH and GP IIb/IIIa inhibitors. The cohort of stable and unstable angina patients in the ISAR-REACT 3 study showed that the administration of bivalirudin was associated with lower bleeding rates when compared to UFH.18 However, it is important to note that the dose of UFH was quite high (140 U/Kg) and patients that had received a lower dose (100 U/Kg) showed similar rates of major bleeding18. Two recent studies performed in stable patients deemed to be at an increased bleeding risk have shown conflicting results. The Novel Approaches for Preventing or Limiting Events (NAPLES) III Trial randomized 837 consecutive, increased bleeding risk, biomarker-negative patients undergoing PCI via the femoral artery to either the recommended dose of UFH or bivalirudin.19 In-hospital major bleeding occurred in 2.6% in the UFH group versus 3.3% in the bivalirudin group (p=0.54). The rate of major and minor bleeding, access site bleeding requiring intervention and clinically overt bleeding were also similar in both groups.19 The Anti-Thrombotic Strategy for Reduction of Myocardial Damage During Angioplasty-Bivalirudin vs. Heparin Study (ARMYDA-7 BIVALVE) randomized 401 patients considered to be at high bleeding risk to bivalirudin or UFH (75 IU/kg) during PCI.20 Although the study showed no difference in 30-day MACCE rates between the two arms, there was a significant reduction in any bleeding or access-site complications after PCI in patient randomized to bivalirudin (1.5% vs. 9.9%, p<0.0001).20
It is important to note that although the ARMYDA-7 BIVALVE study enrolled patients with a variety of clinical syndromes, the large majority (69–73%) had stable angina and 98% underwent the procedure via the femoral artery.20 In contrast, this study enrolled 100% of patients with stable angina and utilized radial access in over 25% of the study population with similar distribution between bivalirudin and UFH. The results showed no differences in the composite bleeding endpoints between the anticoagulants regardless of access site. Furthermore, Valgimigli and colleagues’ recent sub-study of the MATRIX trial randomizing bivlairudin and UFH (70–100 IU/kg) among 7,213 ACS patients, noted there was no indication of an interaction between access site and anticoagulant agent in regards to MACCE, NACE, Bleeding Academic Research Consortium (BARC) type 3 or 5 definition of bleeding, or death.21
Similar to the recent How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention (HEAT-PPCI) trial, two important aspects of our study was the use of GP IIb/IIIa inhibitors as a bail-out option to operators and the dosing of UFH (70 IU/kg). HEAT-PPCI randomized 1,812 patients with ST segment elevation myocardial infarction undergoing primary-PCI to bivalirudin or UFH with provisional GP IIb/IIIa inhibitors. Although different study populations, like HEAT-PPCI the present study minimally utilized GP IIb/IIIa inhibitors and dosed UFH at 70 IU/kg, which is lower than several prior studies.5, 22, 23 Similar to this study, HEAT-PPCI showed bivalirudin was associated with an increased risk in the primary composite efficacy endpoint of all-cause mortality, cerebrovascular accident, re-infarction, or unplanned target lesion revascularization compared with UFH(RR=1.52, 95%CI: 1.09–2.13).22
Acute stent thrombosis has been a source of concern for patients undergoing PCI with bivalirudin. A recent meta-analysis by Piccolo and colleagues of 11 trials, including 16,415 patients showed that treatment with bivalirudin was associated with a significantly higher risk of early stent thrombosis (odds ratio 1.80, CI=1.28–2.52, p=0.0007) and reduced major bleeding (odds ratio=0.64; CI=0.51–0.82, p=0.0003) when compared to UFH ± GP IIb/IIIa administration.24 The increased rate of stent thrombosis was previously noted in multiple trials of patients undergoing primary PCI in the setting of an ST-elevation myocardial infarction. In stable patients enrolled in the NAPLES III study, there was no difference in the rates of stent thrombosis (0.5% in both arms).19 In this study, 2 patients that were randomized to bivalirudin developed sub-acute stent thrombosis vs. none in the UFH group. This overall low rate may be related to the fact that all patients were pretreated with DAPT.
Limitations
As a single center trial, there are inherent limitation. The outcomes presented here may be secondary to specific practices at Stony Brook University and may not be generalizable. Given that the study was terminated early by the DSMB, the sample size to obtain adequate power for the primary endpoint was not achieved. While this study was powered on the composite endpoint of major and minor bleeding, previous studies have demonstrated that minor bleeding is not associated with major adverse events. In addition, there was a moderate attrition rate over the follow-up period. The study was not powered to investigate the impact of access site associated events.
Conclusion
In patients with stable CAD and pre-treated with DAPT, there was no significant difference in bleeding rates with bivalirudin or UFH. There was, however, a significant increase in MACCE with the use of bivalirudin. Bivalirudin was associated with higher odds for NACE among stable ischemic heart disease.
Acknowledgments
Funding sources
This study was supported by grant M01-RR-107-10 (Clinical Research Center) from the National Institutes of Health (Bethesda, MD). The study was also partially supported by a grant from The Medicines Company Inc. (Parsippany, NJ) that supplied study drug for subject randomized to the bivalirudin arm.
Footnotes
Author Disclosure:
The Authors have no conflicts of interest to disclose.
References
- 1.Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–16. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 4.De Luca G, Cassetti E, Verdoia M, Marino P. Bivalirudin as compared to unfractionated heparin among patients undergoing coronary angioplasty: A meta-analyis of randomised trials. Thromb Haemost. 2009;102:428–36. doi: 10.1160/TH09-05-0287. [DOI] [PubMed] [Google Scholar]
- 5.Kastrati A, Neumann FJ, Mehilli J, et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688–96. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 6.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–7. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Mina GS, Gobrial GF, Modi K, Dominic P. Combined Use of Bivalirudin and Radial Access in Acute Coronary Syndromes Is Not Superior to the Use of Either One Separately: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv. 2016;9:1523–31. doi: 10.1016/j.jcin.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med. 2015;373:997–1009. doi: 10.1056/NEJMoa1507854. [DOI] [PubMed] [Google Scholar]
- 9.Kilic S, Van’t Hof AW, Ten Berg J, et al. Frequency and prognostic significance of access site and non-access site bleeding and impact of choice of antithrombin therapy in patients undergoing primary percutaneous coronary intervention. The EUROMAX trial. Int J Cardiol. 2016;211:119–23. doi: 10.1016/j.ijcard.2016.02.131. [DOI] [PubMed] [Google Scholar]
- 10.Hamon M, Coste P, Van’t Hof A, et al. Impact of arterial access site on outcomes after primary percutaneous coronary intervention: prespecified subgroup analysis from the EUROMAX trial. Circ Cardiovasc Interv. 2015;8:e002049. doi: 10.1161/CIRCINTERVENTIONS.114.002049. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–63. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 13.Ndrepepa G, Schulz S, Keta D, et al. Bleeding after percutaneous coronary intervention with Bivalirudin or unfractionated Heparin and one-year mortality. Am J Cardiol. 2010;105:163–7. doi: 10.1016/j.amjcard.2009.08.668. [DOI] [PubMed] [Google Scholar]
- 14.Rao SV, McCoy LA, Spertus JA, et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6:897–904. doi: 10.1016/j.jcin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Task Force on the management of STseamiotESoC. Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 16.Farag M, Gorog DA, Prasad A, Srinivasan M. Bivalirudin versus unfractionated heparin: a meta-analysis of patients receiving percutaneous coronary intervention for acute coronary syndromes. Open Heart. 2015;2:e000258. doi: 10.1136/openhrt-2015-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud AN, Elgendy IY. Bivalirudin versus unfractionated heparin for percutaneous coronary intervention with radial access: A meta-analysis of randomized trials. Int J Cardiol. 2016;216:128–32. doi: 10.1016/j.ijcard.2016.04.140. [DOI] [PubMed] [Google Scholar]
- 18.Schulz S, Mehilli J, Ndrepepa G, et al. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J. 2010;31:582–7. doi: 10.1093/eurheartj/ehq008. [DOI] [PubMed] [Google Scholar]
- 19.Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) III trial: randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting. JACC Cardiovasc Interv. 2015;8:414–23. doi: 10.1016/j.jcin.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Patti G, Pasceri V, D’Antonio L, et al. Comparison of safety and efficacy of bivalirudin versus unfractionated heparin in high-risk patients undergoing percutaneous coronary intervention (from the Anti-Thrombotic Strategy for Reduction of Myocardial Damage During Angioplasty-Bivalirudin vs Heparin study) Am J Cardiol. 2012;110:478–84. doi: 10.1016/j.amjcard.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Valgimigli M, Gagnor A, Calabro P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 22.Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849–58. doi: 10.1016/S0140-6736(14)60924-7. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–30. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 24.Piccolo R, De Biase C, D’Anna C, Trimarco B, Piscione F, Galasso G. Early stent thrombosis with bivalirudin in patients undergoing percutaneous coronary intervention. A meta-analysis of randomised clinical trials. Thromb Haemost. 2015;113:1010–20. doi: 10.1160/TH14-08-0646. [DOI] [PubMed] [Google Scholar]


