Abstract
Background
As a potential tumor suppressor gene, Claudin-7 (Cldn7), which is a component of tight junctions, may play an important role in colorectal cancer occurrence and development.
Aims
To generate a knockout mouse model of inducible conditional Cldn7 in the intestine and analyze the phenotype of the mice after induction with tamoxifen.
Methods
We constructed Cldn7-flox transgenic mice and crossed them with villin-CreERT2 mice. The Cldn7 inducible conditional knockout mice appeared normal and were well-developed at birth. We induced Cldn7 gene deletion by injecting different dosage of Tamoxifen into the mice and then conducted a further phenotypic analysis.
Results
After induction for five days in succession at a dose of 200μl Tamoxifen in sunflower oil at 10 mg/ml per mouse every time, the mice appeared dehydrated, had a lower temperature, and displayed inactivity or death. The results of hematoxylin-eosin staining showed that the intestines of the Cldn7 inducible conditional knockout mice had severe intestinal defects that included epithelial cell sloughing, necrosis, inflammation and hyperplasia. Owing to the death of ICKO mice, we adjusted the dose of Tamoxifen to a dose of 100μl in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) every four days. And we could induce atypical hyperplasia and adenoma in the intestine. Immunofluorescent staining indicated that the intestinal epithelial structure was destroyed. Electron microscopy experimental analysis indicated that the intercellular gap along the basolateral membrane of Cldn7 inducible conditional knockout mice in the intestine was increased and that contact between the cells and matrix was loosened.
Conclusions
We generated a model of intestinal Cldn7 inducible conditional knockout mice. Intestinal Cldn7 deletion induced by tamoxifen initiated inflammation and hyperplasia in mice.
Keywords: Cldn7, ICKO, Cre/LoxP, Inflammation
Introduction
Tight junctions (TJs) are located between epithelial cells and endothelial cells and are the apical component of the epithelial cell junction complex [1]. The main functions of TJs include closing the intercellular gap, maintaining cell adhesion and polarity, assisting signal transduction, etc. [2]. Claudins, a family of cell adhesion molecules, are essential components of TJs, which include 27 claudin members found in mammals [3]. Cldn7 is an important member of the claudin family and is closely related to morphogenesis, invasion and tumor metastasis [1]. We constructed a general Cldn7 deletion in mice and found that Cldn7 had non-tight junction functions, which included maintenance of epithelial cell-matrix interactions and intestinal homeostasis [4]. The Cldn7 general knockout mice induced intestinal inflammatory microenvironment and atypical hyperplasia of the intestinal epithelial cells. Unfortunately, the mice with a Cldn7 deletion only survived for 7 days. In Hiroo Tanaka’s study, the researchers generated an intestinal, conditional deletion of Cldn7 in mice and found that the deletion enhanced paracellular organic solute flux and initiated colonic inflammation [5]. These mice were born with a similar deadly phenotype and survived for just 4 weeks. Based on preliminary results, we considered generating an intestinal, inducible Cldn7 gene-deficient mouse model to continue to investigate the function of Cldn7 in intestinal tumorigenesis. In our study, we obtained mice whose genotype was Cldn7fl/fl; Villin-CreERT2 and induced Cldn7 deletion by injecting tamoxifen. We then achieved a controllable intestinal Cldn7 ICKO mouse. The results showed that inflammation and hyperplasia occurred in the intestines of the Cldn7 ICKO mice.
Materials and Methods
ICKO Mice Construction
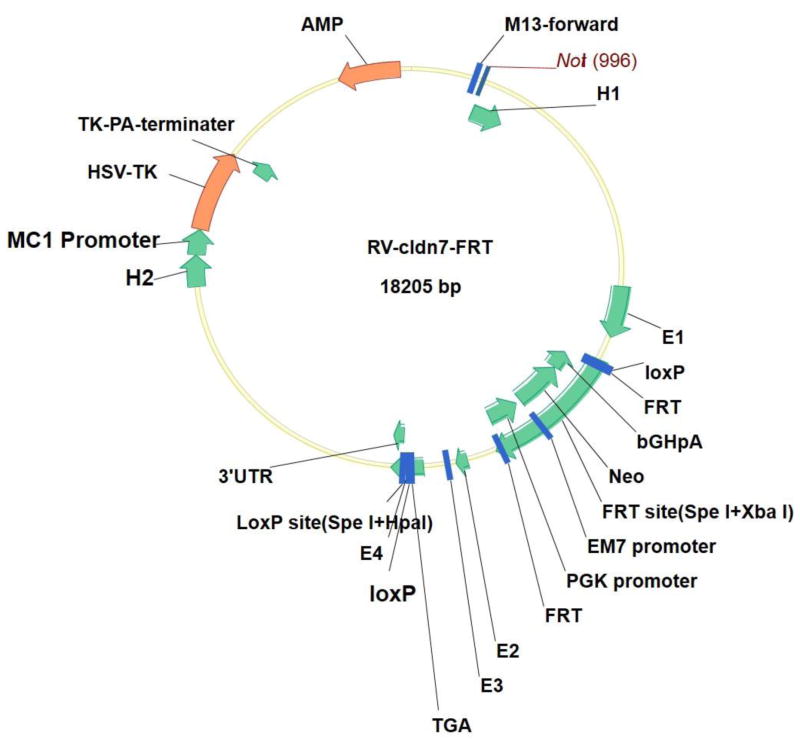
Villin-CreERT2 mice, whose Cre-recombinase fused with the mutational ligand binding domain of the human estrogen receptor to produce a Cre-recombinase that relied on tamoxifen, were obtained from the French professor, Sylvie Robine [6]. Wild type, heterozygote and Cldn7 gene-knockout mice were all fed a specific pathogen free (SPF) standard diet. The study was reviewed and approved by the Medical Ethics Committee of the Beijing Shijitan Hospital Affiliated Capital Medical University Institutional Review Board. First, we constructed the targeting vector for the Cldn7 conditional knockout. The structure of the targeting vector is shown in Figure 1, with relevant references [7, 8]. We inserted a LoxP site into the intron sequence of exon number 4 (69, 967, 555–69, 967, 885) downstream of the Cldn7 gene and inserted the FRT-Neo-FRT-LoxP component into the intron sequence of exon number 2 (69, 966, 943–69, 967, 107) upstream. We utilized Cre-recombinase to specifically recognize the sequence between the LoxP sites of the partial palindrome of 34 bp. The primer sequences were as follows: Cldn7-FRTtF2-CCTGGGATCTGATCTGGGTG; and Cldn7-FRTtR2-GGCAGGTAGCCTTAGGATGG. Second, we transfected the Cldn7 conditional knockout targeting vector to embryonic stem cells by electroporation and screening the clone with Long Range PCR and Southern blot. Third, we injected the blastocyst and prepared chimeric mice. Fourth, chimeric male mice were bred to obtain Cldn7-flox mice. Fifth, we obtained the Cldn7 intestinal ICKO mice. Finally, tamoxifen was used to induce the Cldn7 knockout in the intestine. Cldn7fl/fl; Villin-CreERT2 homozygous mice were given an intraperitoneal injection of tamoxifen, at a dose of 200 μl Tamoxifen in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) for 5 consecutive days [9]. Owing to the death of ICKO mice, we adjusted the dose of Tamoxifen to a dose of 100μl in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) every four days. The control group was given the solvent at the same dose. The number of female and male mice induced by tamoxifen was equal. The wild type mice were injected with the same dose of tamoxifen to determine tamoxifen’s effect on the mouse intestine.
Fig. 1.
The structure of Cldn7 ICKO targeting vector with references to the relevant literature [7, 8].
Western Blot Assay
In accordance with reagent instructions, the total protein was extracted using RIPA lysis buffer (Beyotime, Haimen, China). Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The membrane was blocked for one hour. Next, the membrane was incubated with diluted rabbit polyclonal anti-Cldn7 antibody (1:1000; ab27487; Abcam, USA) at 4°C overnight, followed by donkey anti-rabbit IgG antibody (1:10000; ab175780; Abcam, USA). Finally, the nitrocellulose membrane was scanned using the odyssey infrared imaging system or blots were developed with ECL. The two methods have been used in our experiment. GAPDH was used as the internal reference.
Immunofluorescence Assay
Paraffin sections were deparaffinized, gradient alcohol dehydrated, antigen repaired, and then rinsed in 0.01 M PBST three times at an interval of 5 minutes. The samples were blocked in 5% bovine serum albumin for 60 minutes at room temperature. After blocking, sections were incubated with primary antibodies. All antibodies were diluted in PBS containing 2.5% BSA. After washing, sections were incubated with the corresponding secondary antibody for 45 minutes at room temperature. Samples were photographed using a Nikon A1 laser confocal scanning microscope (Japan Nikon Inc., Tokyo).
Hematoxylin-eosin (HE) Staining Assay
The specimens were made into serial sections. After dewaxing, removing benzene and hydration through a serial alcohol gradient, HE staining was performed. After hematoxylin staining for 5–10 minutes, the sections were rinsed with tap water. Then, the sections were stained with eosin for 1–3 minutes, washed in distilled water, dehydrated using graded ethanol, made transparent with dimethylbenzene and deposited in a sealing agent.
Immunohistochemical Staining Assay
The tissue sections were incubated for 20 minutes in 3% H2O2 to block endogenous peroxidase and rinsed 3 times in 0.01 mol/L TBS, pH 7.4 for 3 minutes with each rinse. Next, sections were incubated with diluted rabbit polyclonal anti-Cldn7 antibody (ab27487; 1:200, Abcam, USA) at 4°C overnight. The sections were washed 3 times in 0.01 mol/L TBS, and incubated with donkey anti-rabbit IgG antibody (ab175780; Abcam, USA) for 20 minutes. Antibody dilutions were 1:1000. Then, all sections were rinsed for 3 min in TBS 3 times and developed in 0.05% 3, 3-diaminobenzidine (DAB) for coloration. Coloration was completed with distilled water.
Results
ICKO Mice Construction
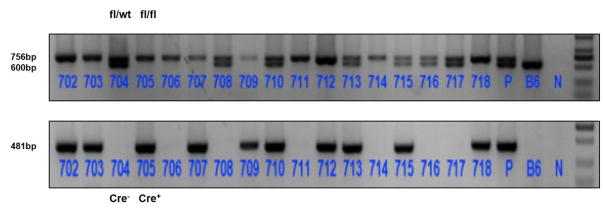
All plasmids were authenticated by PCR, with the fragment between the FRT site and the second LoxP site in the terminal carrier. LoxptF, GTACGAGTTTGGACCTGCCA, was utilized to conduct primer single-track sequencing (approximately 2000 bp), and the testing results showed that the LoxP locus of 34 bp was correct. We created ninety-six drug resistant embryonic stem cells clones after electroporation transfection, which were then authenticated by Long Range PCR and Southern blot. Finally, we selected 6 positive clones. After microinjection, we achieved chimeric mice developed by the gene-knockout embryonic stem cells and donor embryo cells. Mature male chimeric mice whose chimera rate were 50% and above were selected and hybridized with Flper mice. The offspring were mated with wild type B6 mice to produce the F1 generation mice. Finally, we acquired the Cldn7-flox heterozygous mice (genotype: fl/wt) after authenticating the genotype by PCR. Cldn7-flox mice and villin-CreERT2 mice were hybridized, and cut-tail identification after sib-mating was conducted on offspring mice. Finally, we acquired the Cldn7 intestinal ICKO mouse model (Cldn7fl/fl; Villin-CreERT2), and all were confirmed as Cldn7fl/fl; Villin-CreERT2 by genotype identification (Figure 2). The Cldn7 ICKO mice were born with a normal phenotype. Growth and development were good at birth. After induction for five days in succession at a dose of 200μl Tamoxifen in sunflower oil at 10 mg/ml per mouse every time, the mice were dehydrated, thin and weak. In addition, they had a low temperature and displayed inactivity or death. The vehicle control group was well developed and alive after tamoxifen injection on the third day. The control mice did not display any phenotypic abnormalities. Furthermore, no pathological changes were found in the gut. The experimental group mice were sacrificed after five successive days of tamoxifen injection at a dose of 200μl drug in sunflower oil at 10 mg/ml per mouse every time. Owing to the death of ICKO mice, we adjusted the dose of Tamoxifen to a dose of 100μl in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) every four days. And we could induce atypical hyperplasia and adenoma in the intestine (as shown in Figure 7). Moreover, the overall length of the intestinal tract in Cldn7 ICKO mice was significantly shorter compared to the control group. This phenomenon shows that the deletion of Cldn7 in the small intestine affects intestinal morphology (as shown in Figure 3).
Fig. 2.
The PCR sequencing obtained by tail cutting. Cldn7-FRTtF2 primer: CCTGGGATCTGATCTGGGTG; and Cldn7-FRTtR2 primer: GGCAGGTAGCCTTAGGATGG. Wt =600 bp, fl=756 bp; fl/wt=600 bp/756 bp; fl/fl=756 bp; Cre=481 bp. We authenticated the genotype of the neonatal mice and saw that mice with the Cldn7fl/fl CreT genotype were 702, 703, 705, 709, 712 and 718.
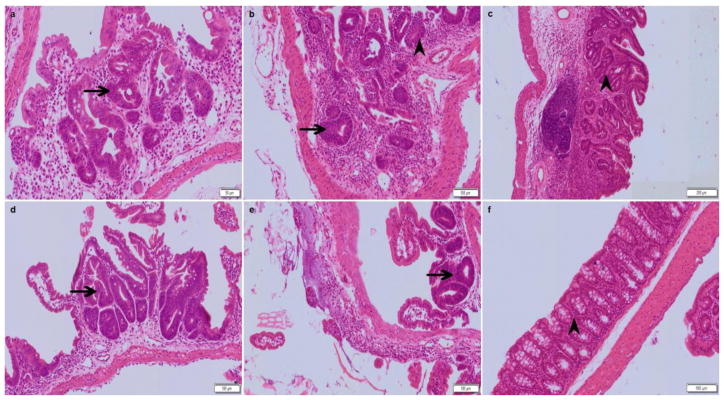
Fig. 7.
Cldn7 deletion in the small intestine and large intestine induced by Tamoxifen caused adenomatous hyperplasia. The arrows show that atypical hyperplasia and adenoma could be observed in the intestinal tract (a–e). We have adjusted Tamoxifen at a dose of 100 μl in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) every four days. The large intestine epithelium structure of the solvent control group was intact and had no pathological changes (f).
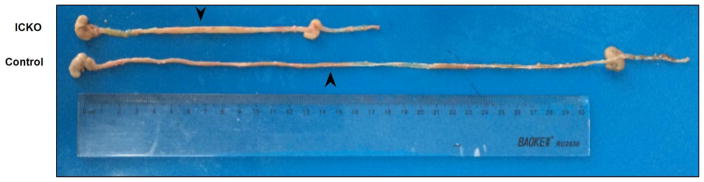
Fig. 3.
Intestine samples from ICKO mice and control group. Compared to the control group, the overall intestinal length in Cldn7 ICKO mice was significantly shorter and with obvious swelling. This phenomenon showed that Cldn7 deletion in the intestine affected intestinal morphology which occurred mainly in the small intestine as shown by the arrowhead in the figure 3.
Western Blot and Immunohistochemical Analysis
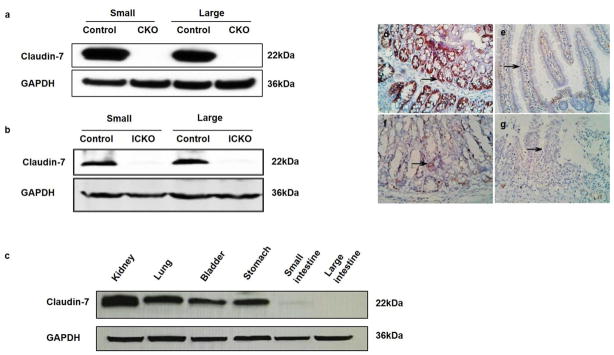
Western blot indicated that intestinal Cldn7 expression was absent in ICKO mice but present in the control vehicle group. In addition, the Cldn7 signal in the kidney, lung, bladder and stomach were still present but absent in the small and large intestine. The results of immunohistochemical staining indicated that a strong Cldn7 signal was present in the vehicle control group and discontinued, weak or absent in ICKO mice (as shown in Figure 4). These results indicated that we had constructed the ICKO mice model successfully.
Fig. 4.
Cldn7 expression in vehicle control, CKO and ICKO mice. The CKO mice lack the expression of Cldn7 in small intestine and large intestine compared to the control group (a). After tamoxifen induction, mice with the Cldn7fl/fl CreERT2 genotype lacked Cldn7 expression, while normal expression was observed in the vehicle control group (b). Cldn7 expression in ICKO mice in the kidney, lung, bladder and stomach was still present, and the Cldn7 signal in the small and large intestine was very weak (c). The blots were developed with ECL (a) and scanned using the odyssey infrared imaging system (b–c). Immunohistochemical staining indicated that a strong Cldn7 signal was present in the vehicle control group and discontinued, weak or absent expression was shown in the induced conditional knockout mice as shown by the arrowhead (d–g). Magnification: × 200. GAPDH is the internal reference control.
HE Staining Analysis
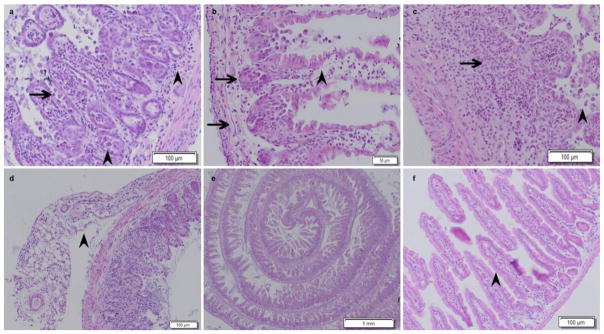
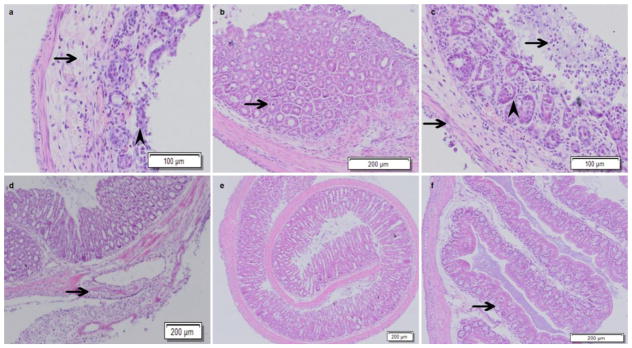
The results of HE staining demonstrated that Cldn7 ICKO mice intestines had severe defects that included epithelial cell sloughing, necrosis, inflammation and hyperplasia. From the HE staining, we could see that the intestinal villi were more sparse and shorter than the control group. Mucosal epithelial cells were jagged with loss of polarity and were disorderly. The intestinal villus necrosed and detached, and we could visualize small goblet cells. The enteraden structure almost disappeared and was replaced by many mesenchymal tissues and inflammatory cells. Necrotic cell fragments and inflammatory cells were found in the glandular cavity. Connective tissue hyperplasia was phanic and broke through the bowel muscle layer to form a neoplasm, which included adipocytes, blood vessels, inflammatory cells, etc. The blood vessels were full of lymphocytes. The muscular layer was thinner and the intercellular gap increased (as shown by the arrowhead in Figure 5 and 6). After adjusting the dose of Tamoxifen, we could induce atypical hyperplasia and adenoma in the intestine of the ICKO mice (as shown by the arrowhead in figure 7). In the vehicle control group, the morphology of the intestinal segment was normal, the epithelium structure was intact and no obvious pathological changes were observed.
Fig. 5.
Cldn7 deletion in the small intestine induced by tamoxifen disrupted the intestinal epithelial structure and led to hyperplasia. The arrowheads indicate that the intestinal mucous membrane epithelial cells were detached, necrotic, arranged loosely, and had lost polarity. Furthermore, the intestinal mucous membrane was indented, submucosae were infiltrated with inflammatory cells, and deciduous glandular epithelial cells and inflammatory cells were observed in the glandular lumens (a). The arrows point to locations where the intestinal mucous membranes were jagged (b). There were glandular epithelial cells, necrotic cell debris and inflammatory cells (macrophage, neutrophil etc.) in the glandular lumens. The intercellular gap increased. Smooth muscle fibers atrophied. The arrows point to locations where the intestinal villus necrosed and detached (c). There were evident pathological manifestations along the intestinal mucosa membrane, which included connective tissue hyperplasia, the disappearance of intestinal gland structure and the infiltration of inflammatory cells. The arrowhead indicates that submucosal connective tissue was hyperplasic, broke through the bowel muscle layer and formed hyperplasia (d). In the photograph, the normal intestinal structure of Cldn7 inducible conditional knockout mice was disordered (e). The intestinal epithelium structure of the solvent control group was intact and had no obvious pathological changes (f).
Fig. 6.
Deletion of Cldn7 in the large intestine induced by tamoxifen disrupted intestinal epithelial structure and induced hyperplasia. The arrow shows where the intestinal mucous membrane epithelial cells detached and necrosed (a). The glandular cavity was full of glandular epithelium cells, which were necrotic, and the glandular cavity almost disappeared. The mucosal epithelium cells and glandular epithelium cells were disorderly in arrangement and the goblet cells were smaller compared to the control group. The arrowhead indicates where local enteraden hyperplasia occurred (b). The arrows show where the mucous membrane epithelium cells were necrotic and detached and where the enteric cavity was mixed with a mucoid substance. The muscular layer was thinner (c). The arrow shows where connective tissue hyperplasia was phanic and broke through the bowel muscle layer to form hyperplasia, which included adipocytes, blood vessels, inflammatory cells, etc. (d). In the photograph, the normal intestinal structure of Cldn7 inducible conditional knockout mice was disordered (e). The intestinal epithelium structure of the solvent control group was intact and had no pathological changes (f).
Transmission Electron Microscopy Analysis
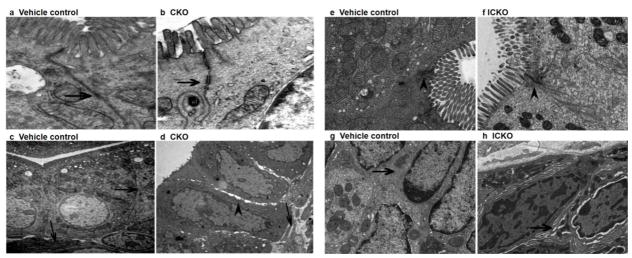
Electron microscopy experimental analysis indicated that the intercellular gap along the basolateral membrane in the intestine of Cldn7 ICKO mice was obvious compared to that of the control group mice. The contact between cells and matrix, which was close in the intestines of control group mice, was loosened in the Cldn7 ICKO mice. These results are consistent with our previous conclusions which were from the conventional knockout (CKO) mice model (as shown in Figure 8).
Fig. 8.
Electron micrographs of the small intestine in Cldn7 CKO mice, ICKO mice and the vehicle control group. The electron micrographs results demonstrated that the intercellular gap along the basolateral membrane of Cldn7 ICKO mice in the intestine was pronounced compared to the control group mice, and the contact between cells and matrix, which was close in the intestines of control group mice, was loosened in the Cldn7 inducible conditional knockout mice (e–h). The result of CKO mice is consist with the ICKO mice (a–d).
Immunofluorescence Analysis
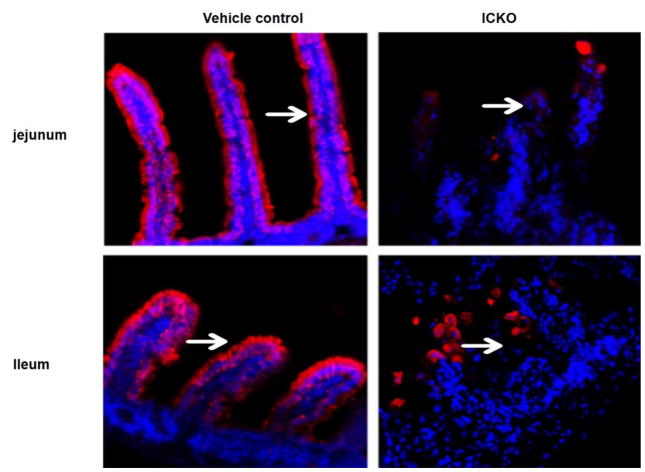
Immunofluorescent staining showed obvious red fluorescence signals arranged along the small intestine villi in the vehicle control group, while the ICKO mice showed weak and disordered signals. This finding indicates that the structure and function of the intestinal epithelial cells in Cldn7 ICKO mice were disrupted (as shown in Figure 9).
Fig. 9.
Immunofluorescent staining of the small intestine villi. The result indicates that obvious red fluorescence signals were arranged along the small intestine villi in the vehicle control group, while the ICKO mice showed weak and absent signals in jejunum and ileum, which means the structure and function of intestinal epithelial cells in Cldn7 ICKO mice was disrupted as shown by the arrowhead.
Discussion
Gene knockout technology is a significant breakthrough and an important tool in gene function research. The basic principle of this technology is to utilize specific methods and means to construct the corresponding targeting vector in vitro and knockout a gene of known sequence but unknown function through homologous recombination. Then, when the function of this specific gene is lost, we can infer the biological function of it [10]. Claudins are a family of cell adhesion molecules including 27 members found in mammals [3]. Cldn7 is a claudin family protein that is strongly distributed along the basolateral membrane. This is in contrast to other claudins, which are localized at apical TJs [11, 12, 13]. In the normal colon, Cldn7 is highly expressed, and Cldn7 knockout in mice results in disordered homeostasis. Cldn7 expression induces mesenchymal to epithelial transformation to inhibit colon tumorigenesis [14]. On the other hand, Cldn7 can promote the epithelial mesenchymal transition in human colorectal cancer [15]. This study indicates that Cldn7 plays a tumor-suppressor role in the colon. We generated a systemic gene knockout of Cldn7 in mice [4] and found that the Cldn7-deficient mice had severe intestinal defects that included mucosal ulcerations, epithelial cell sloughing and inflammation. The Cldn7-deficient mice survived for only seven days, making it difficult to further investigate the function of Cldn7 in the intestine. Additionally, the systemic gene-knockout technology influenced the Cldn7 gene in all cells, making it difficult for us to determine if the action of Cldn7 in the Cldn7-deficient mice was direct or indirect. In conventional systemic gene-knockout technology, the conditional knockout of certain specific gene is more precise in studying the function of a specific gene. The advantage of conditional gene-knockout technology is that it can precisely control the gene-knockout in space and time. Based on this, we generated Cldn7 intestinal conditional knockout mice using the Cre/LoxP system with the deletion of the Cldn7 gene upon induction with tamoxifen. Cre/LoxP technology is a general method of conditional gene-knockout [16, 17]. After inserting the Cre-recombinase gene into the promoter sequence, we cultivated and acquired the mice with Cre-recombinase expression by transgenic technology so that the target gene could be specifically knocked out when the mice were hybridized with transgenic mice whose genomic sequence ends were anchored to a Loxp sequence. Two transgenic mice are required to construct this model of intestinal conditional gene-knockout mice. One is a Cldn7-flox mouse whose targeting gene sequence was inserted into a Loxp sequence, with the Cldn7 gene-knockout mice acquired by deleting the sequence between the two Loxp sites. The other one is a villin-CreERT2 transgenic mouse that expresses Cre-recombinase. This Cre-recombinase is expressed in the intestine specifically. When Cldn7-flox mice are hybridized with villin-CreERT2 transgenic mice, the sequence located in the Cldn7 coding region between the two Loxp sites is deleted, and this phenotype can be inherited in progeny cells. The expression of Cldn7 would have no change in tissues outside of the intestine.
Based on the principle mentioned above, we generated intestinal Cldn7 ICKO mice. We induced Cldn7 deletion by injecting tamoxifen. In our study, we made Cldn7-flox mice hybridized with villin-CreERT2 mice [6, 18] to obtain mice with the genotype Cldn7fl/fl; Villin-CreERT2. The Cre-recombinase in villin-CreERT2 transgenic mice fused with the mutational ligand binding domain of the human estrogen receptor to produce a Cre-recombinase which relies on tamoxifen. The recombinant enzyme is activated by tamoxifen instead of estradiol. The transgenic mice expressed Cre-recombinase, which is controlled by the cytomegalovirus promoter. Tamoxifen induced Cldn7 gene deletion between two Loxp sites in mice whose genotype was Cldn7fl/fl; Villin-CreERT2. The phenotype of the mice with the Cldn7fl/fl; Villin-CreERT2 genotype was normal prior to the tamoxifen injection.
In our study, we generated a mouse model in which the Cldn7 gene was controllable. We could delete the Cldn7 gene in the intestine by injecting tamoxifen. The study demonstrated that the mice induced by tamoxifen had a severe intestinal inflammatory reaction and hyperplasia. The structure of the intestine was disrupted, including the mucous membrane epithelium cells, which were necrotic and detached. The enteric cavity was mixed with a mucoid substance and the glandular cavity was full of necrotic glandular epithelium cells. In comparison to the experiments we performed previously [4], the intestinal Cldn7 ICKO mice showed hyperplasia. We could see that submucosal connective tissues were hyperplasic, broke through the bowel muscle layer and formed hyperplasia as seen in the HE staining photograph (Figure 5–7). Additionally, connective tissue hyperplasia was phanic and broke through the bowel muscle layer to form neoplasms, which included adipocytes, blood vessels, inflammatory cells, etc. Based on the results mentioned above, it is possible to induce adenoma hyperplasia by deleting the Cldn7 gene in the intestine of Cldn7 ICKO mice. We will attempt to explore the minimum tamoxifen dosage to induce Cldn7 so that the Cldn7 ICKO mice can live longer. This optimization will allow us to explore the relationship between the deletion of Cldn7 and intestinal inflammation, hyperplasia or tumors in mice. Further, we can induce tumorigenesis by a carcinogen in both Cldn7 ICKO mice and the control group to explore the function of the Cldn7 gene in tumors. In the conventional Cldn7 knockout mice we constructed in 2012 [4], we found that Cldn7 had non-TJ functions, including maintenance of epithelial cell-matrix interactions and intestinal homeostasis. Compared to the intestine-specific conditional Cldn7 knockout mice [5], the lesions in our inducible intestinal conditional knockout mouse model were mainly in the small intestine. For example, most of ICKO mice had obvious shortening and severe swelling in the small intestine. Other pathological changes include intestinal mucous membrane epithelial cell detachment and necrosis, filling of the glandular cavity with glandular epithelium cells, disorderly mucosal epithelium cells, and smaller goblet cells compared to the control group. However, the intestinal inflammation in Japanese mouse models mainly occurred in the colon [5]. Moreover, we had induced atypical hyperplasia and adenoma successfully in the small intestine and large intestine of ICKO mice after changing the dose of Tamoxifen. We induced the deletion of Cldn7 at a dose of 200 μl Tamoxifen in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) for 5 consecutive days in the beginning. But we could observed obvious weak and death in the mice. After a series of groping and adjustment, we have adjusted Tamoxifen to a dose of 100 μl in sunflower oil at 10 mg/ml per mouse (aged more than 8 weeks old) every four days. After injecting the drug for 10 to 20 times, we could found obvious adenoma in the intestine and the mice were alive as well. We believe this is a novel finding of our research. Furthermore, we will have a deeper mechanism-study of this new finding. This is beneficial for us to investigate the relationship between inflammation and tumor and the important effect of Cldn7 in the development of tumorigenesis.
Conclusions
We generated a model of intestinal Cldn7 inducible conditional knockout mice. Intestinal Cldn7 deletion induced by tamoxifen initiated inflammation and adenoma hyperplasia in mice. This finding demonstrated that Cldn7 is important in maintaining intestinal homeostasis and in the development of tumorigenesis.
Acknowledgments
We would like to thank the Nanjing Biomedical Research Institute of Nanjing University for technical support, including construction of the Cldn7-flox mice and breeding of Cldn7 ICKO mice. We are grateful to the French professor, Sylvie Robine, who provided Villin-CreERT2 mice for us. This work is supported by the National Natural Science Foundation of China (No. 81372585 and No. 81772557), Beijing Municipal Education Commission Science and Technology Program of the Project (No. KM201410025026) and Beijing Health System High Level Training Plan of Health Technical Personnel (2014-3-048). This work is also supported by National Institutes of Health grant DK103166 to Yan-Hua Chen.
Abbreviations
- ICKO
inducible conditional knockout
- HE
hematoxylin-eosin
- TJs
tight junctions
- SPF
specific pathogen free
- PCR
polymerase chain reaction
- CKO
conventional knockout
Footnotes
Author Contributions: Wen-Jing Li, Chang Xu and Kun Wang are the co-author of this paper; Wen-Jing Li is the lead author. Wen-Jing Li analyzed the data and drafted the manuscript; Wen-Jing Li, Chang Xu and Kun Wang performed the experiments; Lei Ding, Hong Gao designed this research and modified the manuscript, and Yan-Hua Chen provides guidance of the experimental design about the suitable dosage of Tamoxifen; Teng-Yan Li, Xiao-Nan Wang, Hui Yang and Tiaosi Xing contributed to breeding mice and providing analytical tools; Wen-Xia Li contributed related reagents. All authors have read and approved the final version.
Compliance with Ethical Standards
Conflict of Interest None.
Ethical Statement All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 2.Koller BH, Hagemann LJ, Doetschman T, et al. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci. 1989;86(22):8927–31. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsujiwaki M, Murata M, Takasawa A, et al. Aberrant expression of claudin-4 and -7 in hepatocytes in the cirrhotic human liver. Med Mol Morphol. 2015;48(1):33–43. doi: 10.1007/s00795-014-0074-z. [DOI] [PubMed] [Google Scholar]
- 4.Ding L, Lu Z, Foreman O, et al. Inflammation and disruption of the mucosal architecture in Cldn7–deficient mice. Gastroenterology. 2012;142(2):305–15. doi: 10.1053/j.gastro.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka H, Takechi M, Kiyonari H, Shioi G, Tamura A, Tsukita S. Intestinal deletion of Cldn7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut. 2015;64(10):1529–38. doi: 10.1136/gutjnl-2014-308419. [DOI] [PubMed] [Google Scholar]
- 6.EIMarjou Fatima, Janssen Klaus-Peter, Chang Benny Hung-Junn, Li Mei, Hindie Valérie, Chan Lawrence, Louvard Daniel, Chambon Pierre, Metzger Daniel, Robine Sylvie. Tissue-Specific and Inducible Cre-Mediated Recombination in the Gut Epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13(3):476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan W, Costantino N, Li R, et al. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res. 2007;35(8):e64. doi: 10.1093/nar/gkm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker Nick, van Es Johan H, Kuipers Jeroen, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;(449):1003–1008. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Liu Guoan, Yang Hong. The research progress of gene targeting technology. Biotechnology Bulletin. 2010;(9):51–55. [Google Scholar]
- 11.Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL. The tight junction proteins Cldn7 and -8 display a different subcellular localization at Henle’s loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant. 2006;21(9):2391–8. doi: 10.1093/ndt/gfl255. [DOI] [PubMed] [Google Scholar]
- 12.Alexandre MD, Lu Q, Chen YH. Overexpression of Cldn7 decreases the paracellular cl-conductance and increases the paracellular Na+ conductance in llc-pk1 cells. J Cell Sci. 2005;118:2683–93. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 13.Mendoza-Rodríguez CA, González-Mariscal L, Cerbón M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res. 2005;319(2):315–30. doi: 10.1007/s00441-004-1010-7. [DOI] [PubMed] [Google Scholar]
- 14.Bhat AA, Pope JL, Smith JJ, et al. Cldn7 expression induces mesenchymal to epithelial transformation (met) to inhibit colon tumorigenesis. Oncogene. 2015;34(35):4570–80. doi: 10.1038/onc.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip R, Heiler S, Mu W, Büchler MW, Zöller M, Thuma F. Cldn7 promotes the epithelial-mesenchymal transition in human colorectal cancer. Oncotarget. 2015;6(4):2046–63. doi: 10.18632/oncotarget.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishihama R, Ishida S, Urawa H, Kamei Y, Kohchi T. Conditional gene expression/deletion systems for marchantia polymorpha using its own heat-shock promoter and cre/loxp-mediated site-specific recombination. Plant Cell Physiol. 2015;57(2):271–80. doi: 10.1093/pcp/pcv102. [DOI] [PubMed] [Google Scholar]
- 17.Okuyama T, Isoe Y, Hoki M, et al. Controlled cre/loxp site-specific recombination in the developing brain in medaka fish, oryzias latipes. PLoS One. 2013;8(6):e66597. doi: 10.1371/journal.pone.0066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinh KR, Morrison SL. Site-specific and directional gene replacement mediated by cre recombinase. J Immunol Methods. 2000;244(1–2):185–93. doi: 10.1016/s0022-1759(00)00250-7. [DOI] [PubMed] [Google Scholar]