Abstract
Pharmacotherapy to treat stimulant use disorders continues to be an unmet medical need. Some evidence supports both the role of opioids in mediating abuse-related amphetamine effects and the potential utility of opioid antagonists as therapeutic candidates for treating amphetamine abuse. This study used intracranial self-stimulation (ICSS) to evaluate effects of exposure to and termination of naltrexone maintenance on rewarding amphetamine effects in an ICSS procedure in rats. Morphine and cocaine were included as a positive and negative controls, respectively. Male Sprague-Dawley rats (N=40) were trained to lever press for electrical brain stimulation to the medial forebrain bundle via an implanted electrode. Rats were then implanted with osmotic pumps delivering naltrexone (0.001, 0.01, or 0.1 mg/kg/h, SC) or saline for 14 days. Cumulative dose-effect curves were determined for amphetamine (0.032–0.32 mg/kg), cocaine (1–10 mg/kg), and morphine (1–10 mg/kg) during the second week of naltrexone maintenance. Additionally, dose-effect curves for morphine and amphetamine were redetermined 24 hours after pump removal. Our results suggest that 1) exposure to and termination of naltrexone maintenance do not affect baseline ICSS responding; 2) naltrexone doses sufficient to antagonize morphine did not alter amphetamine or cocaine effects, and 3) termination of naltrexone treatment produced weak evidence for increased morphine sensitivity but no change in amphetamine effects. Our results do not support naltrexone as a pharmacotherapy for amphetamine and cocaine abuse, and also suggest that termination from chronic naltrexone does not increase sensitivity to abuse-related morphine or amphetamine effects in ICSS.
Keywords: amphetamine, naltrexone, cocaine, morphine, intracranial self-stimulation
Amphetamine and amphetamine-type stimulants continue to be abused world-wide at an increasing rate, and disorders due to their abuse rank second only to opioids (UNODC, 2017). Currently, there are only supportive therapies available for amphetamine abuse, but no approved medications. Amphetamine belongs to a class of compounds that interact with monoamine transporters to ultimately promote monoamine release (Fleckenstein, Volz, Riddle, Gibb, & Hanson, 2007; Sulzer et al., 1995). However, other evidence suggests that amphetamine may also produce some effects mediated by the release of endogenous opioids and subsequent activation of opioid receptors. For example, both acute and chronic amphetamine treatment increased preprodynorphin and preproenkephalin mRNA expression in rat striatal neurons (Wang & McGinty, 1995), and microdialysis studies in rats reported an increase in endorphin levels in nucleus accumbens after amphetamine administration (Olive, Koenig, Nannini, & Hodge, 2001). Behavioral studies suggest a subtle role for opioid signaling in amphetamine effects. For example, effects of the moderately mu-selective opioid antagonist naltrexone were evaluated on amphetamine-induced stimulation of rearing and horizontal locomotor activity and on sensitization to these effects after repeated amphetamine in rats (Balcells-Olivero & Vezina, 1997; Haggkvist et al., 2011). Naltrexone blocked both acute increases in rearing and development of rearing sensitization; conversely, it failed to block acute increases in locomotion or development of locomotor sensitization, although it did attenuate expression of locomotor sensitization.
Intracranial self-stimulation (ICSS) is a behavioral assay that has been used to investigate both abuse-related effects of amphetamine and the role of opioid signaling in mediation of those effects. ICSS is an operant behavioral procedure in which subjects are trained to lever press for pulses of brain stimulation delivered to a brain reward area via a chronically implanted microelectrode, and many drugs of abuse increase (or “facilitate”) low rates of ICSS responding maintained by low frequencies or intensities of brain stimulation (Negus & Miller, 2014). Amphetamine reliably facilitates ICSS (e.g. Bauer, Banks, Blough, & Negus, 2013a), and early studies found that another moderately selective mu antagonist, naloxone, dose-dependently blocked or attenuated amphetamine-induced ICSS facilitation (Esposito, Perry, & Kornetsky, 1980; Holtzman, 1976). Additionally, a more recent study found that an extended-release naltrexone formulation decreased amphetamine effects on ICSS, although the duration of these effects did not correlate with plasma naltrexone levels, and amphetamine effects were not blocked by acute or repeated daily naltrexone doses (Todtenkopf et al., 2009).
The goal of the present study was to build on these previous studies in two ways. First, naltrexone effects were directly compared on changes in ICSS produced by morphine, amphetamine, and cocaine. Naltrexone was delivered continuously via osmotic minipumps because previous evidence indicated that amphetamine effects on ICSS were more sensitive to an extended-release naltrexone formulation than to acute or repeated daily naltrexone doses (Todtenkopf et al., 2009). Morphine was included as a positive control to determine potency of naltrexone maintenance to block mu opioid receptors. Cocaine was included as a negative control given previous evidence that naltrexone does not alter cocaine-induced ICSS facilitation (Pabello et al., 1998). We hypothesized that naltrexone maintenance doses sufficient to block morphine effects would also block or attenuate effects of amphetamine but not cocaine. Second, chronic naltrexone treatment can produce upregulation of mu opioid receptors and increase sensitivity to effects of mu agonists like morphine (Bardo, Neisewander, & Ennis, 1988; Diaz et al., 2002; Lesscher et al., 2003; Zukin et al., 1982). Accordingly, effects of morphine and amphetamine were also evaluated on the day after removal of naltrexone minipumps. We hypothesized that termination of naltrexone treatment could augment ICSS effects of morphine and also of any mu receptor-mediated effects of amphetamine.
METHODS
Subjects
Forty adult male Sprague-Dawley rats (Envigo, Frederick, MD) that weighed at least 290g before surgery were used. Rats were singly housed with free access to food and water and maintained on a 12-hour light/dark cycle (lights on from 6am to 6pm) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Surgery
Surgical procedures were similar to those described previously (Bonano et al., 2015). Briefly, rats were anesthetized with isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ) for stereotaxic implantation of the cathode (0.25 mm diameter, insulated except at tip) of a stainless steel electrode (Plastics One, Roanoke, VA) into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull). Three screws were implanted into the skull, and the anode (0.125 mm diameter, uninsulated) was wrapped around one of the screws to act as a ground. Dental acrylic secured the electrode to the screws and skull. Ketoprofen (5 mg/kg) was administered as a postoperative analgesic immediately and 24 hours post-surgery. Animals were allowed to recover for at least 5 days before initiation of ICSS training.
Apparatus
Studies were conducted in operant conditioning chambers (29.2 cm × 30.5 cm × 24.1 cm) housed in sound-attenuating boxes and equipped with a response lever, three stimulus lights above the lever, a house light, and an ICSS stimulator (Med Associates, St Albans, VT). The intracranial electrode was connected to the stimulator via bipolar cables routed through a swivel-commutator (Model SL2C, Plastics One). Stimulus deliveries were controlled and lever-press responses were recorded with a computer and interface operated by MED-PC IV computer software (Med Associates).
Training
Each behavioral session commenced with illumination of the house light. Rats were trained to lever press under a fixed-ratio 1 (FR 1) schedule for brain stimulation delivered via the intracranial electrode. Each stimulation consisted of a 0.5-s train of square-wave cathodal pulses (0.1 ms per pulse) at a designated frequency and amplitude and was accompanied by illumination of the three stimulus lights over the lever. During initial 60-min training sessions, the stimulation frequency and amplitude were fixed at 126 Hz and 150 μA, respectively. Amplitude was adjusted in each rat to a level sufficient to maintain response rates >30 responses/min. Frequency manipulations were then introduced during 30-min behavioral sessions consisting of three 10-min components, and each component consisted of 10 1-min frequency trials. The frequency of brain stimulation decreased across trials in 0.05 log increments from 2.2 log Hz to 1.75 log Hz. Each trial began with an initial 10-s time out, during which the house light was off and responding had no scheduled consequences, and during the last 5-s of this time-out, 5 noncontingent stimulations at the designated frequency were delivered at 1-s intervals. For the remaining 50 s of each trial, the house light was illuminated, and responding produced both brain stimulation and illumination of the three stimulus lights under an FR1 schedule as described above. ICSS training was considered complete when frequency-rate curves were not statistically different over three consecutive days of training as indicated by lack of a significant effect of ‘day’ in a two-way analysis of variance (ANOVA) with day and frequency as the main effect variables (see Data Analysis below). All training was completed within six-seven weeks of surgery.
Testing
Rats were tested using a 15-day protocol shown in Figure 1. On Day 0, rats were anesthesized with isoflurane for implantation in the midscapular region of subcutaneous osmotic minipumps (Model 2ML2, 5 μl/h flow rate, Alzet, Cupertino, CA, USA) that delivered a continuous 14-day infusion of saline (N=14) or one of three naltrexone doses (0.001 mg/kg/h, N=6; 0.01 mg/kg/h, N=6; or 0.1 mg/kg/h; N=14). Cumulative-dosing test sessions were conducted on Days 7, 9, and 11 with morphine (1–10 mg/kg), amphetamine (0.032–0.32 mg/kg), or cocaine (1–10 mg/kg), and order of testing of these three drugs was counterbalanced across rats using a Latin-square design. Test sessions for each drug began with three baseline ICSS components followed by a series of drug injections and test components. For morphine, sequential doses were administered every 50 min, and pairs of test components began 30 min after each injection. For cocaine and amphetamine, doses were administered every 30 min, and pairs of test components began 10 min after each injection. Each dose increased the total cumulative dose by 0.5 log units. Pumps were removed on Day 14. On Day 15, cumulative dose-effect curves were redetermined for morphine in N=6 rats from all groups and for amphetamine in N=8 rats treated with saline or the highest naltrexone dose (0.1 mg/kg/h). Three-component baseline sessions were conducted on all other days except Days 12–13, when behavioral sessions were omitted. On Day 14, the baseline session was conducted prior to minipump removal. Rats were weighed daily during the 15-day treatment period. All doses and treatment times were based on prior studies and preliminary data (Altarifi & Negus, 2011; Bauer et al., 2013a; Todtenkopf et al., 2009).
Figure 1.

Diagram of experimental timeline.
Data analysis
The primary dependent variable was the reinforcement rate in stimulations per minute during each frequency trial. Data from the first baseline component of each session were discarded. The maximum rate observed during any frequency trial of the second and third baseline components were averaged to yield the Maximum Control Rate (MCR) for that rat on that day. MCR values before minipump implantation were compared across groups by one-way ANOVA, and the criterion of significance for this and all other statistical tests was p<0.05. Additionally, MCR values across days were evaluated by two-way ANOVA with treatment group and experimental day as the two factors, and a significant ANOVA was followed by the Holm-Sidak post hoc test. Because MCR values were similar between groups and stable across time, data from each trial on a given day were then normalized to the MCR for that day using the equation: %MCR = (reinforcement rate during a frequency trial/MCR) × 100. Subsequently, frequency-rate curves that related brain-stimulation frequency to %MCR were determined in each rat under three general conditions. First, a pre-pump baseline was determined in each rat by averaging data from the second and third baseline components for the three consecutive days before pump implantation. Second, daily baseline data were determined on each day after pump implantation by averaging data from the second and third baseline components on that day (except days 12–13, when ICSS sessions were omitted). Lastly, test data after drug administration were determined in each rat by averaging data from the two test components after administration of each drug dose. Data were then averaged across rats in each condition to yield mean frequency-rate curves. To compare effects of saline and naltrexone treatment on baseline ICSS, the pre-pump frequency-rate curves in each group were compared to the daily baselines for treatment days 7, 14 (last day of naltrexone treatment), and 15 (naltrexone termination) using two-way ANOVA, with brain-stimulation frequency and treatment day as the two factors. To evaluate drug effects on ICSS, the daily baseline frequency-rate curve for a given test day was compared to frequency-rate curves collected after administration of each drug dose on that day. These data were analyzed by two-way ANOVA, with brain-stimulation frequency and drug dose as the two factors. A significant ANOVA was followed by the Holm-Sidak post hoc test.
Finally, an additional summary measure of ICSS was used to compare test drug effects across saline/naltrexone treatment groups. For this analysis, the total number of stimulations per component was collapsed across all 10 frequency trials. As with analysis of MCR values, the total numbers of stimulations per component from baseline sessions before minipump implantation were compared across groups by one-way ANOVA, and data across days within each group were compared by two-way ANOVA. Because the total numbers of baseline stimulations per component were similar between groups and stable across time, data for each cumulative test-drug dose were normalized to baseline data on that day using the equation % baseline total stimulations per component = (mean total stimulations per test component/mean total stimulations per baseline component) × 100. Data were then averaged across rats and compared by two-way ANOVA, with cumulative drug dose as a within-subjects factor and naltrexone dose as a between-subjects factor. A significant ANOVA was followed by the Holm-Sidak post hoc test. All statistical analysis was conducted in Prism 7.0 (Graphpad Software, La Jolla, CA).
Drugs
Naltrexone HCl, morphine sulfate, (+)-amphetamine sulfate, and cocaine HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Drug doses are expressed in terms of the salt forms above. Naltrexone was administered SC via minipump. All other drugs were administered IP.
RESULTS
Baseline ICSS performance and effects of naltrexone treatment
Table 1 shows that there were no significant differences across treatment groups in either maximum control rates (MCR) per trial or baseline numbers of stimulations per component before the implantation of minipumps. Additionally, two-way ANOVA of these baseline parameters across groups and time indicated no main effect of treatment group or interaction between treatment group and time. There was a significant main effect of time for both MCR [F(13,468)=3.22, p=0.0001] and stimulations per component [F(13,468)=2.89, p=0.0005] reflecting minor variations in both parameters across days. For example, mean±SEM MCR values varied from a low of 62.2±1.4 on Day 10 to a high of 65.3±1.2 on Day 14, and stimulations per component varied from a low of 262.7±8.8 on Day 10 to a high of 292.2±9.6 on Day 14. However, post hoc analysis indicated that baseline data across days never differed significantly from the prepump values. Supplemental Figure 1 shows that, prior to implantation of minipumps, brain stimulation maintained a frequency-dependent increase in response rates in all treatment groups (open circles in all panels). Supplemental Figure 1 also shows that baseline ICSS frequency-rate curves were not affected by either exposure to or termination of naltrexone treatment.
TABLE 1. Prepump baseline parameters ± SEM of ICSS performance in the different treatment groups.
Naltrexone doses are shown in mg/kg/hr. Maximum Control Rate (MCR) shows the maximum number of stimulations per trial during any trial of each component. Total Stimulations per Component shows the total number of stimulations delivered across all trials within a component. One-way ANOVA indicated no difference across treatment groups for either MCR [F(3,36)=0.71, p=0.55] or Total Stimulations per Component [F(3,36)=0.20, p=0.90].
| Treatment | Maximum Control Rate (MCR) | Stimulations per Component |
|---|---|---|
| Saline | 64.3 ± 2.1 | 285.0 ± 15.8 |
| 0.001 Naltrexone | 58.6 ± 3.5 | 273.6 ± 24.8 |
| 0.01 Naltrexone | 63.9 ± 2.5 | 271.7 ± 22.9 |
| 0.01 Naltrexone | 62.8 ± 2.4 | 286.3 ± 12.2 |
Effects of morphine, amphetamine, and cocaine during saline or naltrexone treatment
Figure 2 shows effects of 1.0–10 mg/kg morphine (A–D), 0.032–0.32 mg/kg amphetamine (E–H), and 1.0–10 mg/kg cocaine (I–L) on full ICSS frequency-rate curves during maintenance on saline or increasing naltrexone doses, and statistical results below show only the dose × frequency interaction effects for brevity. Morphine produced primarily a dose-dependent decrease in ICSS during saline maintenance [F(27,351)=39.48, p<0.0001], and these morphine effects were dose-dependently attenuated by increasing naltrexone maintenance doses [0.001 mg/kg/h, F(27,135)=14.20, p<0.0001; 0.01 mg/kg/h, F(27,135)=2.20, p=0.0017; 0.1 mg/kg/h F(27,351)=1.634, p=0.03]. Conversely, amphetamine dose dependently facilitated ICSS during saline maintenance [F(27,351)=7.95, p<0.0001], and similar amphetamine effects were observed during naltrexone maintenance [0.001 mg/kg/h, F(27,135)=6.18, p<0.0001; 0.01 mg/kg/h, F(27,135)=4.53, p<0.0001; 0.1 mg/kg/h, F(27,351)=12.32, p<0.0001]. Cocaine also dose-dependently facilitated ICSS during saline maintenance [F(27,351)=12.38, p<0.0001], and similar cocaine effects were observed during naltrexone maintenance [0.001 mg/kg/h, F(27,135)=8.0, p<0.0001; 0.01 mg/kg/h, F(27,135)=6.64, p<0.0001; 0.1 mg/kg/h, F(27,135)=14.29, p<0.0001].
Figure 2.

Effects of morphine (A–D), amphetamine (E–H), or cocaine (I–L) on ICSS frequency-rate curves during maintenance on saline or naltrexone (0.001–0.1 mg/kg/hr). Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). All data are mean+/−SEM for N=14 (saline, 0.1 mg/kg/h NTX) or N=6 (0.001 and 0.01 mg/kg/h NTX). Filled points indicate signficantly different from “Test day Baseline” as indicated by a significant two-way ANOVA followed by the Holm-Sidak post hoc test, p<0.05.
Figure 3 shows summary data to permit comparison of test-drug effects across saline/naltrexone treatment groups. Morphine (Figure 3A) dose-dependently decreased this summary measure of ICSS in saline-treated rats, and naltrexone dose-dependently antagonized morphine effects [significant dose × treatment interaction, F(6,72)=23.03, p<0.0001]. Both amphetamine (Figure 3B) and cocaine (Figure 3C) produced dose-dependent facilitation of ICSS in saline-treated rats, but naltrexone did not significantly alter the ICSS facilitating effects of either amphetamine or cocaine [only main effects of amphetamine dose, F(2,72)=134.90, p<0.0001, and cocaine dose, F(2,72)=21.98, p<0.0001].
Figure 3.

Naltrexone blocked the effects of morphine but not of amphetamine or cocaine on the summary measure of ICSS performance. Abscissae: drug dose (mg/kg). Ordinates: percentage of baseline number of stimulations per component delivered across all brain stimulation frequencies (% Baseline total stimulations). All data are mean+/−SEM for N=14 (saline, 0.1 mg/kg/h NTX) or N=6 (0.001 and 0.01 mg/kg/h NTX). Filled points indicate signficantly different from “Saline” as indicated by a significant two-way ANOVA followed by the Holm-Sidak post hoc test, p<0.05.
Effects of morphine and amphetamine after termination of saline or naltrexone treatment
Figure 4 shows effects of 1.0–10 mg/kg morphine (A–B) and 0.032–0.32 mg/kg amphetamine (C–D) on full ICSS frequency-rate curves one day after removal of saline or high-dose (0.1 mg/kg/hr) naltrexone pumps, and statistical results below show only dose × frequency interaction effects for brevity. Morphine primarily decreased ICSS after removal of either saline pumps [F(27,135)=14.01, p<0.0001] or 0.1 mg/kg/hr naltrexone pumps [F(27,135)=21.96, p<0.0001]. Some rats treated with 3.2 mg/kg morphine after termination of 0.1 mg/kg/hr naltrexone treatment displayed extreme sedation usually observed only after administration of 10 mg/kg morphine in saline-treated rats. Amphetamine dose-dependently facilitated ICSS after removal of either saline pumps [F(27,189)=6.48, p<0.0001] or 0.1 mg/kg/hr naltrexone pumps [F(27,189)=10.71, p<0.0001].
Figure 4.

Effects of morphine (A–B) or amphetamine (C–D) on ICSS frequency-rate curves after removal of saline or 0.1 mg/kg/hr naltrexone minipumps. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). All data are mean+/−SEM for N=6 (morphine) or N=8 (amphetamine). Filled points indicate signficantly different from “Test day Baseline” as indicated by a significant two-way ANOVA followed by the Holm-Sidak post hoc test, p<0.05.
Figure 5 shows summary data to permit comparison of test-drug effects across saline/naltrexone treatment groups. After pump removal, morphine produced dose-dependent decreases in this summary measure of ICSS, and there was no difference in morphine effects across treatment groups [only a main effect of morphine dose, F(2,10)=119.9, p<0.0001]. Amphetamine dose-dependently increased ICSS, and there was no difference in amphetamine effects across treatment groups [only a main effect of amphetamine dose F(2,28)=47.48, p<0.0001].
Figure 5.
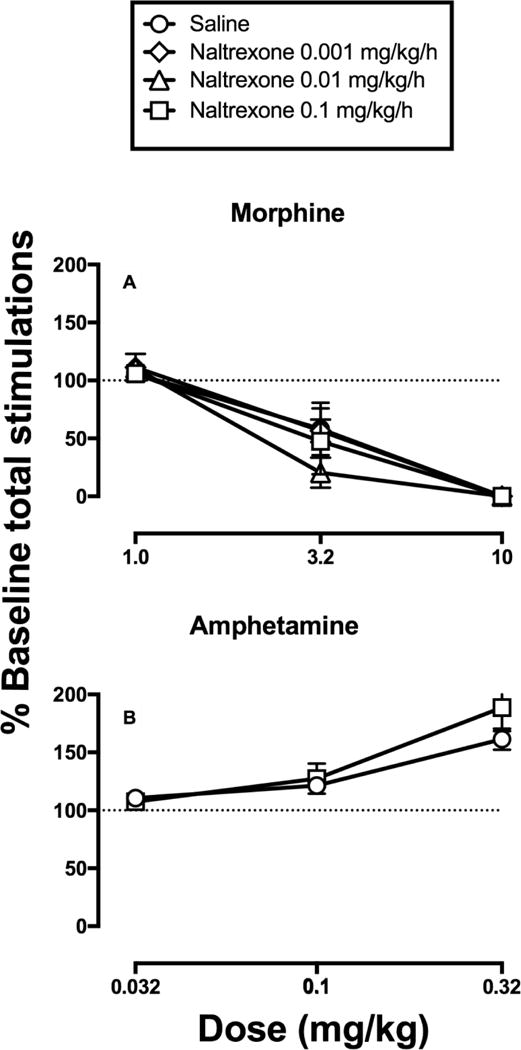
Termination of naltrexone treatment did not alter morphine or amphetamine effects on the summary measure of ICSS performance. Abscissae: drug dose (mg/kg). Ordinates: percentage of baseline number of stimulations per component delivered across all brain stimulation frequencies (% Baseline total stimulations). All data are mean+/−SEM for N=6 (morphine) or N=8 (amphetamine).
DISCUSSION
This study compared the effects of exposure to and termination of naltrexone maintenance on morphine, amphetamine, and cocaine effects in an ICSS procedure. There were three main findings. First, naltrexone maintenance did not alter baseline ICSS performance relative to saline maintenance. Second, naltrexone maintenance dose-dependently antagonized morphine effects on ICSS, and termination of naltrexone treatment produced weak evidence for increased morphine sensitivity. Lastly, neither exposure to nor termination of naltrexone maintenance significantly altered amphetamine-induced ICSS facilitation, and naltrexone maintenance also failed to alter cocaine-induced ICSS facilitation. Overall, these results do not support a role for opioid receptors in amphetamine-induced facilitation of ICSS in rats. Moreover, to the degree that ICSS facilitation is predictive of abuse liability, these results do not support use of naltrexone maintenance as an effective treatment for amphetamine abuse.
The failure of naltrexone maintenance to alter baseline ICSS performance in this study is consistent with previous findings that have shown no effect of acute or chronic mu opioid antagonist treatment on ICSS in non-dependent rats (Altarifi, Miller, & Negus, 2012; Schenk & Nawiesniak, 1985; Todtenkopf et al., 2009; Weibel & Wolf, 1979). These results suggest that endogenous opioids acting at mu opioid receptors do not play a significant role in maintenance of ICSS in the absence of opioid dependence. In contrast, naltrexone and other mu opioid antagonists can precipitate opioid withdrawal and produce withdrawal-associated decreases in ICSS in subjects dependent on mu agonists like morphine (Altarifi et al., 2012; Easterling, Plovnick, & Holtzman, 2000; Schulteis, Markou, Gold, Stinus, & Koob, 1994; Wiebelhaus, Walentiny, & Beardsley, 2016).
Morphine primarily decreased ICSS in saline-treated rats. This agrees with our previous findings that mu agonists primarily decrease ICSS in opioid-naive rats, and that repeated exposure to mu agonists is required to produce tolerance to mu agonist-induced rate-decreasing effects and increased expression of abuse-related rate-increasing effects (Altarifi & Negus, 2011; Miller, Altarifi, & Negus, 2015). Naltrexone maintenance dose-dependently antagonized morphine effects on ICSS, consistent with other evidence for mu opioid receptor mediation of these effects (e.g. (Altarifi et al., 2012).
Chronic naltrexone treatment can increase mu opioid receptor expression (Bardo et al., 1988; Diaz et al., 2002; Schenk & Nawiesniak, 1985; Zukin et al., 1982), and potency of mu agonists can be increased after termination of naltrexone treatment (Bardo et al., 1988; Young, Mattox, & Doty, 1991). However, naltrexone termination in the present study produced only weak evidence for increased morphine potency. For example, Figure 4 shows that 3.2 mg/kg morphine significantly decreased ICSS at 4 frequencies (2.05–2.2 log Hz) after saline treatment but at 6 frequencies (1.95–2.2 log Hz) after 0.1 mg/kg/hr naltrexone treatment. Moreover, some rats treated with 3.2 mg/kg morphine after termination of 0.1 mg/kg/hr naltrexone treatment displayed unusually high levels of sedation. Nonetheless, there was not a significant effect of naltrexone treatment on morphine dose-effect curves to decrease the total number of stimulations per component in Figure 5.
To the degree that a change in morphine effects was observed, there was an increase in morphine potency to produce rate-decreasing effects, not rate-increasing effects. This disagrees with an earlier report that heroin produced greater ICSS facilitation after 20 days of treatment with 10 mg/kg/day naltrexone (administered by daily SC bolus injection) than treatment with repeated saline (Schenk & Nawiesniak, 1985); however, that difference was due more to a decline in heroin-induced facilitation in saline-treated rats than to increased facilitation in naltrexone-treated rats. Moreover, the present study used lower daily naltrexone doses (0.001–0.1 mg/kg/hr = 0.024–2.4 mg/kg/day) validated as sufficient to produce dose-dependent mu-agonist antagonism. Additionally, the present results are consistent with a previous study that reported no change in morphine potency to produce discriminative stimulus or rate-decreasing effects in rats after termination of 3 mg/kg/day naltrexone (administered by minipump as in the present study), although termination of higher naltrexone maintenance doses of 10 and 18 mg/kg/day did increase morphine potency (Young et al., 1991). Overall, these results suggest that if receptor upregulation did occur during naltrexone maintenance, it did not occur in a pattern that would result in increased expression of abuse-related ICSS facilitation by morphine.
In agreement with many previous studies, amphetamine dose-dependently facilitated ICSS in saline-treated rats (Bauer et al., 2013a; Bauer, Banks, Blough, & Negus, 2013b). However, in opposition to our working hypothesis, naltrexone maintenance doses sufficient to produce nearly complete antagonism of morphine effects failed to alter amphetamine-induced ICSS facilitation. Previous studies have reported some evidence to suggest that opioid antagonists can attenuate amphetamine effects on ICSS (Esposito et al., 1980; Holtzman, 1976; Todtenkopf et al., 2009). However, even in these studies, opioid antagonist effects were often small and inconsistent. For example, acute antagonist treatments reduced but did not completely block amphetamine effects and produced an effect that was qualitatively different than that for competitive antagonism (Holtzman, 1976). Also, acute or repeated daily naltrexone treatments failed to alter amphetamine effects, and although treatment with the slow-release naltrexone formulation Vivitrol did decrease amphetamine effects, the duration of that decrease was shorter than the duration of sustained naltrexone plasma levels (Todtenkopf et al., 2009). In the same study, even though the plasma naltrexone levels were similar after acute naltrexone treatment and after two weeks of extended-release naltrexone, only the latter blocked amphetamine effects (Todtenkopf et al., 2009). Taken together with the present findings, these results suggest that naltrexone produces at best only weak and inconsistent decreases in amphetamine-induced ICSS facilitation.
These results do not support utility of naltrexone maintenance to treat amphetamine abuse, and this appears to be consistent with recent clinical trials that reported no significant effect of naltrexone maintenance on metrics of either amphetamine or methamphetamine abuse (Coffin et al., 2017; Runarsdottir et al., 2017). However, some other recent studies have published more encouraging effects of naltrexone. For example, naltrexone pretreatment dose-dependently decreased amphetamine self-administration by rhesus monkeys (Jimenez-Gomez, Winger, Dean, Deaver, & Woods, 2011), although significant decreases were produced only by high doses well above those sufficient to block mu receptors. Similarly, studies in healthy as well as amphetamine-dependent subjects have reported that naltrexone maintenance decreased subjective effects of amphetamine (Jayaram-Lindstrom, Konstenius, et al., 2008; Jayaram-Lindstrom, Wennberg, Hurd, & Franck, 2004), and decreased metrics of amphetamine use in a placebo-controlled clinical trial (Jayaram-Lindstrom, Hammarberg, Beck, & Franck, 2008). Lastly, naltrexone also attenuated the reinforcing effects of methamphetamine in a human laboratory study (Marks et al., 2016), although no effect was observed in another study with a similar experimental design (Stoops, Pike, Hays, Glaser, & Rush, 2015). Overall, these results suggest that naltrexone is less effective, less potent, and less reliable to block abuse-related effects of amphetamine than of mu agonists like morphine.
Consistent with many previous studies, cocaine dose-dependently facilitated ICSS in saline-treated rats (Bauer, Banks, & Negus, 2014; Bonano, Runyon, Hassler, Glennon, & Stevens Negus, 2014). Also, the present results agree with previous findings that naltrexone fails to alter cocaine-induced ICSS facilitation (Pabello et al., 1998). Opioid antagonist treatments also generally fail to alter self-administration of cocaine in research animals (Ettenberg, Pettit, Bloom, & Koob, 1982; Hutsell, Cheng, Rice, Negus, & Banks, 2016; Mello, Mendelson, Bree, & Lukas, 1990; Moerke, Banks, Cheng, Rice, & Negus, 2017; Pettinati et al., 2014; Rowlett, Wilcox, & Woolverton, 1998; Tanda et al., 2016), and Vivitrol failed to alter metrics for cocaine use in a recent clinical trial (Pettinati et al., 2014).
In conclusion, naltrexone doses that were tested in the current investigation were sufficient to dose-dependently antagonize morphine effects but failed to alter effects of amphetamine or cocaine. Also, termination of these naltrexone treatments was not sufficient to produce robust hypersensitivity to morphine effects or to alter amphetamine effects. Overall, our results do not support naltrexone as a pharmacotherapy for amphetamine or cocaine abuse. However, these results also suggest that neither exposure to nor termination of naltrexone maintenance exacerbate abuse-related amphetamine effects.
Supplementary Material
Public significance statement.
With growing global concern due to amphetamine abuse there is a need for development of therapeutics. The current investigation evaluates the mu-opioid receptor antagonist naltrexone as a therapeutic for amphetamine abuse.
Acknowledgments
The authors would like to thank Elizabeth Leggett for technical assistance.
This research is supported by R01DA033930
Footnotes
Disclosures
The authors have no conflict to declare.
References
- Altarifi AA, Miller LL, Negus SS. Role of micro-opioid receptor reserve and micro-agonist efficacy as determinants of the effects of micro-agonists on intracranial self-stimulation in rats. Behavioural Pharmacology. 2012;23(7):678–692. doi: 10.1097/FBP.0b013e328358593c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behavioural Pharmacology. 2011;22(7):663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells-Olivero M, Vezina P. Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology. 1997;131(3):230–238. doi: 10.1007/s002130050288. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Ennis RB. Chronic treatment with naltrexone enhances morphine-stimulated dopamine neurotransmission: neurochemical and behavioral evidence. Neuropharmacology. 1988;27(11):1103–1109. doi: 10.1016/0028-3908(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behavioural Pharmacology. 2013a;24(5–6):448–458. doi: 10.1097/FBP.0b013e328363d1a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British Jornal of Pharmacology. 2013b;168(4):850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology. 2014;231(12):2461–2470. doi: 10.1007/s00213-013-3405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Negus SS. Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. British Jornal of Pharmacology. 2015;172(10):2433–2444. doi: 10.1111/bph.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Runyon SP, Hassler C, Glennon RA, Stevens Negus S. Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats. European Journal of Pharmacology. 2014;743:98–105. doi: 10.1016/j.ejphar.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Hern J, Vittinghoff E, Santos D, Matheson T, Batki SL. Extended-release naltrexone for methamphetamine dependence among men who have sex with men: a randomized placebo-controlled trial. Addiction. 2017 doi: 10.1111/add.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Pazos A, Florez J, Ayesta FJ, Santana V, Hurle MA. Regulation of mu-opioid receptors, G-protein-coupled receptor kinases and beta-arrestin 2 in the rat brain after chronic opioid receptor antagonism. Neuroscience. 2002;112(2):345–353. doi: 10.1016/s0306-4522(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Easterling KW, Plovnick RM, Holtzman SG. Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology. 2000;148(3):263–271. doi: 10.1007/s002130050050. [DOI] [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology. 1980;69(2):187–191. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78(3):204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Haggkvist J, Bjorkholm C, Steensland P, Lindholm S, Franck J, Schilstrom B. Naltrexone attenuates amphetamine-induced locomotor sensitization in the rat. Addiction Biology. 2011;16(1):20–29. doi: 10.1111/j.1369-1600.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Comparison of the effects of morphine, pentazocine, cyclazocine and amphetamine on intracranial self-stimulation in the rat. Psychopharmacologia. 1976;46(3):223–227. doi: 10.1007/BF00421106. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addiction Biology. 2016;21(2):360–373. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. American Journal of Psychiatry. 2008;165(11):1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33(8):1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. Journal of Clinical Psychopharmacology. 2004;24(6):665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Winger G, Dean RL, Deaver DR, Woods JH. Naltrexone decreases D-amphetamine and ethanol self-administration in rhesus monkeys. Behavioural Pharmacology. 2011;22(1):87–90. doi: 10.1097/FBP.0b013e3283423d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Bailey A, Burbach JP, Van Ree JM, Kitchen I, Gerrits MA. Receptor-selective changes in mu-, delta-and kappa-opioid receptors after chronic naltrexone treatment in mice. European Journal of Neuroscience. 2003;17(5):1006–1012. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- Marks KR, Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Separate and Combined Effects of Naltrexone and Extended-Release Alprazolam on the Reinforcing, Subject-Rated, and Cardiovascular Effects of Methamphetamine. Journal of Clinical Psychopharmacology. 2016;36(3):213–221. doi: 10.1097/JCP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1990;254(3):926–939. [PubMed] [Google Scholar]
- Moerke MJ, Banks ML, Cheng K, Rice KC, Negus SS. Maintenance on naltrexone + amphetamine decreases cocaine-vs.-food choice in male rhesus monkeys. Drug and Alcohol Dependence. 2017 doi: 10.1016/j.drugalcdep.2017.09.020. doi: https://doi.org/10.1016/j.drugalcdep.2017.09.020. [DOI] [PMC free article] [PubMed]
- National Research Council. Guide for the care and use of laboratory animals. 8th. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological Reviews. 2014;66(3):869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. Journal of Neuroscience. 2001;21(23):RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabello NG, Hubbell CL, Cavallaro CA, Barringer TM, Mendez JJ, Reid LD. Responding for rewarding brain stimulation: cocaine and isradipine plus naltrexone. Pharmacology Biochemistry and Behavior. 1998;61(2):181–192. doi: 10.1016/s0091-3057(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Dundon WD, Mahoney EM, Wierzbicki MR, O’Brien CP. A pilot trial of injectable, extended-release naltrexone for the treatment of co-occurring cocaine and alcohol dependence. American Journal on Addictions. 2014;23(6):591–597. doi: 10.1111/j.1521-0391.2014.12146.x. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. Journal of Pharmacology and Experimental Therapeutics. 1998;286(1):61–69. [PubMed] [Google Scholar]
- Runarsdottir V, Hansdottir I, Tyrfingsson T, Einarsson M, Dugosh K, Royer-Malvestuto C, Woody GE. Extended-Release Injectable Naltrexone (XR-NTX) With Intensive Psychosocial Therapy for Amphetamine-Dependent Persons Seeking Treatment: A Placebo-Controlled Trial. Journal of Addiction Medicine. 2017;11(3):197–204. doi: 10.1097/ADM.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Nawiesniak E. Chronic naltrexone treatment increases the heroin-produced facilitation of self-stimulation. Pharmacology Biochemistry and Behavior. 1985;22(2):175–177. doi: 10.1016/0091-3057(85)90373-9. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. Journal of Pharmacology and Experimental Therapeutics. 1994;271(3):1391–1398. [PubMed] [Google Scholar]
- Stoops WW, Pike E, Hays LR, Glaser PE, Rush CR. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacology Biochemistry and Behavior. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. Journal of Neuroscience. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL. Lack of Specific Involvement of (+)-Naloxone and (+)-Naltrexone on the Reinforcing and Neurochemical Effects of Cocaine and Opioids. Neuropsychopharmacology. 2016;41(11):2772–2781. doi: 10.1038/npp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, O’Neill KS, Kriksciukaite K, Turncliff RZ, Dean RL, Ostrovsky-Day I, Deaver DR. Route of administration affects the ability of naltrexone to reduce amphetamine-potentiated brain stimulation reward in rats. Addiction Biology. 2009;14(4):408–418. doi: 10.1111/j.1369-1600.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- UNODC. World drug report of the United Nations of Drugs and Crime. 2017 Retrieved from https://www.unodc.org/wdr2017/field/Booklet_4_ATSNPS.pdf.
- Wang JQ, McGinty JF. Alterations in striatal zif/268, preprodynorphin and preproenkephalin mRNA expression induced by repeated amphetamine administration in rats. Brain Research. 1995;673(2):262–274. doi: 10.1016/0006-8993(94)01422-e. [DOI] [PubMed] [Google Scholar]
- Weibel SL, Wolf HH. Opiate modification of intracranial self-stimulation in the rat. Pharmacology Biochemistry and Behavior. 1979;10(1):71–78. doi: 10.1016/0091-3057(79)90171-0. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Walentiny DM, Beardsley PM. Effects of Acute and Repeated Administration of Oxycodone and Naloxone-Precipitated Withdrawal on Intracranial Self-Stimulation in Rats. Journal of Pharmacology and Experimental Therapeutics. 2016;356(1):43–52. doi: 10.1124/jpet.115.228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Mattox SR, Doty MD. Increased sensitivity to rate-altering and discriminative stimulus effects of morphine following continuous exposure to naltrexone. Psychopharmacology. 1991;103(1):67–73. doi: 10.1007/BF02244076. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Sugarman JR, Fitz-Syage ML, Gardner EL, Zukin SR, Gintzler AR. Naltrexone-induced opiate receptor supersensitivity. Brain Research. 1982;245(2):285–292. doi: 10.1016/0006-8993(82)90811-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


